Abstract
Purpose
To evaluate 6-month and 1-year outcomes of every 8 weeks (Q8W) aflibercept in patients with resistant neovascular age-related macular degeneration (AMD).
Design
Retrospective, interventional, consecutive case series.
Methods
Retrospective review of patients with resistance (multiple recurrences or persistent exudation) to every 4 weeks (Q4W) ranibizumab or bevacizumab that were switched to Q8W aflibercept.
Results
Sixty-three eyes of 58 patients had a median of 13 (interquartile range (IQR), 7-22) previous anti Vascular Endothelial Growth Factor (anti-VEGF) injections. At 6-months after changing to aflibercept, 60.3% of eyes were completely dry, which was maintained up to one-year. The median maximum retinal thickness improved from 355 microns to 269 microns at 6 months (p<0.0001) and 248 microns at one year (p<0.0001). There was no significant improvement in ETDRS visual acuity at 6 months (p=0.2559) and one-year follow-up (p=0.1081) compared with baseline. The mean difference in ETDRS visual acuity compared to baseline at 6 months was −0.05 logMAR (+2.5 letters) and 0.04 logMAR at 1 year (−2 letters).
Conclusion
Sixty percent of eyes with resistant AMD while on Q4W ranibizumab or bevacizumab were completely dry after changing to Q8W aflibercept at the 6-month and 1-year follow-ups, but visual acuity did not significantly improve. Only a third of eyes needed to be switched from Q8W to Q4W aflibercept due to persistence of fluid; Q8W dosing of aflibercept without the initial 3 monthly loading doses may be a good alternative in a select group of patients who may have developed ranibizumab or bevacizumab resistance.
INTRODUCTION
Age-related macular degeneration (AMD) is a leading cause of vision loss and blindness in industrialized countries. The most severe vision loss occurs in the neovascular (or wet) form of AMD, involving choroidal neovascularization (CNV) and associated retinal edema.1 The discovery that vascular endothelial growth factor (VEGF) is the driving force behind the CNV and associated edema seen in AMD led to a paradigm shift in the treatment of AMD with anti-VEGF therapy. Monthly intravitreal injections of 0.5 mg ranibizumab, a humanized monoclonal antibody fragment that blocks VEGF, not only prevent vision loss but also lead to significant visual gain in approximately one-third of patients.2, 3 The risk of rare but serious adverse events resulting from the intravitreal procedure, together with the significant burden of making monthly visits to their retinal specialist, have led to extensive efforts to decrease injection and monitoring frequency.1 However, fixed quarterly4, 5 or “as needed” (pro re nata [PRN]) dosing regimens,6, 7 without requiring monthly monitoring visits, were not effective at maintaining vision.
Aflibercept (or VEGF Trap-Eye, Regeneron, Tarrytown, NY) is a soluble decoy receptor fusion protein, consisting of portions of VEGF receptors, VEGFR−1 and VEGFR-2, which binds to all isoforms of VEGF-A, as well as PlGF (placental growth factor), and blocks its activity. One-year follow-up results of two large phase-3 studies (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD [VIEW 1, VIEW 2]) comparing monthly and every-2-month (after 3 initial monthly injections) dosing of intravitreal aflibercept injection with monthly ranibizumab showed that all aflibercept groups were noninferior and clinically equivalent to monthly ranibizumab for the primary end point of maintenance of vision at 52 weeks compared to baseline (the 2q4, 0.5q4, and 2q8 regimens were 95.1%, 95.9%, and 95.1%, respectively, for VIEW 1, and 95.6%, 96.3%, and 95.6%, respectively, for VIEW 2, whereas monthly ranibizumab was 94.4% in both studies). In a prespecified integrated analysis of the 2 studies, all aflibercept regimens were within 0.5 letters of the reference ranibizumab for mean change in BCVA; all aflibercept regimens also produced similar improvements in anatomic measures. Ocular and systemic adverse events were similar across treatment groups.1
The binding affinity of intravitreal aflibercept to VEGF is greater than that of bevacizumab or ranibizumab.8 The greater affinity could translate into a higher efficacy or, as predicted by a mathematic model, into a substantially longer duration of action in the eye,9 allowing for less frequent dosing, as supported by early clinical trials. Because of the higher potency of aflibercept compared to other anti-VEGF agents, we wanted to test whether this higher potency would translate to better efficacy seen clinically as improvements in visual and structural outcomes in patients who developed resistance to other anti-VEGF agents (i.e., ranibizumab or bevacizumab).
In this current study, we retrospectively evaluate the 6-month and 1-year visual and anatomic outcomes of every 8 weeks intravitreal aflibercept injections in patients with ranibizumab- or bevacizumab-resistant neovascular age-related macular degeneration.
MATERIALS AND METHODS
Study Design
This was a retrospective review of patients with neovascular AMD who developed resistance to either intravitreal ranibizumab or bevacizumab monotherapy given every 4 weeks, and were subsequently switched and treated with aflibercept given every 8 weeks (from August 2012 to May 2014) at the Jacobs Retina Center, University of California San Diego (UCSD) Shiley Eye Center. Written informed consent was obtained for each patient prior to the intravitreal injections. UCSD Institutional Review Board (IRB) approval was acquired for the review and analysis of patient data. All procedures adhered to the tenets of the Declaration of Helsinki for research involving human subjects and complied with Health Insurance Portability and Accountability Act (HIPAA) regulations.
