Abstract
Objective
To evaluate treatment outcomes for patients with vulvar cancer with grossly positive pelvic lymph nodes (PLNs).
Methods
From a database of 516 patients with vulvar cancer, we identified patients with grossly positive PLNs without distant metastasis at initial diagnosis. We identified 20 patients with grossly positive PLNs; inclusion criteria included PLN 1.5 cm or larger in short axis dimension on CT/MRI (n=11), FDG-avid PLN on PET/CT (n=3), or biopsy-proven PLN disease (n=6). Ten patients were treated with chemoradiation (CRT) therapy, 4 with RT alone, and 6 with various combinations of surgery, RT or CRT. Median follow-up time for patients who had not died of cancer was 47 months (range, 4-228 months).
Results
Mean primary vulvar tumor size was 6.4 cm; 12 patients presented with 2009 AJCC T2 and 8 with T3 disease. All patients had grossly positive inguinal nodes, and the mean inguinal nodal diameter was 2.8 cm. The 5-year overall survival and disease specific survival rates were 43% and 48%, respectively. Eleven patients had recurrences, some at multiple sites. There were 9 recurrences in the vulva, but no isolated nodal recurrences. Four patients developed distant metastasis within 6 months of starting radiation therapy.
Conclusions
Aggressive locoregional treatment can lead to favorable outcomes for many patients with grossly involved PLNs that is comparable to that of grossly involved inguinal nodes only. We recommend modification of the FIGO stage IVB classification to more accurately reflect the relatively favorable prognosis of patients with PLN involvement.
Keywords: vulva, vulvar cancer, stage IVB, pelvic lymph nodes, radiation therapy
INTRODUCTION
Advanced inguinal and pelvic lymph node (PLN) disease in vulvar cancer has consistently been found to correlate with disease recurrence and death [1-4]. As the number of involved inguinal nodes increases, survival decreases and the likelihood of involved PLNs increases [5].
However, most data defining the prognosis of patients with positive PLNs date from the 1950s through 1980s, before postoperative regional radiation therapy (RT) became standard [6-9]. Gynecologic Oncology Group (GOG-37) reported higher rates of disease-specific survival (DSS) with inguinal and pelvic RT as compared to PLN resection after radical vulvectomy and bilateral inguinal lymphadenectomy [10]. Patients who were treated with surgery alone and were found to have PLN metastases had a very poor survival rate of only 23% at 2 years [10].
As a result, the International Federation of Gynecology and Obstetrics and American Joint Committee on Cancer 2009 (FIGO/AJCC) classified patients with positive PLNs as IVB, grouping these patients with patients that have hematogenous metastases [11, 12]. However, outcome data for patients with positive PLNs treated with post-operative or definitive RT have been derived solely from studies of patients who had PLN dissection, usually without postoperative RT. Following the results of GOG-37, PLN resection fell out of favor for most patients with vulvar cancer, and patients who have inguinal nodal metastases are routinely treated with RT to the groin and pelvis. For this reason, diagnosis of microscopic PLN involvement is rare; patients who are diagnosed with stage IVB disease on the basis of PLN involvement usually have grossly enlarged nodes detected on imaging.
We therefore evaluated outcomes for patients with grossly positive PLNs to determine if classifying such disease as stage IVB remains appropriate in an era when RT is standard for most patients with inguinal node-positive vulvar cancer.
MATERIALS AND METHODS
Patients
From a database of 516 unselected patients with histologically confirmed vulvar cancer treated at The University of Texas MD Anderson Cancer Center during the period from 1980 through 2010, we identified 20 patients who had evidence of gross PLN involvement. Two patients who had para-aortic lymph node metastases, which were defined as metastases in lymph nodes superior to the aortic bifurcation without distant metastases, were included in our analysis. PLNs were considered positive if they contained biopsy-proven disease (6 patients), were fluorodeoxyglucose (FDG) avid on FDG positron emission tomography/computed tomography (PET/CT) (3 patients), or were 1.5 cm or larger in short axis dimension on CT or magnetic resonance imaging (MRI) (11 patients). Patient, tumor, treatment, and follow-up information was abstracted from medical records, and vulvar cancer was staged according to the 2009 FIGO/AJCC staging system [13].
