Abstract
There is an emerging hypothesis that exposure to cadmium (Cd), mercury (Hg), lead (Pb), and selenium (Se) in utero and early childhood could have long-term health consequences. However, there are sparse data on early life exposures to these elements in US populations, particularly in urban minority samples. This study measured levels of Cd, Hg, Pb, and Se in 50 paired maternal, umbilical cord, and postnatal blood samples from the Boston Birth Cohort (BBC). Maternal exposure to Cd, Hg, Pb, and Se was 100% detectable in red blood cells (RBCs), and there was a high degree of maternal–fetal transfer of Hg, Pb, and Se. In particular, we found that Hg levels in cord RBCs were 1.5 times higher than those found in the mothers. This study also investigated changes in concentrations of Cd, Hg, Pb, and Se during the first few years of life. We found decreased levels of Hg and Se but elevated Pb levels in early childhood. Finally, this study investigated the association between metal burden and preterm birth and low birthweight. We found significantly higher levels of Hg in maternal and cord plasma and RBCs in preterm or low birthweight births, compared with term or normal birthweight births. In conclusion, this study showed that maternal exposure to these elements was widespread in the BBC, and maternal–fetal transfer was a major source of early life exposure to Hg, Pb, and Se. Our results also suggest that RBCs are better than plasma at reflecting the trans-placental transfer of Hg, Pb, and Se from the mother to the fetus. Our study findings remain to be confirmed in larger studies, and the implications for early screening and interventions of preconception and pregnant mothers and newborns warrant further investigation.
Keywords: cadmium (Cd), mercury (Hg), lead (Pb) and selenium (Se), maternal–fetal transfer, early life exposure
INTRODUCTION
Chemical exposures during sensitive windows of development, mainly in utero and in the first few years of life, could have a role in chronic disease development.1Cadmium (Cd), mercury (Hg), and lead (Pb) have garnered great attention because of their widespread exposure worldwide, trans-placental passage, evidence of fetotoxicity, multi-organ adverse effects, and ability to interact with the genome and the epigenome.2-11 Numerous studies have provided evidence that prenatal or postnatal exposure to Hg and Pb is associated with neurological dysfunctions in children.12,13 In addition to neurotoxicity, findings from experimental and epidemiological studies suggest that metals such as Cd, Hg, and Pb have a role in cardiometabolic disease.14-16 Increasing evidence also supports that moderate-to-high levels of Se, an essential metalloid with antioxidant properties, could increase cardiometabolic risk.17
The objectives of this study were to evaluate maternal exposure to Cd, Hg, Pb, and Se assessed by concentrations of these elements in plasma and red blood cells (RBCs), and examine the degree of mother-to-fetus trans-placental passage of these elements as measured by the concentration of these elements in umbilical cord plasma and RBCs. Furthermore, we investigated the longitudinal patterns of Se, Cd, Hg, and Pb in maternal, child cord blood, and postnatal RBCs. We studied 50 mother–infant pairs enrolled in the Boston Birth Cohort (BBC), one of the largest longitudinal, predominantly urban African–American birth cohorts in the US.18
Our study aimed to fill in several research gaps. First, there are sparse data on early life metal exposures and maternal–fetal transfer in high-risk US urban African–American populations.19,20 Second, few studies have simultaneously measured concentrations of Cd, Hg, Pb, and Se in plasma and RBCs of mother–newborn pairs at birth. As such, little is known about the relative levels of Cd, Hg, Pb, and Se in maternal and fetal RBCs versus plasma samples, and the utility of RBCs versus plasma to reflect the trans-placental transfer of these elements. Third, few studies have assessed longitudinal changes in metal biomarkers in the first few years of life, especially in urban African–American populations. Dietrich21 and Ernhart22 examined a single Pb exposure of children in predominately African–American populations from Cincinnati and Cleveland, OH, respectively, and found continuously elevated blood Pb levels up to 24 months after birth. Bellinger et al.23 examined prenatal and postnatal Pb exposure of children from birth to 2 years of age in a predominantly white population from Boston, MA, USA, and found a similar trend of continuously elevated blood Pb levels at 6, 12, 18, and 24 months after birth. Sakamoto et al.24further examined changes in Se, Cd, Hg, and Pb concentrations during the first 3 months of life among breastfed Japanese infants. They found dramatically declined Hg and Se levels in infant RBCs as compared with cord RBCs, but constant Pb and Cd levels from birth until the end of the 3-month study period. To date, few studies have examined the dynamic change of multiple metal exposures assessed at birth and in early childhood.
MATERIALS AND METHODS
Study Population and Procedure
We studied 50 African–American mother–infant pairs randomly selected from the BBC cohort. Detailed information on sample enrollment, data collection, and follow-up has been given previously.25 Briefly, the mother–infant pairs were enrolled and maternal venous blood samples were obtained 24–72 h after delivery. Mothers were interviewed by trained research staff using a standardized questionnaire. Umbilical venous blood and maternal blood were collected at birth. Clinical information was obtained by review of maternal and infant medical records. The postnatal follow-up visits were aligned with the pediatric primary care schedule. Venous blood samples were collected at follow-up visits, with an age range of 6–39 months. Written informed consent was obtained from the mothers. The study protocol and informed consent were approved by the Institutional Review Boards of Boston University Medical Center, Ann & Robert H. Lurie Children’s Hospital of Chicago, and Johns Hopkins Bloomberg School of Public Health.
Measurement of Cd, Hg, Pb, and Se
We measured levels of Cd, Hg, Pb, and Se in 50 pairs of maternal and cord blood samples (plasma and RBCs). We also measured 39 child postnatal plasma and RBC samples from the 50 mother–infant pairs. The mean age of the children at the time of taking their postnatal samples was 12.5 months (ranging from 6 to 39 months). In addition, we measured levels of Cd, Hg, Pb, and Se in 17 pairs of cord blood samples, comparing whole blood with RBC samples to evaluate their correlations.