Study Population
All consecutive eyes that developed resistance to multiple intravitreal ranibizumab or bevacizumab injections every 4 weeks were switched to intravitreal aflibercept injections every 8 weeks. The standard protocol that we employ for the treatment of neovascular AMD is a fixed regimen of strict monthly bevacizumab or ranibizumab intravitreal injections until the eye becomes completely dry, after which we give 1-2 bonus injections before going to an observation or holiday phase. The minimum number of injections we gave per treatment cycle was 3 injections even if the eyes become dry after a single injection. When there is evidence of a recurrence (anatomic increase in fluid accompanied by visual symptoms and/or loss of visual acuity), we resume the treatment cycle of strict monthly bevacizumab or ranibizumab injections until the eye becomes dry again. When aflibercept became available for use at our institution (August 2012), we started switching patients that were resistant to other anti-VEGF injections. Since these patients were not newly diagnosed cases of neovascular AMD, and because these were not treatment-naïve eyes and have received a median of 13 previous ranibizumab or bevacizumab injections prior to switching to aflibercept, we decided to commence treatment with aflibercept every 8 weeks without the 3 initial monthly injections.
Resistance was defined as having multiple recurrences (minimum of 2 recurrences after the eyes have been completely dry following a series of at least 3 monthly injections per treatment cycle) or persistence of exudation (poor response to monthly ranibizumab or bevacizumab for at least 5 months) as evident on clinical exam and on imaging studies (leakage on fluorescein angiogram (FA), or fibrovascular pigment epithelial detachment (PED) with intraretinal fluid (IRF) or subretinal fluid (SRF) on spectral-domain optical coherence tomography [SD-OCT]) while on monthly ranibizumab or bevacizumab monotherapy. Poor compliance was not considered resistance. Recurrence was diagnosed as new or increased IRF or SRF with or without vision changes or symptoms. Eyes were excluded if: (1) they have received aflibercept elsewhere prior to the first aflibercept injection at our institution; and (2) they had other retinal conditions other than typical AMD (macular hole, vitreomacular traction (VMT), epiretinal membrane (ERM), retinal detachment, polypoidal choroidal vasculopathy (PCV), pseudovitelliform macular dystrophy, peripapillary CNV). The collected baseline demographic data included patient age; sex; laterality of the treated eye; phakic status; history of previous vitrectomy; number of previous ranibizumab or bevacizumab injections; duration between the last ranibizumab or bevacizumab injection and the first aflibercept injection; number of recurrences or duration of persistent exudation; percentage disruption of the external limiting membrane (ELM) and photoreceptor inner segment/outer segment (IS/OS) layers; type of fluid; ETDRS visual acuity; and maximum retinal thickness.
Ophthalmologic Examination and Imaging Studies
All patients underwent a thorough ophthalmologic examination at baseline and at every-2-month follow-up visits, including visual acuity (VA) testing using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, mydriatic slit-lamp biomicroscopy, and binocular indirect ophthalmoscopy examinations performed by a retina specialist using either a 78- or a 90-diopter indirect slit-lamp lens and a 20-diopter binocular indirect ophthalmoscopy lens, respectively. ETDRS visual acuities were converted to logarithm of the minimum angle of resolution (logMAR) notation, with 0 being the highest score corresponding to 20/20 visual acuity and a value of 1 corresponding to 20/200 visual acuity.
Imaging studies (FA and SD-OCT) using a SD-OCT device coupled with a simultaneous scanning laser ophthalmoscope (cSLO) (Spectralis HRA+OCT, Heidelberg Engineering, Carlsbad, CA) were performed on all patients at baseline (prior to the first aflibercept), and after each aflibercept injection. The OCT images were reviewed using original software from Spectralis. Multiple SD-OCT scans (horizontal and vertical) going through the fovea was obtained, as well as through other areas where the CNV activity was most pronounced. Two experienced observers (CA and FM) masked to visual acuities measured several variables. First, the maximum retinal thickness was measured using the calipers feature on the SD-OCT device in the area where CNV activity was most marked, within an area 2000 micrometers from the fovea, usually where the fibrovascular PED with the greatest amount of overlying IRF or SRF was located. Retinal thickness was measured manually by placing the digital calipers from the outer border of the retinal pigment epithelium (RPE) to the internal limiting membrane (ILM). Second, the presence of IRF and SRF was noted and compared with the baseline using the progression scans to enable point-to-point correspondence between consecutive follow-up scans. Fluid extent was studied in a minimum of two views (horizontal and vertical scans) passing through the fovea. The treatment response was assessed at each of the follow-up visits and graded as being “dry” or not. Eyes were considered “dry” when there was complete resolution of fluid or exudation. Third, the percentage disruption of the external limiting membrane (ELM) and photoreceptor inner segment and outer segment (IS/OS) junction were evaluated 500 micrometers in either direction of the fovea at baseline. A disruption in IS/OS or ELM was defined as the loss of the back-reflection line. Analysis of the retinal structures was done as per our previous publications10, 11. The percentage disruption was averaged to generate a number between 0% (no disruption) and 100% (total loss of the layer in both horizontal and vertical scans).
Treatment Protocol
Intravitreal injection of aflibercept was carried out under aseptic conditions in the clinic. Preservative-free lidocaine gel was instilled in the eye for at least 3 doses five minutes or longer prior to injection. A lid speculum was placed to keep the lids open and 5% povidone iodine solution was instilled on the conjunctival sac prior to injection. The intravitreal injection was performed using a 30-gauge needle in the superotemporal or superonasal quadrant, 3.5-4.0 mm posterior to the limbus depending on phakic status. Patients received a dose of 2 mg/0.05 mL intravitreal aflibercept injections. In vitrectomized eyes, we gave a dose of 4 mg/0.1 mL aflibercept. The eyes were thoroughly washed with sterile balanced salt solution (BSS) after the injection. No topical antibiotic drops were prescribed after the procedure.
Patients were switched from monthly ranibizumab or bevacizumab injections to every 8 weeks aflibercept, without the 3 initial monthly injections. When there was persistent fluid despite strict every 8 week injections (after a median of 4), we shifted the regimen to every 4 weeks aflibercept.
