Abstract
The question of whether prostate cancer is part of the Lynch syndrome spectrum of tumors is unresolved. We investigated the mismatch repair (MMR) status and pathologic features of prostate cancers diagnosed in MMR gene mutation carriers. Prostate cancers (mean age at diagnosis = 62 ± SD = 8 years) from 32 MMR mutation carriers (23 MSH2, 5 MLH1 and 4 MSH6) enrolled in the Australasian, Mayo Clinic and Ontario sites of the Colon Cancer Family Registry were examined for clinico-pathologic features and MMR-deficiency (immunohistochemical loss of MMR protein expression and high levels of microsatellite instability; MSI-H). Tumor MMR-deficiency was observed for 22 cases [69 %; 95 % confidence interval (CI) 50–83 %], with the highest prevalence of MMR-deficiency in tumors from MSH2 mutation carriers (19/23, 83 %) compared with MLH1 and MSH6 carriers combined (3/9, 33 %; p = 0.01). MMR-deficient tumors had increased levels of tumor infiltrating lymphocytes compared with tumors without MMR-deficiency (p = 0.04). Under the assumption that tumour MMR-deficiency occurred only because the cancer was caused by the germline mutation, mutation carriers are at 3.2-fold (95 % CI 2.0–6.3) increased risk of prostate cancer, and when assessed by gene, the relative risk was greatest for MSH2 carriers (5.8, 95 % CI 2.6–20.9). Prostate cancer was the first or only diagnosed tumor in 37 % of carriers. MMR gene mutation carriers have at least a twofold or greater increased risk of developing MMR-deficient prostate cancer where the risk is highest for MSH2 mutation carriers. MMR IHC screening of prostate cancers will aid in identifying MMR gene mutation carriers.
Keywords: Prostate cancer, Lynch syndrome, Mismatch repair deficiency, Mismatch repair gene mutations, Tumor infiltrating lymphocytes
Introduction
Lynch syndrome, formerly known as hereditary non-polyposis colorectal cancer (HNPCC), is an autosomal dominant disorder caused by germline mutations in the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PMS2. Recently, we have shown that MMR gene mutation carriers are at increased risk of developing cancers of the colorectum and endometrium, as well as cancers of the ovary, kidney, pancreas, stomach, urinary bladder and breast [1]. They are also at an increased risk of developing second primary cancers, including those in the breast and prostate [2, 3]. Over 80 % of colorectal cancers diagnosed in individuals with Lynch syndrome have tumor microsatellite instability (MSI) or loss of expression of one or more of the MMR proteins by immunohistochemistry (collectively termed MMR-deficiency) [4, 5]. Morphologically, colorectal cancers in people with Lynch syndrome frequently demonstrate high histologic grade, solid growth pattern and conspicuous lymphocytic infiltration [6].
Recently, sufficient data on Lynch syndrome has been collected to allow rigorous investigation of associations of MMR gene mutations with the more common cancers. Newer molecular and risk estimation studies support the inclusion of breast cancer as part of the Lynch syndrome-associated tumor spectrum [1, 7]. Case reports of uncommon tumors continue to emerge, including sarcomas [8, 9], peritoneal mesothelioma, adrenocortical carcinoma, anaplastic thyroid carcinoma, or neuroendocrine pancreatic tumors [10, 11]. Prostate cancer has not traditionally been considered part of the spectrum of tumors associated with Lynch syndrome, but recent small studies have suggested an increased risk of prostate cancer for people with Lynch syndrome, in particular for MSH2 mutation carriers [3, 12–15]. In addition, MMR-deficiency assessed by loss of immunohistochemical (IHC) expression or by polymerase chain reaction-based methods has been reported several times in prostate cancers in a small number of MMR gene mutation carriers [12, 16–18]. However, to date, no large studies have examined the expression of MMR proteins and pathology features of prostate cancers diagnosed in MMR gene mutation carriers. Consequently, the question of whether prostate cancer is part of the spectrum of tumors is unresolved.
The aim of this study was to investigate the histological features, MSI and MMR IHC expression of prostate cancers in proven MMR gene mutation carriers from the Colon Cancer Family Registry.
Materials and methods
Study sample
Participants were from families recruited between 1997 and 2010 to the Colon Cancer Family Registry via pro-bands who were either recently diagnosed colorectal cancer cases ascertained through the Victorian population-cancer registry in Australia (population-based recruitment) and a state-based population-based registry in the USA (Minnesota Cancer Surveillance System) or they were persons from multiple-case families referred to family cancer clinics in Australia (Melbourne, Adelaide, Perth, Brisbane, Sydney), New Zealand (Auckland), the Mayo Clinic, Rochester, Minnesota, USA (clinic-based recruitment) or the Mount Sinai Hospital, Toronto, Ontario, Canada [19]. Inclusion criteria for this study were: (a) proven to be carrying a pathogenic germline mutation in one of the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2, (b) having a diagnosis of prostate carcinoma confirmed by histological examination, and (c) the availability of archival tissue blocks for additional laboratory testing. Ethics approval was obtained from the relevant institutional Human Research Ethics Committees at recruiting centers including the Queensland Institute of Medical Research under project approval P628.
Germline mutation testing
Mutation testing for MLH1, MSH2, and MSH6 was performed by Sanger sequencing or denaturing high performance liquid chromatography (dHPLC), followed by confirmatory DNA sequencing [7, 19]. Large duplication and deletion mutations were detected by Multiplex Ligation Dependent Probe Amplification (MLPA). PMS2 mutation testing was performed using long-range PCR and MLPA as previously described [20] on individuals demonstrating solitary loss of PMS2 protein expression in a tumor. All donated samples from participants who were relatives of probands with a pathogenic mutation were tested for the same mutation identified in the proband. A pathogenic germline mutation in a DNA mismatch repair genes was defined as a variant causing a stop codon, a large duplication or deletion, a frameshift mutation or a missense mutation previously reported in the scientific literature as being pathogenic [1].
