Abstract
It is well established that exposure to ambient fine particulate matter (PM) is associated with increased cardiovascular morbidity and mortality and that elderly individuals are particularly susceptible to these effects. We speculated that increased susceptibility of the elderly to PM is due to altered production of inflammatory mediators and antioxidants in the lung and pilot studies were performed to test this hypothesis. For these studies we used diesel exhaust, a major component of urban PM as a model. Animals (CB6F1 male mice; 2 m and 18 m) were exposed to air or diesel exhaust at 300 or 1000 µg/m3 for 3 h one time (single) or 3 h/day for 3 consecutive days (repeated). Bronchoalveolar lavage (BAL) fluid, serum and lung tissue were collected 0 and 24 h later. Following single or repeated diesel exhaust exposure, persistent structural alterations and inflammation were observed in the lungs of older mice. This consisted of patchy thickening of alveolar septa and an increase in the number of neutrophils and macrophages in alveolar spaces. In contrast, no major alterations in lung histology were noted in younger mice. In older, but not younger mice, significant increases in expression of the oxidative stress marker, lipocalin 24p3 were also observed. In both younger and older mice, exposure to diesel exhaust was associated with increased expression of TNFα in the lung. However, this response was attenuated in older mice. Exposure to high dose diesel exhaust resulted in significant increases in IL-6 and IL-8 mRNA expression in lungs of older animals which persisted for 24 h. Whereas IL-6 was also upregulated in younger mice after diesel exhaust exposure, no major effects were evident on expression of IL-8 mRNA. Expression of the antioxidant manganese superoxide dismutase (MnSOD) was decreased in lung tissue from younger animals after exposure to DE (single or repeated). In contrast, constitutive expression of MnSOD was not evident in lungs of older mice, and diesel exhaust had no effect on expression of this antioxidant. These preliminary data confirm that older mice are more sensitive to diesel exhaust than younger mice. Moreover, this is associated with altered expression of inflammatory cytokines and the antioxidant, MnSOD. These aberrations may contribute to the increased susceptibility of older mice to inhaled PM.
Introduction
Epidemiologic studies have demonstrated strong associations between hourly or daily changes in air pollution and cardiovascular morbidity and mortality (Dockery et al, 1992; Pope et al, 1992; Mauderly, 2000; Samet et al, 2000; Peters et al, 2001; Brunekreef and Holgate, 2002; Peters et al, 2004; Pope et al, 2004; USEPA, 2004; Forastiere et al, 2005; Park et al, 2005; Rich et al, 2005; Schwartz et al, 2005; Vermylen et al, 2005). Of particular concern is ambient fine particulate matter (PM2.5; particles smaller than 2.5 µm in diameter) as a causal agent, and gases such as ozone, carbon monoxide, sulfur dioxide and nitrogen dioxide. One of the most sensitive populations is the elderly, individuals over 65 years of age. While underlying cardiopulmonary disease is no doubt important, the precise mechanisms mediating increases in the susceptibility of the elderly to the adverse effects of inhaled PM are unknown and this represents the focus of our research. In previous studies using model PM mixtures, we found that lung injury in rodents is associated with increased production of TNFα in the tissue (Morio et al, 2001; Laskin et al, 2003; Sunil et al, 2007a). Interestingly, this response was significantly attenuated in older animals (Sunil et al, 2007a). Although cytokines such as TNFα, IL-1, IL-6 and IL-8 have classically been considered pro-inflammatory produced rapidly in response to tissue injury, recent evidence suggests that they also play an important role in initiating tissue repair later in the inflammatory process, and in regulating inflammatory cell trafficking into tissues (Murtaugh et al, 1996; Wong et al, 1996; Geiser et al, 2001; Chiu et al, 2003a; Chiu et al, 2003b; Geiser, 2003; McClintock et al, 2008). Thus aberrant production of cytokines may be a factor in the enhanced susceptibility of the elderly to PM.
As oxidative stress appears to be a common mechanism underlying the biological effects of PM and other air pollutants, another important contributory factor may be age-related alterations in lung antioxidants (Kelly et al, 2003). These include reduced levels of superoxide dismutase (SOD), vitamin C and dysregultion of vitamin B12 and folic acid metabolism in the lung (Ischiropoulos et al, 1990; van der Loo et al, 2003; Wolters et al, 2004). Aging is also associated with aberrant generation of reactive oxygen and nitrogen species by macrophages resulting in increased oxidative/nitrosative stress (Tasat et al, 2003).
Our overall hypothesis is that inflammatory mediator production by alveolar macrophages and antioxidant defense mechanisms in the lung are altered in the elderly and that this increases their susceptibility to fine PM-induced oxidative stress and cardiopulmonary pathology. In order to carry out mechanistic experiments aimed at testing this hypothesis, a series of pilot studies using inhaled diesel exhaust as a model were performed to generate supportive preliminary data.
Specific Aims
I. Develop and Characterize a Diesel Exhaust Animal Exposure System
Total particle number and mass distribution were evaluated at two diesel exhaust mass concentrations (300 and 1000 µg/m3) in a 17-liter whole body animal exposure chamber. Measurements of PM characteristics and co-pollutant levels were performed. These included particle size distribution and particle mass concentration measurements, as well as analysis of NO/NO2/NOx, CO/CO2 and EC/OC.
II. Determine if Geriatric Mice are More Susceptible to the Adverse Pulmonary Effects of Inhaled Diesel Exhaust
The effects of exposure of younger (2 m) and older (18 m) mice to inhaled diesel exhaust were compared. Lungs of treated animals were examined histologically for evidence of structural alterations and inflammatory cell accumulation. BAL fluid was collected and measurements made of protein, lactate dehydrogenase (LDH), and cell number, as well as lung lipocalin 24p3 expression. Dose response studies were performed using 0, 300, and 1000 µg/m3 mass concentrations of diesel exhaust. The effects of exposure to diesel exhaust for 3 h one time (single) or for 3 h/day for 3 consecutive days (repeated) were evaluated immediately (0 h) and 24 h post exposure.
III. Determine if Increases in the Susceptibility of Geriatric Mice to Inhaled Diesel Exhaust is Associated with Altered Expression of Inflammatory Mediators and Antioxidants
Differences in expression of TNFα, IL-6, IL-8, COX-2 and MnSOD in the lungs, and of TNFα in sera from younger and older mice were compared after exposure to diesel exhaust.
Methods
Exposure System
A Yanmar, Model YDG 5500EE, 5.5 Kw, 406 cc, one-cylinder, two cycle, diesel-powered electrical generator was used as a source of diesel emissions. The generator is located in the penthouse of the Environmental and Occupational Health Sciences Institute (EOHSI) building of Rutgers University/UMDNJ-Robert Wood Johnson Medical School, directly above the ceiling of the animal exposure laboratory. The generator was operated using premium low sulfur (<500 ppm; Petro Inc.) diesel fuel and 40-weight motor oil (Proline HD40). The generator’s load module consists of a bank of ceramic heaters (Maxi Heat Inc.) monitored by an ammeter (Model 1357, Simpson Inc.) to dissipate 5500 watts of generated electricity (100% load). An engine silencer (muffler; URB 2, Cummins Inc.) was installed after the tailpipe to reduce noise.
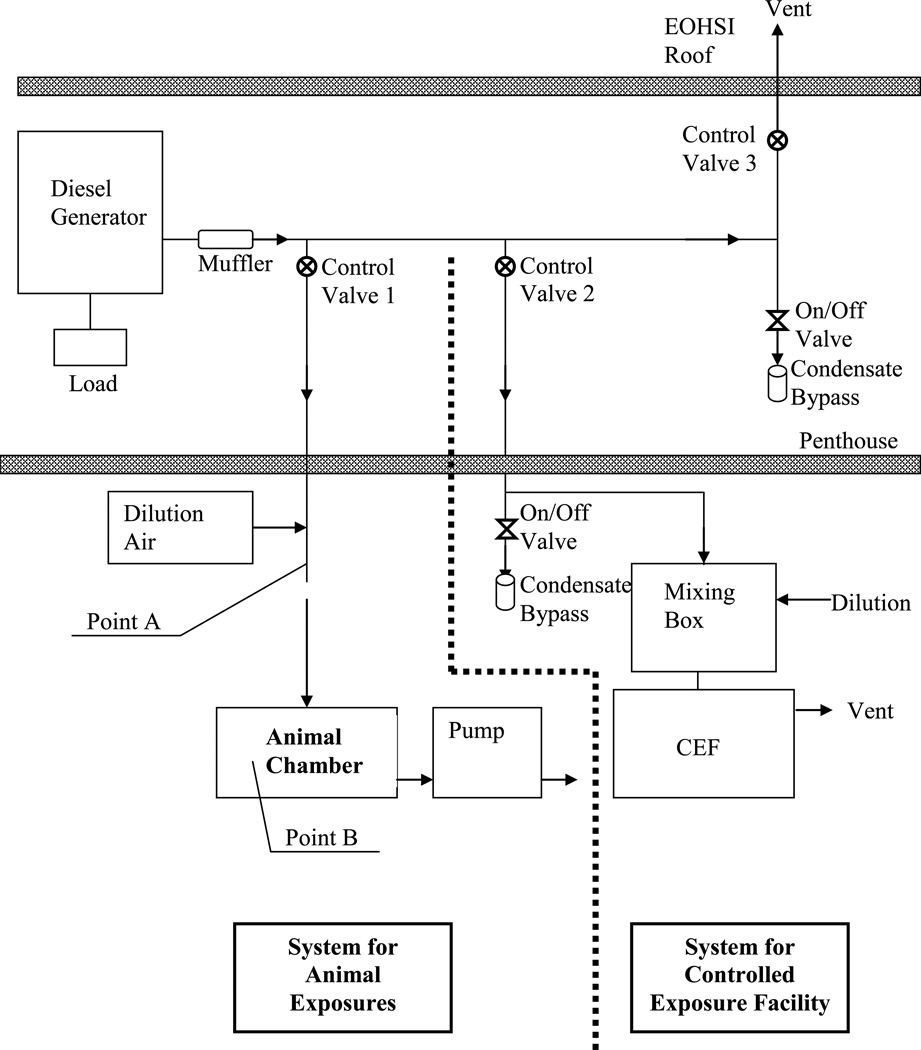
The schematic of the diesel dilution/delivery system used in this pilot study is shown in Figure 1. The components of the overall system can be separated into two parts: one used for animals exposures (left of the dotted line) and the other used for human Controlled Exposure Facility (CEF, right of the dotted line). The system for animal exposures includes a two-stage mass reduction device. The first-stage contains a 10-position butterfly control valve (Control Valve 1 in Figure 1) that can split the diesel exhaust between the laboratory stream and the waste exhaust pipe in 10 different ratios. The emissions diverted to the animal chamber were aged in the delivery system and were then diluted with HEPA-filtered air in the main pipe (Point A in Figure 1), before the diesel exhaust was isokinetically drawn into the exposure chamber (Point B in Figure 1). Five to ten-fold dilution ratios were used depending on desired diesel concentration and the setting of Control Valve 1. At the steady state operating condition, it took the diesel exhaust about five seconds to travel from the exhaust to the dilution point (Point A in Figure 1) and then about an additional second to reach the animal chamber. To minimize cooling, the pipe carrying the exhaust from the diesel generator was insulated up the dilution point. Before animal exposure sessions, the engine was operated for 15–20 minutes to achieve a stable aerosol mass concentration.
Figure 1.
Schematic of Exposure System
Animals were exposed to diesel exhaust in a 17-liter whole body plexiglass divided enclosure (16”L × 8”W × 8”H) with a removable lid. During the exposures, the inside and outside of the lid was sealed with plastic to ensure air tightness of the chamber. To improve distribution of the particles (i.e., mixing), two small fans were installed and operated inside the chamber. In addition to inlet and outlet ports for the diesel exhaust, there were 6 sampling ports available to monitor various characteristics of the exposure aerosol. The flow of the diesel exhaust through the chamber was controlled by a pump downstream of the chamber which maintained air flow at 5 L/min. Air flow was monitored using a rotameter. The diesel exhaust mass concentration in the chamber was controlled by adjusting the fraction of total diesel exhaust directed towards the chamber and by the amount of dilution air. During the exposures, the pressure inside the chamber was approximately −1” H2O relative to room pressure.
Exposure Characterization
The particle mass concentration in the chamber was measured in real-rime by a SidePak personal aerosol mass monitor (TSI Inc.) which was calibrated against gravimetric (filter) measurements of the diesel exhaust. Particle number and mass distributions from 13 nm to 20 µm were obtained with a Scanning Mobility Particle Sizer (SMPS; model 3936, TSI Inc.) and an Aerodynamic Particle Sizer (APS; model 3321, TSI, Inc.). The SMPS uses an electrostatic classifier in combination with a water-based condensation particle counter (model 3786), while the APS utilizes time-of-flight principle and provides high resolution real-time sizing of particles from 0.5 to 20 µm at a sampling flow rate of 1 L/min. The data from the SMPS and the APS were combined using Data Merge Software Module (TSI, Inc.). When merging the data, electrical equivalent diameter was used for the SMPS data and aerodynamic diameter was used for the APS data. As a control, total particle number concentration was monitored in real-time by a condensation particle counter (CPC 3007, TSI Inc.), which measures the concentration of particles larger than 10 nm. Levels of co-pollutant gases (e.g., NO/NO2, CO/CO2) were assessed using an IAQ RAE gas monitor with multi-gas sensors (RAE Systems). To determine PM content (concentration) of elemental carbon (EC), organic carbon (OC), and total carbon (TC = EC + OC), diesel exhaust particulate matter was collected on pre-fired (with organic impurities removed) 25 mm quartz fiber filters (Pall Corp.-Life Sciences). The air through the filter and PM10 pre-classifier (A.D.E. Inc., Naples, Maine) was drawn at a flow rate of 4 L/min by a BGI personal pump for the duration of 1 h. After collecting the samples, the filters were sent to Sunset Laboratory, Inc. (Tigard, Oregon) for EC/OC/TC analysis.
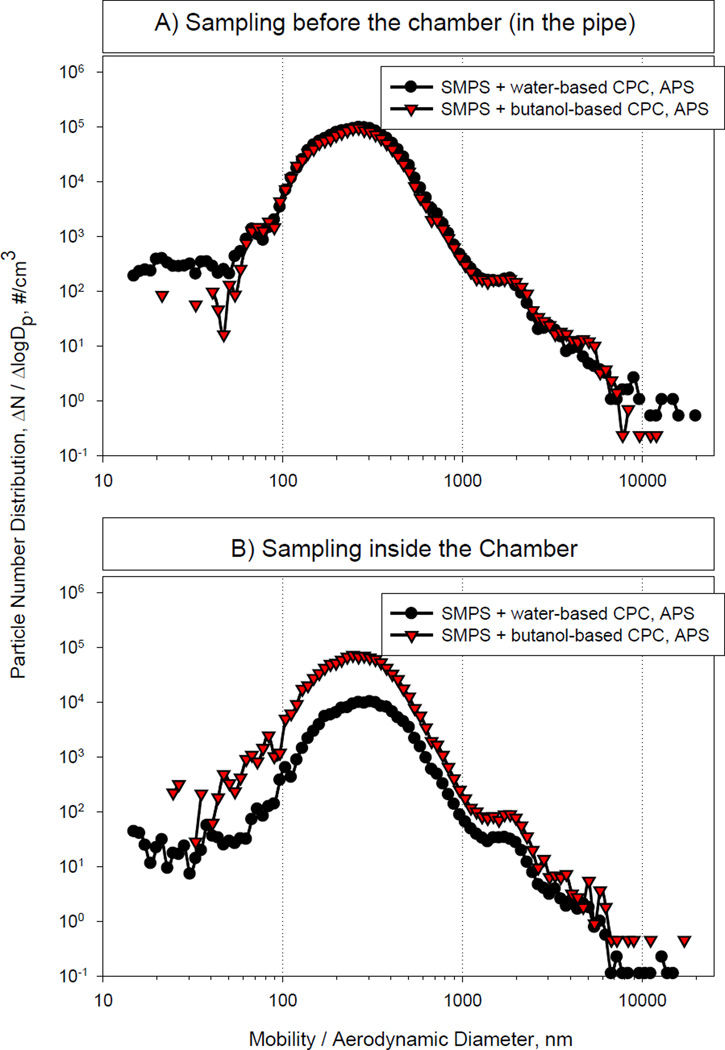
Particle number distribution measurements were performed inside the exhaust pipe (from which diesel is drawn into the chamber) and inside the animal exposure chamber for both DE mass concentrations (300 and 1000 µg/m3). The initial measurements inside the chamber were performed with the SMPS which uses a water-based CPC (model 3786) to count particles in each individual size channel. By adding particle concentrations from each individual size channel, the total particle concentration for the entire size range was obtained. Comparison of the total number provided by the SMPS and the total concentration from the control measurements with the CPC 3007 revealed that the water-based CPC of the SMPS undercounted the number of particles. After consultation with TSI, it was suggested that the undercounting is likely due to slightly negative pressure inside the animal exposure chamber, which reduces the water vapor condensation on the diesel particles. TSI subsequently agreed to provide a butanol-based CPC (3025) for control measurements. Thus, the diesel exhaust characterization was performed with SMPS equipped with both the water-based 3786 and the butanol-based 3025 CPC. Characterization of DE particle size distribution and measurements of co-pollutant gases were performed prior to animal exposures, while mass concentration measurements were made both prior to and during the animal exposures.