Plasma and RBCs were separated by centrifugation and kept frozen at− 80 °C for future metal analyses. The vials used for sample collection and storage were certified to be free of Cd, Hg, Pb, and Se elements. Aliquots of the samples (0.5 ml) were transported on dry ice to the Department of Health and Mental Hygiene (DHMH) State Laboratory in Baltimore, MD, USA, for metal analyses. The DHMH laboratory maintains CLIA and EPA certifications for the analysis of trace metals in blood, urine, and drinking water. All laboratory analyses were blinded to the characteristics of the study participants. Total Cd, Hg (including inorganic and organic Hg), Se, and Pb levels in RBCs, plasma, and whole blood samples were determined by inductively coupled plasma mass spectrometry (ICP-MS), using the Centers for Disease Control and Prevention (CDC) laboratory method CTL-TMS-3.0126 for testing Cd, Hg, and Pb in blood, and adapted for testing other trace metals such as Se in blood. All metals were determined in the same run. Laboratory reagent blanks, laboratory-fortified blanks, laboratory-fortified matrix samples, sample duplicates, reporting level check standards, continuous calibration verification, and external reference materials (Custom Assurance Standard for Pb, Hg, and Cd; Claritas PPT Grade Selenium Standard for Se; both from SPEC Certiprep) were analyzed in every run and met the acceptable criteria. One in every 10 samples was duplicated and spiked. Limits of detection (LOD) for Cd, Hg, Pb, and Se were 0.04 ppb, 0.02 ppb, 0.02 μg/dl, and 4.95 ppb, respectively. The number of samples with Cd, Hg, Pb, and Se levels below the LOD was 22, 0, 5, and 0 for maternal plasma (50 samples in total), 0, 0, 0, and 0 for maternal RBCs (50 samples in total), 31, 1, 3, and 1 for cord blood plasma (50 samples in total), 22, 0, 0, and 0 for cord blood RBCs (50 samples in total), and 12, 0, 0, and 0 for child RBCs (39 samples in total). For samples below the LOD, the metal concentrations were replaced by the LOD divided by the square root of two. The intra-assay coefficients of variation for Cd, Hg, Se, and Pb were 2.1%, 2.4%, 5.0%, and 1.7%, respectively.
Assessment of Birth Outcome
As detailed in our published report,27 gestational age was assessed on the basis of both the first day of the last menstrual period and the early prenatal ultrasonographic results from medical records. Preterm birth is defined as gestational age <37 weeks, whereas term birth is defined as ≥37 weeks of gestation. Birthweight was abstracted from medical records and categorized into two groups: normal birthweight: birthweight ≥2500 g; and low birthweight: birthweight <2500 g.
Statistical Methods
The levels and distributions of Cd, Hg, Pb, and Se in maternal, cord, and postnatal blood were described by geometric mean (95% confidence intervals (CI)), median, interquartile range, and min and max values. Values for Cd, Hg, Pb, and Se were logarithmically transformed for statistical analyses. The comparison was tested using the t-test. Graphic plots, correlation coefficients (r), and linear regression models were used to assess the association between metal levels in maternal and cord blood plasma or RBCs. All statistical analyses were performed using SAS v.9.3 (SAS Institute, Cary, NC, USA).
RESULTS
The characteristics of the 50 mother–infant pairs randomly drawn from the BBC are shown in Table 1. In general, Hg, Se, and Pb levels were much higher than the LOD in both RBCs and plasma of mothers and infants. In contrast, for a large portion of the RBC and plasma samples, the Cd level was close to or below the LOD. As a result, Hg, Se, and Pb were detectable in all RBC and most plasma samples, whereas Cd was undetectable in many RBC and plasma samples. The detailed distributions of Cd, Hg, Pb, and Se in maternal and cord RBCs and plasma can be found in Supplementary Table 1. Spearman’s correlations of Cd, Hg, Pb, and Se in maternal and cord RBC samples are shown in Table 2. There were no strong correlations among any of the elements in either maternal or cord RBCs.
Table 1.
Demographic and clinical characteristics of 50 mother–infant pairs from the Boston Birth Cohort.
| Mean±SD or % | |
|---|---|
| Maternal demographic characteristics | |
| Age (years) | 27.6±6.4 |
| Education (%) | |
| Primary or secondary | 18 |
| High school | 54 |
| Some college | 20 |
| College degree or greater | 8 |
| Ethnicity (%) | |
| African American | 100 |
| Parity (%) | |
| 0 | 38 |
| 1 + | 62 |
| BMI (kg/m2) | 26.5±5.4 |
| Birth outcomes | |
| Gestational age (weeks) | 38.7±2.4 |
| % PTB | 22 |
| Birthweight (g) | 3054±738 |
| % LBW | 18 |
| % Infant gender | |
| Male | 56 |
| Female | 44 |
| % C/S | 30 |
| Postnatal characteristics | |
| Age at sample collection (months) | 12.6±6.1 |
| Breast feeding (%) | |
| No | 22 |
| Yes | 78 |
| BMI (kg/m2) | 17.7±2.1 |
Abbreviations: C/S, cesarean section; LBW, low birthweight; PTB, preterm birth.
Table 2.
Spearman’s correlations of Cd, Hg, Pb, and Se in maternal and cord blood RBCs.
| Cd | Hg | Pb | Se | |
|---|---|---|---|---|
| Maternal RBCs (N=50) | ||||
| Cd | 1.000 | 0.123 (0.396) | 0.381 (0.0063) | 0.293 (0.0391) |
| Hg | 0.123 (0.396) | 1.000 | 0.295 (0.0378) | 0.303 (0.0326) |
| Pb | 0.381 (0.0063) | 0.295 (0.0378) | 1.000 | 0.292 (0.0394) |
| Se | 0.293 (0.0391) | 0.303 (0.0326) | 0.292 (0.0394) | 1.000 |
| Cord RBCs (N=50) | ||||
| Cd | 1.000 | 0.125 (0.389) | 0.246 (0.0849) | 0.0839 (0.562) |
| Hg | 0.125 (0.389) | 1.000 | 0.450 (0.001) | 0.252 (0.0779) |
| Pb | 0.246 (0.0849) | 0.450 (0.001) | 1.000 | 0.168 (0.243) |
| Se | 0.0839 (0.562) | 0.252 (0.0779) | 0.168 (0.243) | 1.000 |
The values in parentheses represent P value.