Pathology review
Paraffin-embedded tissue blocks containing prostate cancer were obtained from relevant clinical pathology departments. Hematoxylin and eosin stained sections were reviewed by one pathologist (CR) to assess tumor histologic type, Gleason score, the presence of capsular and perineural invasion and locoregional lymph node metastases. For four of nine tumors diagnosed in Ontario, pathology review was performed on a digitally scanned hematoxylin and eosin stained section. Tumor infiltrating lymphocytes (TILs) were counted and considered to be ‘significant’ when >4 TILs were identified by high power field [21]. Information on pre-operative prostate specific antigen (PSA) levels were abstracted from the clinical notes on pathology reports or obtained from diagnostic laboratories’ records.
Mismatch repair deficiency testing
Sections from formalin fixed paraffin embedded tissue blocks were used for IHC assessment of the expression of MLH1, MSH2, MSH6 and PMS2 as previously described [22]. For tumors not from Ontario, MSI status was determined by using a 10-loci panel of microsatellite markers in tumor DNA [23] and tumors were deemed to have high levels of microsatellite instability (MSI-H) if ≥30 % of markers were unstable. For tumors from Ontario, MSI was assessed using two mononucleotide markers BAT-25 and BAT-26 and tumors were deemed to be MSI-H if at least one marker was unstable. MMR-deficiency was defined as loss of protein expression by IHC with or without MSI-H where tested. A tumor was defined to be MMR-proficient if it had no loss of MMR protein expression by IHC and, when tested, was microsatellite stable (MSS).
Statistical analysis
Pearson’s Chi squared tests or Fisher’s exact tests were used to test the statistical significance of differences in contingency tables as appropriate. Student’s t test was used to test the statistical significance of differences in the means of continuous variables. Following convention, statistical significance was considered as p < 0.05. 95 % confidence intervals (CIs) of proportions were estimated using binomial exact method. Under the assumption that a MMR-deficient prostate cancer was caused by the MMR gene mutation, the relative risk (RR) of MMR-deficient prostate cancer for men with a germline MMR gene mutation can be estimated by back calculation from the attributable fraction as RR = N/(N–n), where N is the total number of prostate cancer-affected mutation carriers and n is the number of these for which their tumor exhibited MMR-deficiency. The 95 % CI was estimated by assuming that n has a Binomial (N; p) distribution with P = n/N [24, 25].
Results
Clinical and pathological characteristics of prostate cancers in MMR gene mutation carriers
A total of 32 men from 31 families fulfilled the selection criteria and were included in the study as prostate cancer cases. The Amsterdam II criteria (ACII) were met by 25/31 families (81 %). There were 23 × MSH2 mutation carriers (72 %; two from the same family), 5 × MLH1 mutation carriers (16 %), and 4 × MSH6 mutation carriers (12 %; Table 1). No PMS2 gene mutation carriers diagnosed with prostate cancer were identified. Of the 147 population-based families with MMR gene mutations from the Australasian, Ontario and Mayo sites, the distribution of MLH1, MSH2 and MSH6 mutations was 43 % (n = 63), 43 % (n = 63) and 14 % (n = 21) respectively. In these families, there were 351 (151 male) carriers of mutation in a MMR gene (148 × MLH1, 170 × MSH2 and 33 × MSH6) with 58 (39 %), 75 (44 %) and 18 (54 %) males, respectively. Given this distribution of mutation carriers, there was an over-representation of male MSH2 mutation carriers (23/75, 31 %) and an under-representation of male MLH1 mutation carriers (5/58, 9 %) with prostate cancer from these 147 families (p = 0.002). The mean age at diagnosis of prostate cancer was 62 ± 8 years (range 45–74). Information on pre-operative PSA was available for eight carriers with a mean level of 38 µg/l (standard deviation (SD) = 31 µg/l; range 4–81 µg/l). Two other carriers were reported as having “ rising” and “ elevated” PSA values without quantified scores.
Table 1.
