Abstract
BACKGROUND
Donor to recipient lung size matching at lung transplantation (LTx) can be estimated by the predicted total lung capacity (pTLC)ratio (donor pTLC/recipient pTLC). We aimed to determine whether the pTLC-ratio is associated with the risk of primary graft dysfunction (PGD) after bilateral LTx (BLT).
METHODS
We calculated the pTLC-ratio for 812 adult BLTs from the Lung Transplant Outcomes Group between 3/2002-12/2010. Patients were stratified by pTLC-ratio>1.0 (“oversized”) and pTLC-ratio≤1.0 (“undersized”). PGD was defined as any ISHLT grade 3 PGD within 72 hours of reperfusion (PGD 3). We analyzed the association between risk factors and PGD using multivariable conditional logistic regression. As transplant diagnoses can influence the size matching decisions and also modulate the risk for PGD, we performed pre-specified analyses by assessing the impact of lung size mismatch within diagnostic categories.
RESULTS
In univariate analyses oversizing was associated with a 39% lower odds of PGD3 (OR 0.61, 95% CI, p=0.003). In a multivariate model accounting for center effects and known PGD risks, oversizing remained independently associated with a decreased odds of PGD3 (OR 0.58, 95% CI 0.38-0.88, p=0.01). The risk adjusted point estimate was similar for the non-COPD diagnoses groups (OR 0.52, 95%CI 0.32-0.86, p=0.01); however there was no detected association within the COPD group (OR 0.72, 95% CI 0.29-1.78, p=0.5).
CONCLUSION
Oversized allografts are associated with a decreased risk of PGD3 after BLT; this effect appears most apparent in non-COPD patients.
Keywords: Lung transplantation, Lung size mismatch, Primary graft dysfunction
Introduction
Primary graft dysfunction (PGD) is a major cause of early mortality after lung transplantation (LTx) and is associated with the development of bronchiolitis obliterans, which is a major cause of long-term mortality1,2. Improved survival associated with graft oversizing in bilateral lung transplants (BLT) has been demonstrated in several studies3-9. The most common indications for transplantation affect the size matching preference; with a general preference toward oversizing in chronic obstructive pulmonary disease (COPD) and undersizing in idiopathic pulmonary fibrosis (IPF)4,9. However, data from studies limited to patients transplanted for idiopathic pulmonary arterial hypertension (IPAH), a condition in which no sizing preference exists, demonstrate the same association between size matching and survival4.
The mechanisms by which oversizing improves outcomes after transplant remain unknown, but the survival difference may be observed in the early post-transplant period8. It is conceivable that differences in PGD rates are responsible for these early survival differences and that lung undersizing predisposes to PGD through mechanisms involving both the pulmonary vasculature, as well as through potentially injurious tidal volumes during mechanical ventilation. A significantly undersized pulmonary vasculature could result in higher pulmonary arterial pressure at reperfusion, which is a risk factor for PGD2,10. Alternately, variability in size matching could result in different rates of PGD through differences in mechanical ventilation tidal volumes compared to donor lung size during the period of mechanical ventilation during and after lung transplantation. It is common practice to set mechanical ventilation tidal volumes after lung transplantation according to recipient rather than donor characteristics11,12. Therefore, undersized allografts could potentially receive tidal volumes that are high when considered according to donor lung size11,12. Lung protective strategies of low tidal volume ventilation clearly established to be beneficial in acute respiratory distress syndrome (ARDS)13 are also beneficial to patients at risk for ARDS14,15. Likewise, low tidal volume ventilation is also associated with better outcomes even when exposure is limited to the relatively brief intraoperative period in patients undergoing abdominal surgery14.
We hypothesized that an oversized allograft would be associated with a lower risk of PGD. To address this hypothesis, we conducted a study ancillary to the Lung Transplant Outcomes Group (LTOG) to characterize lung size mismatch and its association with the occurrence of PGD.
Methods
Study Design and Subject Selection
LTOG is a US National Institutes of Health sponsored, multicenter, prospective cohort study designed to evaluate risk factors for PGD. Details of the LTOG cohort have been described previously2. In this LTOG ancillary study, we included patients aged 18–80 years undergoing bilateral lung transplantation (BLT) at 10 US transplant centers between March 2002 and December 2010. Clinical parameters were collected prospectively. Additional information was verified from the United Network for Organ Sharing.
Stratification according to lung size matching
Estimates of lung and chest wall size were calculated from sex, age and height, as the predicted total lung capacity (pTLC) (online supplemental information 1)8,16,17. Donor lung size was compared to the size of a recipient's thorax by calculating the ratio of the donor's pTLC to the recipient's pTLC (pTLC-ratio). The pTLC-ratio was analyzed as a continuous variable. To assess for non-linearity, we analyzed according to quintiles of pTLC-ratio. Patients were further stratified by pTLC-ratio ≤ 1.0 (“undersized”) and pTLC-ratio > 1.0 (“oversized”), as previously reported3,9. A propensity score estimating the likelihood of receiving an oversized allograft (pTLC-ratio > 1.0) was created using logistic regression based on 17 potential predictors (online supplemental information 2).
Definition of PGD
PGD was graded according to ISHLT criteria, which is based on PaO2/ FIO2 ratio and the presence of diffuse parenchymal infiltrates in the allograft on chest radiograph1. Chest radiographs were interpreted independently by two physicians unaware of other clinical variables, with adjudication of conflicts by a third reviewer (PGD3 classification kappa = 0.95)1,2. The primary outcome was the presence of grade 3 PGD (PaO2/FIO2 ratio < 200) at any point within 72 hours of transplantation1,2.