Treatment
Patients were selected to receive multimodal therapy on a case-by-case basis after multidisciplinary discussion. Factors taken into consideration in selecting the type of treatment included extent of vulvar, inguinal, and pelvic disease, resectability of disease, performance status, and toxicity of treatment options. Treatment of the vulva, groin, and pelvis was as summarized in Table 1. Patients treated with chemotherapy received a median of 4 cycles (range, 1-7 cycles), consisting in most cases of single-agent cisplatin or a combination of cisplatin and 5-fluorouracil. Six patients did not receive chemotherapy.
Table 1.
Patient, tumor, and treatment characteristics (N=20).
| Characteristic | Median (range) or number of patients |
|---|---|
| Age at diagnosis, years | 61.5 (35-91) |
| Primary tumor size, cm | 5.0 (2.0-20.0) |
| Size of largest inguinal node, cm | 3.0 (1.3-6) |
| Size of largest pelvic node, cm | 2.6 (1.5-4.5) |
| AJCC T category (2009) | |
| T1 | 0 |
| T2 | 12 |
| T3 | 8 |
| AJCC N category (2009) | |
| N1 | 0 |
| N2a | 0 |
| N2b | 14 |
| N2c | 0 |
| N3 | 5 |
| Unknown | 1 |
| Treatment of vulva | |
| Planned preoperative CRT (no surgery)a | 1 |
| Definitive RT alone | 4 |
| Definitive CRT alone | 9 |
| Preoperative CRT + surgeryb | 3 |
| Surgeryb + adjuvant RT | 2 |
| Surgeryb + adjuvant CRT | 1 |
| Treatment of groin/pelvis | |
| CRT | 10 |
| RT alone | 3 |
| LND + RT | 3 |
| LND + CRT | 4 |
CRT, chemoradiation therapy; LND, lymph node dissection or debulking; RT, radiation therapy
Preoperative CRT followed by surgery was planned, but distant metastasis occurred prior to surgery.
Surgery includes either wide local excision or vulvectomy.
All patients received 40-50 Gy of external-beam RT to the vulva, inguinal regions, and pelvis. Regions of gross disease were given additional RT using a variety of techniques, including 3-dimensional conformal RT, integrated or sequential intensity-modulated RT boosts, or brachytherapy. Grossly enlarged nodes and regions of known extracapsular extension were boosted to higher doses (i.e., 60-70 Gy) depending on volume of disease, estimated risk of toxicity, and proximity of critical structures. The superior border for most RT fields was at the L4/5 or L5/S1 interspaces. For patients with para-aortic lymph node involvement, the superior border was raised to include the involved lymph nodes with a margin.
Statistical analysis
Overall survival (OS), progression-free survival, and vulvar, inguinal, and distant disease control were evaluated using Kaplan–Meier estimates and compared using the log-rank test. Clinical and patient factors were compared using Pearson’s chi-squared, Mann–Whitney–Wilcoxon, or Wilcoxon ranked-sign tests as appropriate, and 95% confidence intervals are reported. Statistical analyses were performed with the Stata software package (StataCorp. 2011, Stata Statistical Software: Release 12; College Station, TX). Statistical significance was defined as a p value of 0.5 or less.
RESULTS
Patient, tumor, and treatment characteristics of the 20 patients who met our inclusion criteria are presented in Table 1. Median follow-up time for all patients in the cohort was 21 months and for patients who had not died of vulvar cancer was 47 months (range, 4-228 months). Most patients had extensive primary tumors with grossly involved inguinal nodes. The mean primary tumor size was 6.4 cm (median, 5.0 cm). Twelve patients presented with 2009 AJCC T2 and 8 with T3 vulvar disease. Seventeen patients had palpable inguinal nodes, and all patients presented with at least 2009 AJCC N2b inguinal nodal disease. The mean diameter of the largest inguinal node was 2.8 cm (median, 3.0 cm). For the 14 patients whose positive PLNs were diagnosed clinically (i.e., by CT/MRI [11 patients] or PET/CT without biopsy [3 patients]), the mean PLN size was 3.3 cm (median, 3.0 cm). All 3 patients whose positive PLNs were diagnosed with PET/CT would have also met the CT/MRI inclusion criteria. Nineteen of 20 cancers were squamous cell carcinomas. Of the 7 patients who had an inguinal lymph node dissection or debulking, 6 also had surgical excision of at least 1 PLN.