Animals
Young (2 m; average weight, 24 g; Harlan, Indianapolis, IN) and old (18 m; average weight, 41 g; National Institute of Aging, Bethesda, MD) CB6F1 male mice were used in these studies. Animals were housed in microisolator cages and maintained on sterile food (Picolab Rodent Diet 20, Fisher Scientific, Pittsburgh, PA) and pyrogen-free water ad libitum except during exposures. Animals were acclimatized to housing conditions for 7–10 days prior to initiating the study. Mice were weighed and randomly assigned to treatment groups. The animal rooms were maintained at 20 ± 2°C with 45–55% relative humidity on a 12-h light/dark cycle. All animals received humane care in compliance with the institution’s guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. Animal care personnel followed all applicable standard operating procedures specified by the American Association of Accreditation of Laboratory Animal Care.
Animal Exposures
Animals were exposed for 3 h one time (single) or 3 h per day on three consecutive days (repeated) to ultra-pure air (Messer Gas, Allentown, PA), 300 or 1000 µg/m3 diesel exhaust (as PM2.5). Exposures were performed in a single partitioned chamber without a floor grid. During exposure sessions, six animals per exposure group were placed on each side of the divided chamber.
Sample Collection
Groups of six animals per treatment were sacrificed immediately (0 h) or 24 h after exposure. The 0-h time point was selected for evaluation of inflammatory alterations that occur immediately after exposure, while the 24-h time point assessed the persistence of early inflammatory changes and the appearance of later pro-inflammatory and early repair markers. Nembutal (200 mg/kg body weight) was injected intraperitoneally using a 261/2 gauge needle to euthanize the animals. The body cavity was opened and blood collected from the right ventricle using a 271/2 gauge needle. The volume of blood recovered was recorded and tubes maintained at room temperature until clots formed. Serum samples were centrifuged at 1000 × g for 10 min at 4°C, aliquoted and stored at −80°C.
The thoracic cavity was then opened and the trachea and lungs exposed. The largest lobe was clamped at the bronchus and reserved for histology, immunohistochemistry and RNA analysis (see below). The remaining lobes were lavaged three times through the trachea with one ml sterile, pyrogen-free, ice-cold phosphate buffered saline (PBS). The volume of recovered lavage fluid was recorded and transferred to tubes containing 5 mM diethylenetriaminepentaacetic acid (DTPA). Tubes were centrifuged at 300 × g for 8 min at 4°C and supernatants transferred to fresh tubes for analysis of protein and LDH activity. Cell pellets were resuspended in 50 µl of PBS. Ten µl of each sample were stained with trypan blue and analyzed using a hemocytometer to determine cell number and viability. PBS (160 µl) was then added to the cells which were cytocentrifuged (600 × g, 8 min, room temperature; Shannon Southern Products, England) onto slides. Slides were stained with Giemsa (Lab Chem Inc., Pittsburgh, PA) and analyzed at X400 by light microscopy. A total of 300 cells per sample were counted to determine the percentage of alveolar macrophages, monocytes, neutrophils and lymphocytes. For all treatment groups, greater than 98% of the cells were identified as alveolar macrophages and 1–2% as lymphocytes (data not shown).
For RNA analysis (3/6 mice per group), the largest lobe was removed, and stored at −80°C in tubes containing 200 µl of RNALater (Sigma-Aldrich Corp., St Louis, MO). For histological analysis and immunohistochemistry (3/6 mice per group), the largest lobe was instilled with 3% cold paraformaldehyde in PBS, removed and placed on ice for 4 h. Samples were then transferred to 50% ethanol.
Measurement of BAL Protein
Total protein content was quantified in cell-free preparations of BAL fluid using a BCA Protein Assay kit (Pierce Biotechnologies Inc., Rockford, IL) with bovine serum albumin (BSA) as the standard. Ten µl of undiluted sample from each animal was analyzed in triplicate at 540 nm. Data were normalized to the volume of BAL collected. Samples from 5–6 animals per treatment group were assayed.
Measurement of BAL Albumin
A mouse albumin quantitative ELISA (Bethyl Laboratories, Montgomery, TX) was used to assay albumin content in BAL fluid. For the assay, 100 µl of red blood cell free undiluted BAL were analyzed in duplicate at 450 nm. Data were normalized to the volume of BAL collected. Samples from 5–6 animals per treatment group were assayed.
Measurement of LDH Activity
LDH activity in BAL was determined using the CytoTox 96 non-radioactive cytotoxicity assay kit from Promega Corporation (Madison, WI). Each sample (50 µl undiluted BAL) was analyzed in triplicate at 490 nm. Data were normalized to the volume of BAL collected. Samples from 6 animals per treatment group were assayed.
Histological Analysis
Histological sections (4 µm) were stained with hematoxylin and eosin. Specimens were analyzed by light microscopy using the ProgRes Capture Pro v2.5 software. The extent of inflammatory changes including alveolar macrophage and neutrophil accumulation, alterations in alveolar epithelial barriers, and edema, were assessed by a board certified veterinary pathologist (Sherritta Ridgely, D.V.M., Ph.D., Clinical Laboratory Animal Veterinarian, Laboratory Animal Services, Rutgers University). Three mice per treatment group were analyzed.
Immunohistochemistry
Tissue sections (4 µm) were deparaffinized, blocked in 100% serum (room temperature, 3–4 h) and incubated overnight at 4°C with rabbit IgG or anti-MnSOD (1:400, Stressgen Biotechnologies Inc., San Diego, CA.) antibody, followed by 30 min incubation with biotinylated secondary antibody (Vector Labs, Burlingame, CA). Binding was visualized using a Peroxidase Substrate Kit DAB (Vector Labs). Three mice per treatment group were analyzed.
Measurement of Serum TNFα
TNFα levels in serum were quantified using a quantikine immunoassay kit from R&D Systems (Minneapolis, MN). Serum (50 µl) from each sample was diluted two-fold and analyzed in duplicate. Plates were analyzed at 450 nm and 540 nm and a standard curve was generated using a four parametric logistic (4-PL SoftMax Pro v4 software, R&D Systems) curve fit. Samples from 2–4 animals per treatment group were assayed.
Quantitative Real Time Polymerase Chain Reaction
Total mRNA was isolated from lung tissue using the RNeasy Mini kit (Qiagen, Valencia, CA). RNA was reverse-transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Standard curves were generated using serial dilutions from pooled cDNA samples. Real time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on a 7900HT thermocycler according to manufacturer protocol. All PCR primers were generated using Primer Express 3.0 (Applied Biosystems). Samples from 2–3 animals per treatment group were analyzed and presented relative to GAPDH mRNA expression. Primer sequences were as follows:
24p3 Forward: 5’ AGG AAC GTT TCA CCC GCT TT 3’
24p3 Reverse: 5’ TGT TGT CGT CCT TGA GGC C 3’
TNF-α Forward: 5’ AGG GAT GAG AAG TTC CCA AAT G 3’
TNF-α Reverse: 5’ TGT GAG GGT CTG GCG CAT A 3’
IL-6 Forward: 5’ CCA CGG CCT TCC CTA CTT C 3’
IL-6 Reverse: 5’ GTT GGG AGT GGT ATC CTC TGT GA 3’
IL-8 Forward: 5’ CAG CTG CCT TAA CCC CAT CA 3’
IL-8 Reverse: 5’ CTT GAG AAG TCC ATG GCG AAA 3’
COX-2 Forward: 5’CAT TCT TTG CCC AGC ACT TCA C 3’
COX-2 Reverse: 5’GAC CAG GCA CCA GAC CAA AGA C 3’
GAPDH Forward: 5’ TGA AGC AGG CAT CTG AGG G 3’
GAPDH Reverse: 5’ CGA AGG TGG AAG AGT GGG AG 3’
Statistical Methods and Data Analysis
Statistical design and analysis were performed in consultation with Rebecka Jornsten, Ph.D., Department of Statistics, Rutgers University. Separate data sets were constructed for each type of exposure (single or repeated) and for each post exposure time (0 h or 24 h). For each separate data set, a two-way analysis of variance was used to test the impact of age (2 m vs. 18 m) on each response variable (e.g., BAL volume, cell number, etc.), at each level of exposure (0, 300 and 1000 µg/m3). Thus, the two common factors in each model were age and exposure level. Two-way ANOVA models were fit to each data set using the SAS software (which is a licensed software product). The validity of each model was carefully assessed using residual diagnostics. Specifically, the underlying modeling assumption was examined using normal Quantile-Quantile plots. Extreme observations, i.e. outliers, were identified from the QQ-plots. All values are presented for all assays with the exception of BAL total protein where either one outlier (single 0 h: 18 m, 0 µg/m3 and 2 m, 1000 µg/m3 DE; single 24 h: 18 m, 0 µg/m3 DE and 2 m, 1000 µg/m3 DE; repeated 0 h: 2 m, 0 µg/m3 DE; repeated 24 h: 2m, 0 µg/m3, 1000 µg/m3 DE) or two outliers (repeated 24 h, 2 m, 300 µg/m3 DE) were excluded prior to analysis.
Specific hypotheses that were tested included age effect at each level of exposure. P-values of the age effect at each level of exposure (0, 300 and 1000 µg/m3 DE) are reported for each separate data set. To examine the impact of exposure concentration on the outcome at each type of exposure, time of exposure and age of animal, one-way ANOVA models were used. Modeling assumptions were examined using QQ-plots, and extreme observations were removed prior to hypothesis testing. Specific hypotheses for 2 m and 18 m that were tested include: (a) that 0 and 300 µg/m3 DE result in the same expected outcome; (b) that 0 and 1000 µg/m3 DE result in the same outcome; (c) that 300 and 1000 µg/m3 DE result in the same outcome when compared to 0 µg/m3 (air control). Separate P-values are reported for each of these hypotheses. A p-value of <0.05 was considered statistically significant. Changes in gene expression >2-fold were considered biologically significant.
Results
I. Characterization of Diesel Exhaust Animal Exposure System
Particle Characterization and Measurement
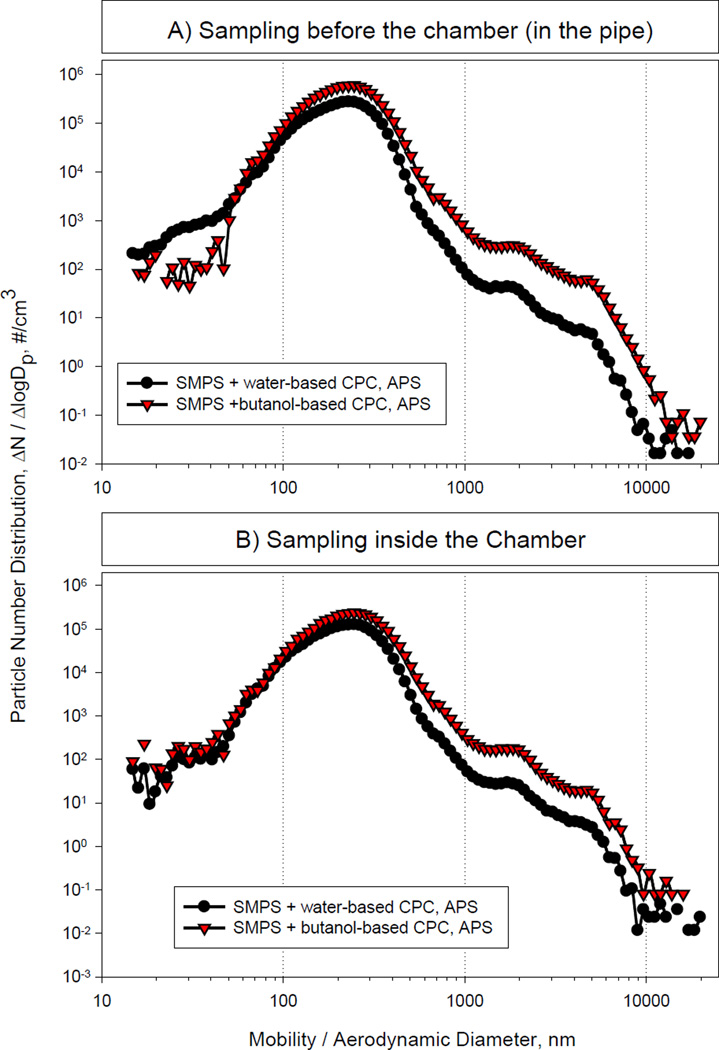
The first part of our studies was designed to assess the stability of the diesel exhaust mass concentrations within the chamber over the planned 3 h experimental exposure period. We found that particle mass concentrations from 300 to 1000 µg/m3 (PM2.5) remained stable over several hours with coefficient of variation ≤15%. In contrast, under the conditions used, we were unable to maintain stable particle mass concentrations of 100 µg/m3 in the animal exposure chamber. This is most likely due to the high sensitivity of the animal exhaust to engine variations and wind drafts. Thus, in further pilot characterization and exposure studies we limited our analysis to diesel exhaust concentrations of 300 and 1000 µg/m3. The measured particle number distributions at these two different mass concentrations are shown in Figures 2 and 3. A summary of measured atmosphere characteristics is presented in Table 1. Based on the particle size distribution analysis with the butanol-based CPC placed inside the animal chamber, at the 300 µg/m3 mass concentration, the median diameter was approximately 255 nm, the mean, approximately 273 nm, and the mode 246 nm, with a total particle number of approximately 3.6 × 104/cm3. For the 1000 µg/m3 mass concentration, the median diameter was 230 nm, the mean 242 nm, and the mode 246 nm, while the total particle number was >1 × 105/cm3. These values are greater than those reported previously by McDonald et al. (2004) which is most likely due to differences in the design of the diesel exposure systems. In our system, it takes a few seconds for the diesel exhaust to reach the dilution point. This time may be sufficient for nano-sized particles to agglomerate causing a shift in median particle diameters.
Figure 2.
DE Particle Size Distribution at 300 µg/m3 Mass Concentration
Figure 3.