Analytical plan
Logistic regression was used to estimate odds ratios (OR) for the association between pTLC-ratio and PGD. Transplant center was evaluated as a fixed effect using conditional logistic regression. As transplant diagnoses can influence the size matching decisions and also modulate the risk for PGD, we performed pre-specified analyses assessing the impact of lung size mismatch within the following transplant indications: A) within chronic obstructive pulmonary disease (COPD) versus non-COPD; B) within COPD, idiopathic pulmonary fibrosis (IPF) and idiopathic pulmonary arterial hypertension (IPAH). An interaction analysis was performed between diagnoses [A) COPD versus non-COPD and B) COPD, IPF, IPAH, and “other”] and pTLC-ratio upon the outcome of PGD3 (online supplemental information 3). Potential confounders were selected into multivariable models based on risk factors previously identified in the literature and plausible association with pTLC-ratio or PGD (2). Recipient body mass index (BMI) was included as a categorical variable (quintiles of BMI) in multivariable modeling because of its observed nonlinearity18. The risk of PGD by pTLC-ratio strata was further assessed in a second multivariable model incorporating ordinal quintiles of the propensity score (likelihood of receiving an oversized allograft based on 17 predictors) (online supplemental information 2). Potential problems of missing data were approached as described previously (2).
Characteristics of mechanical ventilation at reperfusion
Recipient tidal volumes (TVs) at reperfusion were expressed as absolute values in milliliters (ml). As a general goal is to set tidal volume to match lung size, TVs are commonly set according to predicted body weight (PBW), and not measured body weight13. TVs were expressed in relation to PBW of the recipient and PBW of the donor (online supplemental information 1).
Results
Of 1255 total lung transplants, there were 812 BLT subjects, and PGD3 developed in 31%. The histogram of the pTLC-ratio is shown in online supplemental figure 2.
In univariate conditional logistic regression models, each 0.1 increase in pTLC-ratio was associated with a 14% lower odds of PGD3 (OR 0.86, 95% CI, 0.78-0.96, p = 0.005, table 1). The unadjusted relationship between pTLC-ratio and probability of PGD3 is shown in figure 1. To address the possibility of a non-linear relationship between pTLC-ratio and risk for PGD3, quintiles of pTLC-ratio were included in the analyses (table 1).
Table 1.
Odds ratio's for the association between pTLC-ratio and any grade 3 Primary Graft Dysfunction within 72 hours after lung transplantation for the overall study population.
| Univariate N = 812 | Multivariate 1 † N = 798 | Multivariate 2 τ N = 798 | ||||
|---|---|---|---|---|---|---|
| pTLC-ratio based parameter | OR (95% CI) | value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| pTLC-ratioLinear Cont. | ||||||
| Per 0.1 increase | 0.86 (0.78-0.96) | 0.005 | 0.89 (0.77-1.02) | 0.09 | 0.88 (0.76-1.01) | 0.07 |
| By Quintile [pTLC-ratio-range] | ||||||
| Quintile 1 [0.65 - 0.90] | Reference | - | Reference | - | Reference | - |
| Quintile 2 [0.91 - 1.00] | 0.67 (0.42-1.09) | 0.1 | 0.79 (0.44-1.34) | 0.4 | 0.75 (0.42-1.33) | 0.3 |
| Quintile 3 [1.01 - 1.08] | 0.55 (0.34-0.91) | 0.02 | 0.48 (0.26-0.88) | 0.02 | 0.48 (0.26-0.89) | 0.02 |
| Quintile 4 [1.09 - 1.18] | 0.42 (0.26-0.70) | 0.001 | 0.47 (0.25-0.90) | 0.02 | 0.42 (0.22-0.82) | 0.01 |
| Quintile 5 [1.19 - 1.64] | 0.53 (0.32-0.87) | 0.01 | 0.52 (0.26-1.05) | 0.07 | 0.49 (0.24-1.00) | 0.05 |
| By undersized vs. oversized [pTLC-ratio] | ||||||
| “Undersized” [0.89 ± 0.08] | Reference | - | Reference | - | Reference | |
| “Oversized” [1.16 ± 0.13] | 0.61 (0.45-0.85) | 0.003 | 0.58 (0.38-0.88) | 0.01 | 0.57 (0.38-0.88) | 0.01 |
final multivariate conditional (center effect) logistic regression model adjusted for diagnosis [CF,COPD,IPF,IPAH, Sarcoidosis] , recipient gender (and stratified by number of pregnancies in female recipients), BMI [recipient in quintiles], cardiopulmonary bypass, ischemic time, blood product use [PRBCs transfused, as categorical groups: None, < 1 liter and > 1 liter], pulmonary artery systolic pressure, donor smoking history, FiO2, tidal volume at reperfusion (in ml/kg donor predicted body weight) and transplant year.
Same multivariate conditional (center effect) logistic regression model as in †, additionally adjusted for quintiles of the propensity score.
pTLC = predicted total lung capacity.