Treatment Outcomes
The primary treatment outcomes of this study included change in visual acuity (VA) and maximum retinal thickness at 6 months and 1 year compared with baseline. Secondary outcomes included proportion of eyes that were dry at 6 months and 1 year; proportion of eyes that had stable VA, gain of ≥ 1 line, and loss of ≥ 1 line of ETDRS VA at 6 months and 1 year; and survival analysis of the time to recurrence after being dry, and time to switching to every 4 weeks aflibercept.
Statistical analysis
Descriptive statistical analyses using Fisher-exact test or Chi-squared test was used for categorical variables. For continuous variables, Kruskal Wallis test was performed when comparing VA improvement (stable, gain, or loss). Wilcoxon rank sum test was performed when comparing eyes that were dry or not dry at the follow-up time points. Wilcoxon signed test was used to compare the paired difference between baseline and follow-ups. For the survival analysis, Kaplan-Meier curves were generated. Cox proportional hazard model (using Breslow method for handling ties) was used, with data for both eyes of the subject included in the model. The method of Lee, Wei, and Amato was used to adjust for the correlation of the two eyes from the same subject. Robust sandwich variance estimators were desired. For all hypothesis tests, statistical significance was set at a level of p<0.05. Statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, North Carolina, USA) and R version 3.0.0 (http://www.r-project.org/).
RESULTS
Sixty-three eyes of 58 patients with neovascular AMD resistant to ranibizumab and/or bevacizumab were switched to every 8 weeks aflibercept during the study period and were included in the review and analysis of data after they have met the inclusion/exclusion criteria. Patient characteristics at the time of aflibercept conversion are summarized in Table 1. The median age at the time of switching to aflibercept was 81 years (interquartile range (IQR), 76-87). Most of the eyes were pseudophakic (69.8%) and had no history of prior vitrectomy (90.5%). Eyes received a median of 13 (IQR, 7-22) previous bevacizumab and/or ranibizumab injections before being considered resistant and before switching to aflibercept. There were 45 eyes that had multiple recurrences, with a median of 2 recurrences (IQR, 2-3). Thirty-two eyes had poor response with persistent fluid despite strict Q4W ranibizumab or bevacizumab injections, with a median duration of 5 months (IQR, 5-7). The median duration between the last bevacizumab or ranibizumab injection and the first aflibercept injection was 6 weeks (IQR, 4-18). Eyes at baseline had approximately equal distribution of fluid type, with 28 eyes (44.4%) having intraretinal fluid (IRF) and 30 eyes (47.6%) with subretinal fluid (SRF).
Table 1.
Baseline demographic characteristics of patients with recurrent or persistent neovascular age-related macular degeneration that were switched to intravitreal aflibercept injections
| Patients (eyes) Median age, years (IQR) Female, n (%) Right eye, n (%) |
58 (63) 81 (76-87) 34 (58.6) 31 (49.2) |
| Phakic status Phakic, n (%) Pseudophaldc, n (%) |
19 (30.2) 44 (69.8) |
| History of vitrectomy Non-vitrectomized, n (%) Vitrectomized, n (%) |
57 (90.5) 6 (9.5) |
| Multiple recurrences Eyes, n (%) Number of recurrences, median (IQR) Persistent fluid (in months) Eyes, n (%) Duration, median (IQR) |
45 (71.4) 2 (2-3) 32 (50.8) 5 (5-7) |
| Previous injections,* median (IQR) Bevacizumab Ranibizumab |
13 (7-22) 13 (7-21) 5 (3-8) |
| Duration between last bevacizumab or ranibizumab injection and first aflibercept (in weeks), median (IQR) |
6 (4-18) |
| ELM disruption (%), mean (median; IQR) IS-OS disruption (%), mean (median; IQR) |
51.7 (50; 0-100) 71.2 (75; 20-90) |
| Type of fluid, n (%) Intraretinal fluid (IRF) Subretinal fluid (SRF) Both (IRF and SRF) |
28 (44.4) 30 (47.6) 5 (7.9) |
Includes bevacizumab and/or ranibizumab
IQR, interquartile range; ELM, external limiting membrane; IS-OS, inner segment-outer segment
The 6-month follow-up visit took place at a median of 24.57 weeks (IQR, 23.71-25.57). Thirty-eight eyes (60.3%) were completely dry at the 6-month follow-up visit, which was maintained up to the one-year follow-up visit (median of 68.86 weeks, IQR 33.00-80.71). Majority (97.4%) of eyes that were dry at the last follow-up had no previous history of vitrectomy (p=0.0323). Neither the number of previous recurrences nor the duration of resistance prior to switching to aflibercept predicted whether or not the eyes were dry at the 6-month and one-year follow-up visits. Illustrative cases are shown in figures 1 and 2.
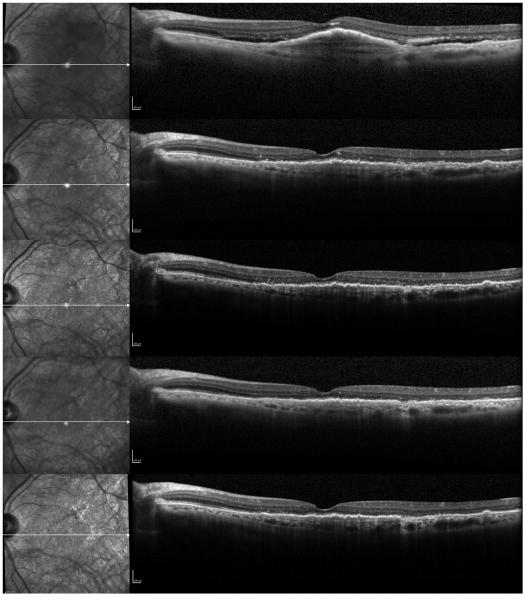
Figure 1.