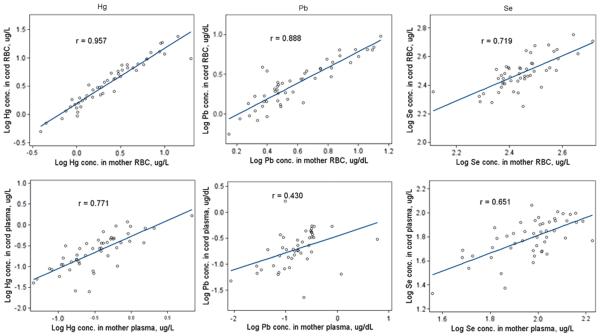
Maternal–fetal transfer was assessed by both maternal/cord blood metal exposure correlations and cord/maternal blood ratios. Cd, Hg, Pb, and Se concentrations were higher in RBCs than in plasma for both maternal and cord blood samples (Table 3). Hg, Pb, and Se concentrations in maternal and cord RBCs were strongly correlated, with Hg having the highest correlation (r = 0.96) and Se the lowest (r = 0.72) (Figure 1). The corresponding correlations in plasma were weaker, ranging from 0.43 for Pb to 0.77 for Hg. Correlations between maternal and cord concentrations of Cd were 0.24 in RBCs and 0.45 in plasma. As presented in Table 3, cord/maternal ratios for Cd, Hg, Pb, and Se in RBCs were 0.07, 1.53, 0.61, and 1.09, respectively. For each of these four elements, cord/maternal ratios in plasma varied from those found in RBCs. For example, the cord/maternal Hg ratio was 1.53 in RBCs but was much lower in plasma (0.67).
Table 3.
Cd, Hg, Pb, and Se concentrations in 50 pairs of maternal and cord RBCs and plasma.
| Geometric mean (95% CI) |
||||
|---|---|---|---|---|
| Cd (μg/l) | Hg (μg/l) | Pb (μg/dl) | Se (μg/l) | |
| Maternal RBCs | 0.86 (0.70–1.07) | 2.35 (1.82–3.03) | 3.93 (3.33–4.64) | 278.12 (259.64–297.90) |
| Cord RBCs | 0.06 (0.05–0.09) | 3.58 (2.76–4.65) | 2.41 (2.00–2.90) | 303.99 (282.04–327.64) |
| Cord/maternal RBC ratio | 0.07 (0.05–0.10) | 1.53 (1.41–1.64) | 0.61 (0.56–0.67) | 1.09 (1.04–1.15) |
| Maternal plasma | 0.06 (0.04–0.09) | 0.32 (0.24–0.42) | 0.13 (0.09–0.18) | 92.41 (84.31–101.30) |
| Cord plasma | 0.04 (0.03–0.05) | 0.21 (0.16–0.29) | 0.16 (0.12–0.22) | 57.44 (48.65–67.82) |
| Cord/maternal plasma ratio | 0.62 (0.42–0.91) | 0.67 (0.55–0.81) | 1.27 (0.91–1.78) | 0.62 (0.53–0.73) |
Figure 1.
Correlations between maternal and cord concentrations of Hg, Pb, and Se in RBCs (top panels) and plasma (bottom panels).
As shown in Supplementary Figures 2 and 3, Pearson’s correlation coefficients between gestational age and Hg concentration in maternal plasma, cord plasma, maternal RBCs, and cord RBCs were − 0.507 (0.0002), − 0.420 (0.0024), − 0.315 (0.0257), and − 0.293 (0.0392), respectively; Pearson’s correlation coefficients between birthweight and Hg concentration in maternal plasma, cord plasma, maternal RBCs, and cord RBCs were − 0.372 (0.0078), − 0.273 (0.0554), − 0.200 (0.163), and − 0.191 (0.185), respectively. The geometric means of Hg concentrations for those in the preterm birth category were higher than for those in the corresponding term birth category, and the geometric means of Hg concentrations for those in the low birthweight category were higher than for those in the corresponding normal birthweight category (Table 4).
Table 4.
Hg concentrations and P value in 50 pairs of maternal and cord plasma and RBCs.
| Birth outcome | Geometric mean (95% CI) (μg/l) |
|||
|---|---|---|---|---|
| Mother plasma | Cord plasma | Mother RBCs | Cord RBCs | |
| Gestational age | ||||
| < 37 weeks | 0.93 (0.78–1.10) | 0.83 (0.73–0.94) | 1.86 (1.49–2.33) | 2.22 (1.67–2.96) |
| ≥ 37 weeks | 0.55 (0.48–0.63) | 0.46 (0.40–0.53) | 1.37 (1.21–1.55) | 1.65 (1.46–1.86) |
| P for trenda | 0.0002 | 0.0024 | 0.026 | 0.039 |
| Birthweight | ||||
| <2500 g | 0.91 (0.77–1.08) | 0.79 (0.69–0.90) | 1.86 (1.51–2.30) | 2.24 (1.71–2.94) |
| ≥2500 g | 0.55 (0.48–0.63) | 0.46 (0.40–0.53) | 1.37 (1.21–1.55) | 1.65 (1.46–1.86) |
| P for trend | 0.0078 | 0.055 | 0.16 | 0.19 |
P for trend was tested using linear regression model: Y=Hg, X=gestational age or birthweight.
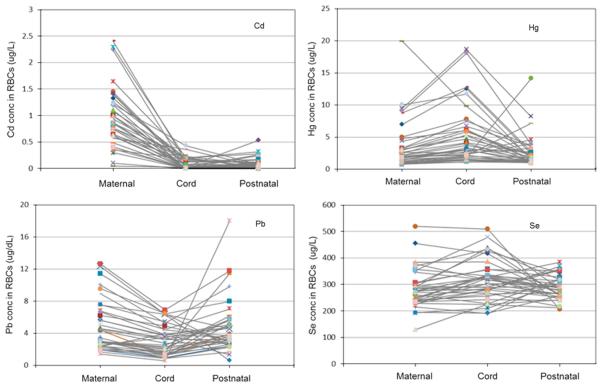
Figure 2 shows the longitudinal patterns of Cd, Hg, Pb, and Se concentrations in maternal, cord, and early childhood (6–39 months of age) RBCs among 39 mother–infant pairs who had complete exposure data. Geometric means of Cd, Hg, Pb, and Se in maternal, cord, and early childhood RBCs among these 39 mother–infant pairs are shown in Table 5, and the results of the other distributions (minimum, Q1, median, Q3, and maximum) can be found in Supplementary Table 2. Hg and Se concentrations in cord RBCs were approximately 54% and 12% higher, respectively, as compared with maternal RBCs, and then declined by approximately 43% and 8%, respectively, in postnatal RBCs. Cd and Pb concentrations in cord RBCs were 93% and 38% lower, respectively, as compared with maternal RBCs, and then increased by 17% and 50%, respectively, in postnatal RBCs.