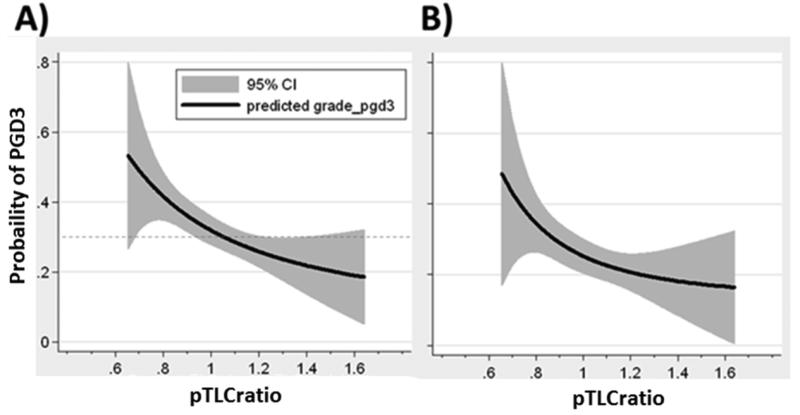
Figure 1.
Lung size mismatch (pTLCratio) is associated with the probability of PGD3. The relationship of pTLCratio (pTLCdonor / pTLCrecipeint) and predicted probability of any grade PGD3 within 72 hours is shown using a fractional polynomial fit with 95% CIs (gray area).
A) Unadjusted analysis. The dashed line represents the occurrence of PGD3 in all bilateral lung transplant patients (31%).
B) Multivariate adjusted analysis (as described in table 2 as multivariate model 1)
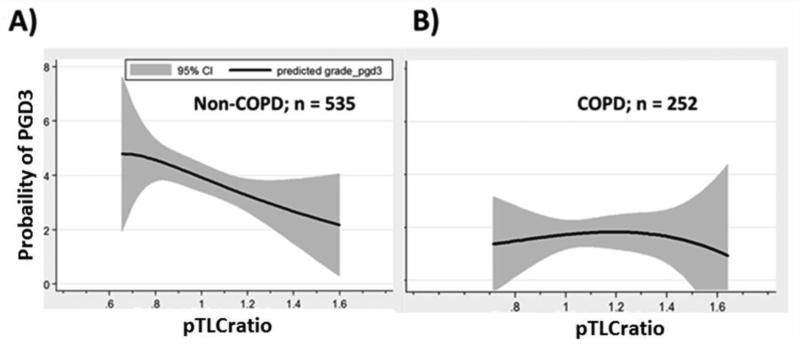
There were 330 subjects classified as undersized, with a mean pTLC-ratio of 0.89 ± 0.08 (range 0.64-1.00), and 482 subjects classified as oversized, with a mean pTLC-ratio of 1.16 ± 0.13 (range 1.01-1.64). Baseline characteristics by undersized and oversized groups are shown in table 2. Subjects in the undersized group were more often male and had IPF as diagnosis, while subjects in the oversized group more often had COPD as diagnosis. PGD3 developed in 37.6% of the undersized and in 26.7% of the “oversized” subjects (p=0.001). In univariate analyses oversizing was associated with a lower odds of PGD3 (OR 0.61, 95% CI, p=0.003), table 1. The unadjusted relationships between the pTLC-ratio and probability of PGD3 according to COPD and non-COPD transplant diagnoses are shown in figure 2. In univariate conditional logistic regression models, the odds ratio for PGD3 for the “oversized” group was 0.64 (95% CI, 0.44-0.94, p = 0.02) for subjects with non-COPD diagnoses (n=535) and 1.03 (95% CI 0.50-2.1, p = 0.9) for subjects with COPD (n=252). The results for the univariate analysis within IPF and IPAH are shown in online supplemental information 4. There was no statistical interaction identified between diagnostic groups (both COPD versus Non-COPD (p=0.2) and COPD vs. IPF vs. IPAH vs. other (p=0.6)) and pTLC-ratio regarding their impact on PGD3.
Table 2.
Baseline characteristics for “undersized” and “oversized” cohorts.
| Undersized pTLC-ratio ≤1.0 | Oversized pTLC-ratio >1.0 | p-value | |
|---|---|---|---|
| Number | 330 | 482 | |
| Size matching characteristics | Mean (SD) | Mean (SD) | |
| pTLC-ratio, mean ± SD | 0.89 ± 0.08 | 1.16 ± 0.13 | <0.001 |
| pTLC-ratio, range | 0.65 to 1.00 | 1.01 to 1.64 | <0.001 |
| Recipient | |||
| Gender Male | 75.4% | 40.2% | <0.001 |
| Age | 51.5 ± 13 | 48.7 ± 14 | 0.004 |
| BMI- Recipient | 25.6±4.8 | 23.5±4.9 | <0.001 |
| Diagnosis | |||
| COPD | 23.9% | 40.9% | <0.001 |
| IPAH | 3.9% | 4.4% | 0.7 |
| Cystic Fibrosis | 15.7% | 25.8% | <0.001 |
| Idiopathic Pulmonary Fibrosis | 43.3% | 18.3% | <0.001 |
| Sarcoidosis | 6.6% | 4.1% | 0.1 |
| Other | 6.4% | 6.4% | 0.9 |
| Operative Characteristics | |||
| Cardiopulmonary Bypass | 51.8% | 48.9% | 0.4 |
| Ischemic time (1st / 2nd implant) | 264/361 | 260/351 | 0.6 / 0.2 |
| PRBC transfused in cc | 893±1128 | 980±1136 | 0.3 |
| Pulmonary artery systolic pressure | 45.3±19 | 43.2±19 | 0.1 |
| Tidal Volume at reperfusion (in ml) | 495 ± 203 | 470 ± 188 | 0.07 |
| FiO2 at reperfusion | 52.5 ± 29.6 | 51.4 ± 29 | 0.6 |
| Donor Characteristics | |||
| BMI- Donor | 25.8 ± 5.5 | 25.5 ± 5.2 | |
| Donor smoking history | 37.5% | 37.1% | 0.9 |
| Transplant related | |||
| Transplant after 2005 | 79% | 82% | 0.3 |
| Center with high volume (inclusion of > 100 BLTs during study period) | 62% | 57% | 0.2 |
pTLC= predicted total lung capacity; BMI = body mass index; COPD = chronic obstructive pulmonary disease; IPAH = idiopathic pulmonary arterial hypertension; PRBC = pack red blood cell concentrates; FiO2 = fraction of inspired oxygen; BLT = bilateral lung transplant.