DE Particle Size Distribution at 1000 µg/m3 Mass Concentration
Summary Of Findings
| Single | Repeated | |||
|---|---|---|---|---|
| Marker | Young | Old | Young | Old |
| Histology (Neutrophils/Macrophages) |
No Change | Increase | No Change | Increase |
| BAL Cells | Increase | Decrease | Increase | Decrease |
| BAL Protein | Increase | No Change | Increase | No Change |
| BAL LDH Activity | Decrease | No Change | Increase | No Change |
| Lipocain 24p3 Expression | No Change | Increase | Small Increase | Increase |
| TNFα Expression | Increase | Small Increase | No Change | Small Increase |
| Serum TNFα | Decrease | Increase | Small Increase | Small Decrease |
| Mn SOD Expression Constitutive DE |
+ − |
− − |
+ − |
− − |
| IL-6 & IL-8 Expression | Increase | Increase | Increase | Increase |
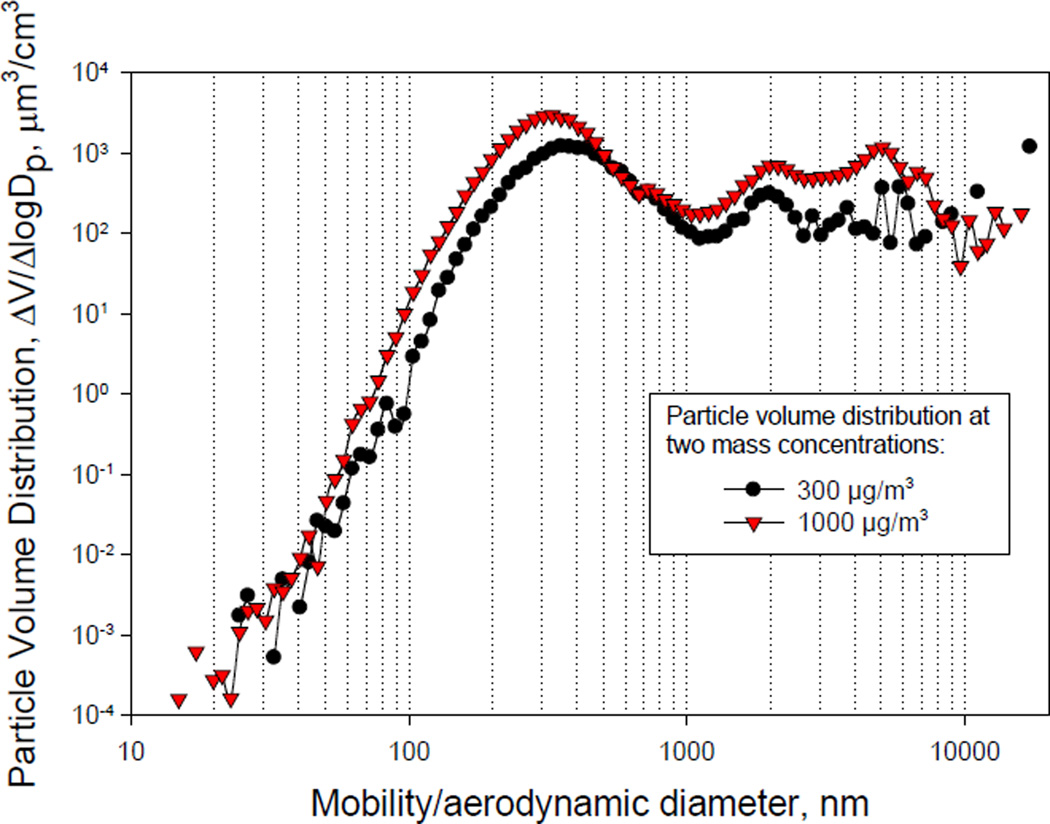
As shown in Figures 2 and 3, data obtained with the water-based CPC and the butanol-based CPC sampled from the exhaust pipe were similar. In contrast, the water-based CPC underestimated the concentration of the particles inside the exposure chamber when compared to butanol-CPC at the 300 µg/m3 concentration. For all distributions, the geometric standard deviation was 1.5. Aerosols with geometric standard deviations <2 are considered monodisperse. According to the particle volume data shown in Figure 4, the median mass diameter was 487 nm and 411 nm, and the mean mass diameter, ~2,200 nm and 1676 nm for the 300 µg/m3 and the 1000 µg/m3 mass concentrations, respectively. Taken together, these results indicate that the number and mass concentration in the animal exposure chamber can be varied within these particle mass concentrations, without substantially changing the particle size distribution.
Figure 4.
Particle Volume Distribution
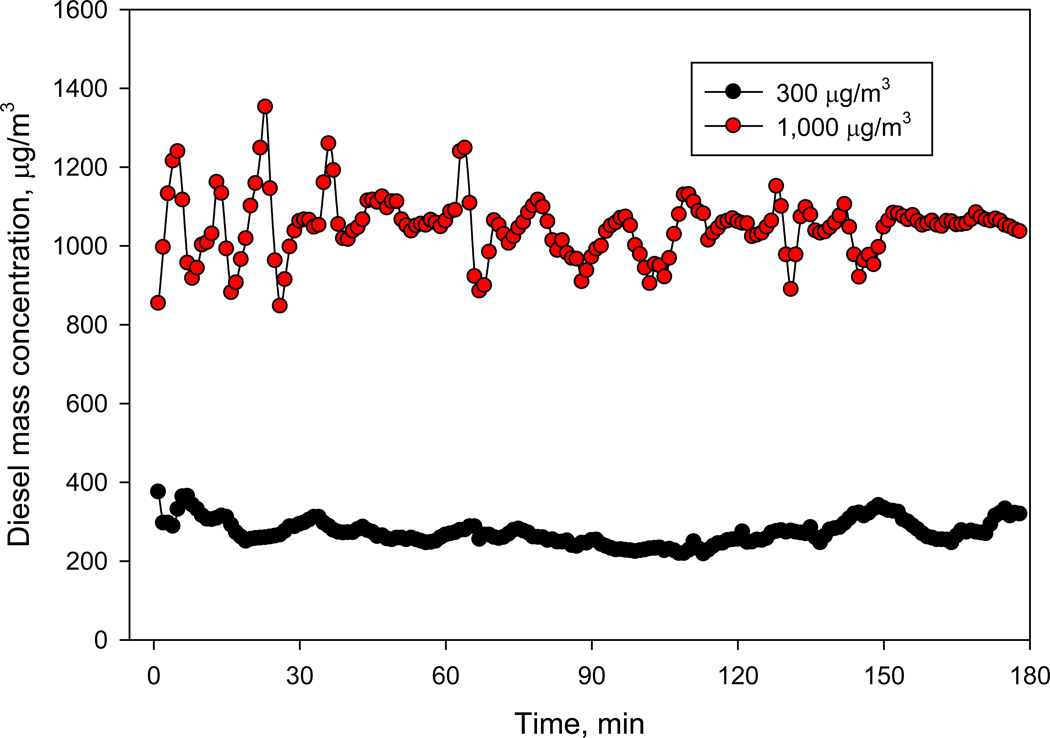
An example of diesel exhaust mass concentration changes in the exposure chamber over time is presented in Figure 5. For both particle mass concentrations (300 and 1000 µg/m3), the coefficient of variation over 3 h period was ≤15%, which indicates stable aerosol concentration during the exposures.
Figure 5.
Mass Concentration Changes Over Time
We also measured gas concentrations inside the chamber (Table 1). Since gaseous co-pollutants are generated by combustion, it was expected that their concentrations would change with the same ratio as the diesel exhaust mass concentration due to the introduction of dilution air. As the PM concentration increased from 300 µg/m3 to 1000 µg/m3, the concentration of NOx (NO + NO2) increased by a factor of 3.3 as expected, from 4.28 ppm to 14.47 ppm. The increase was largely due to an increase in NO2 (from 3.86 to 14 ppm), while the concentration of NO remained relatively stable. The CO concentration was found to increase from 4 ppm at a PM concentration of 300 µg/m3 to 6.6 ppm at 1000 µg/m3, which is less than a factor of two. The CO2 concentration increased by more than a factor of two, from 1200 to 2500 ppm, as the PM concentration increased. By comparison, the CO concentration in the dilution air was < 1 ppm, the CO2 concentration, approximately 350 ppm, and the NOx concentration, <0.02 ppm (data not shown).
EC/OC/TC levels in diesel exhaust were quantified by a commercial laboratory. Preliminary results for the mass concentration of 300 µg/m3 indicated an OC concentration of 124 µg/m3, an EC concentration of 115 µg/m3 and the resulting TC concentration of 239 µg/m3. This is in reasonable agreement with OC/EC ratios reported in diesel vehicle emissions studies, which are quite variable (Zielinska et al, 2004; Fujita et al, 2007). The carbon content analysis at the 1000 µg/m3 mass concentration was not performed due to technical difficulties.
II. Effects of Inhaled Diesel Exhaust on Markers of Lung Injury in Young and Old Mice
The next series of studies were undertaken to determine if older animals are more susceptible to the adverse effects of inhaled diesel exhaust when compared to younger mice. For these studies, we exposed groups of six young (2 m) and six old (18 m) CB6F1 male mice to ultra pure air or diesel exhaust (300 or 1000 µg/m3) for 3 h once (single) or 3 h/day on three consecutive days (repeated). Although we originally proposed to collect samples only at 24 h post exposure, recent findings by our laboratory suggested that fine PM-induced injury and inflammatory changes in the lung may occur more rapidly (Sunil et al, 2007a). Thus a 0 h post exposure time point was included in our analysis. Lung tissue was evaluated histologically for structural and morphological alterations. Additional markers of lung injury that were assessed included BAL cell number, protein and albumin content, LDH activity and expression of the acute phase protein, lipocalin 24p3. For the purposes of clarity, data on single and repeated exposure to diesel exhaust are described separately.
Effects of Diesel Exhaust on Lung Histology
Single Exposure
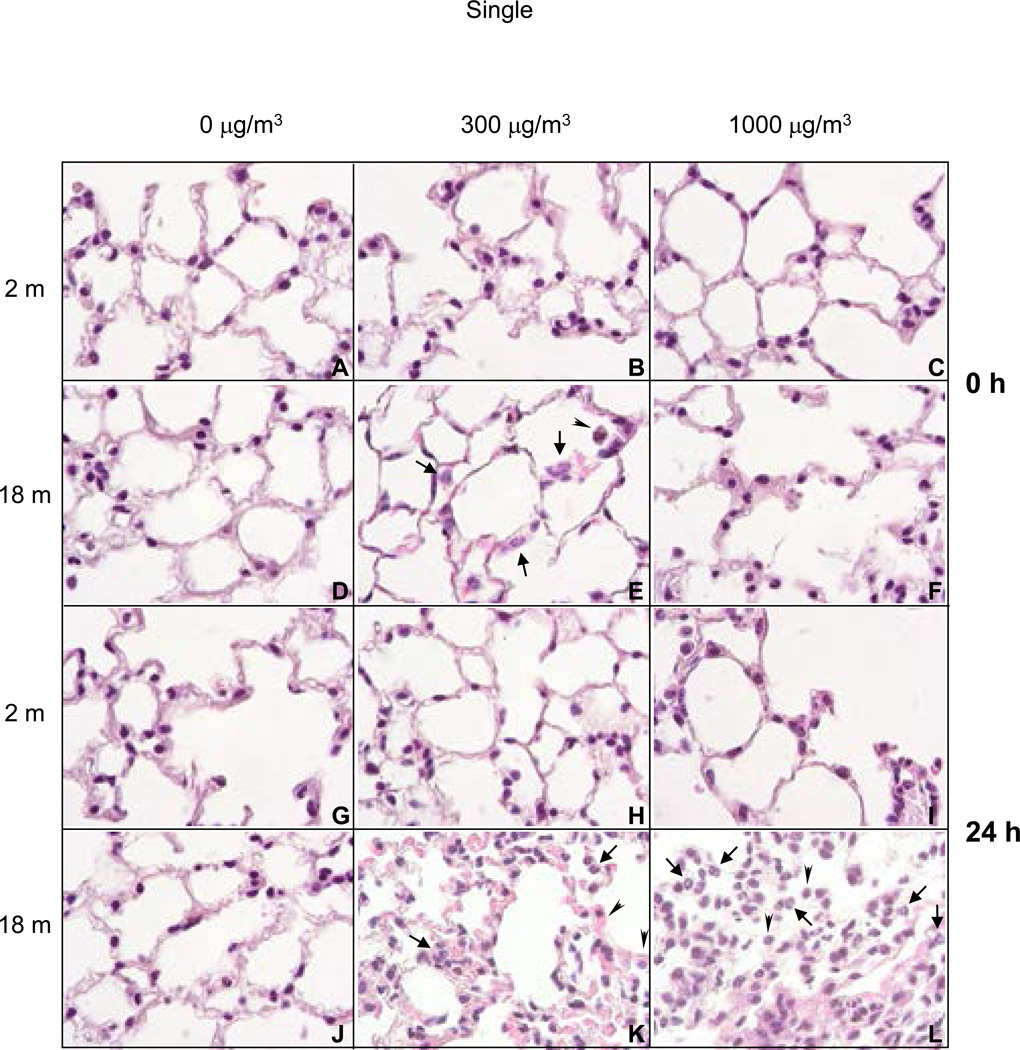
A single acute exposure of young mice to either low (300 µg/m3) or high dose (1000 µg/m3) diesel exhaust had no significant effects on lung histology (Figure 6, panels B, C, H, and I). In older animals however, after low dose diesel exhaust exposure, an immediate increase in neutrophils was noted in alveolar spaces and capillaries which became more pronounced after 24 h (Figure 6, panels E and K). Inflammatory changes were more marked in older mice exposed to high dose diesel exhaust, and included greater numbers of neutrophils in alveolar spaces and capillaries, and focal inflammatory infiltrates consisting predominantly of plasma cells and macrophages (Figure 6, panels F and L). These changes became more prominent 24 h post exposure to high dose diesel exhaust.
Figure 6.
H and E Staining of Lung
Repeated Exposure
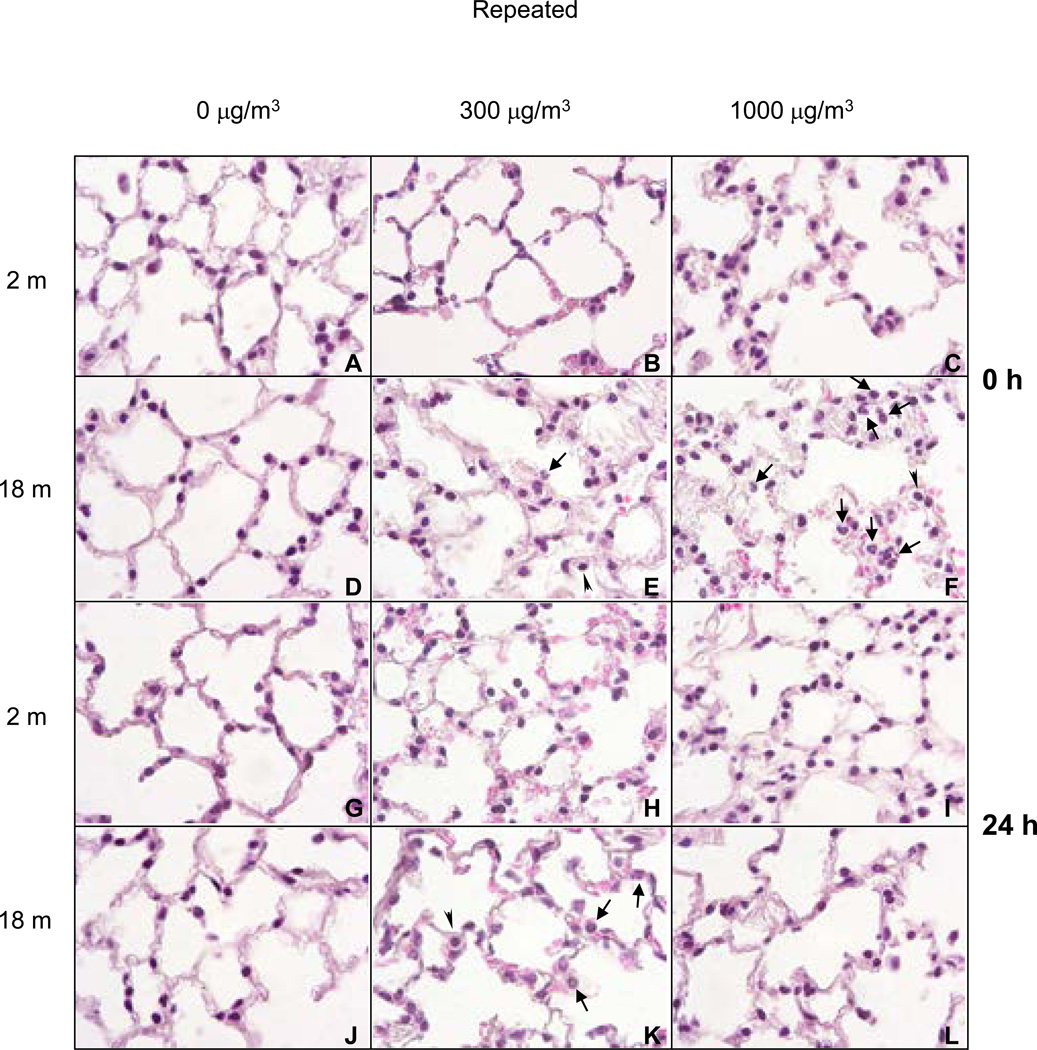
In older animals, repeated exposure to low dose diesel exhaust resulted in patchy thickening of alveolar septa, cytomegaly in alveolar septal walls and an increase in the number of macrophages in alveolar spaces immediately after exposure (Figure 7, panel E). By 24 h post exposure, focal thickening of alveolar septal walls and increased numbers of neutrophils and erythrocytes were evident (Figure 7, panel K). Structural alterations in younger mice following low dose exposure to diesel exhaust were minimal when compared to changes observed in older animals (Figure 7, panels B, C, H and I). However, the most significant and consistent inflammatory changes were noted in older animals exposed to repeated high dose diesel exhaust. Thus, in older animals, greater numbers of neutrophils were observed in alveolar spaces and capillaries. Focal infiltrates consisting predominantly of plasma cells and macrophages were also increased in older animals (Figure 7, panels F and L). Unlike the effects of a single exposure to high dose diesel exhaust, histologic changes in lungs of older animals exposed to repeated diesel exhaust were more prominent immediately after exposure.
