Abstract
Purpose
To measure oxygen (pO2) in eyes of patients undergoing intraocular surgery and identify correlations with central corneal thickness (CCT).
Design
Prospective, cross-sectional study
Methods
Setting: Institutional
Patient Population: 124 patients undergoing cataract and/or glaucoma surgery
Observation Procedure: Prior to surgery, an oxygen sensor was introduced into the anterior chamber (AC) via peripheral corneal paracentesis. The tip of the flexible fiberoptic probe was positioned for three measurements in all patients: (1) near central corneal endothelium, (2) in mid-AC and (3) in AC angle. In patients undergoing cataract extraction, additional measurements were taken (4) at the anterior lens surface and (5) in the posterior chamber.
Main Outcome Measures: pO2 measurements at five locations within the eye were compared to central corneal thickness measurements by multivariate regression analyses.
Results
There was a statistically significant inverse correlation between CCT and pO2 in the anterior chamber angle (p=0.048). pO2 was not significantly related to CCT at any other location, including beneath the central cornea. Regression analysis relating CCT to age, race and oxygen levels in all five locations in the anterior segment revealed an association of a thinner cornea with increasing age (p=0.007).
Conclusions
Physiologic correlations with central corneal thickness may provide clues to understanding why a thinner cornea increases the risk of open glaucoma. Associations between glaucoma risk, CCT and pO2 in the AC angle suggest that exposure of the outflow system to increased oxygen or oxygen metabolites may increase oxidative damage to the trabecular meshwork cells, resulting in elevation of intraocular pressure.
Introduction
Although elevated intraocular pressure (IOP) is the most significant risk factor for the development of glaucoma, recent studies revealed an association between central corneal thickness (CCT) and the risk of developing glaucoma,1,2 the leading cause of irreversible blindness worldwide. However, the importance of CCT in glaucoma was not studied more closely until validated in the Ocular Hypertension Treatment Study (OHTS).3 Measurement of CCT is now considered to be the standard of care in the evaluation of a patient at risk for glaucomatous optic neuropathy. The Early Manifest Glaucoma Trial (EMGT),2 which enrolled subjects with glaucoma damage not based on IOP measurement, correlated the effect of CCT on glaucomatous progression. In spite of the small size, homogeneity of the study population and the relatively short follow-up, CCT correlated with progression. A thinner cornea is not only a strong predictive risk factor for progression of ocular hypertension to open angle glaucoma (OAG), but also correlates with the severity of visual field damage and more rapid progression of visual field loss.4,5 In addition, a recent publication6 from the OHTS trial data indicated that utilizing a formula to adjust the IOP for CCT does not improve the prediction model for open angle glaucoma. Although this does not prove a role for CCT as an independent risk factor in this population, it remains an important risk factor without a physiologic explanation.
Although Jonas et al7 confirmed that thinner CCT correlated significantly with the area of the neuroretinal rim and inversely with visual field loss at the time of referral, they noted progression of the neuropathy to be independent of CCT. These investigators did not find a correlation with CCT and thickness of the lamina cribrosa8 or development of glaucomatous optic disc hemorrhages.9
Anatomic and mechanical links between corneal thickness and the structural properties of the optic disc and lamina cribrosa have been identified. Disc area, cup depth and elasticity of the lamina cribrosa have been shown to be associated with CCT. A statistically significant correlation of scleral thickness and CCT has been described among patients with normal tension glaucoma, but not in controls or patients with ocular hypertension or primary OAG.10 Genome-wide association studies11 identified a significant association between CCT and variants linked to the α2 chain of collagen VIII and the α1 chain of collagen V. Although this statistical association is robust, the mechanistic link between collagen expression or function and glaucoma pathogenesis remains unclear.
In spite of these clinical, anatomic, and genetic correlates, no physiologic associations with CCT have been identified. We report that thinner corneas are associated with increased partial pressure of oxygen (pO2) in the anterior chamber angle in patients undergoing cataract and/or glaucoma surgery.
Methods
Study Design
The Human Resource Protection Office and the Institutional Review Board of the Washington University School of Medicine prospectively approved this study which is compliant with HIPAA guidelines and conforms to the tenets of the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. The prospective, cross-sectional study was designed to measure oxygen distribution in different regions of the eye in a reference group, with no previous cataract or vitrectomy surgery, and evaluate correlations with patient characteristics.
Patients and oxygen measurements
Patients undergoing cataract and/or open-angle glaucoma surgery in a single subspecialty practice (CJS) were eligible for this study. Patients were excluded from the study if they had evidence of corneal endothelial dysfunction, ischemic ocular disease, anterior chamber angle closure, inflammatory disease, ocular neoplasia, shallow anterior chamber, or monocular status. Patients with prior ocular surgery, except for laser therapy and glaucoma filtering surgery, were also excluded from participation.