Figure 2.
Lung size mismatch (pTLCratio) is associated with the probability of PGD3 in Non-COPD subjects, but not COPD. Unadjusted relationship via fractional polynomial regression (95% confidence intervals [CI] as gray area) of the pTLC-ratio (pTLCdonor / pTLCrecipeint) and the predicted probability of any grade PGD3 within 72 hours analyzed within individual diagnoses. A) Subjects with Non- Chronic obstructive pulmonary disease (COPD), B) Subjects with COPD.
Multivariate analyses
For the overall study population, in a multivariate model accounting for center effects, diagnosis, ischemic time, use of cardiopulmonary bypass, transfusions, pulmonary artery systolic pressure, BMI, donor smoking history, and both FiO2 as well as tidal volume at reperfusion, “oversizing” remained independently associated with a decreased odds of PGD3 with a similar point estimate (OR 0.58, 95%CI 0.38-0.88, p=0.01), table 1. The adjusted relationship between pTLC-ratio and predicted probability of PGD3 is shown in figure 1B. When stratified according to non-COPD versus COPD diagnoses the risk adjusted point estimate was similar for the non-COPD diagnoses group (OR 0.52, 95%CI 0.32-0.86, p=0.01, online supplemental table 1). However there was no significant association within the COPD group alone (HR 0.72, 95% CI 0.29-1.78, p=0.5; online supplemental table 2). After propensity score quintiles were included in a separate multivariable analysis the results were comparable (table 1 and supplemental online information 2, supplemental table 1 and 2).
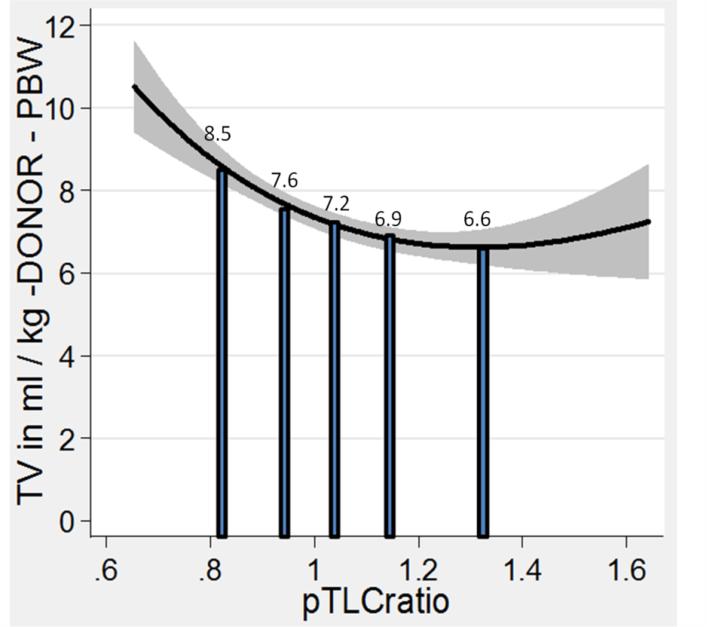
Tidal volumes (TVs) recorded at the time of reperfusion were expressed in relation to an estimate of the allograft size as ml/kg predicted body weight (PBW) of the donor. Undersized subjects received larger donor-based TVs than oversized subjects (8.1±3.3 ml/kg-donor-PBW vs. 6.9±2.7 ml/kg-donor-PBW, p < 0.001; table 3). Figure 3 shows the fractional polynomial regression of the TV at reperfusion in ml/kg donor-PBW plotted against the pTLC-ratio and the mean values for the pTLC-ratio quintiles. Limiting the TV analysis to only patients with PGD3 yielded comparable results (table 3). However, none of the TV parameters were independent predictors of PGD3 and did not change the association between the pTLC-ratio and PGD3 (supplemental online table 3).
Table 3.