Figure 7.
H and E Staining of Lung
Effects of Diesel Exhaust on BAL Cell Number
Single Exposure
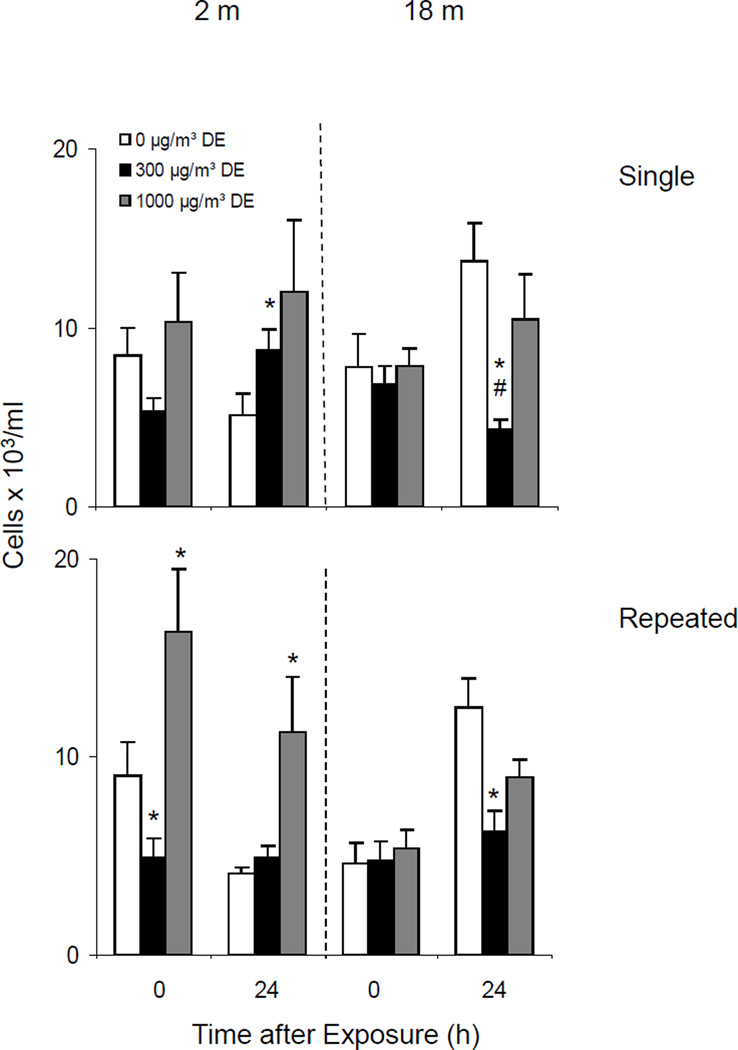
In further studies, we compared the number of cells recovered in BAL fluid from younger and older mice following a single acute exposure to diesel exhaust. In general, these values were highly variable which most likely reflects variations in quantities of lavage fluid recovered from the mice. Similar numbers of cells were recovered from younger and older animals immediately after exposure to air control (Figure 8, upper panel). Whereas in younger animals no significant effects were observed immediately after exposure to low or high dose diesel exhaust, after 24 h, a small increase in BAL cell number was noted. In contrast, a decrease in cell number was observed in the older animals at low dose diesel exhaust.
Figure 8.
Effects of DE on BAL Cell Number
Repeated Exposure
Repeated exposure of younger animals to high dose diesel exhaust resulted in an immediate increase in BAL cell number which persisted for at least 24 h. As observed with single diesel exhaust exposure, in older animals, cell number decreased after exposure to diesel exhaust (Figure 8, lower panel).
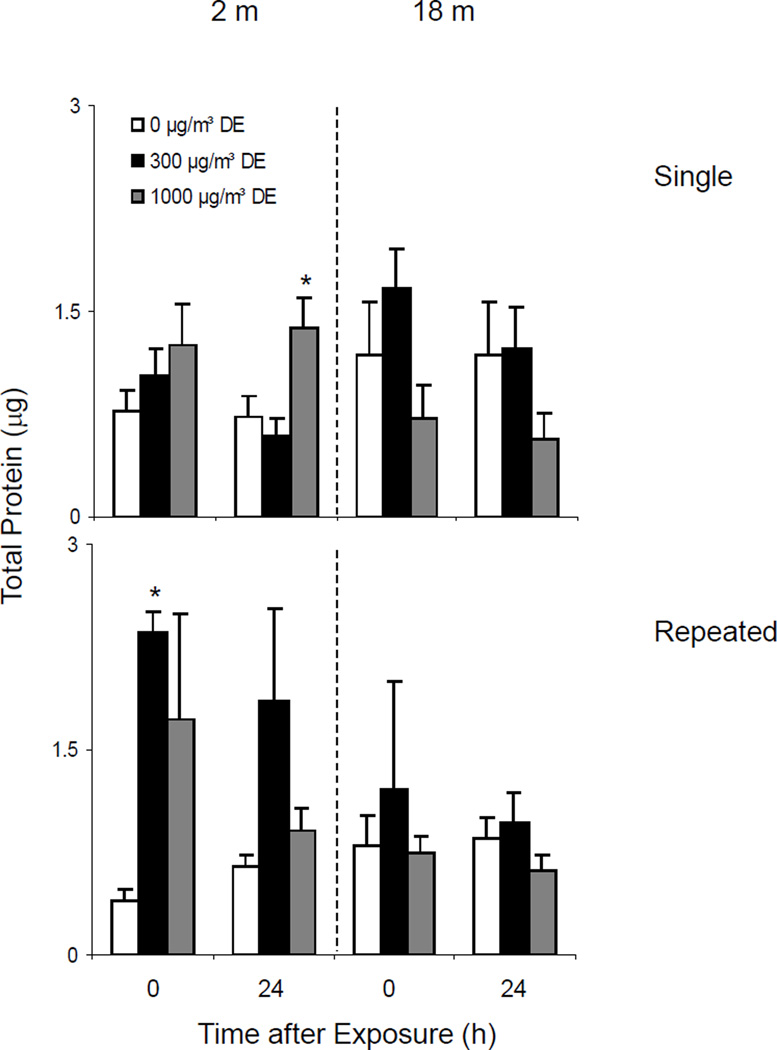
Effects of Diesel Exhaust on BAL Protein Levels
Single Exposure
Increased BAL protein content indicates enhanced alveolar epithelial permeability and is a marker of acute injury to the lower lung (Bhalla, 1999). Low levels of protein were detected in BAL from both young and old animals. Whereas a single exposure of mice to high dose diesel exhaust increased BAL protein in younger animals, no significant effects were noted in older animals (Figure 9, upper panel).
Figure 9.
Effects of DE on BAL Protein Levels
Repeated Exposure
Increases in BAL protein were also observed in younger animals following repeated exposures to both low and high dose diesel exhaust. In general, this trend persisted for at least 24 h (Figure 9, lower panel). In contrast, no significant changes were noted in BAL protein in older animals after repeated exposure to diesel exhaust.
We also quantified albumin levels in BAL from young and older mice after single and repeated exposures to diesel exhaust. No significant effects were observed at either 300 or 1000 µg/m3 DE (data not shown).
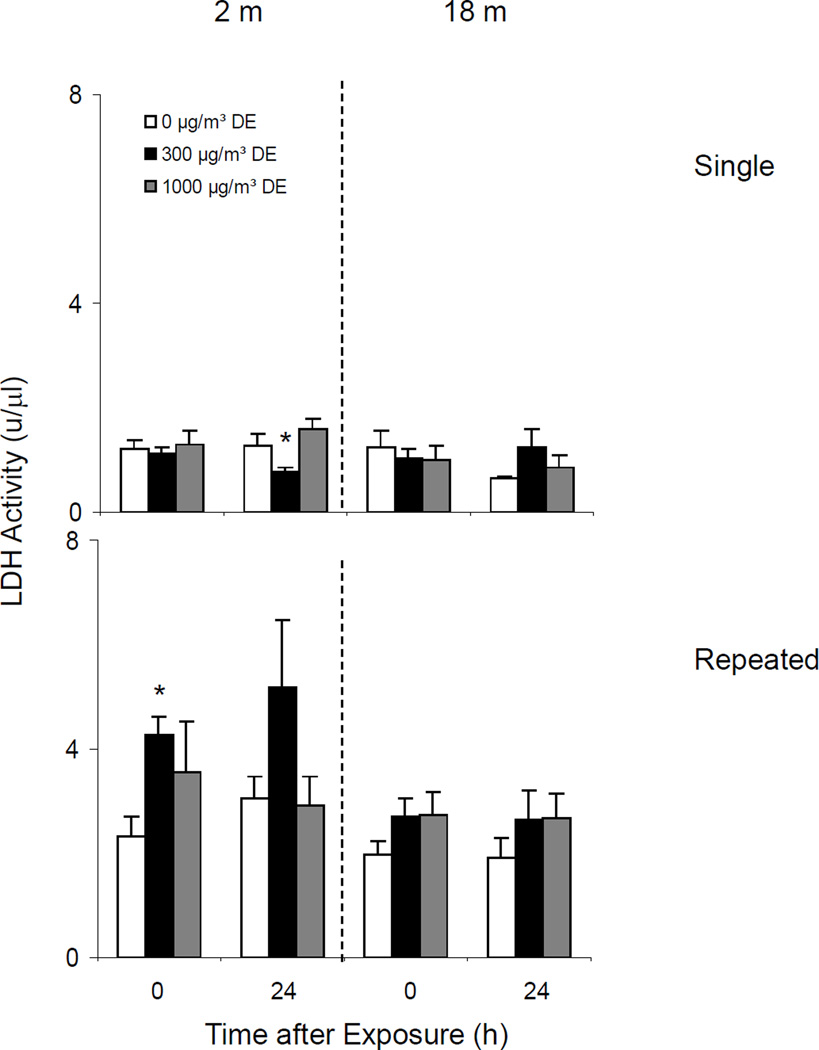
Effects of Diesel Exhaust on BAL LDH Activity
Single Exposure
In our next set of experiments, we quantified LDH activity in BAL as a marker of cytotoxicity. Constitutive LDH activity was detected in BAL from both younger and older mice (Figure 10, upper panel). Whereas exposure of younger mice to low dose diesel exhaust resulted in a small decrease in LDH activity at 24 h, no significant effect was seen in older animals.
Figure 10.
Effects of DE on BAL LDH Activity
Repeated Exposure
In contrast to results observed following a single diesel exhaust exposure, after repeated exposure of younger mice to low dose diesel exhaust, an increase in LDH activity was noted (Figure 10, lower panel). However, these changes were not observed in older mice.
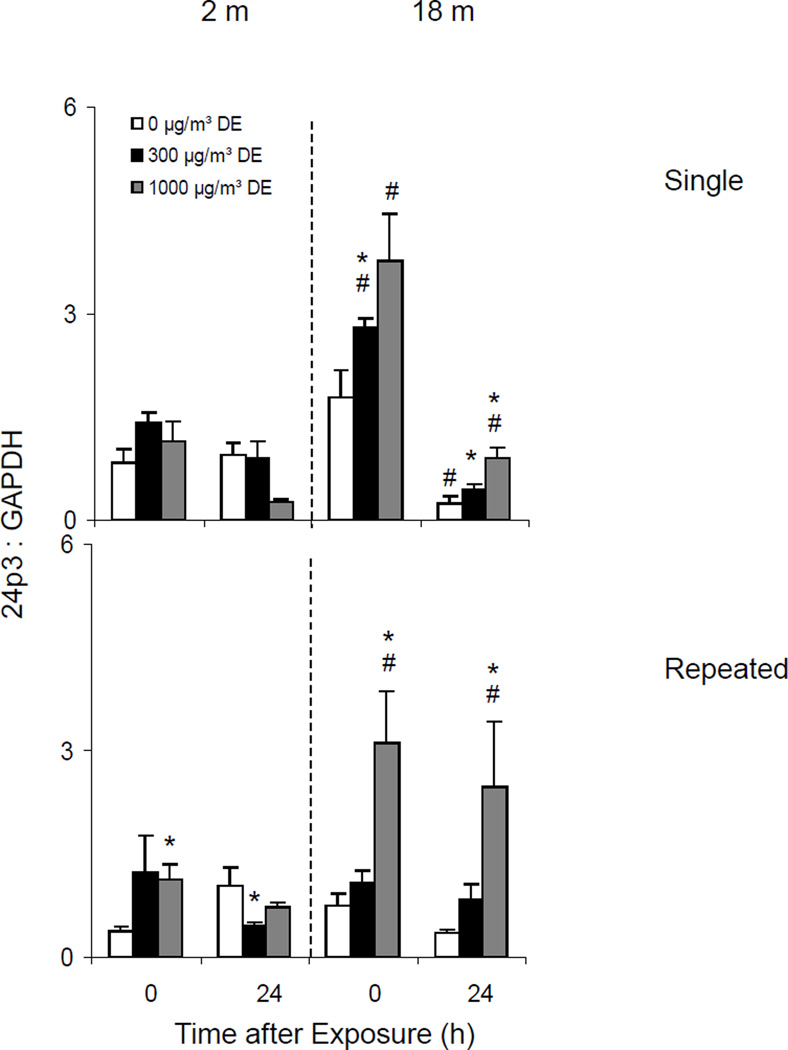
Effects of Diesel Exhaust on Expression of Lipocalin 24p3
Acute phase proteins such as lipocalin 24p3 are rapidly produced in response to oxidative stress and tissue injury (Roudkenar et al, 2007). In further studies we analyzed the effects of diesel exhaust on expression of 24p3 in the lung.