Survival outcomes
The 5-year OS rate for the 20 patients was 43% (95% CI, 20%-64%) (Fig. 1). Two patients with para-aortic lymph node disease died at 4 and 12 months from beginning RT. At last follow-up, 8 patients were alive and had remained continuously without evidence of disease at a median of 47 months (range, 23-137 months). Of the 12 patients who died, 10 died from progressive or recurrent disease and 1 from a cardiac cause with no evidence of disease at 10 months after treatment. There was a trend toward improved 5-year OS for the 14 patients treated after 1995 compared to those treated before 1995 (57% vs 17%, log-rank p = 0.06).
Fig. 1.
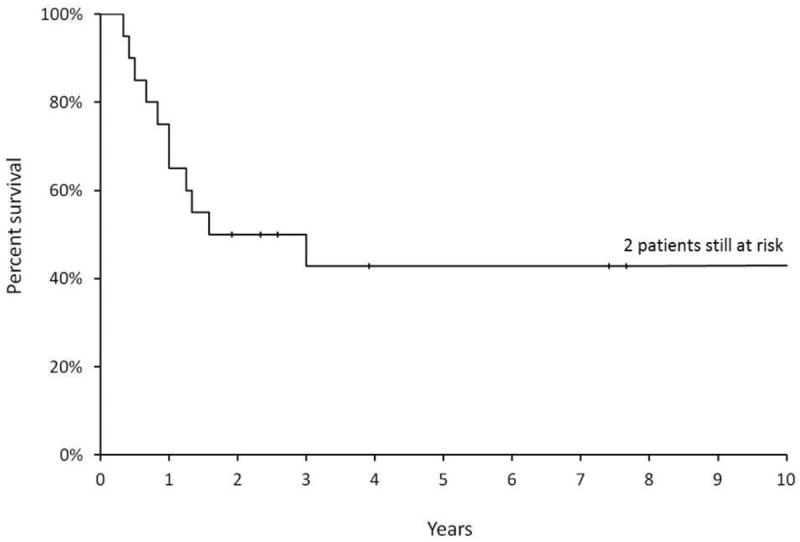
Kaplan–Meier curves showing overall survival for all 20 patients in this cohort in the first 10 years of follow-up. Forty-three percent of patients remained alive at 10 years. Two patients remained at risk after 10 years of follow-up.
Local, regional, and distant progression/recurrence
Eleven patients had recurrences at a median of 6 months after treatment (range, 1-223 months). Four of 6 patients with biopsy proven PLN disease recurred, and 7 out of 11 patients with CT/MRI diagnosed PLN disease recurred. None of the 3 patients with PET/CT diagnosed PLN disease recurred in this series. Patients with recurrence had a median OS of 12 months (range, 4-228) from the end of RT and just 5 months (range, 1-11 months) from first recurrence (Supplementary Table 1). Table 2 details the locations of recurrence for these 11 patients. All patients with recurrence died.
Table 2.
Locations of recurrences in the 11 patients with recurrence.
| Location of recurrence or progression | No. of patients |
|---|---|
| First recurrences | |
| Vulva | 7 |
| Inguinal nodes | 1 |
| Distant site | 2 |
| Unknown | 1 |
| All recurrences | |
| Vulva | 9 |
| Inguinal nodes | 3 |
| Pelvic nodes/sidewall | 1 |
| Lungs | 5 |
| Supraclavicular fossa | 1 |
| Abdominal skin | 1 |
| Peritoneal carcinomatosis | 1 |
| Bone | 1 |
| Sites of recurrence by patient | |
| Vulva only | 2 |
| Vulva + inguinal nodes | 1 |
| Vulva + distant metastasis | 4 |
| Vulva + inguinal nodes + distant metastasis | 2 |
| Inguinal nodes only | 0 |
| Distant metastasis only | 1 |
| Unknown | 1 |
Most of the patients in this study had extensive local disease, and 9 of 11 patients with relapse had a vulvar recurrence. Most vulvar recurrences were detected within 1 year of RT; the median time to detection of vulvar recurrence was 6 months. Patients with a vulvar progression or recurrence died at a median of 6 months (range, 1-11 months) after first progression/recurrence. However, one of these patients developed either a vulvar recurrence as first local recurrence or, more likely, a second primary vulvar tumor at 223 months and died of an unknown cause 5 months later.