Tidal volumes in absolute volume (milliliters), related to recipient predicted body weight and related to donor predicted body weight.
| Tidal Volume in ml | Tidal Volume in ml/kg-Recipient | Tidal Volume in ml/kg-Donor | |
|---|---|---|---|
| All BLTs (n = 812) | Mean (SD) | Mean (SD) | Mean (SD) |
| By undersized vs. oversized [pTLC-ratio] | |||
| “Undersized” [0.89 ± 0.08] | 495 ± 203 | 7.3 ± 2.9 | 8.1±3.3 |
| “Oversized” [1.16 ± 0.13] | 470 ± 188 | 8.0 ± 2.9 | 6.9±2.7 |
| By Quintile [pTLC-ratio-range] | |||
| Quintile 1 [0.65 - 0.90] | 502 ±205 | 7.3 ± 2.8 | 8.5 ± 3.4 |
| Quintile 2 [0.91 - 1.00] | 488 ± 203 | 7.3 ± 3.1 | 7.6 ± 3.2 |
| Quintile 3 [1.01 - 1.08] | 475 ± 195 | 7.6 ± 3.0 | 7.2 ± 2.9 |
| Quintile 4 [1.09 - 1.18] | 474 ± 180 | 7.9 ± 3.0 | 6.9 ± 2.6 |
| Quintile 5 [1.19 - 1.64] | 462 ± 191 | 8.6 ± 3.8 | 6.6 ± 2.8 |
| Only PGD3 (n = 253) | Mean (SD) | Mean (SD) | Mean (SD) |
|---|---|---|---|
| By under vs. over [pTLC-ratio] | |||
| “Undersized” [0.89 ± 0.08] | 461 ± 207 | 7.0 ± 3.0 | 7.8±3.5 |
| “Oversized” [1.16 ± 0.13] | 424 ± 182 | 7.2 ± 3.1 | 6.2±2.6 |
| By Quintile [pTLC-ratio-range] | |||
| Quintile 1 [0.65 - 0.90] | 478 ± 207 | 7.0 ± 2.9 | 8.3 ± 3.6 |
| Quintile 2 [0.91 - 1.00] | 442 ± 208 | 6.9 ± 3.3 | 7.2 ± 3.4 |
| Quintile 3 [1.01 - 1.08] | 406 ± 177 | 6.5 ± 2.8 | 6.2 ± 2.7 |
| Quintile 4 [1.09 - 1.18] | 445 ± 174 | 7.2 ± 3.0 | 6.3 ± 2.4 |
| Quintile 5 [1.19 - 1.64] | 425 ± 197 | 7.9 ± 3.5 | 6.1 ± 2.8 |
Vol. = volume; BLT= bilateral lung transplant; ml = milliliter; pTLC = predicted total lung capacity; PGD = primary graft dysfunction;
Figure 3.
Lung size mismatch (pTLCratio) is associated with the mechanical ventilation tidal volumes (TV) at reperfusion, when the TV is related to the size of the allograft. Fractional polynomial regression of the TV in ml/kg donor-predicted body weight (PBW) plotted against the pTLCratio (pTLCdonor / pTLCrecipeint). The solid vertical bars represent the mean values of the TV in ml/kg donor-PBW according to pTLCratio-quintiles.
Discussion
In this ancillary study to the Lung Transplant Outcomes Group study, a pTLC-ratio >1.0 (suggestive of an oversized allograft) was associated with a lower odds of PGD3 after bilateral lung transplantation (BLT). Our findings are consistent with prior studies, including a single center study of BLT patients3, a prior study of living lobar lung transplantation in pediatric patients19, and a prior registry study by our group8. While our sample was not powered to detect an interaction by diagnosis group, our results seem to have the most apparent effect among non-COPD patients. Therefore, if confirmed, our results may have important implications for decisions regarding allograft sizing at the time of listing, and may also inform the approach to mechanical ventilation tidal volumes at the time of transplantation.
Data from ARDS research suggest that ventilator-induced alveolar stretch and shear force can induce inflammation and injury leading to capillary leak13,15,20. Higher tidal volumes could contribute to such injury in lung transplant recipients leading to clinical PGD. Mechanical ventilation tidal volumes are commonly set according to an estimate of patient size in milliliters (ml) per kilogram (kg) body weight13. After LTx, however, tidal volumes are usually set according to recipient characteristics rather than donor lung size11,12. We have previously described the relationship between donor–recipient lung size mismatch and postoperative mechanical ventilation tidal volumes according to recipient- and donor-predicted body weights in a cohort of BLT subjects12. Tidal volumes in ml/kg-donor-predicted body weights revealed significant differences between undersized, matched, and oversized subsets (11.4 ± 3.1 vs 9.4 ± 1.2 vs 8.1 ± 2.1, respectively; p < 0.05). During mechanical ventilation after BLT, undersized allografts received relatively higher tidal volumes compared with oversized allografts when the tidal volumes were related to donor-predicted body weights.
When the tidal volumes at reperfusion in this investigation were expressed in relation to an estimate of the allograft size (as ml per kg-donor-predicted-body-weight) undersized allografts also received significantly larger tidal volumes compared to oversized allografts (table 3 and figure 3). Low tidal volume ventilation is associated with improved survival in patients with ARDS13. In patients without lung injury, but with risk factors for pulmonary complications, a lung protective low TV ventilation strategy during surgery was associated with lower rates of pulmonary complications after surgery14. Moreover, in patients with no prior lung injury who received mechanical ventilation during bypass for cardiac surgery, larger tidal volumes were associated with higher circulating inflammatory mediator levels21. Furthermore, larger tidal volumes were associated with the development of ARDS in patients who came to the ICU without ARDS, but had risk factors for ARDS15. In a randomized controlled trial comparing a low tidal volume with a standard ventilation strategy in subjects who would soon donate their lungs, a significantly higher proportion of donor lungs could be utilized from the low tidal volumes group22. If a lower tidal volumes approach, based on donor characteristics (i.e. donor predicted body weight) is protective before transplantation, the same may be true during and after transplantation. Additional studies of tidal volumes in relation to donor predicted body weight and of mechanical ventilation strategies in this high-risk setting are needed.