A case of a 79-year-old female with recurrent fluid who has received 27 previous bevacizumab injections, and was subsequently switched to every 8 weeks intravitreal aflibercept injections. Progression scans going through the fovea at baseline and subsequent follow-ups at 1 month, 2 months, 4 months, and 6 months after switching to aflibercept show a decrease in the height of the subfoveal fibrovascular pigment epithelial detachment (PED), resolution of the subretinal fluid (SRF), and focal disruption of the photoreceptor layer.
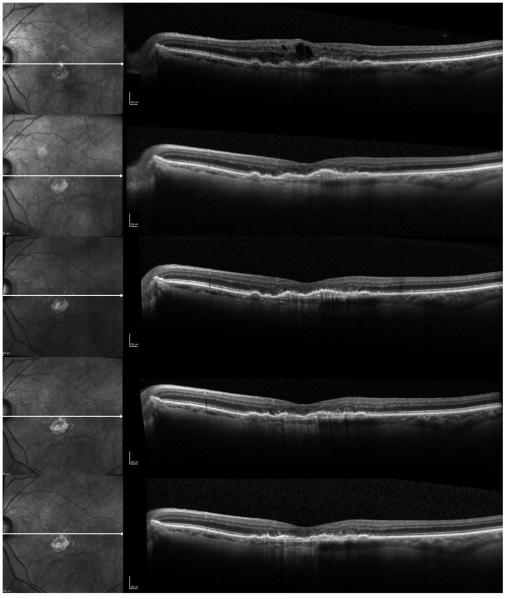
Figure 2.
A case of an 82-year-old female with recurrent fluid who has received 13 previous bevacizumab injections, and was subsequently switched to every 8 weeks intravitreal aflibercept injections. Progression scans going through the fovea at baseline and at subsequent follow-ups at 1 month, 2 months, 4 months, and 6 months after switching to aflibercept show progressive flattening of the subfoveal fibrovascular pigment epithelial detachment (PED) and resolution of the intraretinal fluid (IRF). Photoreceptor layer appears disrupted in the fovea.
The median maximum retinal thickness improved from 355 microns to 269 microns at 6 months (p<0.0001) and 248 microns at one year (p<0.0001). Despite the good anatomic response, there was no significant improvement in ETDRS visual acuity at 6 months (p=0.2559) and up to the one-year follow-up (p=0.1081). The mean difference in ETDRS visual acuity compared to baseline at 6 months was −0.05 logMAR (+2.5 letters) and 0.04 logMAR at 1 year (−2 letters). The anatomic and functional outcomes at 6 months and 1 year are summarized in Table 2.
Table 2.
Anatomic and functional treatment response after switching to every 8 weeks intravitreal aflibercept injections in recurrent or persistent neovascular age-related macular degeneration
| Median (IQR) | P value* | |
|---|---|---|
| Maximum retinal thickness (in microns) Baseline 6 months 1 year |
355 (325-427) 269 (227-325) 248 (201-279) |
<0.0001
<0.0001 |
| ETDRS visual acuity (in logMAR) Baseline 6 months 1 year |
0.40 (0.30-0.70) 0.40 (0.20-0.70) 0.40 (0.20-0.90) |
0.2559 0.1081 |
Wilcoxon signed test
ETDRS, Early Treatment Diabetic Retinopathy Study; logMAR, logarithm of the minimum angle of resolution
At 6 months, 24 eyes (38.1%) had stable VA, 36.5% gained at least 1 line of ETDRS VA, and 23.8% lost at least 1 line. Table 3 summarizes the descriptive characteristics of eyes according to the distribution of visual acuity at 6 months. One hundred percent of gainers at the 6 months follow-up visit were non-vitrectomized eyes. All of the vitrectomized eyes (n=5) at 6 months had stable VA. Excluding vitrectomized eyes, analysis at 6 months showed a trend towards visual improvement but was not significant. Among eyes that gained VA, 52.2% had SRF and 39.1% had IRF. In contrast to this, among eyes that lost at least 1 line, 53.3% had IRF and 33.3% had SRF. Gainers had less ELM disruption (median of 35%, IQR 10-90), compared with eyes that had stable VA (median of 50%, IQR 15-95) and eyes that lost VA (median of 60%, IQR 25-80) (p=0.7960). There was no statistically significant trend in the degree of photoreceptor inner segment/outer segment disruption and visual acuity at the 6 months follow-up.
Table 3.
Descriptive table of the distribution of visual acuity at 6 months follow-up after switching to every 8 weeks intravitreal aflibercept injections in recurrent or persistent neovascular age-related macular degeneration
| Stable VA (n=24) |
Gain ≥ 1 line (n=23) |
Loss ≤ 1 line (n=15) |
P value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 79.5 (77-86.5) | 81 (73-85) | 83 (76-89) | 0.3999 |
| Female sex, n (%) | 5 (20.8) | 12 (52.2) | 6 (40.0) | 0.0822 |
| Phakic status, n (%) Phakic Pseudophakic |
9 (37.5) 15 (62.5) |
7 (30.4) 16 (69.6) |
3 (20.0) 12 (80.0) |
0.5357 |
| History of vitrectomy, n (%) Vitrectomized Non-vitrectomized |
19 (79.2) 5 (20.8) |
23 (100.0) 0 (0.0) |
15 (100.0) 0 (0.0) |
0.0221 |
| Number of recurrences, median (IQR) |
2 (2-2.5) | 2 (2-3) | 2 (2-3) | 0.8392 |
| Duration of persistent fluid (in months), median (IQR) |
5 (5-9) | 5 (5-9) | 5 (5-6) | 0.8739 |
| Previous injections,*
median (IQR) |
12 (7-21) | 13 (10-26) | 12 (8-22) | 0.7552 |
| Type of fluid, n (%) Intraretinal fluid (IRF) Subretinal fluid (SRF) Both (IRF and SRF) |
10 (41.7) 13 (54.2) 1 (4.2) |
9 (39.1) 12 (52.2) 2 (8.7) |
8 (53.3) 5 (33.3) 2 (13.3) |
0.6493 |
| ELM disruption (%), mean (median; IQR) |
50 (15-95) | 35 (10-90) | 60 (25-80) | 0.7960 |
| IS-OS disruption (%), mean (median; IQR) |
77.5 (52.5-100) | 75 (70-90) | 70 (50-100) | 0.9222 |
Includes bevacizumab and/or ranibizumab
IQR, interquartile range; ELM, external limiting membrane; IS-OS, inner segment-outer segment
At the one-year follow-up, 17 eyes (27.0%) had stable VA, 25.4% gained, and 44.4% lost at least 1 line of ETDRS VA. Descriptive characteristics of eyes according to the distribution of visual acuity at 1 year are summarized in Table 4. Gainers were younger (median age 75 years, IQR 72.5-82) compared with the patients that had stable VA (median age 82 years, IQR 77-87) or lost at least 1 line (median age 83 years, IQR 78-89) (p=0.0113). Similar to the 6-month follow-up, one hundred percent of gainers at the one-year follow-up visit were non-vitrectomized eyes, and 80.0% of vitrectomized eyes (n=4) at still had stable VA at 1 year. Among eyes that gained VA, 81.3% had SRF and 12.5% had IRF, and among eyes that lost at least 1 line, 53.6% had IRF and 35.7% had SRF. Gainers had significantly less ELM disruption (median of 20%, IQR 10-55), compared with eyes that had stable VA (median of 60%, IQR 20-100) and eyes that lost VA (median of 67.5%, IQR 27.5-95) (p=0.0393). A similar non-significant trend in the degree of photoreceptor inner segment/outer segment disruption was noted with gainers having less IS/OS disruption (p=0.2018).