Figure 2.
Longitudinal patterns of Cd, Hg, Pb, and Se concentrations in maternal, cord, and early childhood RBCs among 39 mother–infant pairs.
Table 5.
Concentrations of Cd, Hg, Pb, and Se in maternal, cord, and early childhood (6–39 months) RBCs among 39 mother–infant pairs.
| Geometric mean (95% CI) |
||||
|---|---|---|---|---|
| Cd (μg/l) | Hg (μg/l) | Pb (μg/dl) | Se (μg/l) | |
| Maternal RBCs | 0.81 (0.62–1.05) | 2.50 (1.90–3.29) | 4.09 (3.34–5.03) | 277.81 (255.51–302.05) |
| Cord RBCs | 0.06 (0.04–0.08) | 3.84 (2.93–5.04) | 2.55 (2.04–3.21) | 311.10 (286.48–337.84) |
| Postnatal RBCs | 0.07 (0.04–0.11) | 2.17 (1.74–2.70) | 3.83 (3.13–4.69) | 286.84 (272.93–301.45) |
Finally, we examined the concentrations of these elements in 17 subjects with paired whole blood and RBC samples. As shown in Supplementary Figure 1, high correlations between RBCs and whole blood (r>0.97) were observed for Hg and Pb. Se showed a reasonable correlation (r = 0.8) (data not shown). Cd showed no correlation (r = 0.190) in part due to Cd values in RBCs and whole blood, which were either undetectable or close to the LOD (data not shown).
DISCUSSION
To our knowledge, this is the first study to examine maternal exposure to multiple metals (including Cd, Hg, Pb, and Se) and trans-placental transfer of these elements from the mother to the fetus in a variety of blood mediums in a US urban minority birth cohort. We showed that maternal exposure to Cd, Hg, Pb, and Se was widespread in the BBC, and maternal–fetal transfer was a major source of early life exposure to these elements. In addition, our results suggest that RBCs are better than plasma at reflecting the trans-placental transfer of Hg, Pb, and Se from the mother to the fetus. Finally, this study found significantly higher levels of Hg in maternal and cord plasma and RBCs in preterm or low birthweight births, compared with that in term or normal birthweight births. This pilot study has laid a foundation for future investigations.
First, we demonstrated that maternal exposure to Hg, Pb, and Se was widespread in our study samples, drawn primarily from urban African–American populations. By converting the RBC concentrations to whole blood concentrations using the equation reported elsewhere,28 we compared metal levels in 50 mother– infant paired samples using the current thresholds for intervention suggested by the US government. Our results show that Hg, Pb, and Se were detectable in all whole blood and RBC samples and in most plasma samples. In contrast, Cd was not detectable in a large portion of the samples. The EPA reference value for Hg in blood based on a recommended reference dose for methylmercury is 5.8 μg/l. We found that, after converting to a whole blood equivalent, approximately 12% of cord RBCs and 6% of maternal RBCs had Hg levels above the EPA reference value. However, there have been many arguments for lowering this limit. Jedrychowski’s study indicated delays in neurocognitive performance as a result of low-level prenatal exposure to Hg (greater than 0.80 mg/l in cord blood or greater than 0.50 mg/l in maternal blood).29 The Occupational Safety and Health Administration (OSHA) suggests a threshold of 5 mg/l for the intervention of Cd, and none of the 50 mother–infant pairs were above this level. CDC thresholds for Pb intervention in children and pregnant women are both 5 μg/dl;30 after converting Pb concentrations in RBCs to a whole blood equivalent, 2% of cord blood samples and 6% of maternal blood samples were above 5 μg/dl.
Supplementary Table 3 shows the estimation of whole blood equivalents based on Cd, Hg, Pb, and Se concentrations in 50 pairs of maternal and cord RBCs and plasma. Overall, the blood Pb and Se levels in this population were either below or comparable to national levels.31,32 However, the mean blood Hg level was comparatively higher than the NHANES 2001–2002 estimate (0.83 μg/l in whole blood). Our results are consistent with previous reports of higher blood mercury levels in the Eastern coastal region of the US as compared with the US as a whole.33,34 One possible explanation may be that those who reside along the coast have higher exposure because they eat much more fish and seafood compared with non-coastal residents.34
We further examined the correlation of metal exposure with gestational age and birthweight in this population. Our results show that low birthweight and preterm delivery are both associated with Hg burden in mothers and cord blood. Xue et al.35 reported similar results of the observed relationship between elevated Hg levels and increased risk of very preterm delivery. Foldspang et al.5 reported that high maternal and offspring methylmercury (MeHg) blood concentrations were associated with low mean birthweight. The implications of these results on efforts to prevent low birthweight and preterm birth by screening the mercury burden in maternal blood before or during pregnancy warrant more investigation.
Second, by comparing paired RBC and whole blood data, our study showed that RBCs may serve as an alternative medium to whole blood for metal exposure assessment. Over the past decades, whole blood has been the primary medium for assessing metal exposure, particularly for Hg and Pb.36 The US government currently uses the concentrations found in whole blood for determining the thresholds for intervention for metal exposure. We evaluated an additional 17 whole blood/RBC pairs to obtain the correlation between whole blood and RBCs. As shown in Supplementary Figure 1, in our study Hg and Pb concentrations in RBCs were considerably higher than those found in whole blood, and consistent with the cumulative effect of Hg and Pb in RBCs. There were high correlations between RBCs and whole blood for both Hg and Pb (r = 0.98, P<0.0001 for Hg and r = 1.0, P<0.0001 for Pb). The coefficient of correlation between RBCs and whole blood for Se and Cd were 0.80 (P = 0.0001) and 0.19 (P = 0.4641), respectively (data not shown). The low correlation of Cd is likely because the Cd concentration in many of our samples was either close to or below the limit of detection. Our results suggest that RBCs may serve as an alternative medium to whole blood for metal exposure assessment.