Despite the extensive nodal disease in these patients, only 4 patients had regional nodal recurrences. Three patients had inguinal nodal recurrences. The first patient presented with a 6-cm inguinal node and received only 40 Gy of planned preoperative chemoradiation therapy (CRT) and never completed planned locoregional treatment because of interval development of distant metastasis. The second patient underwent preoperative CRT to 50 Gy to the vulva, inguinal nodes, and PLNs followed by wide local excision and inguinal lymph node debulking. The third patient underwent definitive CRT to 64 Gy but had groin recurrence. Additionally, 1 patient had recurrence in the pelvic sidewall despite PLN debulking and RT to 45 Gy. All of these patients with regional nodal recurrences also had recurrent vulvar disease. Only 1 of 13 patients treated with definitive RT or CRT without lymph node resection had an inguinal nodal recurrence.
Seven patients experienced distant metastasis at a median of 6 months after treatment (range, 2-35 months). Four patients had distant metastasis within 6 months of completing RT, and the median survival of patients with distant metastasis was only 5 months (range, 1-11 months) after the first recurrence. Six of 7 patients with distant metastasis also had recurrent vulvar disease.
DISCUSSION
Our data demonstrate that locoregional treatment with definitive or adjuvant RT can be curative for many patients with PLN-positive stage IVB vulvar cancer. Indeed, OS and disease-free survival for these patients approached those of patients with M0 disease with positive inguinal nodes (i.e., FIGO stage III disease) [14, 15].
The FIGO stage IVB designation for patients with positive PLNs initially resulted from the poor survival rates of a limited number of patients with positive PLNs described in older, retrospective surgical series of patients treated without RT [5-10] (Table 3). These series, published between the 1950s and 1980s, reported results in selected patients whose treatment included PLN dissection. At the time, RT was rarely used and was felt to have little or no role in vulvar cancer [7]. Five-year OS rates for patients with positive PLNs in these studies ranged from 13-38% [5-9], but the numbers of patients with positive PLNs were small, ranging from 3 to 16.
Table 3.
Older surgical series reporting outcomes of patients with stage IVB vulvar cancer with pelvic lymph node (PLN) disease.
| Series | Year of publication | Number of patients with PLN disease | 5-year survival rate, % | Adjuvant or definitive radiation therapy included? |
|---|---|---|---|---|
| Green et al [7] | 1958 | 16 | 13 | No |
| Way [9] | 1960 | 8 | 38 | No |
| Collins et al [6] | 1963 | 6 | 17 | No |
| Morris [8] | 1977 | 3 | 33 | Yes, for some patients with recurrence or second primary cancer, but not specified for patients with PLN |
| Curry et al [5] | 1980 | 9 | 22 | Yes, but for selected patients and not specified for patients with PLN unspecified |
| Homesley et al [10] | 1986 | 15 | 23 (2-year survival data only) | No |
| Current study | 2013 | 20 | 43 | Yes |
In an attempt to improve outcomes for patients with inguinal metastases, the GOG-37 trial compared the efficacy of inguinal and pelvic RT with PLN resection after radical vulvectomy and bilateral inguinal lymphadenectomy [10]. The authors reported improved outcomes in the RT arm [1, 10]. This study formed the basis for the current standard of care, and the use of pelvic dissection in cases of inguinal metastasis subsequently declined [10, 16]. The 15 patients entered on GOG-37 in whom PLN metastases were detected had a poor survival rate of only 23% at 2 years [10]. However, all of the patients known to have PLN metastases underwent PLN dissection and were therefore on the control arm that received no adjuvant RT. Although there undoubtedly were PLN-positive patients on the experimental and ultimately superior RT arm, these were unidentifiable in a treatment schema that included no surgical or clinical method of PLN evaluation in the RT arm. It is important to note that the dose of pelvic RT given in this early trial also was likely inadequate to control PLN that were grossly involved with cancer. This may explain some of the pelvic recurrences in the RT arm. With postoperative RT, the likelihood of inguinal recurrence was decreased, making it more likely that uncontrolled pelvic disease would be detected as the site of first recurrence.