Table 4.
Descriptive table of the distribution of visual acuity at 1 year follow-up after switching to every 8 weeks intravitreal aflibercept injections in recurrent or persistent neovascular age-related macular degeneration
| Stable VA (n=17) |
Gain ≥ 1 line (n=16) |
Loss ≤ 1 line (n=28) |
P value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 82 (77-87) | 75 (72.5-82) | 83 (78-89) | 0.0113 |
| Female sex, n (%) | 6 (35.3) | 9 (56.3) | 7 (25.0) | 0.1107 |
| Phakic status, n (%) Phakic Pseudophakic |
4 (23.5) 13 (76.5) |
6 (37.5) 10 (62.5) |
8 (28.6) 20 (71.4) |
0.7199 |
| History of vitrectomy, n (%) Vitrectomized Non-vitrectomized |
13 (76.5) 4 (23.5) |
16 (100.0) 0 (0.0) |
27 (96.4) 1 (3.6) |
0.0331 |
| Number of recurrences, median (IQR) | 2 (2-2) | 2 (2-2) | 2 (2-3.5) | 0.3071 |
| Duration of persistent fluid (in months), median (IQR) |
5 (5-9) | 5 (5-9.5) | 5 (5-6.6) | 0.8184 |
| Previous injections,*
median (IQR) |
10 (7-14) | 12.5 (7.5-15.5) | 15.5 (7.5-26) | 0.1517 |
| Type of fluid, n (%) Intraretinal fluid (IRF) Subretinal fluid (SRF) Both (IRF and SRF) |
9 (52.9) 7 (41.2) 1 (5.9) |
2 (12.5) 13 (81.3) 1 (6.3) |
15 (53.6) 10 (35.7) 3 (10.7) |
0.0279 |
| ELM disruption (%), mean (median; IQR) |
60 (20-100) | 20 (10-55) | 67.5 (27.5- 95) |
0.0393 |
| IS-OS disruption (%), mean (median; IQR) |
80 (50-100) | 72.5 (40-82.5) | 80 (60-100) | 0.2018 |
Includes bevacizumab and/or ranibizumab
IQR, interquartile range; ELM, external limiting membrane; IS-OS, inner segment-outer segment
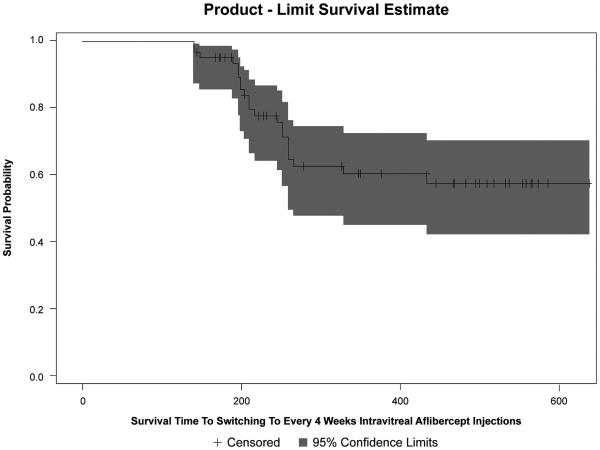
At 1 year, the median number of every 8 weeks aflibercept injections received was 4 (IQR, 4-6). Twenty-one eyes (33.3%) were switched to every 4 weeks aflibercept during the study period due to persistence of fluid despite strict every 8 weeks aflibercept. The Kaplan-Meier curve for time to switching to every 4 weeks aflibercept is shown in Figure 3. The median time to switching to monthly aflibercept was 37 weeks (IQR, 28.43–71.29). The estimated probability that a patient will switch to monthly aflibercept treatment for ~266 days or 38 weeks (i.e. where the Kaplan-Meier curve starts to stabilize) is 0.37. The more number of every 8 weeks aflibercept injections received, the less likely for the need to switch to monthly aflibercept (hazard ratio (HR) 0.747, 95% confidence interval (CI) 0.590-0.945; p=0.015). Hazard ratios for the need to switch to monthly or every 4 weeks aflibercept are summarized in Table 5.
Figure 3.
Kaplan-Meier curve of the survival time to switching to every 4 weeks intravitreal aflibercept injections in eyes with recurrent or persistent neovascular age-related macular degeneration.