Third, our data showed that RBCs better reflect the mother-to-fetus transfer of Hg, Pb, and Se compared with plasma. Hg, Pb, and Se all demonstrated strong correlations between maternal and cord blood concentrations of Hg, Pb, and Se in RBCs (r = 0.96, 0.89, and 0.72, respectively; P<0.0001), as shown in Figure 1. Cd showed a much weaker maternal/cord blood correlation (data not shown) because of the limited trans-placental passage. These results are consistent with those of a similar study reported elsewhere.37 Our results for cord/maternal ratios in RBCs for Cd, Hg, Pb, and Se (Table 3) are consistent with the results from numerous studies showing that Hg, Pb, and Se exhibit free trans-placental passage from the mother to the fetus, wherease Cd cannot freely pass the placental barrier.38,39 Pb transport into placenta cells has been reported to be through passive diffusion.6 Methylmercury (MeHg) can easily pass through the placenta via active transport by amino acid carriers,40 which may explain the bioaccumulation of Hg in cord blood as demonstrated by the dramatically higher cord/maternal ratio in RBC samples.
However, the results that we obtained using the plasma samples varied from those we obtained using RBC samples. While the results from the RBC samples unambiguously demonstrated free trans-placental transfer of Hg, Pb, and Se, this was less well-reflected in plasma. This finding suggests that RBCs are a better medium than plasma for measuring the trans-placental transfer of metals. For example, we found a cord/maternal RBC ratio of Hg of 1.53 but a cord/maternal plasma ratio of Hg of 0.67, as shown in Table 3. Sakamoto et al.41 reported the mean cord/maternal RBC ratio of Hg to be 1.63, which is close to our result in RBCs. A higher Hg concentration in cord blood compared with maternal blood has also been reported in several other studies.37,42,43
Hg in blood is composed of an organic (methylmercury), metallic (mercury vapor Hg0), and inorganic form of Hg. Compared with the metallic and inorganic forms of Hg, which mostly come from industrial/occupational exposure, MeHg represents the focus of our concern from a public health standpoint because the majority of Hg exposure in the general population is likely from MeHg.44,45 Typically, MeHg exposure comes from sources such as fish and seafood intake, which has been reported by numerous studies to cause severe neurotoxicity in children at a blood MeHg level much higher than those observed in our cohort study.12 However, the health effects of lower level exposure to MeHg remain to be determined. As mentioned in the Methods section, the ICP-MS used in our assessment measured the total metal level regardless of its inorganic or organic form. Therefore, one possible explanation for the considerably lower cord/maternal Hg ratio in plasma, as shown in Table 3, is that inorganic Hg accumulates in the plasma of neonates and young offspring46,47 and tends to be trapped in the placenta when trying to pass through the placental barrier.48 In contrast, the methyl form of Hg is capable of readily crossing the placenta,42 and is mostly stored in RBCs (more than 90% of Hg in RBCs is known to be in the methyl form).49 It appears then that measuring Hg levels in plasma underestimates the mother-to-fetus transfer of the more toxic MeHg. Therefore, our data underscores that an assessment of Hg in RBCs better reflects the trans-placental transfer of MeHg (the more toxic form of Hg) from the mother to the fetus.
Meanwhile, the correlations between maternal and cord plasma for Hg, Pb, and Se (bottom panels) were all lower than those between maternal and cord RBCs (top panels), as shown in Figure 1. This difference is likely due to the fact that most transferred metals are stored in RBCs in the fetus, as evidenced by our results, as shown in Table 3, that the concentrations in RBCs were much higher than in plasma for all of the tested metals. Therefore, the metal concentrations in plasma would be subject to transient variation,50 again implying that RBCs may be a better medium than plasma for reflecting trans-placental passage.
Lastly, we examined the longitudinal pattern of prenatal and postnatal exposures by linking levels of Cd, Hg, Pb, and Se in maternal, cord blood, and postnatal blood samples. Although breastfeeding can be a major source of maternal–fetal transfer that contributes to postnatal exposure, metal levels in breast milk are noticeably lower than in blood.24 The decline in Hg levels in children 1 year after birth can be attributed to several factors: (1) the gradually decaying metals accumulate in fetal RBCs over time (half-life is 40–50 days for MeHg, 3–4 months for Cd, and 35 days for Pb); (2) the ceasing trans-placental transport of toxic metals from the mother to the fetus after birth; and (3) the lower possibility of Hg intake during the first year of life—for example, no or very little fish consumption in the daily diet and comparatively lower Hg levels in breast milk (demographic characteristics in Table 1 show that 80% of the children in our study used breastfeeding as a food source in early childhood). On the other hand, Pb levels were elevated by ~50% in postnatal samples as compared with those from the fetus.
Sakamoto et al.24studied 16 mother–child pairs from the population in Fukuoka, Japan, for a study of postnatal Cd, Hg, Pb, and Se levels. Compared with our study population, the mothers and babies included in Sakamoto’s study both had much higher Pb and Cd levels. Their study showed that, after 3 months of breastfeeding, Hg and Se levels declined by ~40% and 25% in postnatal blood, respectively, as compared with that in cord RBCs, which is consistent with the trend shown in our results. However, whereas our results show an elevation in Pb levels at the postnatal data point compared with fetal levels, the subjects in their study experienced almost no change in Pb level. It is well known that populations from different ethnic groups and different geographic regions can experience completely different levels of metal exposures due to different dietary habits and proximate sources of exposure. In addition, genetic and epigenetic factors may also contribute to variations in metal exposures.