Clinical and genetic characteristics of 32 men with a germline mutation in a mismatch repair gene diagnosed with prostate carcinoma
| Carrier # |
Gene | Variant | Amsterdam II criteria |
Age at diagnosis |
PSA levels (ug/ L) |
Other malignancy (age at diagnosis)a |
|---|---|---|---|---|---|---|
| 1 | MSH2 | c.1865C > T p.Pro622Leu | Yes | 71 | NA | Colorectal × 2 (44) |
| 2 | MSH2 | c.892C > T p.Gln298X | Yes | 58 | NA | Colorectal × 4 (38, 45, 51, 68); Small intestinal (64); Renal pelvis (68) |
| 3 | MSH2 | c.892C > T p.Gln298X | Yes | 73 | 81 | Pancreatic (75) |
| 4 | MSH2 | c.1-?_1386 + ?del p.Met1_Gln462del | Yes | 53 | 35 | |
| 5 | MSH6 | c.3439-1 G > T r.spl? p.? | Yes | 64 | 3.6 | |
| 6 | MSH2 | c.942 + 3A > T r.793_942del p.Val265_Gln314del | Yes | 63 | 54 | |
| 7 | MSH2 | c.645 + 1G > A r.spl? p.? | Yes | 59 | NA | Colorectal (54); Melanoma (57) |
| 8 | MSH2 | c.1165C > T p.Arg389X | Yes | 70 | “Rising” | Colorectal × 2 (50, 61) |
| 9 | MLH1 | c.588delA p.Lys196AsnfsX6 | Yes | 68 | 6.9 | Colorectal × 2 (34, 59) |
| 10 | MSH2 | c.1277-?_1386 + ?del p.Lys427GlyfsX4 | Yes | 69 | NA | Colorectal (61); Bladder (69) |
| 11 | MSH2 | c.792 + 1G > A r.spl? p.? | Yes | 55 | 26 | Colorectal (42) |
| 12 | MLH1 | c.117-2A > G r.spl?p.? | Yes | 68 | NA | Colorectal (44) |
| 13 | MSH2 | c.1147C > T p.Arg383X | Yes | 71 | NA | Melanoma (50); Renal pelvis (59); Colorectal × 2 (64, 68) |
| 14 | MSH2 | c.942 + 3A > T r.793_942del p.Val265_Gln314del | Yes | 61 | NA | |
| 15 | MSH2 | c.1046C > T p.Pro349Leu | Yes | 47 | NA | |
| 16 | MSH2 | c.645-1967_1076 + 5075del10166 p.Ile216_Arg359 > Ilefs × 29 | Yes | 57 | NA | Colorectal (50); sebaceous skin tumor (63) |
| 17 | MLH1 | c. 350C > T p.Thr117Met | Yes | 62 | NA | Colorectal (46) |
| 18 | MSH6 | c.3261_3262insC p.Phe1088LeufsX5 | No | 59 | NA | Colorectal (61); sebaceous skin tumor (65) |
| 19 | MSH6 | c.2731C > T p.Arg911X | No | 60 | NA | |
| 20 | MLH1 | c. 1852_1854delAAG p.Lys618del | No | 61 | NA | Colorectal × 2 (34, 59); Leiomyosarcoma (67) |
| 21 | MSH2 | c.942 + 3A > T r.793_942del p.Val265_Gln314del | Yes | 50 | “Elevated” | Colorectal (52) |
| 22 | MSH2 | c.1889_1892delGAAG p.Gly630GlufsX4 | Yes | 45 | 80 | |
| 23 | MSH2 | c.1865C > T p.Pro622Leu | Yes | 72 | NA | Colorectal (54) |
| 24 | MSH2 | c.1591_1611del p.Lys531_537del | Yes | 73 | NA | Jejunal (46), Colorectal × 4 (49, 63, 63, 63), sebaceous adenoma (63) |
| 25 | MSH2 | c.1786_1788delAAT p.Asn596del | No | 74 | NA | Colorectal × 3 (60, 61, 61), ureter (73) |
| 26 | MSH2 | c.1864C > A p.Pro622Thr | Yes | 68 | NA | Colorectal (54), Bladder (67), ureter (78) |
| 27 | MSH6 | c.3336_3337insATGA p.Ile1113MetfsX7 | Yes | 70 | 19 | Colorectal × 2 (61, 65) |
| 28 | MSH2 | c.475_476insA p.Arg159LysfsX19 | Yes | 53 | NA | Colorectal (51) |
| 29 | MSH2 del exons | 9–12 | No | 59 | NA | Sebaceous adenoma (61) |
| 30 | MSH2 | c.1906G > C p.Ala636Pro | No | 60 | NA | Colorectal (64) |
| 31 | MLH1 | c.2038_2063del p.Cys680AlafsX5 | Yes | 47 | NA | Colorectal × 4 (35, 35, 51, 53), Melanoma (53) |
| 32 | MSH2 | c.1-?_2805 + ?del | Yes | 60 | NA | Colorectal × 2 (45, 61), Basal cell carcinoma (69), Sebaceous adenoma (70) |
Other malignancies that are underlined have been tested by immunohistochemistry for mismatch repair proteins and demonstrated pattern of loss of protein concordant with underlying germline mutation. This information was not available on cases 24–32 from Ontario
The pathology specimens were transrectal ultrasound biopsies (TRUS Bx; n = 9), transurethral resection of the prostate (TURP) specimens (n = 5) and radical prostatectomy specimens (n = 18) (Table 2). All tumors were prostatic adenocarcinomas of acinar type. Total Gleason scores (GS) ranged from 5 to 10; two tumors had a GS of 5, twenty-two had a GS of 6 or 7, and eight had a GS ≥ 8 (including one case reported as poorly differentiated). There was some evidence for an association between the gene mutated (MSH2 vs. MLH1 and MSH6 combined) and a GS ≥ 8, however, this was not nominally significant (8/23 vs. 0/9; p = 0.07). Of the assessable tumors, perineural invasion was identified in 12/18 (67 %) and extracapsular invasion was identified in 9/19 (47 %). The nodal status was known for nine carriers, one of whom had metastatic disease (11 %).
Table 2.