Single Exposure
A single acute exposure to diesel exhaust caused a dose related increase in lipocalin 24p3 mRNA expression in the lung, but only in older animals (Figure 11, upper panel). The effects of diesel exhaust were most pronounced immediately after exposure.
Figure 11.
Effects of DE on 24p3 mRNA Expression
Repeated Exposure
In younger animals, repeated exposure to diesel exhaust resulted in a small, but transient increase in 24p3 expression (Figure 11, lower panel). By comparison, in older mice, repeated exposure to high dose diesel exhaust caused a 3-fold increase in expression of this acute phase protein. This was observed immediately after exposure and persisted for at least 24 h.
III. Effects of Inhaled Diesel Exhaust on Expression of Inflammatory Mediators and Antioxidants in the Lungs of Younger and Older Mice
According to our hypothesis, increases in the sensitivity of the elderly to inhaled diesel exhaust is due to age-related alterations in expression of inflammatory mediators such as TNFα and antioxidants, and this was analyzed in our next series of pilot studies. Although we originally proposed to evaluate the effects of diesel exhaust on TNFR1 using ELISA, we were unable to complete these studies since we did not have sufficient quantities of BAL and serum to perform the assays. We were also unable to assess TNFα or TNFR1 protein expression in the lung due to technical difficulties which we are continuing to overcome. As an alternative, we analyzed the effects of diesel exhaust on TNFα, Interleukin-6 (IL-6), Interleukin-8 (IL-8), and cyclooxygenase-2 (COX-2) mRNA expression in the lung by real-time PCR and TNFα protein in serum by ELISA.
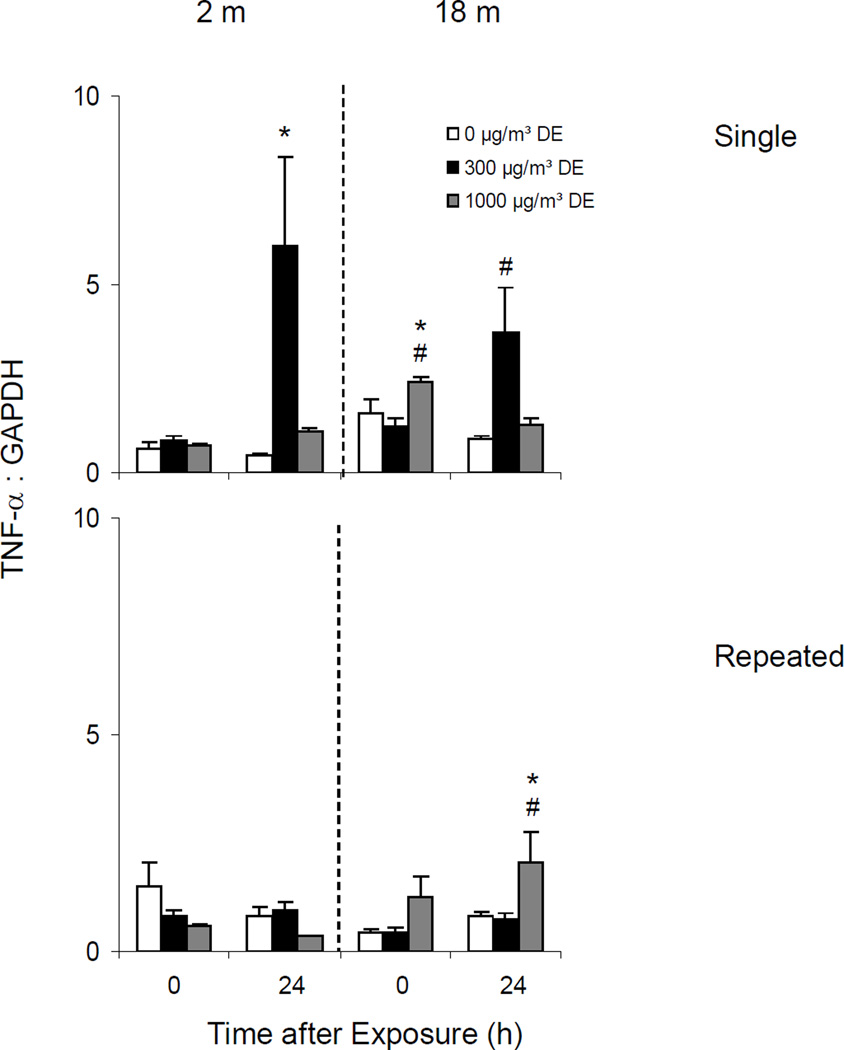
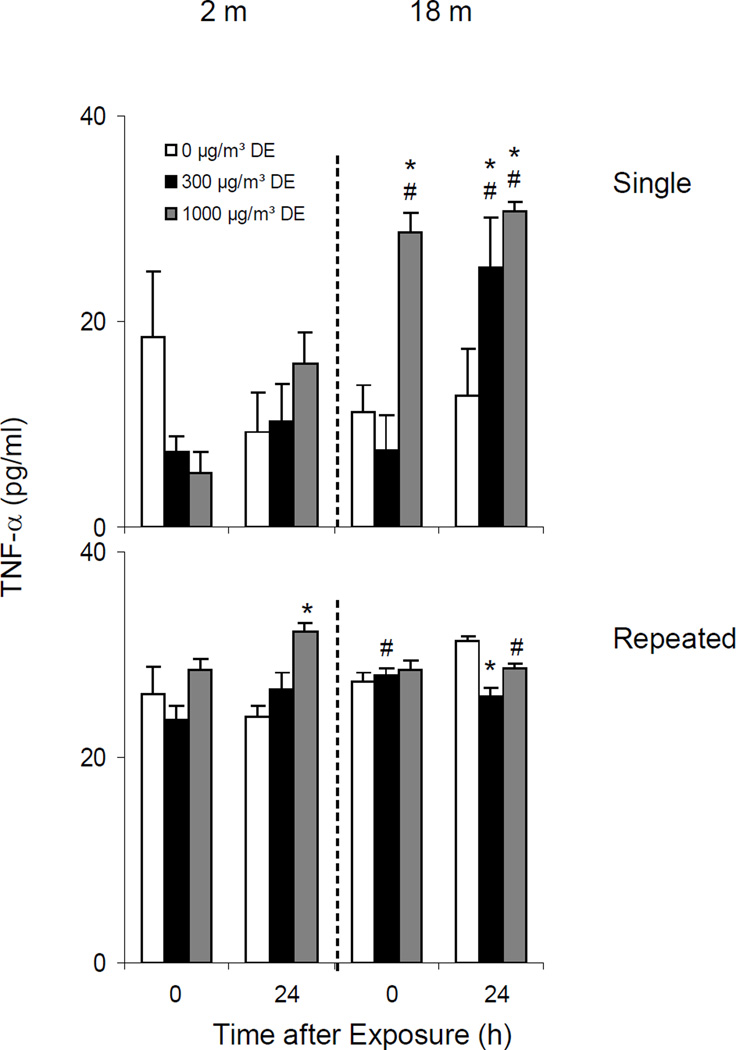
Effects of Diesel Exhaust on TNFα Expression
Single Exposure
Both younger and older animals constitutively expressed low levels of TNFα mRNA in the lungs. Treatment of younger mice with diesel exhaust had no effect on TNFα expression immediately after exposure (Figure 12, upper panel). In contrast, a five-fold increase in lung TNFα was noted 24 h post exposure to low dose diesel exhaust. Although increases in TNFα mRNA were also observed at this time in older animals after exposure to low dose diesel exhaust, this response was significantly attenuated.
Figure 12.
Effects of DE on TNF-α mRNA Expression
Repeated Exposure
No significant change in TNFα expression was noted in younger animals following repeated exposure to diesel exhaust. In older animals however, a small but significant increase in TNFα mRNA expression was observed after high dose diesel exhaust exposure, but this was only evident after 24 h (Figure 12, lower panel).
Effects of Diesel Exhaust on Serum TNFα Levels
Single Exposure
We next assessed the effects of diesel exhaust on serum TNFα levels. Low levels of TNFα were detected in sera from both younger and older animals (Figure 13, upper panel). Diesel exhaust caused a marked increase in serum TNFα in older but not younger animals, an effect that persisted for 24 h in older mice.
Figure 13.
Effects of DE on Serum TNF-α Levels
Repeated Exposure
In general the effects of repeated exposure of younger and older mice to diesel exhaust were quite modest. Thus, while a small but significant increase in serum TNFα was noted in younger mice 24 h post exposure, in older mice serum TNFα levels decreased (Figure 13, lower panel).
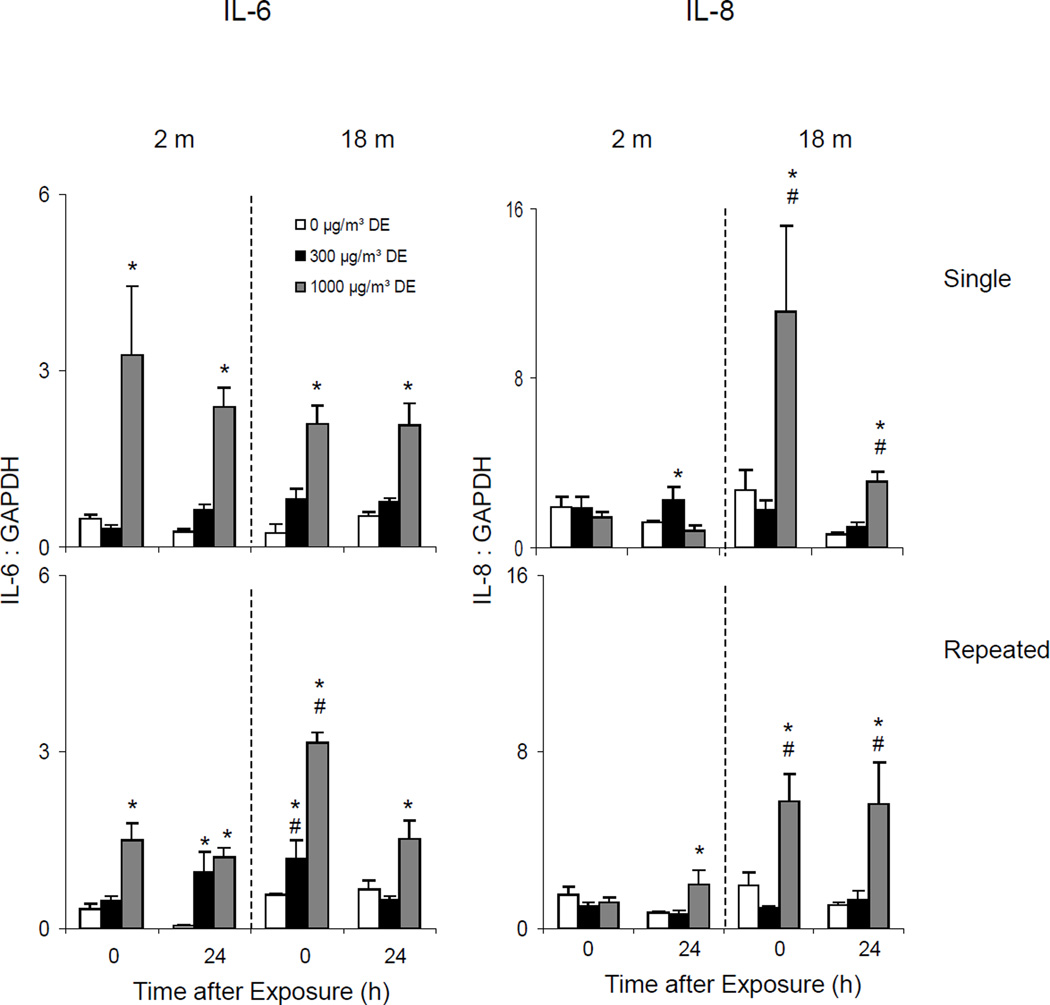
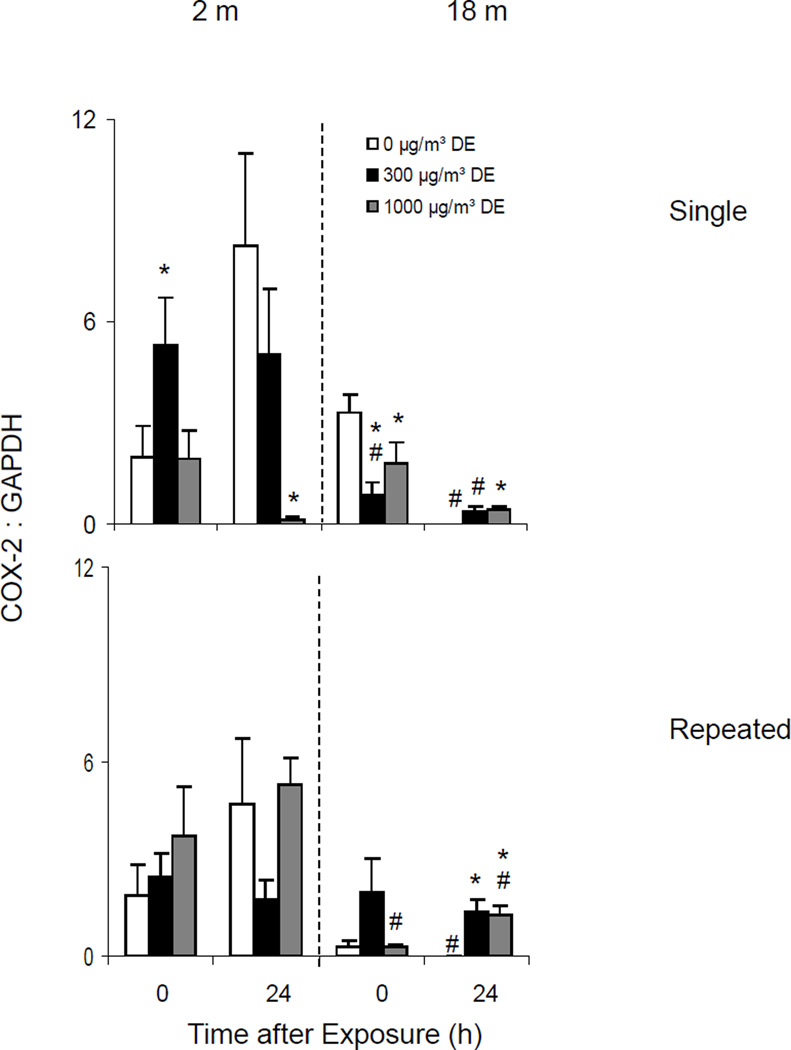
Effects of Diesel Exhaust on IL-6, IL-8 and COX-2 expression
Single Exposure
In further studies we determined if exposure of younger and older mice to diesel exhaust differentially modified expression of other inflammatory proteins known to be important in the lung response to pulmonary irritants. These included IL-6, IL-8, and COX-2.
High dose exposure to diesel exhaust resulted in significant increases in IL-6 and IL-8 mRNA expression in lungs of older animals which persisted for 24 h (Figure 14, upper panels). Although IL-6 was also upregulated in younger mice after a single low dose diesel exhaust exposure, no major changes were noted in IL-8 mRNA expression. A single low dose exposure to diesel exhaust also resulted in increased COX-2 mRNA expression in younger animals, which was observed immediately after exposure (Figure 15, upper panel). In contrast, COX-2 mRNA expression decreased in lungs of older animals exposed to a single dose of diesel exhaust.
Figure 14.
Effects of DE on IL-6 and IL-8 mRNA Expression
Figure 15.
Effects of DE on COX-2 mRNA Expression
Repeated Exposure
Generally similar effects of diesel exhaust on IL-6 and IL-8 expression were observed after repeated exposure (Figure 14, lower panels). In contrast, repeated exposure to diesel exhaust had no consistent effects on COX-2 expression in the lung (Figure 15, lower panel).