To our knowledge, ours is the first paper to describe the outcomes of patients with stage IVB vulvar cancer with grossly positive PLNs treated with RT. Clinically negative PLNs are rarely dissected and typically are irradiated prophylactically at the same time as inguinal RT is delivered. Microscopic PLN involvement is therefore rarely diagnosed. Although only 6 patients in our cohort had histological proof of PLN positivity, the others had clinical evidence of PLN involvement according to stringent imaging criteria. Modern radiologic studies suggest that PET/CT has approximately 95% specificity in detecting pelvic or para-aortic lymph node metastases in patients with pelvic malignancies [17, 18]. Additionally, we chose a size threshold of 1.5 cm for CT/MRI diagnosis because PLNs of this size were unlikely to be only inflammatory in nature [19]. In fact, most of the positive PLNs in our patients were much larger than 1.5 cm, and the median size of positive PLNs was 3.0 cm. Histological confirmation of all PLNs would indeed strengthen the results of our study, since clinical evaluation with CT or PET/CT is not gold standard. However, the median size of the largest inguinal node in this cohort was 3.0 cm, and the median size of the largest PLN node was 2.6 cm. These nodes are likely larger than nodal enlargement caused by an inflammatory change alone.
Patients with stage IVB disease in this series had a remarkable 5-year OS rate of 43% despite their advanced primary tumors and nodal involvement. This is markedly better than the expected OS rate for patients who have hematogenous metastases from vulvar cancer [14]; it also compares favorably with the 5-year OS rate for patients with positive PLNs treated with lymph node dissection only.
Most patients in this study had extensive inguinal and PLN disease treated with CRT or RT alone rather than with a combination of lymph node dissection and RT. Despite this advanced presentation, only 4 patients had evidence of regional nodal recurrence. All 4 of these patients also had recurrent or progressive vulvar disease, and at least 2 received a lower dose than would now be recommended for definitive management. Whether or not surgical resection of positive PLNs would be of benefit is difficult to know, but our series suggests that there is little room for improvement over the regional control rates achieved with carefully planned definitive RT. Although resection may be considered if the nodes appear resectable, this should only be attempted after careful consideration of the overall disease burden, the potential for postoperative complications (including dehiscence, infection, lymphocyst, and lymphedema), and the potential for delays in definitive RT [20, 21]. The location of the involved nodes may also influence management decisions; the distal iliac lymph nodes that are most commonly involved are often sufficiently distant from critical structures that they can be safely treated to a high RT dose using modern imaging and treatment planning methods (Fig. 2). In fact, our data suggest that vulvar disease control posed the greatest challenge for patients with positive PLNs, who frequently had massive, unresectable disease. Regional recurrence and isolated distant failure rarely occurred in these patients. However, it is uncertain if early vulvar recurrence actually represented progression of persistent disease. Regardless, all patients with LR or local progression of disease died.
Fig. 2.
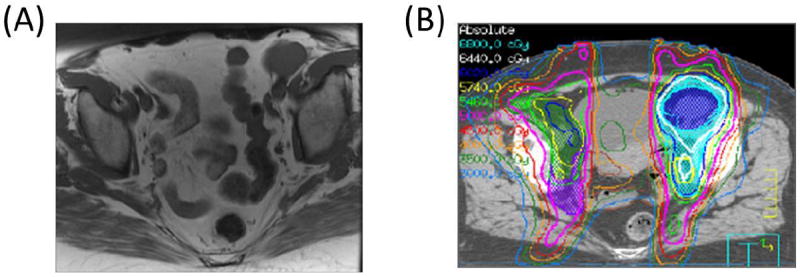
The location of the involved PLNs is important when definitive treatment options are being considered. (A) Axial T1 magnetic resonance imaging scan shows a large left distal external iliac lymph node measuring approximately 3.5 cm in maximal dimension. (B) This node is sufficiently far from critical structures that a simultaneous integrated boost and sequential boost to 64 Gy could be safely delivered. This patient is currently without evidence of disease 7.7 years after RT, with normal bladder and bowel function.