Pathological features and mismatch repair status of men with prostate carcinoma
| Carrier # | Gene | Specimen type |
Gleason score |
TILs | Perineural invasion |
Capsular invasion |
Node metastasis |
Pattern of MMR protein loss by IHC |
MSI status |
MMR status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MSH2 | TURP | 9 | Present | NA | NA | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 2 | MSH2 | TRUS Bx | 7 | Present | NA | NA | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 3 | MSH2 | TRUS Bx | 7 | NA | NA | NA | NA | MSH2/MSH6 loss | MSI-H | MMR-deficient |
| 4 | MSH2 | Radical prostatectomy | 8 | Present | Present | Present | Present | MSH2/MSH6 loss | NT | MMR-proficient |
| 5 | MSH6 | TRUS Bx | 6 | Absent | NA | NA | NA | Normal expression | NT | MMR-proficient |
| 6 | MSH2 | TURP | 10 | Present | NA | NA | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 7 | MSH2 | TRUS Bx | 7 | Present | Absent | NA | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 8 | MSH2 | TURP | 9 | Absent | NA | NA | NA | Normal expression | MSS | MMR-proficient |
| 9 | MLH1 | TRUS Bx | 7 | Absent | Present | NA | NA | MLH1/PMS2 loss | NT | MMR-proficient |
| 10 | MSH2 | Radical prostatectomy | 9 | Present | NA | Present | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 11 | MSH2 | TRUS Bx | 6 | Absent | NA | NA | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 12 | MLH1 | Radical prostatectomy | 7 | Present | NA | Present | Absent | MLH1 lossa | MSI-H | MMR-deficient |
| 13 | MSH2 | Radical prostatectomy | 7 | Present | Present | Present | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 14 | MSH2 | Radical prostatectomy | 7 | Present | Present | Present | Absent | MSH2/MSH6 loss | NT | MMR-deficient |
| 15 | MSH2 | Radical prostatectomy | 7 | Absent | Absent | Absent | Absent | Normal expression | NT | MMR-proficient |
| 16 | MSH2 | Radical prostatectomy | 6 | Present | Absent | Absent | Absent | Normal expression | NT | MMR-proficient |
| 17 | MLH1 | Radical prostatectomy | 7 | Absent | Absent | Absent | NA | Normal expression | NT | MMR-proficient |
| 18 | MSH6 | Radical prostatectomy | 5 | Present | Present | Absent | NA | MSH2/MSH6 loss | NT | MMR-deficient |
| 19 | MSH6 | Radical prostatectomy | 7 | Absent | NA | Absent | Absent | Normal expression | NT | MMR-proficient |
| 20 | MLH1 | Radical prostatectomy | 5 | Present | Present | Present | Absent | Normal expression | NT | MMR-proficient |
| 21 | MSH2 | Radical prostatectomy | 7 | Present | Present | Absent | NA | MSH2/MSH6 loss | NT | MMR-proficient |
| 22 | MSH2 | TRUS Bx | 9 | Absent | Present | NA | NA | MSH2/MSH6 loss | NT | MMR-proficient |
| 23 | MSH2 | TURP | 7 | Absent | NA | NA | NA | MSH2/MSH6 loss | NT | MMR-proficient |
| 24 | MSH2 | TRUS Bx | Poor diff | NA | NA | NA | NA | MSH2/MSH6 loss | MSI-H | MMR-deficient |
| 25 | MSH2 | TURP | 9 | NA | NA | NA | NA | MSH2/MSH6 loss | MSI-H | MMR-proficient |
| 26 | MSH2 | Radical prostatectomy | 7 | NA | Present | Present | NA | MSH2/MSH6 loss | MSI-H | MMR-proficient |
| 27 | MSH6 | TRUS Bx | 6 | Absent | Absent | Absent | NA | Normal expression* | NT | MMR-proficient |
| 28 | MSH2 | Radical prostatectomy | 7 | NA | NA | No | NA | MSH2/MSH6 loss | NA | MMR-deficient |
| 29 | MSH2 | Radical prostatectomy | 7 | Present | Present | Present | Absent | MSH2/MSH6 loss | MSS | MMR-deficient |
| 30 | MSH2 | Radical prostatectomy | 7 | Present | Present | Present | Absent | Normal expression | MSS | MMR-proficient |
| 31 | MLH1 | Radical prostatectomy | 7 | Absent | Present | Absent | NA | Normal expression | MSS | MMR-proficient |
| 32 | MSH2 | Radical prostatectomy | 7 | NA | Absent | Absent | NA | MSH2/MSH6 loss | MSS | MMR-deficient |
TRUS Bx transrectal ultrasound biopsy, TURP transurethral resection of the prostate, TILs tumor infiltrating lymphocytes, MMR IHC mismatch repair immunohistochemistry, MSI microsatellite instability, MSS microsatellite stable, NA not available, NT not tested
PMS2 not tested
Mismatch repair status of prostate cancers
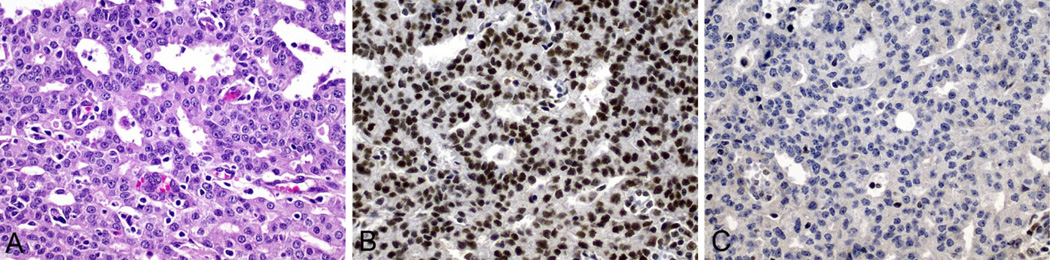
Immunohistochemical expression of MMR proteins was assessed for all 32 prostate cancer tumors and MSI status was determined for 10 tumors (Table 2). Loss of expression of MMR proteins by IHC was observed for 22 tumors (69 %; 95 % CI 50–84 %) and, when evident, the pattern of loss of protein expression was 100 % concordant with that of the underlying germline mutation (Fig. 1). The tumors from MSH2 mutation carriers had the highest proportion of MMR-deficiency (19 in 23 (83 %; 95 % CI 61–95 %)) compared with tumors in MLH1 mutation carriers (2 in 5 (40 %; 95 % CI 5–85 %)) and tumors in MSH6 carriers (1 in 4 (25 %; 95 % CI 1–81 %)). This variation was inconsistent with chance (p = 0.01).
Fig. 1.
Prostate carcinoma from carrier #4 who had a pathogenic germline mutation in MSH2 (MSH2 del × 1–8). a Hematoxylin and eosin stained sections showing Gleason 8 adenocarcinoma with tumor infiltrating lymphocytes; b and c Immunohistochemistry showing normal nuclear expression of MLH1 in tumor cells (b) and loss of nuclear expression of MSH2 in tumor cells (c). All images magnification ×400
Under the assumption that tumor MMR-deficiency occurs only because the cancer was caused by the underlying germline mutation, the RR of prostate cancer for all MMR gene mutation carriers was estimated to be 3.2-fold (95 % CI 2.0–6.3). When broken down by gene, the RR was estimated to be 5.8-fold (95 % CI 2.6–20.9) for MSH2 mutation carriers, 1.7-fold (95 % CI 1.1–6.7) for MLH1 mutation carriers and 1.3-fold (95 % CI 1.1–5.3) for MSH6 mutation carriers. The difference in RR between MSH2 and other gene mutation carriers was significant (p = 0.01).