Effects of Diesel Exhaust on MnSOD Expression
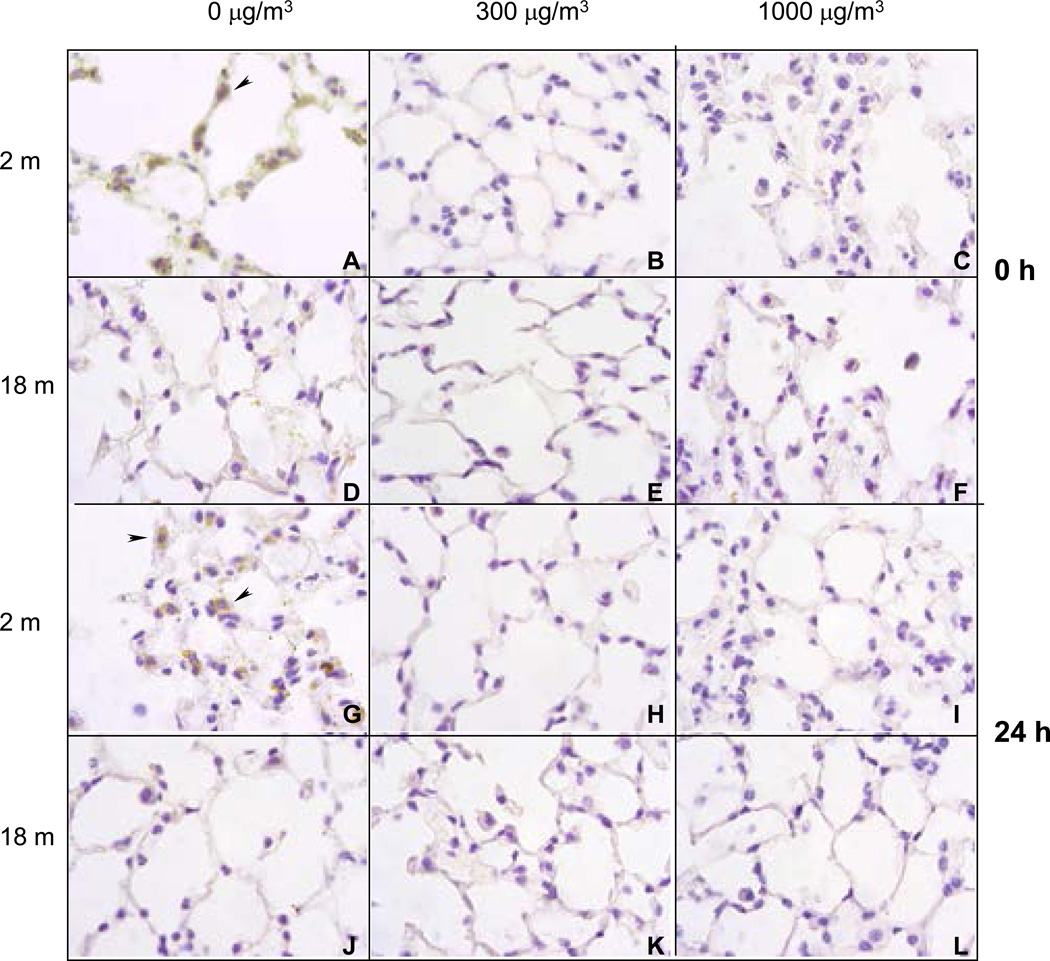
Single Exposure
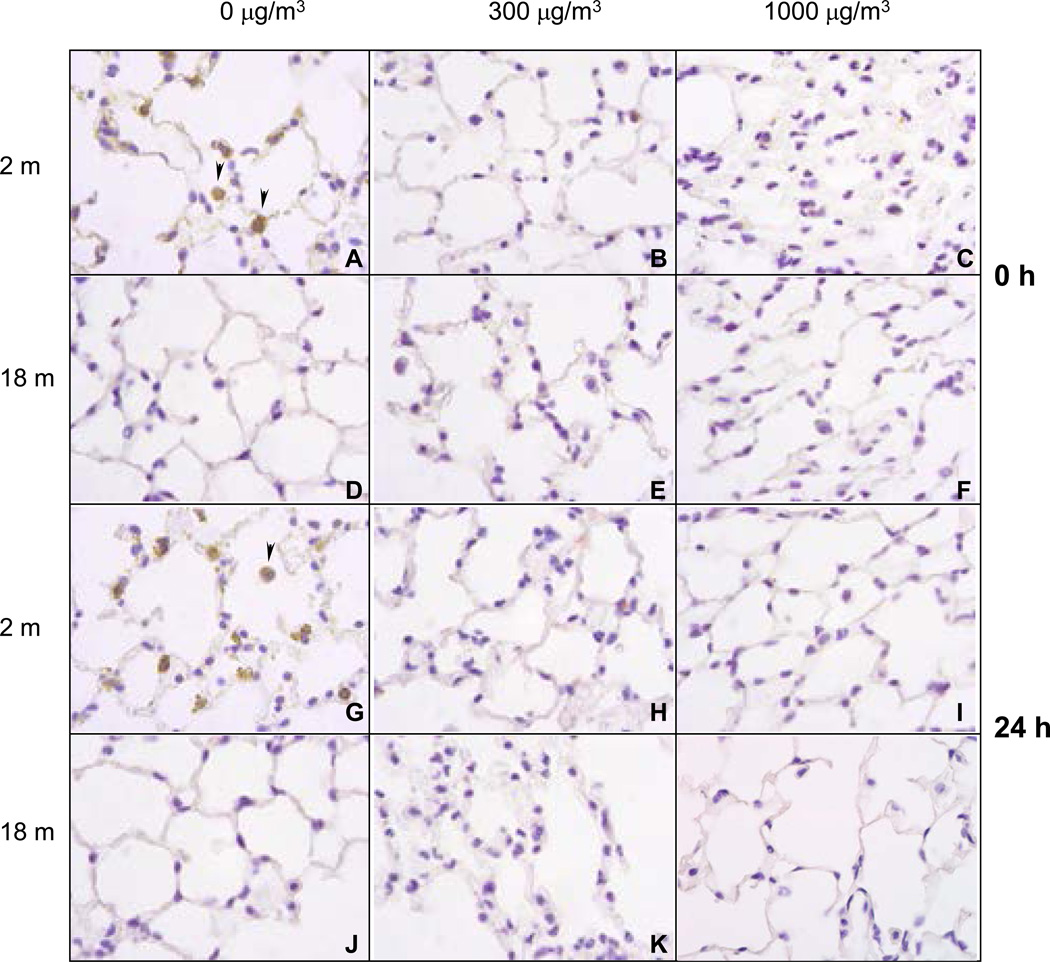
In our next series of experiments, we evaluated the effects of diesel exhaust on expression of the antioxidant, MnSOD in the lung. Immunohistochemical analysis revealed that younger, but not older mice, constitutively expressed MnSOD (Figure 16, panels A, and G). This was most prominent in alveolar macrophages (indicated by arrowhead). While exposure to diesel exhaust at both 300 and 1000 µg/m3 resulted in a persistent decrease in MnSOD expression in younger animals (Figure 16, panels B, C, H and I), no changes were seen in older animals (Figure 16, panels E, F, K, and L).
Figure 16.
Effects of Single DE Exposure on Mn SOD Expression
Repeated Exposure
A similar antioxidant response was noted in younger mice after repeated exposure of diesel exhaust. Thus, a persistent decrease in constitutive expression of MnSOD was observed following repeated exposure to DE (Figure 17, panels A, B, C, G, H, and I). In contrast, MnSOD levels remained undetectable in older animals following repeated exposure to diesel exhaust (Figure 17, panels D, E, H, J, K and L).
Figure 17.
Effects of Repeated DE Exposure Mn SOD Expression
Discussion
The aims of this study were to develop and characterize a diesel exhaust animal exposure system and to compare the effects of inhaled diesel exhaust on younger and older mice. We hypothesized that increased susceptibility of older animals to the adverse cardiopulmonary effects of inhaled diesel exhaust is due to altered expression of inflammatory mediators and antioxidants in the lung. Our overall goal in these pilot studies was to generate preliminary data to support this hypothesis.
The animal chamber that was constructed consisted of a 17-liter whole body Plexiglas divided enclosure that allowed simultaneous exposure of 12 mice (2 groups of six mice on each side of the enclosure). Using a SidePack personal aerosol mass monitor, we found that particle mass concentrations from 300 µg/m3 to 1000 µg/m3 could be maintained stably in the chamber during the 3 h animal exposure period. Moreover, the mass concentrations in the chamber could be varied without substantially changing the particle size distribution. The particle size median diameter distribution within the chamber was found to be 200–300 nm which is larger than previously reported (McDonald et al, 2004). This may be due to differences in the animal exposure systems. It is likely that the larger particle size distribution of our diesel exhaust resulted in lower deposition in the lung. Co-pollutant measurements assessed using an IAQ RAE gas monitor revealed relatively low levels of CO and NO in the chamber, and markedly higher concentrations of CO2 and NO2. Although concentrations of NO remained relatively stable, CO2 and NO2 levels increased 2–3 fold as PM mass increased from 300 to 1000 µg/m3. These increases are likely due to the fact that a larger fraction of the primary diesel exhaust was directed into the delivery system to achieve the desired 1000 µg/m3 mass concentration when compared to the 300 µg/m3 concentration and not to contaminants in the dilution air.
For our animal studies, three different atmospheres (0 µg/m3 diesel exhaust [air], 300 µg/m3 diesel exhaust and 1000 µg/m3 diesel exhaust), two exposure regimens (single and repeated), and two post-exposure time points (0 h and 24 h) were evaluated. In our first series of experiments we determined if older mice were more susceptible to the adverse pulmonary effects of diesel exhaust when compared to younger mice. Markers of lung injury including structural and morphological alterations, BAL cell number, protein and albumin content and LDH activity, and lung expression of lipocalin 24p3 were assessed. In older, but not younger mice, exposure to diesel exhaust was associated with rapid and progressive morphological and structural alterations in the lung. These included neutrophil accumulation in alveolar spaces and capillaries, as well as focal infiltrates consisting predominantly of plasma cells and macrophages, patchy thickening of alveolar septa, and an increase in the number of macrophages in alveolar spaces. Similar morphological changes have previously been described in older animals after exposure to PM (Sunil et al, 2007a). The effects of diesel exhaust on lung pathology in older mice were dose related and more pronounced 24 h post exposure. Interestingly, no major differences were noted between single and repeated exposure to diesel exhaust, suggesting that the effects of diesel exhaust on lung morphology are not cumulative. The fact that significant changes in lung pathology were not observed in younger mice is consistent with our hypothesis that older mice are more susceptible to the toxic effects of inhaled diesel exhaust.
A characteristic feature of injury to the lower lung is an accumulation of protein and inflammatory cells in BAL fluid, and an increase in LDH activity (Bhalla, 1999). Despite significant evidence of lung pathology in histological sections, no changes were noted in levels of protein or LDH activity in BAL fluid from older mice. This may reflect the relative insensitivity of these measures as markers of lung injury and inflammation (Li et al, 1999). Alternatively, diesel exhaust-induced injury may not be prominent in the lower lung of older mice. In contrast, some increases in cell number, protein and LDH activity in BAL fluid were detected in younger animals. In general these were greater after repeated, when compared to a single diesel exhaust exposure, and for protein and LDH activity were more pronounced at the 300 µg/m3 dose of DE. However, no major structural alterations were noted in the lungs of younger mice. These findings further indicate the limitations in the use of BAL protein and cell number as general markers of acute lung injury. Our studies also suggest that diesel exhaust induces a distinct spectrum of toxic effects in younger versus older mice. Of note was our finding that BAL cell number was reduced in older mice 24 h following single or repeated diesel exhaust exposure. This is most likely due to increased adherence of inflammatory cells to alveolar epithelium, which is consistent with our histologic findings.
Lipocalin 24p3 (Lcn2, NGAL) is a 25 kDa member of the lipocalin family of proteins. Originally identified as an acute phase protein produced in the liver (Liu and Nilsen-Hamilton, 1995), it has since been demonstrated that 24p3 is upregulated in a number of pathologic states including cancer, acute endotoxemia, ischemia-reperfusion injury, β-thalassemia, infection, inflammation, kidney, lung and heart injury, as well as burn and radiation-induced injury (Nielsen et al, 1996; Friedl et al, 1999; Mishra et al, 2004; Missiaglia et al, 2004; Vemula et al, 2004; Giordano, 2005; Mishra et al, 2006; Roudkenar et al, 2007; Sunil et al, 2007b; Stevens et al, 2008). The fact that each of these conditions is characterized by excessive production of reactive oxygen species has led to the suggestion that 24p3 is important in the response of cells and tissues to oxidative stress (Roudkenar et al, 2007). This is supported by findings that hydrogen peroxide-induced 24p3 expression is inhibited by antioxidants (Roudkenar et al, 2007). The present studies demonstrate that exposure of older mice to diesel exhaust is associated with a rapid dose related induction in 24p3 expression in the lung. This occurred immediately after a single or repeated exposure to diesel exhaust, and persisted for 24 h, although at reduced levels. These data suggest that 24p3 may be a highly sensitive biomarker of early oxidative stress in the lung. In contrast to older mice, increases in 24p3 expression in younger animals were relatively small and only noted immediately after repeated exposure to diesel exhaust. Similar, relatively small increases in 24p3 expression, have also been reported in lungs of young mice following exposure to the combination of diesel exhaust and LPS (Yanagisawa et al, 2004). These data provide additional evidence that older mice are more sensitive than younger mice to inhaled diesel exhaust.
In our next series of studies we analyzed potential mechanisms underlying increased susceptibility of older mice to the untoward effects of inhaled diesel exhaust. We speculated that altered production of inflammatory cytokines like TNFα, IL-6 and IL-8 and reduced expression of antioxidants such as SOD, following diesel exhaust exposure may underlie this response. TNFα is a macrophage-derived cytokine implicated in the pathogenesis of lung injury induced by a number of air pollutants (Kelley, 1990; Dinarello, 1997; Schins and Borm, 1999; Gowdy et al, 2008). Although TNFα possesses proinflammatory and cytotoxic activity, it also plays an important role in initiating tissue repair. This is thought to be due to upregulation of antioxidants, like SOD, catalase and HO-1, and stimulation of epithelial cell proliferation and extracellular matrix turnover (Tsan et al, 1990; Sasaki et al, 2000; Ryter et al, 2002; Alam and Cook, 2003; Chiu et al, 2003b; Guo et al, 2003). In accord with previous studies (Saber et al, 2006; Gowdy et al, 2008), we found significant increases in expression of TNFα in lungs of younger animals 24 h after exposure to diesel exhaust. Interestingly, this response was most notable after exposure of younger mice to 300 µg/m3 diesel exhaust, which is consistent with our findings on diesel exhaust-induced increases in BAL cell and protein content and LDH activity. It may be that higher doses of diesel exhaust cause suppression of inflammatory responses in the lung (Yin et al, 2002).
Although TNFα expression also increased in lungs of older animals 24 h following exposure to 300 µg/m3 diesel exhaust, this response was attenuated relative to younger mice. A similar attenuation of TNFα in lung or BAL fluid has previously been described in older animals after induction of endotoxin shock and after exposure to silica or terpene oxidation products (Corsini et al, 2003; Corsini et al, 2004; Ito et al, 2007; Sunil et al, 2007a). Age-related decreases in production of TNFα have also been described in isolated monocytes and macrophages (Lloberas and Celada, 2002; Renshaw et al, 2002; Boehmer et al, 2004). Of note, this response appears to be tissue specific, with the lung showing the greatest deficit in TNFα activity (Bradley et al, 1989; Higashimoto et al, 1993; Shimada and Ito, 1996). It remains to be determined if these aberrations in TNFα production in older mice are important in their susceptibility to inhaled diesel exhaust.