At the same time that improved therapies may offer better outcomes for patients with PLN metastases, modern diagnostic techniques may allow for more accurate staging than in previous eras and can improve patient selection for aggressive treatments. Three patients in this series developed distant metastasis within 3 months of the start of treatment; all 3 died within 5 to 8 months. Three of these patients were diagnosed and treated in the 1980s and 1990s, and it is possible that with modern imaging techniques, these patients would have been more appropriately staged and selected for palliative treatment. In our series, there was a trend toward better OS in patients treated after 1995 (5-year OS rate, 57%) than in those treated earlier. More patients treated after 1995 received CT/MRI or PET/CT staging than those treated earlier, which could lead to more accurate identification of DM at presentation. Additionally, intensity-modulated RT (IMRT) was introduced at our institution after 1999 and has allowed dose escalation with reduced toxicity compared to 3-dimensional conformal RT techniques. Over time, chemotherapy was integrated concurrently with RT and was given more frequently in weekly doses. Earlier treatment regimens also consisted of pre-operative RT that was later abandoned due to higher failure rate. Later treatment approaches moved to definitive concurrent chemoRT. However, given the small number of patients in each subgroup, an OS difference did not reach statistical significance.
Our findings suggest that aggressive locoregional management with RT is a vital component of treatment for PLN-positive stage IVB vulvar cancer. The role of RT has significantly changed since Green et al [7] stated in 1958 that “radiation is of no value in the primary treatment of cancer of the vulva, and is of little benefit in its palliation.” GOG-37 clearly demonstrated that RT played an important role in the management of locoregionally advanced vulvar cancer and confirmed that even patients with extensive inguinal node involvement could often be cured. Our study carries this 1 step further and demonstrates that several decades of advances in multimodality treatment have not only led to better results for patients with inguinal nodal metastases but also made it possible to cure many patients with PLN involvement. In this context, it seems inappropriate to group patients who have PLN involvement with patients who have hematogenous metastases, which are nearly always incurable. For this reason, we strongly recommend modification of the FIGO IVB classification to more realistically reflect the relatively favorable prognosis of patients with PLN involvement.
Supplementary Material
RESEARCH HIGHLIGHTS.
Definitive treatment for patients with involved pelvic nodes led to favorable outcomes.
Overall survival was similar to that of patients with positive inguinal nodes.
We recommend modification of the FIGO Stage IVB classification.
Footnotes
Conflicts of Interest
MF reports personal fees from Novadaq and Ethicon. Otherwise, the authors declare that there are no other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kunos C, Simpkins F, Gibbons H, Tian C, Homesley H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstetrics and gynecology. 2009;114:537–46. doi: 10.1097/AOG.0b013e3181b12f99. [DOI] [PubMed] [Google Scholar]
- 2.Backstrom A, Edsmyr F, Wicklund H. Radiotherapy of carcinoma of the vulva. Acta obstetricia et gynecologica Scandinavica. 1972;51:109–15. doi: 10.3109/00016347209154004. [DOI] [PubMed] [Google Scholar]
- 3.Frankendal B, Larsson LG, Westling P. Carcinoma of the vulva. Results of an individualized treatment schedule. Acta radiologica: therapy, physics, biology. 1973;12:165–74. doi: 10.3109/02841867309130391. [DOI] [PubMed] [Google Scholar]