Ronchetti et al.51 suggest that rapid bone turnover, which takes place during the first 12 months of life, causes elevated Pb levels in blood as a result of the mobilization of bone Pb stored during pregnancy back into circulating fluids.52 A previous study reported that Pb can be retained in bone with a half-life on the order of decades.53 However, it appears that a shorter half-life of Pb should be expected in children’s bone because of the rapid bone remodeling that takes place during the early years.53,54 Although there is ambiguity regarding how much the mobilization of bone Pb contributes to Pb levels in infant blood, the results of our study, showing that young children in early childhood experience elevated Pb levels, are consistent with those of Ronchetti.51 Both studies imply that an elevated Pb level after birth is likely to be related not only to metal exposure after birth but also to metal exposure before birth—that is, maternal exposure during or before pregnancy.
It is clear that the accumulation of Hg in the fetus as a result of trans-placental passage and the elevation of Pb levels in children during early childhood are both related to maternal prenatal exposure. Furthermore, numerous studies have shown that maternal exposure to Hg and Pb is positively related to neurotoxicity in children.55-57 The research of Hu et al.58 showed that first-trimester measures of Pb in maternal blood were predictive of adverse neurodevelopment in children later in life. Ronchetti et al.51 further proposed that maternal blood Pb levels represent the existing equilibrium between endogenous and exogenous sources of Pb in which endogenous sources, such as lifetime maternal Pb storage in bone and correspondent Pb mobilization from bone to blood during pregnancy, may prominently contribute to neurotoxicity in young children.
Clinical and Public Health Implications
In 2012, the CDC lowered the “level of concern” for Pb in children’s blood from 10 to 5 μg/dl. In 2010, the CDC issued the first guidelines regarding the screening and management of pregnant and lactating women who have been exposed to Pb, and recommended follow-up blood Pb testing for pregnant women with BLL ≥5 μg/dl and their newborn infants. Meanwhile, the American College of Obstetricians and Gynecologists (ACOG) recommends evaluating pregnant and breastfeeding women for their individual risk for environmental Pb exposure. However, more action is needed to urge public concern on this issue. To date, only New York State, New York City, and Minnesota have issued Pb screening regulations and follow-up recommendations for pregnant women by physicians or other health-care providers.30The results from our prospective cohort study support the CDC’s new guidelines regarding the screening and management of pregnant and lactating women who had been exposed to Pb. As our study is based on a predominantly urban African–American population, which has not been well studied, our results may be particularly relevant to this high-risk population.
Early blood Pb testing not only facilitates the identification of an exposure source but also represents an important step toward early intervention and clinical decision-making. Previous studies48 have shown that prenatal calcium supplements when given to pregnant women may limit Pb mobilization in bone and thus provide a solution to reduce the adverse effects of circulating maternal Pb on neurodevelopment of the fetus during pregnancy. Therefore, our results suggest that, once risk to exposure is indicated, blood Pb testing in pregnant women or those planning for pregnancy should take place as early as possible, ideally at preconception or during early pregnancy.
Our results, along with those from the other studies noted above, also raise a question as to whether screening women for Hg at preconception or during early pregnancy should be considered to protect the growing fetus and young children from exposure to toxic Hg. Although pregnant women are advised to be careful of Hg intake (e.g., to choose fish and seafood that are lower in MeHg), currently there are no guidelines regarding blood Hg screening in pregnant women or children. One possible reason is the uncertainty on the adverse effects such as developmental neurotoxicity of low-level prenatal exposure to MeHg.59 However, our results show that, in this urban African–American population, Hg levels in cord blood were 50% higher than in maternal blood. As a result, blood Hg level in 12% of newborn infants in our study exceeded the EPA reference value as compared with 3% of the mothers. More importantly, we found significantly higher levels of Hg in maternal and cord plasma and RBCs in preterm or low birthweight births, compared with that in term or normal birthweight births. Both preterm and low birthweight births are associated with high infant mortality and a wide range of postnatal morbidity.60Although the adverse effects of lower blood Hg levels remain to be determined, our results showed significant fetal bioaccumulation of Hg from the trans-placental passage of maternal Hg exposure to the fetus, which puts infants at a much higher risk than mothers for adverse outcomes. Future studies are needed to generate additional evidence and develop cost-effective strategies, including how to identify preconception and pregnancy women at high risk of Hg exposure; what would be safe and effective interventions during pregnancy for women with high blood level of Hg; and how to minimize adverse health effects of in utero Hg exposure on the offspring.
In addition to the well-known neurotoxic effects of high levels of Cd, Hg, and Pb, there is growing evidence that Hg, Pb, Cd, and Se exposures are associated with the risk for diabetes, hypertension or other metabolic diseases.14-16,61 Environmental exposures to these elements may in part explain the rising prevalence of pre- and peri-natal metabolic abnormalities seen in vulnerable populations. For example, in the BBC, over 50% of mothers are overweight, 15% of mothers have hypertensive disorder, and 4.6% had gestational diabetes during pregnancy. While the Risk Characterization Handbook of the US Environmental Protection Agency recommends risk assessment in vulnerable populations,62 there has been a particular lack of prospective birth cohort studies in predominately African–American populations to assess exposure to Cd, Hg, Se, and Pb in utero and during early childhood, which have been recognized as the most vulnerable periods for environmental exposures and epigenetic alterations, as well as critical stages for adipose tissue development, metabolic programming, and subsequent risk of metals.63-71
A major limitation of this study was the small sample size. A similar study with a larger sample size should be conducted in the future.