Table 5.
Univariate and multivariate survival analysis: Switch to every 4 weeks intravitreal aflibercept injections for persistent fluid despite every 8 weeks aflibercept injections
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% confidence interval) |
P Value | Hazard Ratio (95% confidence interval) |
P Value |
| Age | 0.991 (0.927-1.059) | 0.787 | 0.997 (0.924-1.075) | 0.930 |
| Female sex | 0.967 (0.386-2.420) | 0.943 | N/A | |
| Phakic at baseline | 0.926 (0.348-2.467) | 0.878 | N/A | |
| Vitrectomized | 1.910 (0.516-7.065) | 0.332 | 1.674 (0.368-7.611) | 0.505 |
| Previous injections |
0.952 (0.908-0.999) | 0.043 | 0.962 (0.912-1.015) | 0.159 |
| Number of recurrences |
0.299 (0.117-0.764) | 0.012 | N/A | |
| Duration of resistance |
1.011 (0.952-1.073) | 0.732 | N/A | |
| Type of fluid | 1.178 (0.587-2.363) | 0.645 | N/A | |
| IS-OS disruption | 0.998 (0.985-1.012) | 0.791 | N/A | |
| ELM disruption | 0.988 (0.975-1.001) | 0.062 | N/A | |
| Total number of Q8W IAI |
0.708 (0.570-0.878) | 0.002 | 0.747 (0.590-0.945) | 0.015 |
ELM, external limiting membrane; IS-OS, inner segment-outer segment; Q8W, every-8-weeks; IAI, intravitreal aflibercept injection
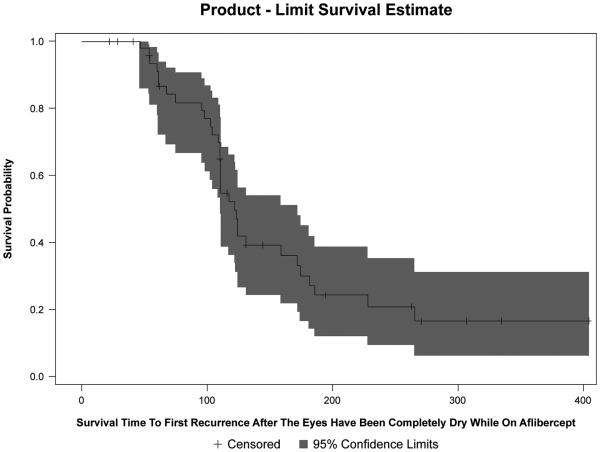
The median time to recurrence after the eye has been completely dry was 14.71 weeks (IQR, 5.86–18.71). Figure 4 displays the Kaplan-Meier curve for time to first recurrence after being dry. The estimated probability that a patient will have a recurrence at 6 months (~26 weeks or 183 days) is 0.73. The estimated probability that a patient will have a recurrence at 1 year (~52 weeks or 365 days) is 0.83. Among the variables studied in the univariate and multivariate analyses, only the duration of resistance prior to switching to aflibercept was significantly associated with recurrence risk. The longer the duration of resistance, the greater the risk of getting a recurrence after the eye has been treated dry (HR 1.084, 95% CI 1.016-1.156; p=0.014). The variables and corresponding hazard ratios analyzed for time to recurrence is shown in Table 6.
Figure 4.
Kaplan-Meier curve of the survival time to first recurrence after the eyes have been completely dry while on intravitreal aflibercept injections in eyes with recurrent or persistent neovascular age-related macular degeneration.
Table 6.
Univariate and multivariate survival analysis: Recurrence after being completely dry while on intravitreal aflibercept injections
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% confidence interval) |
P Value | Hazard Ratio (95% confidence interval) |
P Value |
| Age | 1.012 (0.972-1.054) | 0.562 | 1.016 (0.975-1.060) | 0.451 |
| Female sex | 1.005 (0.545-1.853) | 0.987 | N/A | |
| Phakic at baseline | 1.145 (0.619-2.121) | 0.666 | N/A | |
| Vitrectomized | 0.830 (0.225-3.070) | 0.781 | 0.906 (0.274-2.999) | 0.872 |
| Previous injections |
1.000 (0.974-1.027) | 0.977 | 0.967 (0.919-1.018) | 0.205 |
| Number of recurrences |
1.017 (0.846-1.222) | 0.857 | 1.235 (0.900-1.694) | 0.192 |
| Duration of resistance |
1.044 (1.009-1.081) | 0.014 | 1.084 (1.016-1.156) | 0.014 |
| Type of fluid | 1.315 (0.759-2.279) | 0.329 | N/A | |
| IS-OS disruption | 1.002 (0.990-1.013) | 0.771 | N/A | |
| ELM disruption | 0.994 (0.985-1.004) | 0.257 | N/A | |
| Total number of Q8W IAI |
1.132 (0.998-1.284) | 0.053 | N/A | |
ELM, external limiting membrane; IS-OS, inner segment-outer segment; Q8W, every-8-weeks; IAI, intravitreal aflibercept injection
There were no observed significant ocular adverse events such as ocular inflammation, sterile and infectious endophthalmitis, vitreous hemorrhage, retinal detachment, or sustained increase in intraocular pressure (IOP) with the use of intravitreal aflibercept injections in this series.
DISCUSSION
The objective of this study was to evaluate the 6 months and 1 year anatomic and functional response of eyes that had ranibizumab or bevacizumab resistance and were switched to every 8 weeks intravitreal aflibercept injections. Our cohort of patients was comprised of highly resistant difficult cases that did not respond to multiple anti-VEGF (ranibizumab and/or bevacizumab) injections, but more than half had excellent anatomic response with injections every 8 weeks (even without the 3 initial monthly loading doses). However, with longer follow-up to 1 year, these eyes tended to recur, and a third of eyes needed monthly aflibercept injections as discussed below.