An oversized allograft was associated with lower pulmonary artery pressures and lower pulmonary vascular resistance23. In contrast, elevated pulmonary artery pressures were reported in the setting of undersized pediatric and adult lobar lung transplantation 19,24. Elevated pulmonary artery pressures at the time of transplant were identified as a risk factor for PGD2,10. Furthermore, pulmonary vascular resistance is related to the inflation state of the lung. Above functional residual capacity, pulmonary vascular resistance progressively increases25,26. An undersized allograft would be expected to ventilate at a higher volume and thus at higher pulmonary vascular resistance than would be predicted based on the reduced volume of vasculature alone. Hyperinflation of undersized allografts during mechanical ventilation after LTx has been reported and was associated with early graft failure27.
A key finding of our study is that the majority of the effect occurred in non-COPD subjects. Diagnosis can significantly affect the size matching preference, with a general preference toward oversizing in COPD4,8,9,17. The association between lung size mismatch and PGD in this study was most apparent in non-COPD subjects, whereas within COPD no significant association was found. There is some suggestion that the higher the risk for the occurrence of PGD, the more the pTLCratio modulates this risk (figure 2 and supplemental figure 3). While there is a general preference to undersize recipients with IPF, and IPF may be a clinical risk factor for PGD, the preference of undersizing subjects with IPF is not supported by this study. On the contrary, the benefits of oversizing seem to be most apparent in IPF recipients. Thus, within surgically feasible limits, similar sized or oversized allografts for recipients with IPF could potentially reduce PGD rate and improve outcomes.
However, there are opinions published that significantly oversized allografts are associated with increased perioperative complications and worse outcomes24,28. Contrary to these concerns, in a porcine model of LTx, a 1.8-fold oversized allograft was compared with a size-matched allograft and the oversized graft was associated with superior function23. In prior human studies, oversized allografts were not associated with an increase in post-LTx complications, and were associated with a shorter hospital length of stay after LTx and lower resource utilization3. In that single center study, higher acuity in the undersized group might explain these findings; however, multivariate models suggested an independent association between oversizing and lower resource utilization3.
An important question is, whether pulmonary pathology, such as interstitial lung disease, permanently reduces chest cavity size. LTx candidates with COPD, IPF and cystic fibrosis evaluated with opto-electronic plethysmography before transplant showed disease-specific adaptations of the respiratory system and chest wall volume during exercise29. However, the same assessment post-transplant demonstrated that, irrespective of transplant indication, the respiratory system and the chest wall volume response to exercise was not different from normal controls29. This would suggest that the underlying diagnosis does not permanently long term alter post-transplant chest wall mechanics. However intra-operatively and in the early post-LTx period hemodynamic compromise could occur in the setting of a profoundly oversized allograft secondary to a compartment-syndrome-like picture occurring after chest wall closure. A strategy of delayed chest wall closure allowed for the transplant of a 2.07 fold oversized allograft with good long-term outcomes30. The impact of delayed chest closure with skin closure on outcomes after lung transplantation was recently systematically evaluated by the University of Pittsburgh group31. In a matched cohort study to compare the results of a delayed chest closure technique with skin closure to similarly matched controls with primary closure, delayed chest closure as compared with primary closure contributed to significantly lower incidence of severe PGD , as well as improved post-transplant survival and functional status 31. The optimal strategy for size matching decisions, especially for recipient with restrictive lung disease, remains to be defined. In the USA, candidates for LTx are listed for acceptable donor height ranges.32 However lung size is determined by height, sex, age and race; all of these determinants could be accounted for by the pTLC-ratio16. Thus listing recipients for acceptable donor pTLC ranges rather than height ranges might be a useful step to optimize the size matching process3,4,7-9,16,33-36.
This investigation is limited by the imprecision of the pTLC-ratio as a parameter of allograft–thorax mismatch. The pTLC is calculated via regression equations based on sex, height and age and is derived from population norms16. The pTLC of the donor is likely reflective of the allograft size. However, the pTLC of the recipient might not accurately reflect the thorax size of a patient with end-stage lung disease of different etiologies. Techniques such as optoelectronic plethysmography or computed tomographic volumetry could provide a more precise measurement37. However, our findings of oversizing and risk of PGD within diagnostic categories account for recipient differences. This study was limited in power to detect interactions; however, in pre-specified subgroup analyses we were able to demonstrate effects in non-COPD patients. Although we used standard extraction techniques, information bias is possible in the way the tidal volume variables are extracted in the operating room, and may have been related to dynamic changes in ventilation related to elevated airway pressures in more injured lungs. However, this effect would likely cause less injured lungs to receive higher tidal volumes, and thus would bias our results in the opposite direction of our observed effect. Information on lung trimming or lobar resection was not captured in the dataset; however, lobar lung transplants were excluded a priori. Confounding by unmeasured trimming would likely bias oversizing towards an increased risk of PGD3 in oversized lungs24,28. Furthermore, uncontrolled confounding is possible; however, we included all known PGD risk factors in this analysis.
The possible mechanisms linking a higher pTLC-ratio to a decreased risk for PGD3 cannot be answered by this investigation. In the setting of donor organ shortage and waitlist mortality it is not practical to intentionally oversize all allografts; however, the pTLC-ratio could provide further refinement in the peri-transplant risk assessment32-36.