In conclusion, this study showed that maternal exposure to Hg, Pb, and Se was widespread in the study samples from the BBC. We found a high degree of maternal–fetal transfer for Hg, Pb, and Se. In particular, the Hg level in the cord RBCs was 1.5 times higher than that found in their mothers (an evidence of fetal bioaccumulation). Correlations between maternal and cord, Hg, Pb, and Se levels were higher in RBCs than in plasma. This study also showed changes in metal concentrations in the first few years of life, and found decreased levels of these elements, except for Pb, in RBCs during early childhood. Our results suggest that maternal–fetal transfer is a major source of early life exposure to Hg, Pb, and Se. Moreover, higher maternal exposure to Hg had adverse effects on birthweight and gestational age. Our study findings remain to be confirmed in larger studies, and the implications for future screening and interventions of preconception and pregnant women and newborns warrant further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all of the study participants, and the Boston Medical Center Labor and Delivery Nursing Staff for their support and help with the study. We thank Lingling Fu, MS, for data management, and Ann Ramsey for administrative support. We are also grateful for the dedication and hard work of the field team at the Department of Pediatrics, Boston University School of Medicine. The Boston Birth Cohort (the parent study) is supported in part by the March of Dimes PERI grants (20-FY02-56, 21-FY07-605), the Food Allergy Initiative, the Department of Defense (W81XWH-10-1-0123), and the National Institutes of Health (NIH) grants (R21 ES011666, R01 HD041702, R21HD066471, R21AI088609, U01AI090727).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
REFERENCES
- 1.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry Toxicological profile for mercury. 1999:1–676. [Google Scholar]
- 3.Sikorski R, Paszkowski T, Szprengier-Juszkiewicz T. Mercury in neonatal scalp hair. Sci Total Environ. 1986;57:105–110. doi: 10.1016/0048-9697(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 4.Lucas M, Dewailly E, Muckle G, Ayotte P, Bruneau S, Gingras S, et al. Gestational age and birth weight in relation to n-3 fatty acids among Inuit (Canada) Lipids. 2004;39:617–626. doi: 10.1007/s11745-004-1274-7. [DOI] [PubMed] [Google Scholar]
- 5.Foldspang A, Hansen JC. Dietary intake of methylmercury as a correlate of gestational length and birth weight among newborns in Greenland. Am J Epidemiol. 1990;132:310–317. doi: 10.1093/oxfordjournals.aje.a115660. [DOI] [PubMed] [Google Scholar]
- 6.Goyer RA. Transplacental transport of lead. Environ Health Perspect. 1990;89:101–105. doi: 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen PC, Pan IJ, Wang JD. Parental exposure to lead and small for gestational age births. Am J Ind Med. 2006;49:417–422. doi: 10.1002/ajim.20313. [DOI] [PubMed] [Google Scholar]
- 8.Rayman MP, Wijnen H, Vader H, Kooistra L, Pop V. Maternal selenium status during early gestation and risk for preterm birth. CMAJ. 2011;183:549–555. doi: 10.1503/cmaj.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai Y, Ohgane J, Yagi S, Ito R, Iwasaki Y, Saito K, et al. Epigenetic assessment of environmental chemicals detected in maternal peripheral and cord blood samples. J Reprod Dev. 2011;57:507–517. doi: 10.1262/jrd.11-034a. [DOI] [PubMed] [Google Scholar]
- 10.Hanna CW, Bloom MS, Robinson WP, Kim D, Parsons PJ, vom Saal FS, et al. DNA methylation changes in whole blood is associated with exposure to the environmental contaminants, mercury, lead, cadmium and bisphenol A, in women undergoing ovarian stimulation for IVF. Hum Reprod. 2012;27:1401–1410. doi: 10.1093/humrep/des038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zahir F, Rizwi SJ, Haq SK, Khan RH. Low dose mercury toxicity and human health. Environ Toxicol Pharmacol. 2005;20:351–360. doi: 10.1016/j.etap.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 13.Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations<10 mu g/dl and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, et al. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett. 2003;137:65–83. doi: 10.1016/s0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- 15.He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA trace element study. Diabetes Care. 2013;36:1584–1589. doi: 10.2337/dc12-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in US adults. Diabetes Care. 2007;30:829–834. doi: 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 18.Mestan K, Yu Y, Matoba N, Cerda S, Demmin B, Pearson C, et al. Placental inflammatory response is associated with poor neonatal growth: preterm birth cohort study. Pediatrics. 2010;125:e891–e898. doi: 10.1542/peds.2009-0313. [DOI] [PubMed] [Google Scholar]
- 19.Wells EM, Jarrett JM, Lin YH, Caldwell KL, Hibbeln JR, Apelberg BJ, et al. Body burdens of mercury, lead, selenium and copper among Baltimore newborns. Environ Res. 2011;111:411–417. doi: 10.1016/j.envres.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones EA, Wright JM, Rice G, Buckley BT, Magsumbol MS, Barr DB, et al. Metal exposures in an inner-city neonatal population. Environ Int. 2010;36:649–654. doi: 10.1016/j.envint.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich KN, Douglas RM, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 22.Ernhart CB, Morrow-Tlucak M, Marler MR, Wolf AW. Low level lead exposure in the prenatal and early preschool periods: early preschool development. Neurotoxicol Teratol. 1987;9:259–270. doi: 10.1016/0892-0362(87)90011-0. [DOI] [PubMed] [Google Scholar]
- 23.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med. 1987;316:1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto M, Chan HM, Domingo JL, Kubota M, Murata K. Changes in body burden of mercury, lead, arsenic, cadmium and selenium in infants during early lactation in comparison with placental transfer. Ecotoxicol Environ Saf. 2012;84:179–184. doi: 10.1016/j.ecoenv.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Ouyang F, Story RE, Pongracic JA, Hong X, Wang G, et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J Allergy Clin Immunol. 2009;124:1031–8. doi: 10.1016/j.jaci.2009.06.052. e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC . Laboratory Procedure Manual: Lead and Cadmium in Whole Blood. Division of Laboratory Sciences National Center for Environmental Health; Atlanta, GA: 2008. [Google Scholar]