Despite the significant decrease in maximum retinal thickness at 6 months and 1 year, there was no significant improvement in VA. There was a non-significant mild visual gain at 6 months (−0.05 logMAR or +2.5 letters), but this was not sustained at 1 year (0.04 logMAR or −2 letters). This may be due to our study population who already had a median of 13 prior injections of another anti-VEGF drug; this population may have limited potential to improve vision. It has been reported that visual and anatomic outcomes can be distinct, and OCT measurements may not be robust markers for visual function.12 It is still unclear whether extinguishing all VEGF activity and drying the macula more with any anti-VEGF agent would result in better visual acuity in the long term, or may be more damaging by causing retinal thinning and geographic atrophy (GA). Pooled CATT and aflibercept data showed increased GA risk with monthly injections, but ranibizumab was not associated with a higher GA risk than bevacizumab.13
In our study, we found that 36.5% of eyes gained ≥ 1 line of ETDRS VA at 6 months follow-up. However, this dropped down to 25.4% at 1 year. Despite the strict regimen of every 8 weeks aflibercept injections, and even escalating to monthly aflibercept in a third of eyes, the visual gains were not sustained at 1 year, with a greater proportion of eyes losing vision (44.4%) compared to eyes that had stable VA (27.0%) or gained vision (25.4%). The vision may have not changed significantly because we gave the patients a holiday once they were dry and retreated as needed (PRN). There may have been more recurrences than if they were treated continuously. Eyes with SRF had greater tendency to gain vision compared with eyes with IRF. Previous presentations in meetings have alluded to this association between the presence of SRF and better vision. We also found that eyes that gained vision had less ELM disruption. We have previously published that preoperative ELM integrity was a better predictor for vision improvement than central macular thickness or IS/OS junction integrity. The ELM is not a true membrane, but is formed by a tangentially oriented series of adhesions, zonulae adherens, connecting apical processes of the Muller cells with inner segments of the photoreceptors. This layer may help to maintain the alignment and orientation of the photoreceptors. Thus, the ELM is an OCT landmark of integrity of inner segments of the photoreceptors.11
Despite our treatment protocol of strict every 8 weeks aflibercept injections, we decided to shift to monthly aflibercept injections in one third of eyes with persistent fluid. The statistical analysis was done before the change to monthly aflibercept, as well as after. This shows that only a select number of eyes needed monthly injections; or stated in another way, aflibercept injections every 8 weeks worked well in approximately two-thirds of eyes. This regimen may decrease treatment burden and may reduce the risk of getting complications from frequent intravitreal injections. We plan longer follow-up of our cohort to determine the efficacy or added benefit of switching to monthly aflibercept injections.
Most eyes with neovascular AMD, including eyes that have had an excellent response, will have recurrent exudation when anti-VEGF therapy is discontinued, regardless of the agent being used (i.e., ranibizumab, bevacizumab, or aflibercept). Stopping treatment and having recurrent exudation does not constitute either "resistance" or "poor response" but simply the natural history of untreated neovascular AMD. Because of this reason, we felt it important to exclude people who had responded excellently to anti-VEGF agents, and included only eyes that we considered truly resistant. In the majority of eyes, every 8 weeks aflibercept may be a good option, and this may even be extended up to 12 weeks, because the median time to recurrence was approximately 15 weeks in this study. The longer the duration of resistance to other anti-VEGF agents prior to switching to aflibercept, the greater the risk of getting a recurrence after the eye becomes dry with aflibercept. These eyes may be harder to treat and may require more frequent injections or may not be amenable to injection-free holidays or extending treatment interval. This finding may give us an idea of who may benefit from a treat-and-extend regimen, and who may need a continuous regimen.
In the setting of previously treated neovascular AMD, better efficacy of a given drug can only be inferred if the degree of persistent exudation with continuous, adequately-dosed therapy (i.e. sub-optimal or incomplete response) then improves with a comparable dosing regimen of the comparator drug. Although comparative or better efficacy cannot be inferred from stopping the previous treatment and having recurrent exudation that subsequently improves on the new drug, we can infer comparative efficacy if we look at those eyes that never responded, specifically the eyes with persistent fluid that never resolved. In this study, eyes with persistent fluid did respond, and only a third of eyes needed to be switched to monthly aflibercept injections due to persistent fluid.
Another issue is the possibility of tachyphylaxis in some cases. It is known that improvement in exudation can occur in the setting of tachyphylaxis with any switch among the three anti-VEGF agents (ranibizumab, bevacizumab, or aflibercept). Only a randomized trial of switching amongst different agents can prove this, but there does seem to be some benefit of aflibercept on bevacizumab incomplete responders.
Numerous short-term studies evaluating the response of resistant or recalcitrant neovascular AMD cases that were switched to aflibercept have all shown anatomic improvements with decreased macular thickness and resolution of fluid 14-18, but almost all have observed no significant change in visual acuity, except for Kumar et al18 reporting significant visual improvement at 6 months of follow-up. Compared with the previous studies, our present study had stricter criteria for resistance (minimum of 2 recurrences after the eyes have been completely dry following a series of at least 3 monthly injections per treatment cycle) and longer duration of resistance (i.e. poor response to, or persistence of exudation despite, monthly ranibizumab or bevacizumab for at least 5 months). We have employed strict inclusion criteria and tried to make the study population as homogenous as possible, given the retrospective nature of this study. We also used the standard ETDRS chart for all measurements of VA at all follow-up visits; and we imaged patients regularly with SD-OCT and FA. The treatment regimen that we employed in our study that is different from all previous studies is the strict every 8 weeks aflibercept injection regimen without the initial 3 monthly loading doses; this approach enabled us to filter out the patients that actually needed monthly injections, while lowering the treatment burden for a majority of patients that responded well to injections every 8 weeks. Also, our present study had a longer follow-up period (median of 68.86 weeks) compared with previous studies. Another noticeable difference from previous studies is that even with our initial every 8 weeks aflibercept injection regimen, there was a higher proportion of success (i.e., 60% of eyes were completely dry as measured by SD-OCT) at 6 months and 1 year. However, we did not perform subgroup analysis comparing recurrent cases to persistent cases because the study population included 63 eyes, and by further dividing the population, this will not yield any statistical significance. Previous similar studies14, 16 published in this journal included both persistent and recurrent cases as a whole, without comparing outcomes between the two groups.