In contrast to the effects of diesel exhaust on TNFα expression in the lung, serum TNFα levels were increased in older mice, but not younger mice after a single diesel exhaust exposure. Whereas at the higher dose of diesel exhaust, this was observed immediately after exposure, at the lower dose, the response was delayed for 24 h. The origin of TNFα in serum from older mice is unknown. It may reflect TNFα rapidly released in the lung following diesel exhaust exposure. Alternatively, TNFα may be released by extrapulmonary tissues responding to diesel exhaust - induced oxidative stress. Additional studies are required to explore these possibilities.
IL-6 and IL-8 are known to be important in regulating inflammatory cell trafficking into injured tissues (Murtaugh et al, 1996; McClintock et al, 2008). Previous studies have described diesel exhaust-induced increases in IL-6 and IL-8 in rodent lung (Gowdy et al, 2008) and in cultured bronchial epithelial cells (Steerenberg et al, 1998). Similarly, we noted significant increases in IL-6 and IL-8 mRNA expression in lungs of younger and older animals after both single and repeated exposure to diesel exhaust. Whereas the kinetics of IL-6 mRNA expression appeared to be independent of age, IL-8 expression differed markedly between younger and older animals. Thus, IL-8 levels increased in older animals immediately after diesel exhaust exposure while expression of this cytokine was delayed for 24 h in younger animals. A number of studies have described increased production of IL-8 with advancing age (Esposito et al, 1989; Himi et al, 1997; Pulsatelli et al, 2000). Our finding that IL-8 is generated more rapidly in the lung in older mice following diesel exhaust exposure is in accord with these studies. IL-8 is a potent chemoattractant for neutrophils (Kafoury and Kelley, 2005; Matsuzaki et al, 2006). These cells are known to be a major source of reactive oxygen species. Our findings of increased IL-8 in older mice following exposure to diesel exhaust are consistent with our histologic data of increased accumulation of neutrophils in the lung. These cells may contribute to excessive oxidative stress in the elderly in response to inhaled diesel exhaust.
We also analyzed expression of COX-2, an inducible enzyme mediating the production of prostaglandins during inflammation and immune responses (Park and Christman, 2006). COX-2 expression has been reported to be induced in lung cells following exposure to diesel exhaust particles (Inoue et al, 2004; Cao et al, 2007; Ahn et al, 2008). Similarly, we found that a single diesel exhaust exposure was associated with upregulation of COX-2 expression in younger mice. In contrast, COX-2 expression decreased in older animals after diesel exhaust. These results were surprising since COX-2 expression and PGE2 production have been reported to be upregulated in elderly animals (Go et al, 2007; Meydani and Wu, 2007; Wu et al, 2007; Tang and Vanhoutte, 2008). These findings suggest that in our system, the proinflammatory activity of COX-2 may not play a major role in determining susceptibility to diesel exhaust; however this remains to be determined.
Li et al. (2004) have suggested that oxidative stress is a key mechanism by which ambient PM induces adverse health effects. According to these investigators, PM-induced oxidative stress is a multi-tier response in which cytoprotective responses transition to injurious effects as the level of oxidative stress increases. Antioxidants play a critical role in host defense by scavenging and detoxifying oxidants. Thus, they minimize tissue damage and the development of diseases associated with oxidative stress (Heffner, 1991; Nel et al, 1998; Dhalla et al, 2000; Lang et al, 2002). One antioxidant that plays a key role in protecting cells and tissues from oxidative stress is SOD. Three isoforms of SOD have been identified, MnSOD, copper-zinc SOD (CuZnSOD) and extracellular SOD (EC-SOD), each with different structures and tissue distributions (Kinnula and Crapo, 2003). The important protective role of SOD is most evident from studies demonstrating reduced mortality and tissue injury in transgenic mice overexpressing CuZnSOD or EC-SOD following treatment with hypoxia, ozone, PM, radiation or bleomycin (Janssen et al, 1993; Weinberger et al, 1998; Fakhrzadeh et al, 2004). With increasing age, constitutive levels of tissue antioxidants and the capacity to respond to oxidative stress declines (Lykkesfeldt and Ames, 1999; Squier, 2001; Thomas and Mallis, 2001; Servais et al, 2005). Thus, while constitutive expression of antioxidant enzymes, such as SOD rapidly decreases in younger animals as it is utilized following exposure to PM, in older mice this response is reduced or absent (Elsayed et al, 1982; Sagai et al, 1993; Lim et al, 1998; Ghio et al, 2002; Sunil et al, 2007a). We found that younger mice constitutively expressed MnSOD, which was predominantly localized in alveolar macrophages. Exposure of younger mice to single or repeated diesel exhaust resulted in a rapid reduction in MnSOD expression which persisted for at least 24 h. In contrast constitutive expression of MnSOD was not evident in lungs of older mice and diesel exhaust did not alter its expression. These findings are consistent with our hypothesis that increased susceptibility of older mice to diesel exhaust is due to aberrant antioxidant defense. Further studies are necessary in order to elucidate mechanisms regulating expression of antioxidants such as SOD in the lung.
In summary, data presented in this pilot study provide strong support for our hypothesis that there are significant aberrations in production of inflammatory mediators and antioxidant defenses in the lungs of elderly mice in response to inhaled diesel exhaust. Further studies are needed to directly test our hypothesis that these alterations underlie increases in susceptibility of the elderly to PM.
Problems Encountered.
In the original project plan, we proposed to evaluate the effect of diesel exhaust in younger and older mice using 4 test atmospheres i.e. clean air, 100 µg/m3, 300 µg/m3 and 1000 µg/m3 and one post-exposure analysis time (24 h). Using the existing diesel exhaust generation system in EOHSI that was modified for animal exposures, we found that we were unable to maintain stable particle mass concentrations of 100 µg/m3 in the animal chamber. The instability is likely due to the fact that at this concentration only a small fraction of the total diesel exhaust is directed towards the animal exposure system relative to the amount that is diverted to the exhaust vent (see Figure 1). As a consequence, the animal chamber exhaust became highly susceptible to concentration variations in the engine exhaust, as well as to the effects of wind drafts at the vent. Because we could not overcome this difficulty within the time frame and budget constraints of the pilot project, we were forced to limit our studies to concentrations of diesel exhaust of 300 and 1000 µg/m3. However, since there were extra mice available, we included an additional post exposure analysis time of 0 h which we previously found was sufficient to observe biological effects.
We originally proposed to assess TBARS as a measure of lipid peroxidation in BAL fluid, as well as TNFR1 in serum using ELISA. However, due to insufficient quantities of BAL and serum recovered from some of the mice, and the fact that some of the samples were contaminated with red blood cells, we were unable to perform these assays in sufficient replicates that would permit statistical analysis of the data.
In the original project plan, we proposed to assess TNFα and TNFR1 protein expression in the lung. Unfortunately we encountered technical problems and were unable to measure these proteins in lung tissue by immunostaining or by western blotting. The reason for these technical problems is unclear. It may be that the commercial antibodies that we tested (from three different vendors) did not recognize the exposed antigenic determinants in mouse lung. Alternatively, the post exposure analysis times that we chose (0 h and 24 h) may not have been appropriate to detect changes in these proteins. It is also possible that exposure to diesel exhaust may have caused the release of TNFα from the cells. Immunohistochemistry and western blotting are not suitable techniques for the detection of secreted proteins. Thus in future studies, we may need to use an ELISA.
Future Studies.
The overall hypothesis underlying our research is that production of inflammatory proteins such as TNFα, IL-6, IL-8 and COX-2 by alveolar macrophages and antioxidant defense mechanisms in the lung are altered in the elderly and this increases their susceptibility to fine PM-induced oxidative stress and tissue injury. Studies described in this report provide preliminary data that support this hypothesis. Thus following diesel exhaust exposure we found reduced expression of TNFα, and increased expression of IL-6 and IL-8 in lungs of older mice relative to younger mice; moreover antioxidant defense responses (e.g., MnSOD expression) were impaired. These preliminary data provide a strong basis and rationale for continuing studies aimed at testing our hypothesis. These studies will include assessing the injurious/inflammatory effects of diesel exhaust on the lung, as well as the heart, at lower concentrations (30 and 100 µg/m3) and at additional post exposure times (6 h, 48 h, 72 h, 7 d, 14 d). In addition to structural evaluation by light and electron microscopy, biochemical markers of tissue injury will be assessed including troponin C and I in the heart, as well as levels of IgM and fibrin in the lung, potentially more specific markers of alveolar epithelial injury.
We will also assess expression of mRNA and protein for 24p3 in the lung and heart, as an early marker of oxidative stress. The expression of various cytokines (TNFα, TNFR1, IL-1, IL-6, IL-8, IL-10), chemokines (MCP-1, MIP-2), and antioxidants SOD, ascorbate, glutathione S-transferase,, NADPH quinine oxidoreductase (NQO1), catalase, glutathione peroxidase (GPx), and heme oxygenase (HO-1) in lungs and hearts of younger and older mice will be evaluated after diesel exhaust exposure to determine if these are important in the increased susceptibility of the elderly to PM. A unique aspect of our future studies will be to include a comparative analysis of the effects of diesel exhaust in a transgenic animal model of chronic emphysema (surfactant protein D-deficient mice). These studies are particularly relevant since chronic emphysema is a common lung disease observed in the elderly, particularly in those individuals who have smoked cigarettes. We predict that older mice lacking the gene for surfactant protein D, which have developed emphysema will be more susceptible to diesel exhaust than their younger counterparts or wild type mice.
The results of these studies are important since they will provide mechanistic data that may be useful in designing efficacious strategies aimed at preventing or reducing cardiopulmonary morbidity and mortality in the geriatric population.
Implication of Findings.
The mechanisms underlying increases in susceptibility of elderly individuals to the adverse effects of inhaled PM are unknown. Data generated in these pilot studies suggest that aberrant production of inflammatory cytokines such as TNFα, IL-6, IL-8 and downstream events such as expression of antioxidants may be key determinants of susceptibility to diesel exhaust. Further studies are necessary in order to define the precise relationship between inflammatory mediators and antioxidant defense in the lung and potentially the heart of the elderly. Results from these studies will provide mechanistic insights into the pathogenesis of toxicity that may be useful in designing effective treatment or preventative strategies that limit PM-induced cardiopulmonary morbidity and mortality in susceptible populations.
Acknowledgements
The authors acknowledge Sherritta Ridgely, D.V.M., Ph.D., Clinical Laboratory Animal Veterinarian, Laboratory Animal Services, Rutgers University, for her help in evaluating lung histology. We also thank Rebecka Jornsten, Ph.D., Department of Statistics, Rutgers University, for statistical analysis of the data.
Abbreviations
- DE
diesel exhaust
- BAL
Bronchoalveolar lavage
- LDH
Lactate dehydrogenase
- SOD
Superoxide dismutase
- PM
Particulate matter
- TNFα
Tumor necrosis factor-α
- TBARS
Thiobarbituric acid reactive substance
- TNFR1
TNF-receptor-1
- EOHSI
Environmental and Occupational Health Sciences Institute
- PBS
Phosphate buffered saline
- DTPA
Diethylenetriaminepentaacetic acid
- BSA
Bovine serum albumin
- COX
Cyclooxygenase
- IL-8
Interleukin-8
- IL-6
Interleukin–6
Biographies
Debra L. Laskin is Professor II (Distinguished Professor) and Chair of the Department of Pharmacology and Toxicology and the Roy A Bowers Endowed Chair at Rutgers University Ernest Mario School of Pharmacy. She is also a member of the Toxicology division of the Environmental and Occupational Health Sciences Institute (EOHSI) at Rutgers University/University of Medicine and Dentistry of New Jersey (UMDNJ)-Robert Wood Johnson Medical School. She received her Ph.D. in Pharmacology and Toxicology in 1980 from the Medical College of Virginia (Richmond, VA). After postdoctoral studies in immunology at the Wistar Institute of the University of Pennsylvania, she joined the faculty of Rutgers University in 1982. Her research is focused on analyzing inflammatory mechanisms mediating lung injury induced by environmental pollutants.
Gediminas Mainelis is an Associate Professor in the Department of Environmental Sciences of Rutgers University and a member of the Environmental Sciences Division of EOHSI. He received his Ph.D. in Environmental Health from the University of Cincinnati in 2000. After one year of post-doctoral studies at the same University, he joined the faculty of Rutgers with a goal of establishing an innovative research program in biological and non-biological aerosols. His research is focused on the development of bioaerosol sampling, control, and generation methods; bioaerosol exposure assessment; and exposure and health effects of nanoparticles.
Barbara J. Turpin is a Professor in the Department of Environmental Sciences of Rutgers University and a member of the Environmental Sciences Division of EOHSI. She received her Ph.D. in Environmental Science and Engineering in 1990 from Oregon Graduate Institute at the Oregon Health Sciences University. After postdoctoral studies at the University of Minnesota in the Particle Technology Laboratory, she joined the faculty of Rutgers University in 1994. Her research interests are focused on atmospheric aerosols and include particulate matter exposure, organic aerosol measurement and atmospheric chemistry leading to secondary organic aerosol formation.
Vasanthi R. Sunil received her M.S. and Ph.D. from the Department of Microbiology and Molecular Genetics, Rutgers University and UMDNJ-Robert Wood Johnson Medical School in 2001. After a postdoctoral fellowship in the Department of Pharmacology and Toxicology at Rutgers University, she was appointed Assistant Research Professor in the same department. Her areas of interest include inflammation and the effects of toxicants on the immune system.
Kinal J. Patel received her B.A. in Biological Sciences from Rutgers University and an MPH in Epidemiology from UMDNJ School of Public Health in 2008. She is currently working as a Research Assistant in the Department of Pharmacology and Toxicology.