The prostate tumor from one MSH6 mutation carrier also had loss of MSH2 expression which was consistently shown on repeated testing. A subsequent colorectal carcinoma from this carrier had loss of expression of MSH6 only. Of the ten tumors tested for MSI, five were MSI-H and also had loss of expression by IHC and five were not MSI-H of which three were MMR-proficient by IHC. There were two carriers whose tumors had loss of concordant MMR proteins that were not MSI-H.
There was no difference in the mean age at diagnosis of prostate cancer between carriers with a MMR-deficient tumor compared with those with a MMR-proficient tumor (63 ± 8 years vs. 60 ± 8 years; p = 0.4) (Table 3). Compared with MMR-proficient tumors, MMR-deficient tumors were more likely to have tumor infiltrating lymphocytes (p = 0.04) but there was no difference in the presence of high histologic grade (GS ≥ 8) (p = 0.4), perineural invasion (p = 0.1) or capsular invasion (p = 0.2). All the high grade prostate cancers were diagnosed in MSH2 mutation carriers. Regional lymph node status was assessed for only seven carriers, and the single tumor with involved lymph nodes was MMR-deficient.
Table 3.
Comparison between mismatch repair (MMR) deficient and MMR proficient prostate carcinomas
| Features | MMR-deficient tumors n/N (%) |
MMR-proficient tumors n/N (%) |
p values |
|---|---|---|---|
| Age at diagnosis, years (mean ± SD) | 63 ±8 | 60 ± 8 | 0.35 |
| Gleason score ≥ 8 | 7/22 (32 %) | 1/10 (10 %) | 0.38 |
| Presence of TILs | 12/16 (75 %) | 3/10 (30 %) | 0.04 |
| Perineural invasion | 9/11 (82 %) | 3/7 (43 %) | 0.14 |
| Capsular invasion | 7/11 (64 %) | 2/8 (25 %) | 0.17 |
| History of other malignancies | 18/22 (82 %) | 7/10 (70 %) | 0.65 |
Personal history of other malignancies
Twenty-three carriers (72 %, 95 % CI 53–86 %) had a diagnosis of colorectal cancer (Table 1). For twenty of these the colorectal cancer preceded the prostate cancer, by on average 16 ± 8 years (range 2–34). The prostate cancer was diagnosed 2 years prior to the colorectal cancer for two carriers, and 4 years prior for one carrier. Prostate cancer was the first (n = 5) or only (n = 7) tumor diagnosed for 37 % of carriers (95 % CI 22–56 %). There was no difference in the history of other malignancies between carriers with a MMR-deficient tumor compared with carriers with a MMR-proficient tumor (p = 0.7) (Table 3).
Discussion
We observed that 69 % of 32 prostate cancers diagnosed in MMR gene mutation carriers had MMR-deficiency, consistent with the 88 and 100 % reported by two previous studies of a total of 10 MMR gene mutation carriers [12, 18]. MSI has been detected in prostate cancer cell lines and in some studies of primary tumors with a wide range of frequencies (20–65 %) [26–29]. MMR-deficiency and/or high levels of MSI are the hallmarks of Lynch syndrome-associated tumors. Our demonstration of this phenotype in a large proportion of prostate cancers from mutation carriers adds weight to the argument that prostate cancers can develop as a result of MMR gene mutations. However, further evidence is needed to determine whether MMR-deficiency is a driver that initiates the carcinogenesis of these tumors or is a passenger molecular alteration with little effect on tumor initiation and development.
We observed an equal proportion of families with mutations in MSH2 and MLH1 overall from the Australasian, Ontario and Mayo sites of 43 %. However, when comparing the prevalence of mutation carriers with prostate cancer with male mutation carriers overall, we identified a significant over-representation of prostate cancer-affected MSH2 mutation carriers (31 %) while prostate cancer-affected MLH1 mutation carriers were under-represented (9 %). MSH2 mutation carriers also demonstrated a higher prevalence of tumor MMR-deficiency when compared with MLH1 and MSH6 mutation carriers. Previous studies have also reported an over-representation of MSH2 mutations in carriers with a prostate cancer [12–14, 16, 17]. Among MMR gene mutation carriers with a diagnosis of prostate cancer, the MSH2 mutation has been reported as the putative cause for 6/9 tumors by Grindedal et al. [12] and 7/8 by Barrow et al. [14]. However, unlike these studies, we found that prostate cancer with MMR-deficiency was not restricted to MSH2 and MSH6 mutation carriers: we found five cases in MLH1 mutation carriers, two of which had loss of MLH1 protein expression in tumor cells. Together these data suggest gene specific differences in the risk of prostate cancer with MSH2 mutation carriers more likely to develop prostate cancer. We did not find any case of prostate cancer in PMS2 mutation carriers. Most published studies did not include PMS2 mutation carriers in their Lynch syndrome patient cohorts. Only one prostate cancer in an obligate PMS2 mutation carrier has been reported [12]; however, immunohistochemistry has not been performed to demonstrate loss of PMS2 expression in tumor cells.
In addition to MMR-deficiency, tumors associated with Lynch syndrome often have a particular pathological phenotype including high histological grade and a pronounced lymphocytic immune response with the presence of tumor infiltrating lymphocyte. These morphological characteristics are exemplified in colorectal and endometrial carcinomas and can be used to predict MMR-deficiency in these tumor types [21, 22]. This study is the first to demonstrate that prostate cancers with MMR-deficiency more frequently showed tumor infiltrating lymphocytes than tumors that did not display MMR-deficiency. However, the prevalence of high histological grade (Gleason score ≥ 8) was not significantly different between the two groups. In the series of prostate cancers in proven or obligate MMR gene mutation carriers reported by Grindedal et al. [12] all 5 tumors with a Gleason score of 8 or more were identified in MSH2 mutation carriers. Similarly, we found that all 6 MMR-deficient prostate cancers with a Gleason score ≥ 8 were diagnosed in MSH2 mutation carriers. However, having a MSH2 mutation or MMR-deficiency was not associated with a high Gleason score in prostate cancers in our study.