- 4.van der Steen S, de Nieuwenhof HP, Massuger L, Bulten J, de Hullu JA. New FIGO staging system of vulvar cancer indeed provides a better reflection of prognosis. Gynecologic oncology. 2010;119:520–5. doi: 10.1016/j.ygyno.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Curry SL, Wharton JT, Rutledge F. Positive lymph nodes in vulvar squamous carcinoma. Gynecologic oncology. 1980;9:63–7. doi: 10.1016/0090-8258(80)90009-8. [DOI] [PubMed] [Google Scholar]
- 6.Collins CG, Collins JH, Barclay DL, Nelson EW. Cancer Involving the Vulva. A Report on 109 Consecutive Cases. American journal of obstetrics and gynecology. 1963;87:762–72. [PubMed] [Google Scholar]
- 7.Green TH, Jr, Ulfelder H, Meigs JV. Epidermoid carcinoma of the vulva; an analysis of 238 cases. II. Therapy and end results. American journal of obstetrics and gynecology. 1958;75:848–64. doi: 10.1016/0002-9378(58)90667-7. [DOI] [PubMed] [Google Scholar]
- 8.Morris JM. A formula for selective lymphadenectomy. Its application to cancer of the vulva. Obstetrics and gynecology. 1977;50:152–8. [PubMed] [Google Scholar]
- 9.Way S. Carcinoma of the vulva. American journal of obstetrics and gynecology. 1960;79:692–7. doi: 10.1016/0002-9378(60)90626-8. [DOI] [PubMed] [Google Scholar]
- 10.Homesley HD, Bundy BN, Sedlis A, Adcock L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstetrics and gynecology. 1986;68:733–40. [PubMed] [Google Scholar]
- 11.Hopkins MP, Reid GC, Johnston CM, Morley GW. A comparison of staging systems for squamous cell carcinoma of the vulva. Gynecologic oncology. 1992;47:34–7. doi: 10.1016/0090-8258(92)90071-p. [DOI] [PubMed] [Google Scholar]
- 12.Hacker NF. Revised FIGO staging for carcinoma of the vulva. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105:105–6. doi: 10.1016/j.ijgo.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Maisonneuve P, et al. Carcinoma of the vulva. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics; FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer; 2006. pp. S7–27. [DOI] [PubMed] [Google Scholar]
- 15.Tabbaa ZM, Gonzalez J, Sznurkowski JJ, Weaver AL, Mariani A, Cliby WA. Impact of the new FIGO 2009 staging classification for vulvar cancer on prognosis and stage distribution. Gynecologic oncology. 2012;127:147–52. doi: 10.1016/j.ygyno.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Woelber L, Trillsch F, Kock L, Grimm D, Petersen C, Choschzick M, et al. Management of patients with vulvar cancer: a perspective review according to tumour stage. Therapeutic advances in medical oncology. 2013;5:183–92. doi: 10.1177/1758834012471699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MC, Chen JH, Liang JA, Yang KT, Cheng KY, Kao CH. 18F-FDG PET or PET/CT for detection of metastatic lymph nodes in patients with endometrial cancer: a systematic review and meta-analysis. European journal of radiology. 2012;81:3511–7. doi: 10.1016/j.ejrad.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Lv K, Guo HM, Lu YJ, Wu ZX, Zhang K, Han JK. Role of 18F-FDG PET/CT in detecting pelvic lymph-node metastases in patients with early-stage uterine cervical cancer: comparison with MRI findings. Nuclear medicine communications. 2014;35:1204–11. doi: 10.1097/MNM.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 19.Hedgire SS, Pargaonkar VK, Elmi A, Harisinghani AM, Harisinghani MG. Pelvic nodal imaging. Radiologic clinics of North America. 2012;50:1111–25. doi: 10.1016/j.rcl.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Burke TW, Levenback C, Coleman RL, Morris M, Silva EG, Gershenson DM. Surgical therapy of T1 and T2 vulvar carcinoma: further experience with radical wide excision and selective inguinal lymphadenectomy. Gynecologic oncology. 1995;57:215–20. doi: 10.1006/gyno.1995.1128. [DOI] [PubMed] [Google Scholar]
- 21.Montana GS, Thomas GM, Moore DH, Saxer A, Mangan CE, Lentz SS, et al. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. International journal of radiation oncology, biology, physics. 2000;48:1007–13. doi: 10.1016/s0360-3016(00)00762-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