We conclude that a pTLC-ratio >1.0, suggestive of an oversized allograft is associated with a decreased risk of PGD3 after BLT. An oversized allograft may receive relatively smaller tidal volumes and operate at lower pulmonary vascular resistance. These possible physiological advantages of oversizing could be a mechanistic link to the lower risk of PGD3. The general preference of undersizing subjects with IPF is not supported by our findings. Thus, extending the acceptable donor size ranges to oversized allografts within surgically feasible limits for recipients with IPF could potentially both decrease waitlist mortality and improve post-transplant outcomes for IPF recipients.
Supplementary Material
Acknowledgements
This work was presented at the Thirty-third Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation, Montreal, Quebec, Canada, April24– 27, 2013.
Funding: The Lung Transplant Outcomes Group Study is supported by NIH grants RO1 HL087115, RO1 HL081619, and RO1 HL096845.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented at the Thirty-third Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation, Montreal, Quebec, Canada, April24– 27, 2013.
Disclosures: No relevant disclosures.
Participating Centers and Investigators in the Lung Transplant Outcomes Group:
University of Pennsylvania (coordinating site): Jason Christie, M.D., M.S. (PI), Steven M. Kawut, M.D., M.S., Alberto Pocchetino, M.D., Y. Joseph Woo, M.D., Ejigayehu Demissie, M.S.N., Robert M. Kotloff, M.D., Vivek N. Ahya, M.D., James Lee, M.D., M.S., Denis Hadjiliadis, M.D., M.H.S., Melanie Rushefski, B.S., Richard Aplenc, M.D., Clifford Deutschman, M.D., M.S., Benjamin Kohl, M.D., Edward Cantu III, M.D., Joshua M. Diamond, M.D., M.S., Rupal J. Shah, M.D., M.S, and Laurel Kalman.
Columbia University: David Lederer, M.D., M.S. (PI), Selim Arcasoy, M.D., Joshua Sonett, M.D., Jessie Wilt, M.D., Frank D'Ovidio, M.D., Lori Shah, M.D., Hilary Robbins, M.D., Matthew Bacchetta, M.D., Nilani Ravichandran, N.P., Genevieve Reilly, N.P., Jeffrey Okun, M.D., Debbie Rybak, B.A., Michael Koeckert, B.A., Robert Sorabella, B.A., Nisha Ann Philip, M.B.B.S., Nadine Al-Naamani, M.D., Matthew LaVelle, B.S., Megan Larkin, M.P.H., and Shefali Sanyal, B.S.
Vanderbilt University: Lorraine Ware, M.D. (PI), Aaron Milstone, M.D., Jean Barnes, R.N., Stephanie Logan, R.N., Carla Ramsey, R.N., Thelma Walden, and Shaquita Claybrooks, R.N. Stanford University: Ann Weinacker, M.D. (PI), Susan Spencer Jacobs, M.S.N., Val Scott, M.S.N., and Tal Alfasi, M.S.
University of Alabama, Birmingham: Keith Wille, M.D. (PI), and Necole Harris, R.N.
Johns Hopkins University: Jonathan Orens, M.D. (PI), Ashish Shah, M.D., John McDyer, M.D., Christian Merlo, M.D., M.P.H., Matthew Pipeling, M.D., Reda Girgis, M.D., Karen Oakjones, R.N., and April Thurman.
University of Michigan: Vibha Lama, M.D., M.S. (PI), Fernando Martinez, M.D., M.S., Emily Galopin, Douglas R. Armstrong R.N., M.S., and Mary Maliarik, B.S.
Duke University: Scott M. Palmer, M.D., M.H.S. (PI), David Zaas, M.D., M.B.A., R. Duane Davis, M.D., Ashley Finlen-Copeland, M.S.W., Jessica Martissa, and William A. Davis. University of Chicago: Sangeeta Bhorade, M.D. (PI), and Mark Lockwood, R.N., M.S.N. University of Pittsburgh: Maria Crespo, M.D. (PI), Joseph Pilewski, M.D., Christian Bermudez, M.D., and Kathleen Hanze.
Indiana University: David S. Wilkes, M.D., David Wilson Roe, M.D., Thomas Wozniak, M.D., Ronda L. McNamee, R.N., Kim A. Fox, R.N., Danyel F. Gooch, R.N., and Tonya Isaacs, R.N.