Previous studies utilizing the Colon Cancer Family Registry have investigated the risk of prostate cancer for MMR gene mutation carriers compared with men from the general population. In a retrospective study, Dowty et al. [30] observed no evidence of an increased risk of prostate cancer as a first cancer diagnosis in mutation carriers: hazard ratio (HR) of 0.79 (95 % CI 0.25–2.5) for men with MLH1 mutations and 1.0 (95 % CI 0.47–2.3) for men with MSH2 mutations. In a prospective study, Win et al. [1] estimated the increased risk of prostate cancer for mutation carriers by a standardized incidence ratio (SIR) of 2.49 (95 % CI 0.51–7.28). However, for men with Lynch syndrome with a previous diagnosis of colorectal cancer, Win et al. [3] estimated a two-fold increased risk of prostate cancer for all mutation carriers combined, compared with the general population (SIR, 2.05; 95 % CI 1.23–3.01). In that study, most prostate cancers (15/19) were in men with MSH2 mutations, for whom the SIR was 3.62 (95 % CI 2.07–5.36) compared with 0.87 (95 % CI 0.00–2.19) for men with MLH1 mutations. Three other independent studies found an increased risk of prostate cancer for MMR gene mutation carriers compared with the general population with SIRs of 2.5 (95 % CI 1.2–4.0) [13] and 5.1 (95 % CI 4.1–17.1) [12] and a RR estimated to 10.4 (95 % CI 2.80–26.65) for MSH2 mutation carriers [14]. A further recent study of 198 families carrying MMR gene mutations reported a two-fold increased risk of prostate cancer in mutation carriers compared with the general population (HR = 1.99, 95 % CI 1.31–3.03, p = 0.0013) [15]. We observed that 69 % of prostate cancers in carriers of MMR gene mutations had MMR-deficient tumors, and thus demonstrated a potential link between the germline mutation and prostate tumor initiation. Based on this high prevalence of MMR-deficiency and the assumption that tumors with MMR-deficiency were caused by the underlying germline mutation (and a somatic mutation as the second hit), we estimated the RR of MMR-deficient prostate cancer for all mutation carriers combined and for MSH2 mutation carriers alone to be 3.2 (95 % CI 2.0–6.3) and 5.8 (95 % CI 2.6–20.9), respectively, providing further support for the inclusion of prostate cancer as part of the Lynch syndrome-associated tumor spectrum. However, the issue of whether prostate cancer risk is increased for men with Lynch syndrome is still debatable as other studies have not found evidence for an increased risk [31, 32]. Therefore, future studies using large prospective studies of known mutation carriers with long follow-up will be needed to conclusively resolve the issue of risk of prostate cancer for MMR gene mutation carriers.
An interesting finding from this study was the diagnosis of prostate cancer in 12 of 32 mutation carriers (37 %) as the first or only diagnosed malignancy. A similar finding was reported for a series of breast cancers diagnosed in women with Lynch syndrome, in which 44 % of those with a MMR-deficient tumor had no previous history of malignancy [7]. This suggests that testing for MMR protein expression in tumors currently not considered part of the Lynch syndrome spectrum, such as breast or prostate cancers, can identify people with Lynch syndrome, even when there is no suspicion of Lynch syndrome, as well as in families with a known or suspected MMR gene germline mutation when no colorectal tumors are available for testing.
This study has some limitations. We selected only cases for which paraffin tissue blocks were available for additional testing and, therefore, were not able to assess all the prostate tumors from mutation carriers within the Colon Cancer Family Registry. MSI status by PCR-based methods was determined for only 10 tumors. It is possible that some additional MMR-deficient tumors not tested for MSI may have been missed. Two tumors showed loss of MSH2/MSH6 by immunohistochemistry but no evidence of MSI-H. This discordance may be caused by insufficient proportion of tumor cells in DNA to demonstrate the MSI-H phenotype. Also, our RR calculations were based on the assumption that tumors with MMR-deficiency were caused by the underlying germline mutation and an unmeasured second somatic hit. We did not confirm the presence or type of this second hit, however, the fact that inactivation of both alleles is needed to cause loss of MMR function is a well-established tumorigenic mechanism in Lynch syndrome. Given that all the men in the study were MMR gene mutation carriers, other mechanisms of MMR-deficiency such as tumor DNA promoter methylation is less likely. For 14 of the 32 prostate cancers (44 %), the pathologic evaluation was performed from biopsy specimens (TRUS or TURP) which may affect the overall Gleason score, and this precluded a complete assessment of other pathologic features in relation to the MMR status of the tumor.
In conclusion, to the best of our knowledge this is the largest study of prostate cancers in proven MMR gene mutation carriers for whom pathology and MMR status has been characterized. We found MMR protein loss of expression in 69 % of tumors. We observed, for the first time, tumor infiltrating lymphocytes more often in MMR-deficient tumors than in MMR-proficient prostate tumors, similar to what is observed for other Lynch syndrome spectrum tumors. These findings suggest that defective mismatch repair is involved in prostate cancer development in men who carry a MMR gene mutation, in particular a MSH2 gene mutation, and together with other recent evidence of an increased risk of prostate cancer for mutation carriers suggests that this malignancy be considered part of the spectrum of tumors in Lynch syndrome. Furthermore, screening for MMR-deficiency in men presenting with prostate cancer, especially those with other indications of Lynch syndrome, could identify MMR gene mutation carriers and provide the opportunity to target cancer prevention strategies to carriers and their relatives.