The time to switching to aflibercept was variable in this study because of insurance issues. The eyes truly had persistent fluid that was not responding to strict monthly ranibizumab or bevacizumab anymore, and when we decided to switch them, they had to coordinate with their insurance companies prior to the aflibercept injections. We do not know how the duration of the switch affected the eventual clinical course of aflibercept but this was real-world situation, and not controlled study conditions, hence the limitation of this being a retrospective study.
Vitrectomized eyes may behave and respond differently compared with non-vitrectomized eyes, and this may confound the interpretation of both efficacy and durability of aflibercept. We tried to address any pharmacokinetic issues of faster clearance by doubling the dose of aflibercept in vitrectomized eyes. We cannot conclude if this had any variable effect because there were less than 10% of eyes that were vitrectomized in the study population. Nevertheless, this was not an outcome of the study.
As stated earlier, the differences from previous studies and the strengths of the present study include the strict inclusion and exclusion criteria, use of the patients as their own control, fixed treatment interval with strict every 8 weeks dosing, use of best-available ETDRS visual acuity, use of FA and high-resolution spectral domain OCT images with manual measurements of maximum retinal thickness and grading of ELM and photoreceptor disruption, and longer follow-up. Limitations include retrospective study design and small cohort of patients.
In summary, sixty percent of eyes with resistant or persistent exudation while on monthly ranibizumab or bevacizumab had complete anatomic response after changing to every 8 weeks aflibercept at the 6-month and 1-year follow-ups. Although visual acuity did not significantly improve, there was a suggestion that eyes with better ELM preservation would have more improvement; we note that we were treating chronic advanced cases that were poorly responsive and had limited visual potential. Only a third of eyes needed to be switched from every 8 weeks to every 4 weeks aflibercept injections due to persistence of fluid; dosing of aflibercept every 8 weeks without the 3 initial monthly loading doses may be a good alternative in a select group of patients who may have developed ranibizumab or bevacizumab resistance. There were no observed significant ocular adverse events with the use of intravitreal aflibercept injections in this series.
ACKNOWLEDGMENTS
a. Funding/Support: This study was supported by NIH grants R01EY007366 and R01EY018589, NEI vision core grant P30EY022589, Research to Prevent Blindness, and a grant from Regeneron Pharmaceuticals, Inc. The funding organizations had no role in the design or conduct of this research.
d. Other Acknowledgments: None.
Biographies
Biosketch (Dr. Arcinue)
Cheryl A. Arcinue, MD, is currently in private practice in Manila, Philippines. She is a graduate of the University of the Philippines College of Medicine and she finished her ophthalmology residency training at the Philippine General Hospital. She completed her fellowship in uveitis and ocular immunology at the Massachusetts Eye Research and Surgery Institution and her fellowship in vitreoretinal disease at the University of California San Diego Shiley Eye Center.

Biosketch (Dr. Freeman)
William R. Freeman, MD, is a Distinguished Professor of Ophthalmology and Vice-Chairman of the Department of Ophthalmology at the University of California San Diego Shiley Eye Center, and Director of the Jacobs Retina Center. He earned his graduate degree at Mount Sinai School of Medicine in New York City before completing his residency at Lenox Hill Hospital and fellowships at the University of California San Francisco and the University of Southern California.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
b. Financial Disclosures: No financial disclosures.
c. Contributions to Authors in each of these areas: Concept and design of the study (CAA, FM, WRF); Literature search (CA, FM); Data collection (CAA, FM, GB); Analysis and interpretation of data (LS, MLG, WRF); Writing the manuscript (CA, FM); Critical revision of the manuscript (GB, WRF); Final approval of the manuscript (WRF). Each author meets the 4 criteria set by the ICMJE required to claim authorship.
REFERENCES
- 1.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118(5):831–9. doi: 10.1016/j.ophtha.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239–48. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731–9. doi: 10.1016/j.ophtha.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175–83. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92(5):667–8. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- 10.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150(1):63–7. doi: 10.1016/j.ajo.2010.01.039. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhablani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250(10):1415–20. doi: 10.1007/s00417-012-1968-x. [DOI] [PubMed] [Google Scholar]
- 12.Moutray T, Alarbi M, Mahon G, Stevenson M, Chakravarthy U. Relationships between clinical measures of visual function, fluorescein angiographic and optical coherence tomography features in patients with subfoveal choroidal neovascularisation. Br J Ophthalmol. 2008;92(3):361–4. doi: 10.1136/bjo.2007.123976. [DOI] [PubMed] [Google Scholar]
- 13.Grunwald JE, Daniel E, Huang J, et al. Risk of Geographic Atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2014;121(1):150–61. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho VY, Yeh S, Olsen TW, et al. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol. 2013;156(1):23–8. doi: 10.1016/j.ajo.2013.02.009. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonekawa Y, Andreoli C, Miller JB, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156(1):29–35. doi: 10.1016/j.ajo.2013.03.030. e2. [DOI] [PubMed] [Google Scholar]
- 16.Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156(1):15–22. doi: 10.1016/j.ajo.2013.02.017. e1. [DOI] [PubMed] [Google Scholar]
- 17.Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97(8):1032–5. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Marsiglia M, Mrejen S, et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33(8):1605–12. doi: 10.1097/IAE.0b013e31828e8551. [DOI] [PubMed] [Google Scholar]