References
- 1.Christie JD, Bellamy S, Ware LB, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1231–1239. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberlein M, Arnaoutakis GJ, Yarmus L, et al. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:492–500. doi: 10.1016/j.healun.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Eberlein M, Diehl E, Bolukbas S, et al. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32:1172–1178. doi: 10.1016/j.healun.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Eberlein M, Permutt S, Brown RH, et al. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011;183:79–87. doi: 10.1164/rccm.201004-0593OC. [DOI] [PubMed] [Google Scholar]
- 6.Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451–460. doi: 10.1378/chest.11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg. 2013;96:457–463. doi: 10.1016/j.athoracsur.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 8.Eberlein M, Reed RM, Maidaa M, et al. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc. 2013;10:418–425. doi: 10.1513/AnnalsATS.201301-008OC. [DOI] [PubMed] [Google Scholar]
- 9.Eberlein M, Reed RM, Permutt S, et al. Parameters of donor-recipient size mismatch and survival after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:1207–1213. e1207. doi: 10.1016/j.healun.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Whelan TP, Dunitz JM, Kelly RF, et al. Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. J Heart Lung Transplant. 2005;24:1269–1274. doi: 10.1016/j.healun.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Beer A, Reed RM, Bolukbas S, et al. Mechanical ventilation after lung transplantation. An international survey of practices and preferences. Ann Am Thorac Soc. 2014;11:546–553. doi: 10.1513/AnnalsATS.201312-419OC. [DOI] [PubMed] [Google Scholar]
- 12.Dezube R, Arnaoutakis GJ, Reed RM, et al. The effect of lung-size mismatch on mechanical ventilation tidal volumes after bilateral lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:275–281. doi: 10.1093/icvts/ivs493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 14.Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 15.Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 16.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 17.Mason DP, Batizy LH, Wu J, et al. Matching donor to recipient in lung transplantation: How much does size matter? J Thorac Cardiovasc Surg. 2009;137:1234–1240. e1231. doi: 10.1016/j.jtcvs.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184:1055–1061. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lillehei CW, Shamberger RC, Mayer JE, Jr., et al. Size disparity in pediatric lung transplantation. J Pediatr Surg. 1994;29:1152–1155. doi: 10.1016/0022-3468(94)90299-2. discussion 1155-1156. [DOI] [PubMed] [Google Scholar]
- 20.Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zupancich E, Paparella D, Turani F, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2005;130:378–383. doi: 10.1016/j.jtcvs.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 22.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. Jama. 2010;304:2620–2627. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 23.Binns OA, DeLima NF, Buchanan SA, et al. Use of over-sized mature pulmonary lower lobe grafts results in superior pulmonary function. Ann Thorac Surg. 1997;64:307–312. doi: 10.1016/S0003-4975(97)00565-1. [DOI] [PubMed] [Google Scholar]
- 24.Mitilian D, Sage E, Puyo P, et al. Techniques and results of lobar lung transplantations. Eur J Cardiothorac Surg. 2014;45:365–369. doi: 10.1093/ejcts/ezt353. discussion 369-370. [DOI] [PubMed] [Google Scholar]
- 25.Permutt S, Riley RL. HEMODYNAMICS OF COLLAPSIBLE VESSELS WITH TONE: THE VASCULAR WATERFALL. J Appl Physiol. 1963;18:924–932. doi: 10.1152/jappl.1963.18.5.924. [DOI] [PubMed] [Google Scholar]
- 26.Whittenberger JL, Mc GM, Berglund E, et al. Influence of state of inflation of the lung on pulmonary vascular resistance. J Appl Physiol. 1960;15:878–882. doi: 10.1152/jappl.1960.15.5.878. [DOI] [PubMed] [Google Scholar]
- 27.Kozower BD, Meyers BF, Ciccone AM, et al. Potential for detrimental hyperinflation after lung transplantation with application of negative pleural pressure to undersized lung grafts. J Thorac Cardiovasc Surg. 2003;125:430–432. doi: 10.1067/mtc.2003.139. [DOI] [PubMed] [Google Scholar]
- 28.Shigemura N, D'Cunha J, Bhama JK, et al. Lobar lung transplantation: a relevant surgical option in the current era of lung allocation score. Ann Thorac Surg. 2013;96:451–456. doi: 10.1016/j.athoracsur.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 29.Wilkens H, Weingard B, Lo Mauro A, et al. Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis, pulmonary fibrosis and COPD before and after lung transplantation. Thorax. 2010;65:808–814. doi: 10.1136/thx.2009.131409. [DOI] [PubMed] [Google Scholar]
- 30.Chen F, Matsukawa S, Ishii H, et al. Delayed chest closure assessed by transesophageal echocardiogram in single-lobe lung transplantation. Ann Thorac Surg. 2011;92:2254–2257. doi: 10.1016/j.athoracsur.2011.05.102. [DOI] [PubMed] [Google Scholar]
- 31.Shigemura N, Orhan Y, Bhama JK, et al. Delayed chest closure after lung transplantation: Techniques, outcomes, and strategies. J Heart Lung Transplant. 2014 doi: 10.1016/j.healun.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Eberlein M, Garrity ER, Orens JB. Lung allocation in the United States. Clin Chest Med. 2011;32:213–222. doi: 10.1016/j.ccm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Eberlein M, Bolukbas S, Reed RM. eComment. Gender mismatching in lung transplantation: lung size mismatch is the issue! Interact Cardiovasc Thorac Surg. 2013;16:435–436. doi: 10.1093/icvts/ivt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eberlein M, Bolukbas S, Reed RM. Bilateral lobar lung transplantation and size mismatch by pTLC-ratio. Eur J Cardiothorac Surg. 2013;44:394–395. doi: 10.1093/ejcts/ezt004. [DOI] [PubMed] [Google Scholar]
- 35.Eberlein M, Reed RM. Letter by Eberlein and Reed regarding article, “transplantation for idiopathic pulmonary arterial hypertension: improvement in the lung allocation score era”. Circulation. 2014;129:e457. doi: 10.1161/CIRCULATIONAHA.113.004888. [DOI] [PubMed] [Google Scholar]
- 36.Reed RM, Eberlein M. Sizing strategies in heart and lung transplantation: you cannot manage what you do not measure. Future Cardiol. 2014;10:303–306. doi: 10.2217/fca.14.17. [DOI] [PubMed] [Google Scholar]
- 37.Chen F, Fujinaga T, Shoji T, et al. Perioperative assessment of oversized lobar graft downsizing in living-donor lobar lung transplantation using three-dimensional computed tomographic volumetry. Transpl Int. 2010;23:e41–44. doi: 10.1111/j.1432-2277.2010.01123.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.