Acknowledgments
This work was supported by the National Cancer Institute, National Institutes of Health under RFA # CA-95-011 and through cooperative agreements with members of the Colon Cancer Family Registry and PIs of the Australasian Colorectal Cancer Family Registry (U01 CA097735), Familial Colorectal Neoplasia Collaborative Group (U01 CA074799) [USC], Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01 CA074800), Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783), Seattle Colorectal Cancer Family Registry (U01 CA074794), and University of Hawaii Colorectal Cancer Family Registry (U01 CA074806). The authors thank all study participants of the Colon Cancer Family Registry and staff for their contributions to this project. Prostate cancer tissue samples in this study were obtained from the Jeremy Jass Memorial Tissue Pathology Bank. The authors are grateful to the many pathology laboratories involved for supply of archived prostate tissue for analysis. Thanks are due to Judi Maskiell, Leanne Prior, Maggie Angelakos and Kelly Aujard, for data and pedigree retrieval. This work was supported by the National Cancer Institute, National Institutes of Health under RFA # CA-95-011 and through the Australasian Colorectal Cancer Family Registry (U01 CA097735), the Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01 CA074800) and the Ontario Colon Cancer Family Registry (U01 CA074783) cooperative agreements. Christophe Rosty is the Jeremy Jass Pathology Fellow. John L. Hopper is an NHMRC Senior Principal Research Fellow and Distinguished Visiting Professor at Seoul National University, Korea. Mark A. Jenkins is an NHMRC Senior Research Fellow. Aung Ko Win is an NHMRC Early Career Fellow.
The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Cancer Family Registries, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the Cancer Family Registry. Authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
Footnotes
Conflict of interest The authors declare they hold no conflict of interest with respect to this work.
Ethics approval Written informed consent was obtained from all study participants and the study protocol was approved by the QIMR HREC under protocol P628.
Contributor Information
Christophe Rosty, Oncogenomics Group, Genetic Epidemiology Laboratory, Department of Pathology and Centre for Epidemiology and Biostatistics, University of Melbourne, Parkville, VIC 3010, Australia; Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia; Envoi Specialist Pathologists, Herston, QLD 4006, Australia; School of Medicine, University of Queensland, Herston, QLD 4006, Australia.
Michael D. Walsh, Oncogenomics Group, Genetic Epidemiology Laboratory, Department of Pathology and Centre for Epidemiology and Biostatistics, University of Melbourne, Parkville, VIC 3010, Australia Department of Histopathology, Sullivan Nicolaides Pathology, Taringa, QLD 4068, Australia.
Noralane M. Lindor, Department of Health Science Research, Mayo Clinic Arizona, Scottsdale, AZ 85259, USA
Stephen N. Thibodeau, Department of Laboratory Medicine, Mayo Clinic, Rochester, MN 59095, USA
Erin Mundt, Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA.
Steven Gallinger, Cancer Care Ontario, Toronto, ON, Canada; Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON, Canada; Zane Cohen Centre for Digestive Diseases, Mount Sinai Hospital, Toronto, ON, Canada.
Melyssa Aronson, Zane Cohen Centre for Digestive Diseases, Mount Sinai Hospital, Toronto, ON, Canada.
Aaron Pollett, Zane Cohen Centre for Digestive Diseases, Mount Sinai Hospital, Toronto, ON, Canada.
John A. Baron, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
Sally Pearson, Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia.
Mark Clendenning, Oncogenomics Group, Genetic Epidemiology Laboratory, Department of Pathology and Centre for Epidemiology and Biostatistics, University of Melbourne, Parkville, VIC 3010, Australia; Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia.
Rhiannon J. Walters, Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia
Belinda N. Nagler, Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia
William J. Crawford, Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia
Joanne P. Young, Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia
Ingrid Winship, Department of Medicine, The University of Melbourne, Parkville, VIC 3010, Australia; Genetic Medicine, The Royal Melbourne Hospital, Parkville, VIC 3010, Australia.
Aung Ko Win, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Parkville, VIC 3010, Australia.
John L. Hopper, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Parkville, VIC 3010, Australia Seoul National University, Seoul, Korea.
Mark A. Jenkins, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Parkville, VIC 3010, Australia
Daniel D. Buchanan, Email: daniel.buchanan@unimelb.edu.au, Oncogenomics Group, Genetic Epidemiology Laboratory, Department of Pathology and Centre for Epidemiology and Biostatistics, University of Melbourne, Parkville, VIC 3010, Australia; Cancer and Population Studies Group, Bancroft Centre, Queensland Institute of Medical Research, Herston, QLD 4006, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Parkville, VIC 3010, Australia.