References Cited
- Ahn EK, Yoon HK, Jee BK, Ko HJ, Lee KH, Kim HJ, Lim Y. COX-2 expression and inflammatory effects by diesel exhaust particles in vitro and in vivo. Toxicol Lett. 2008;176:178–187. doi: 10.1016/j.toxlet.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Bradley SF, Vibhagool A, Kunkel SL, Kauffman CA. Monokine secretion in aging and protein malnutrition. J Leukoc Biol. 1989;45:510–514. doi: 10.1002/jlb.45.6.510. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Cao D, Bromberg PA, Samet JM. COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol. 2007;37:232–239. doi: 10.1165/rcmb.2006-0449OC. [DOI] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, Brittingham JA, Durham SK, Laskin JD, Laskin DL. Role of p55 tumor necrosis factor receptor 1 in acetaminophen-induced antioxidant defense. Am J Physiol Gastrointest Liver Physiol. 2003a;285:G959–G966. doi: 10.1152/ajpgi.00219.2003. [DOI] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, Durham SK, Brittingham JA, Laskin JD, Laskin DL. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol Appl Pharmacol. 2003b;193:218–227. doi: 10.1016/j.taap.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Corsini E, Giani A, Lucchi L, Peano S, Viviani B, Galli CL, Marinovich M. Resistance to acute silicosis in senescent rats: role of alveolar macrophages. Chem Res Toxicol. 2003;16:1520–1527. doi: 10.1021/tx034139+. [DOI] [PubMed] [Google Scholar]
- Corsini E, Giani A, Peano S, Marinovich M, Galli CL. Resistance to silica-induced lung fibrosis in senescent rats: role of alveolar macrophages and tumor necrosis factor-alpha (TNF) Mech Ageing Dev. 2004;125:145–146. doi: 10.1016/j.mad.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Dinarello C. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinincal findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- Dockery DW, Schwartz J, Spengler JD. Air pollution and daily mortality: associations with particulates and acid aerosols. Environ Res. 1992;59:362–373. doi: 10.1016/s0013-9351(05)80042-8. [DOI] [PubMed] [Google Scholar]
- Elsayed N, Mustafa M, Postlethwait E. Age-dependent pulmonary response of rats to ozone exposure. J Toxicol Environ Health. 1982;9:835–848. doi: 10.1080/15287398209530206. [DOI] [PubMed] [Google Scholar]
- Esposito AL, Poirier WJ, Clark CA, Brown ML. The release of neutrophil chemoattractant activity by bronchoalveolar macrophages from adult and senescent mice. J Gerontol. 1989;44:B93–B99. doi: 10.1093/geronj/44.4.b93. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Gardner CR, Laskin DL. Superoxide dismutase-overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-alpha. Am J Respir Cell Mol Biol. 2004;30:280–287. doi: 10.1165/rcmb.2003-0044OC. [DOI] [PubMed] [Google Scholar]
- Forastiere F, Stafoggia M, Picciotto S, Bellander T, D’Ippoliti D, Lanki T, von Klot S, Nyberg F, Paatero P, Peters A, Pekkanen J, Sunyer J, Perucci CA. A Case-Crossover Analysis of Out-of-Hospital Coronary Deaths and Air Pollution in Rome, Italy. Am J Respir Crit Care Med. 2005;172:1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31:433–441. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- Fujita EM, Zielinska B, Campbell DE, Arnott WP, Sagebiel JC, Mazzoleni L, Chow JC, Gabele PA, Crews W, Snow R, Clark NN, Wayne WS, Lawson DR. Variations in speciated emissions from spark-ignition and compression-ignition motor vehicles in California’s south coast air basin. J Air Waste Manag Assoc. 2007;57:705–720. doi: 10.3155/1047-3289.57.6.705. [DOI] [PubMed] [Google Scholar]
- Geiser T. Mechanisms of alveolar epithelial repair in acute lung injury--a translational approach. Swiss Med Wkly. 2003;133:586–590. doi: 10.4414/smw.2003.10267. [DOI] [PubMed] [Google Scholar]
- Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. Am J Respir Crit Care Med. 2001;163:1384–1388. doi: 10.1164/ajrccm.163.6.2006131. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Suliman HB, Carter JD, Abushamaa AM, Folz RJ. Overexpression of extracellular superoxide dismutase decreases lung injury after exposure to oil fly ash. Am J Physiol Lung Cell Mol Physiol. 2002;283:L211–L218. doi: 10.1152/ajplung.00409.2001. [DOI] [PubMed] [Google Scholar]
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go EK, Jung KJ, Kim JM, Lim H, Lim HK, Yu BP, Chung HY. Betaine modulates age-related NF-kappaB by thiol-enhancing action. Biol Pharm Bull. 2007;30:2244–2249. doi: 10.1248/bpb.30.2244. [DOI] [PubMed] [Google Scholar]
- Gowdy K, Krantz QT, Daniels M, Linak WP, Jaspers I, Gilmour MI. Modulation of pulmonary inflammatory responses and antimicrobial defenses in mice exposed to diesel exhaust. Toxicol Appl Pharmacol [Epub ahead of print] 2008 doi: 10.1016/j.taap.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Guo Z, Boekhoudt GH, Boss JM. Role of the intronic enhancer in tumor necrosis factor-mediated induction of manganous superoxide dismutase. J Biol Chem. 2003;278:23570–23578. doi: 10.1074/jbc.M303431200. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Repine JE. Antioxidants in the lung. In: Crystal RG, West JB, editors. The Lung. New York: Raven Press; 1991. pp. 1811–1820. [Google Scholar]
- Higashimoto Y, Fukuchi Y, Shimada Y, Ishida K, Ohata M, Furuse T, Shu C, Teramoto S, Matsuse T, Sudo E, et al. The effects of aging on the function of alveolar macrophages in mice. Mech Ageing Dev. 1993;69:207–217. doi: 10.1016/0047-6374(93)90024-l. [DOI] [PubMed] [Google Scholar]
- Himi T, Yoshioka I, Kataura A. Influence of age on the production of interleukin-8-like chemokine (GRO/CINC-1) in rat nasal mucosa. Eur Arch Otorhinolaryngol. 1997;254:101–104. doi: 10.1007/BF01526189. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yamaki K, Uchiyama K, Yoshikawa T. Components of diesel exhaust particles differentially affect lung expression of cyclooxygenase-2 related to bacterial endotoxin. J Appl Toxicol. 2004;24:415–418. doi: 10.1002/jat.984. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Nadziejko CE, Kikkawa Y. Effect of aging on pulmonary superoxide dismutase. Mech Ageing Dev. 1990;52:11–26. doi: 10.1016/0047-6374(90)90141-2. [DOI] [PubMed] [Google Scholar]
- Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp Lung Res. 2007;33:375–384. doi: 10.1080/01902140701634843. [DOI] [PubMed] [Google Scholar]
- Janssen YM, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Lab Invest. 1993;69:261–274. [PubMed] [Google Scholar]
- Kafoury RM, Kelley J. Ozone enhances diesel exhaust particles (DEP)-induced interleukin-8 (IL-8) gene expression in human airway epithelial cells through activation of nuclear factors- kappaB (NF-kappaB) and IL-6 (NF-IL6) Int J Environ Res Public Health. 2005;2:403–410. doi: 10.3390/ijerph2005030004. [DOI] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am Rev Respir Dis. 1990;141:765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Dunster C, Mudway I. Air pollution and the elderly: oxidant/antioxidant issues worth consideration. Eur Respir J Suppl. 2003;40:70s–75s. doi: 10.1183/09031936.03.00402903. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- Lang JD, McArdle PJ, O’Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Morio L, Hooper K, Li TH, Buckley B, Turpin B. Peroxides and macrophages in the toxicity of fine particulate matter in rats. Res Rep Health Eff Inst. 2003:1–51. discussion 53–63. [PubMed] [Google Scholar]
- Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, Wang M, Miguel AH, Cho A, Sioutas C, Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- Li XY, Brown D, Smith S, MacNee W, Donaldson K. Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats. Inhal Toxicol. 1999;11:709–731. doi: 10.1080/089583799196826. [DOI] [PubMed] [Google Scholar]
- Lim HB, Ichinose T, Miyabara Y, Takano H, Kumagai Y, Shimojyo N, Devalia JL, Sagai M. Involvement of superoxide and nitric oxide on airway inflammation and hyperresponsiveness induced by diesel exhaust particles in mice. Free Radic Biol Med. 1998;25:635–644. doi: 10.1016/s0891-5849(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. J Biol Chem. 1995;270:22565–22570. doi: 10.1074/jbc.270.38.22565. [DOI] [PubMed] [Google Scholar]
- Lloberas J, Celada A. Effect of aging on macrophage function. Exp Gerontol. 2002;37:1325–1331. doi: 10.1016/s0531-5565(02)00125-0. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Ames BN. Ascorbic acid recycling in rat hepatocytes as measurement of antioxidant capacity: decline with age. Methods Enzymol. 1999;299:83–88. doi: 10.1016/s0076-6879(99)99011-0. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Amakawa K, Yamaguchi K, Ishizaka A, Terashima T, Matsumaru A, Morishita T. Effects of diesel exhaust particles on human neutrophil activation. Exp Lung Res. 2006;32:427–439. doi: 10.1080/01902140601047641. [DOI] [PubMed] [Google Scholar]
- Mauderly JL. Animal models for the effect of age on susceptibility to inhaled particulate matter. Inhal Toxicol. 2000;12:863–900. doi: 10.1080/08958370050123216. [DOI] [PubMed] [Google Scholar]
- McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12:R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JD, Barr EB, White RK. Design, characterization, and evaluation of a small-scale diesel exhaust exposure system. Aerosol Science and Technology. 2004;38:62–78. [Google Scholar]
- Meydani SN, Wu D. Age-associated inflammatory changes: role of nutritional intervention. Nutr Rev. 2007;65:S213–S216. doi: 10.1111/j.1753-4887.2007.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- Missiaglia E, Blaveri E, Terris B, Wang YH, Costello E, Neoptolemos JP, Crnogorac-Jurcevic T, Lemoine NR. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer. 2004;112:100–112. doi: 10.1002/ijc.20376. [DOI] [PubMed] [Google Scholar]
- Morio LA, Hooper KA, Brittingham J, Li TH, Gordon RE, Turpin BJ, Laskin DL. Tissue injury following inhalation of fine particulate matter and hydrogen peroxide is associated with altered production of inflammatory mediators and antioxidants by alveolar macrophages. Toxicol Appl Pharmacol. 2001;177:188–199. doi: 10.1006/taap.2001.9316. [DOI] [PubMed] [Google Scholar]
- Murtaugh MP, Baarsch MJ, Zhou Y, Scamurra RW, Lin G. Inflammatory cytokines in animal health and disease. Vet Immunol Immunopathol. 1996;54:45–55. doi: 10.1016/s0165-2427(96)05698-x. [DOI] [PubMed] [Google Scholar]
- Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290:L797–L805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Schwartz J, Ransom MR. Daily mortality and PM10 pollution in Utah Valley. Arch Environ Health. 1992;47:211–217. doi: 10.1080/00039896.1992.9938351. [DOI] [PubMed] [Google Scholar]
- Pulsatelli L, Meliconi R, Mazzetti I, Dolzani P, Meneghetti A, Neri S, Silvestri T, Ravaglia G, Forti P, Facchini A, Mariani E. Chemokine production by peripheral blood mononuclear cells in elderly subjects. Mech Ageing Dev. 2000;121:89–100. doi: 10.1016/s0047-6374(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, Speizer FE, Dockery DW. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Kuwahara Y, Baba T, Roushandeh AM, Ebishima S, Abe S, Ohkubo Y, Fukumoto M. Oxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditions. J Radiat Res (Tokyo) 2007;48:39–44. doi: 10.1269/jrr.06057. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Xi S, Hartsfield CL, Choi AM. Mitogen activated protein kinase (MAPK) pathway regulates heme oxygenase-1 gene expression by hypoxia in vascular cells. Antioxid Redox Signal. 2002;4:587–592. doi: 10.1089/15230860260220085. [DOI] [PubMed] [Google Scholar]
- Saber A, Jacobson N, Bornholdt J, Kjaer S, Dybdahl M, Risom L, Loft S, Vogel U, Wallin H. Cytokine expression in mice exposed to diesel exhaust particles by inhalation: Role of tumor necrosis factor. Part Fibre Toxicol. 2006;3:4. doi: 10.1186/1743-8977-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagai M, Saito H, Ichinose T, Kodama M, Mori Y. Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse. Free Radic Biol Med. 1993;14:37–47. doi: 10.1016/0891-5849(93)90507-q. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, Schwartz J, Zanobetti A. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst. 2000;94:5–70. discussion 71–79. [PubMed] [Google Scholar]
- Sasaki M, Kashima M, Ito T, Watanabe A, Izumiyama N, Sano M, Kagaya M, Shioya T, Miura M. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1beta and TNF-alpha. Mediators Inflamm. 2000;9:155–160. doi: 10.1080/09629350020002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schins RP, Borm PJ. Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg. 1999;43:7–33. doi: 10.1016/s0003-4878(98)00069-6. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, Nearing B, Verrier R, Stone P, MacCallum G, Speizer FE, Gold DR. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais S, Boussouar A, Molnar A, Douki T, Pequignot JM, Favier R. Age-related sensitivity to lung oxidative stress during ozone exposure. Free Radic Res. 2005;39:305–316. doi: 10.1080/10715760400011098. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Ito H. Heterogeneous aging of macrophage-lineage cells in the capacity for TNF production and self renewal in C57BL/6 mice. Mech Ageing Dev. 1996;87:183–195. doi: 10.1016/0047-6374(96)01704-6. [DOI] [PubMed] [Google Scholar]
- Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, Scheepers PT, Van Loveren H. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res. 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- Stevens T, Krantz QT, Linak WP, Hester S, Gilmour MI. Increased transcription of immune and metabolic pathways in naive and allergic mice exposed to diesel exhaust. Toxicol Sci. 2008;102:359–370. doi: 10.1093/toxsci/kfn006. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Laumbach RJ, Patel KJ, Turpin BJ, Lim HJ, Kipen HM, Laskin JD, Laskin DL. Pulmonary effects of inhaled limonene ozone reaction products in elderly rats. Toxicol Appl Pharmacol. 2007a;222:211–220. doi: 10.1016/j.taap.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil VR, Patel KJ, Nilsen-Hamilton M, Heck DE, Laskin JD, Laskin DL. Acute endotoxemia is associated with upregulation of lipocalin 24p3/Lcn2 in lung and liver. Exp Mol Pathol. 2007b;83:177–187. doi: 10.1016/j.yexmp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Tasat DR, Mancuso R, O’Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell. 2003;2:159–164. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Mallis RJ. Aging and oxidation of reactive protein sulfhydryls. Exp Gerontol. 2001;36:1519–1526. doi: 10.1016/s0531-5565(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Tsan MF, White JE, Santana TA, Lee CY. Tracheal insufflation of tumor necrosis factor protects rats against oxygen toxicity. J Appl Physiol. 1990;68:1211–1219. doi: 10.1152/jappl.1990.68.3.1211. [DOI] [PubMed] [Google Scholar]
- USEPA. Air quality criteria for particulate matter. Research Triangle Park, NC: U. S. Environmental Protection Agency; 2004. [Google Scholar]
- van der Loo B, Bachschmid M, Spitzer V, Brey L, Ullrich V, Luscher TF. Decreased plasma and tissue levels of vitamin C in a rat model of aging: implications for antioxidative defense. Biochem Biophys Res Commun. 2003;303:483–487. doi: 10.1016/s0006-291x(03)00360-7. [DOI] [PubMed] [Google Scholar]
- Vemula M, Berthiaume F, Jayaraman A, Yarmush ML. Expression profiling analysis of the metabolic and inflammatory changes following burn injury in rats. Physiol Genomics. 2004;18:87–98. doi: 10.1152/physiolgenomics.00189.2003. [DOI] [PubMed] [Google Scholar]
- Vermylen J, Nemmar A, Nemery B, Hoylaerts MF. Ambient air pollution and acute myocardial infarction. J Thromb Haemost. 2005;3:1955–1961. doi: 10.1111/j.1538-7836.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Fakhrzadeh L, Heck DE, Laskin JD, Gardner CR, Laskin DL. Inhaled nitric oxide primes lung macrophages to produce reactive oxygen and nitrogen intermediates. Am J Respir Crit Care Med. 1998;158:931–938. doi: 10.1164/ajrccm.158.3.9708014. [DOI] [PubMed] [Google Scholar]
- Wolters M, Strohle A, Hahn A. Age-associated changes in the metabolism of vitamin B(12) and folic acid: prevalence, aetiopathogenesis and pathophysiological consequences. Z Gerontol Geriatr. 2004;37:109–135. doi: 10.1007/s00391-004-0169-6. [DOI] [PubMed] [Google Scholar]
- Wong GH, Kaspar RL, Vehar G. Tumor necrosis factor and lymphotoxin: protection against oxidative stress through induction of MnSOD. Exs. 1996;77:321–333. doi: 10.1007/978-3-0348-9088-5_21. [DOI] [PubMed] [Google Scholar]
- Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- Yanagisawa R, Takano H, Inoue K-I, Ichinose T, Yoshida S-I, Sadakane K, Takeda K, Yoshino S, Yamaki K, Kumagai Y, Yoshikawa T. Complementary DNA microarray analysis in acute lung injury induced by lipopolysaccharide and diesel exhaust particles. Exp Biol Med. 2004;229:1081–1087. doi: 10.1177/153537020422901013. [DOI] [PubMed] [Google Scholar]
- Yin XJ, Schafer R, Ma JY, Antonini JM, Weissman DD, Siegel PD, Barger MW, Roberts JR, Ma JK. Alteration of pulmonary immunity to Listeria monocytogenes by diesel exhaust particles (DEPs). I. Effects of DEPs on early pulmonary responses. Environ Health Perspect. 2002;110:1105–1111. doi: 10.1289/ehp.021101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska B, Sagebiel J, McDonald JD, Whitney K, Lawson DR. Emission rates and comparative chemical composition from selected in-use diesel and gasoline-fueled vehicles. J Air Waste Manag Assoc. 2004;54:1138–1150. doi: 10.1080/10473289.2004.10470973. [DOI] [PubMed] [Google Scholar]