- 27.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311:587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deSilva PE. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Br J Ind Med. 1981;38:209–217. doi: 10.1136/oem.38.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jedrychowski W, Jankowski J, Flak E, Skarupa A, Mroz E, Sochacka-Tatara E, et al. Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: epidemiologic cohort study in Poland. Ann Epidemiol. 2006;16:439–447. doi: 10.1016/j.annepidem.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 30.CDC Guideline for the Identification and Management of Lead Exposure in Pregnant and Lactating Women. 2010 Available at: http://www.cdc.gov/nceh/lead/publications/LeadandPregnancy2010.pdf. (accessed December 2013)
- 31.US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry Toxicological profile for selenium. 2003:1–418. [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry Toxicological profile for lead. 2007:1–528. [PubMed] [Google Scholar]
- 33.Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, et al. A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environ Health Perspect. 2007;115:1435–1441. doi: 10.1289/ehp.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect. 2007;115:42. doi: 10.1289/ehp.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagerkvist BJ, Soderberg H-A, Nordberg GF, Ekesrydh S, Englyst V. Biological monitoring of arsenic, lead and cadmium in occupationally and environmentally exposed pregnant women. Scand J Work Environ Health. 1993;19(Suppl 1):50–53. [PubMed] [Google Scholar]
- 37.Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11:1322–1330. doi: 10.1039/b903805a. [DOI] [PubMed] [Google Scholar]
- 38.Gundacker C, Hengstschläger M. The role of the placenta in fetal exposure to heavy metals. Wiener Med Wochenschr. 2012;162:201–206. doi: 10.1007/s10354-012-0074-3. [DOI] [PubMed] [Google Scholar]
- 39.Lagerkvist BJ, Sandberg S, Frech W, Jin T, Nordberg GF. Is placenta a good indicator of cadmium and lead exposure? Arch Environ Health. 1996;51:389–394. doi: 10.1080/00039896.1996.9934427. [DOI] [PubMed] [Google Scholar]
- 40.Kajiwara Y, Yasutake A, Adachi T, Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996;70:310–314. doi: 10.1007/s002040050279. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto M, Murata K, Kubota M, Nakai K, Satoh H. Mercury and heavy metal profiles of maternal and umbilical cord RBCs in Japanese population. Ecotoxicol Environ Saf. 73:1–6. doi: 10.1016/j.ecoenv.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Stern AH, Smith AE. An assessment of the cord blood:maternal blood methyl-mercury ratio: implications for risk assessment. Environ Health Perspect. 2003;111:1465–1470. doi: 10.1289/ehp.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuchiya H, Mitani K, Kodama K, Nakata T. Placental transfer of heavy metals in normal pregnant Japanese women. Arch Environ Health. 1984;39:11–17. doi: 10.1080/00039896.1984.10545827. [DOI] [PubMed] [Google Scholar]
- 44.US Environmental Protection Agency (USEPA) Mercury. Human exposure. 2009 Available at: http://www.epa.gov/mercury/exposure.htm (accessed December 2013)
- 45.Freire C, Ramos R, Lopez-Espinosa M-J, Díez S, Vioque J, Ballester F, et al. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ Res. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Sundberg J, Jonsson S, Karlsson MO, Hallen IP, Oskarsson A. Kinetics of methyl-mercury and inorganic mercury in lactating and nonlactating mice. Toxicol Appl Pharmacol. 1998;151:319–329. doi: 10.1006/taap.1998.8456. [DOI] [PubMed] [Google Scholar]
- 47.Feng WY, Wang M, Li B, Liu J, Chai ZF, Zhao JJ, et al. Mercury and trace element distribution in organic tissues and regional brain of fetal rat after in utero and weaning exposure to low dose of inorganic mercury. Toxicol Lett. 2004;152:223–234. doi: 10.1016/j.toxlet.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida M, Satoh M, Shimada A, Yamamoto E, Yasutake A, Tohyama C. Maternal-to-fetus transfer of mercury in metallothionein-null pregnant mice after exposure to mercury vapor. Toxicology. 2002;175:215–222. doi: 10.1016/s0300-483x(02)00084-7. [DOI] [PubMed] [Google Scholar]
- 49.Kershaw TG, Clarkson TW, Dhahir PH. The relationship between blood levels and dose of methylmercury in man. Arch Environ Health. 1980;35:28–36. doi: 10.1080/00039896.1980.10667458. [DOI] [PubMed] [Google Scholar]
- 50.Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113:1669–1674. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ronchetti R, van den Hazel P, Schoeters G, Hanke W, Rennezova Z, Barreto M, et al. Lead neurotoxicity in children: is prenatal exposure more important than post-natal exposure? Acta Paediatr Suppl. 2006;95:45–49. doi: 10.1080/08035320600886224. [DOI] [PubMed] [Google Scholar]
- 52.Gulson BL, Mizon KJ, Palmer JM, Patison N, Law AJ, Korsch MJ, et al. Longitudinal study of daily intake and excretion of lead in newly born infants. Environ Res. 2001;85:232–245. doi: 10.1006/enrs.2000.4223. [DOI] [PubMed] [Google Scholar]
- 53.Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993;101:598. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellinger DC, Stiles KM, Needleman HL. Low-level lead exposure, intelligence and academic achievement: a long-term follow-up study. Pediatrics. 1992;90:855–861. [PubMed] [Google Scholar]
- 56.Canfield RL, Henderson CR, Jr., Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 mu g/dL. Neurotoxicology. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu H, Tellez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Behrman RE, Butler AS. Preterm Birth: Causes, Consequences, and Prevention. National Academies Press; Washington DC: 2007. p. 381. [PubMed] [Google Scholar]
- 61.Baker JR, Satarug S, Edwards RJ, Moore MR, Williams DJ, Reilly PE. Potential for early involvement of CYP isoforms in aspects of human cadmium toxicity. Toxicol Lett. 2003;137:85–93. doi: 10.1016/s0378-4274(02)00382-x. [DOI] [PubMed] [Google Scholar]
- 62.USEPA (US Environmental Protection Agency) Risk Characterization, Science Policy Council Handbook. EPA/100/B-00/002.United States Environmental Protection Agency, Office of Science Policy, Office of Research and Development; Washington, DC: 2000. Available at: http://www.epa.gov/OSA/spc/pdfs/rchandbk.pdf, (accessed April 26 2010) [Google Scholar]
- 63.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 65.Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol. 1992;99:275–276. doi: 10.1111/j.1471-0528.1992.tb13719.x. [DOI] [PubMed] [Google Scholar]
- 66.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barker DJ. The long-term outcome of retarded fetal growth. Clin Obstet Gynecol. 1997;40:853–863. doi: 10.1097/00003081-199712000-00019. [DOI] [PubMed] [Google Scholar]
- 68.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128. [PubMed] [Google Scholar]
- 69.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 70.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.