References
- 1.Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:64–958. doi: 10.1200/JCO.2011.39.5590. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Win AK, Lindor NM, Winship I, et al. Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome. J Natl Cancer Inst. 2013;105:9–274. doi: 10.1093/jnci/djs525. doi: 10.1093/jnci/djs525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Win AK, Lindor NM, Young JP, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst. 2012;104:72–1363. doi: 10.1093/jnci/djs351. doi: 10.1093/jnci/djs351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:8–261. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins MA, Hayashi S, O’Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh MD, Buchanan DD, Cummings MC, et al. Lynch syndrome-associated breast cancers: clinicopathologic characteristics of a case series from the colon cancer family registry. Clin Cancer Res. 2010;16:24–2214. doi: 10.1158/1078-0432.CCR-09-3058. doi: 10.1158/1078-0432.CCR-09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brieger A, Engels K, Schaefer D, et al. Malignant fibrous histiocytoma is a rare Lynch syndrome-associated tumor in two German families. Fam Cancer. 2011;10:5–591. doi: 10.1007/s10689-011-9455-9. doi: 10.1007/s10689-011-9455-9. [DOI] [PubMed] [Google Scholar]
- 9.Nilbert M, Therkildsen C, Nissen A, Akerman M, Bernstein I. Sarcomas associated with hereditary nonpolyposis colorectal cancer: broad anatomical and morphological spectrum. Fam Cancer. 2009;8:13–209. doi: 10.1007/s10689-008-9230-8. doi: 10.1007/s10689-008-9230-8. [DOI] [PubMed] [Google Scholar]
- 10.Broaddus RR, Lynch PM, Lu KH, Luthra R, Michelson SJ. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod Pathol. 2004;17:11–981. doi: 10.1038/modpathol.3800150. [DOI] [PubMed] [Google Scholar]
- 11.Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol. 2012;43:87–1677. doi: 10.1016/j.humpath.2011.12.012. doi: 10.1016/j.humpath.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Grindedal EM, Moller P, Eeles R, et al. Germ-line mutations in mismatch repair genes associated with prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:7–2460. doi: 10.1158/1055-9965.EPI-09-0058. doi: 10.1158/1055-9965.EPI-09-0058. [DOI] [PubMed] [Google Scholar]
- 13.Engel C, Loeffler M, Steinke V, et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol. 2012;30:15–4409. doi: 10.1200/JCO.2012.43.2278. doi: 10.1200/JCO.2012.43.2278. [DOI] [PubMed] [Google Scholar]
- 14.Barrow PJ, Ingham S, O’Hara C, et al. The spectrum of urological malignancy in Lynch syndrome. Fam Cancer. 2013;12:57–63. doi: 10.1007/s10689-012-9573-z. doi: 10.1007/s10689-012-9573-z. [DOI] [PubMed] [Google Scholar]
- 15.Raymond VM, Mukherjee B, Wang F, et al. Elevated risk of prostate cancer among men with lynch syndrome. J Clin Oncol. 2013;31:8–1713. doi: 10.1200/JCO.2012.44.1238. doi: 10.1200/JCO.2012.44.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soravia C, van der Klift H, Brundler MA, et al. Prostate cancer is part of the hereditary non-polyposis colorectal cancer (HNPCC) tumor spectrum. Am J Med Genet A. 2003;121A:62–159. doi: 10.1002/ajmg.a.20106. doi: 10.1002/ajmg.a.20106. [DOI] [PubMed] [Google Scholar]
- 17.Wagner DG, Gatalica Z, Lynch HT, Kohl S, Johansson SL, Lele SM. Neuroendocrine-type prostatic adenocarcinoma with microsatellite instability in a patient with lynch syndrome. Int J Surg Pathol. 2010;18:3–550. doi: 10.1177/1066896910379406. doi: 10.1177/1066896910379406. [DOI] [PubMed] [Google Scholar]
- 18.Bauer CM, Ray AM, Halstead-Nussloch BA, et al. Hereditary prostate cancer as a feature of Lynch syndrome. Fam Cancer. 2011;10:37–42. doi: 10.1007/s10689-010-9388-8. doi: 10.1007/s10689-010-9388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:43–2331. doi: 10.1158/1055-9965.EPI-07-0648. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 20.Clendenning M, Walsh MD, Gelpi JB, et al. Detection of large scale 30 deletions in the PMS2 gene amongst Colon-CFR participants: have we been missing anything? Fam Cancer. 2013;12:6–563. doi: 10.1007/s10689-012-9597-4. doi: 10.1007/s10689-012-9597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young J, Simms LA, Biden KG, et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001;159:16–2107. doi: 10.1016/S0002-9440(10)63062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh MD, Cummings MC, Buchanan DD, et al. Molecular, pathologic, and clinical features of early-onset endometrial cancer: identifying presumptive Lynch syndrome patients. Clin Cancer Res. 2008;14:700–1692. doi: 10.1158/1078-0432.CCR-07-1849. doi: 10.1158/1078-0432.CCR-07-1849. [DOI] [PubMed] [Google Scholar]
- 23.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:8–1043. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd edn. Philadelphia: Lippincott; 2008. [Google Scholar]
- 25.Ahlbom A, Norell S. Introduction to modern epidemiology. 1st edn. Stockholm: Epidemiology Resources Inc.; 1990. [Google Scholar]
- 26.Chen Y, Wang J, Fraig MM, et al. Defects of DNA mismatch repair in human prostate cancer. Cancer Res. 2001;61:21–4112. [PubMed] [Google Scholar]
- 27.Cunningham JM, Shan A, Wick MJ, et al. Allelic imbalance and microsatellite instability in prostatic adenocarcinoma. Cancer Res. 1996;56:82–4475. [PubMed] [Google Scholar]
- 28.Egawa S, Uchida T, Suyama K, et al. Genomic instability of microsatellite repeats in prostate cancer: relationship to clinicopathological variables. Cancer Res. 1995;55:29–2418. [PubMed] [Google Scholar]
- 29.Gao X, Wu N, Grignon D, et al. High frequency of mutator phenotype in human prostatic adenocarcinoma. Oncogene. 1994;9:2999–3003. [PubMed] [Google Scholar]
- 30.Dowty JG, Win AK, Buchanan DD, et al. Cancer risks for MLH1 and MSH2 mutation carriers. Hum Mutat. 2013;34:7–490. doi: 10.1002/humu.22262. doi: 10.1002/humu.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pande M, Wei C, Chen J, et al. Cancer spectrum in DNA mismatch repair gene mutation carriers: results from a hospital based Lynch syndrome registry. Fam Cancer. 2012;11:7–441. doi: 10.1007/s10689-012-9534-6. doi: 10.1007/s10689-012-9534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott RJ, McPhillips M, Meldrum CJ, et al. Hereditary nonpolyposis colorectal cancer in 95 families: differences and similarities between mutation-positive and mutation-negative kindreds. Am J Hum Genet. 2001;68:27–118. doi: 10.1086/316942. [DOI] [PMC free article] [PubMed] [Google Scholar]