A complete general medical and ophthalmic history was obtained as well as a complete ophthalmic examination. Central corneal thickness (CCT) was measured by ultrasound (DGH 55 Pachmate, DGH Technology, Inc., Exton, PA). Axial length measurements were recorded on patients undergoing cataract extraction (Zeiss IOLMaster, Carl Zeiss Meditec, Germany). Race was self-reported using a standardized questionnaire. As per usual surgical protocol, the patient was placed in the supine position and intravenous sedation was administered. Supplemental oxygen was provided by nasal cannula. The surgical field was completely separated from the cannula using an adhesive surgical drape to avoid any additional oxygen exposure to the eye. Blood oxygen saturation (SaO2) monitoring was performed by continuous pulse oximetry and maintained between 95 and 100%. The surgical eye was prepped and draped and a lid speculum was placed. A sub-Tenon’s injection of 3 ml of 2% lidocaine and 0.375% bupivacaine (50:50) was performed to provide local anesthesia. Prior to the planned surgical procedure, a 30-gauge needle was used for entry into the anterior chamber to fashion a peripheral corneal paracentesis and the Oxylab pO2™ optical oxygen sensor (optode; Oxford Optronix, Oxford, United Kingdom) was carefully introduced into the anterior chamber without leakage of aqueous humor (Figure-top). Instrument calibration was checked prior to and following each set of measurements. The tip of the flexible fiberoptic probe was positioned for three measurements in all patients by the surgeon (CJS; Figure-bottom): (1) near the central corneal endothelium, (2) in the mid-anterior chamber (AC) and (3) in the AC angle. In patients scheduled to undergo cataract extraction, additional measurements were taken at (4) the anterior lens surface and (5) in the posterior chamber just behind the iris. This avoided risk of damage to the lens in patients who were to remain phakic after the operative procedure. Each measurement required a mean of 46.2 seconds for accurate probe positioning and stabilization of the pO2 level. Patients were monitored postoperatively for any complications.
FIGURE.
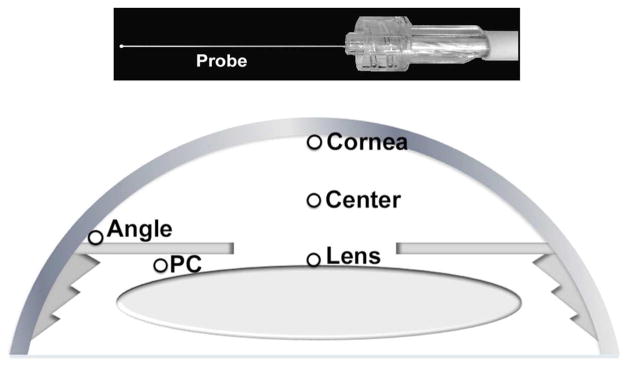
Measurement of Oxygen Levels in the Human eye in vivo Oxylab pO2™ optical oxygen sensor device with flexible fiberoptic probe is introduced into the eye via 30-gauge corneal paracentesis site (Top). Open circles indicate locations for measurement of oxygen (pO2) within the eye (Bottom).
PC = Posterior chamber
Statistical analysis
Results are expressed as mean values ± standard deviation (SD). Statistical analyses were performed using SPSS software Version 17.0 (SPSS, Chicago, IL, USA). Multivariate regression analyses were performed with adjustment for all potential confounding variables measured. Probability values less than 0.05 were considered statistically significant.
Results
A total of 124 patients participated. There were 53 male and 71 female subjects, 37 African American and 87 Caucasian. The mean age was 70.4 years. The pO2 in different regions of the eye was not related to surgical diagnosis or surgical procedure performed. The study group included patients with no previous intraocular ocular surgery (77 eyes) and patients with prior laser (22 eyes) or glaucoma filtering surgery (25 eyes). Glaucoma patients were divided into those with medically controlled IOP and those for whom medical control was not adequate. Glaucoma patients were also subdivided into three groups based on their visual field loss, as a measure of glaucoma severity. The oxygen levels at the five locations, patient age, axial length and CCT are shown in Table 1. There was no significant difference in the two groups (glaucoma plus cataract versus cataract only).
Table 1.
Comparison of baseline patient characteristics and intraocular oxygen measurements at five locations based on preoperative diagnosis
| Glaucoma + Cataract (N=107) | Cataract only (N=17) | p value | |
|---|---|---|---|
| Age (years) | 70.2 ± 10.0 | 71.2 ± 13.8 | 0.785 |
| CCT (μm) | 549.6 ± 38.0 | 556.3 ± 41.7 | 0.566 |
| Axial Length (mm) | 24.0 ± 1.4 | 24.5 ± 2.0 | 0.329 |
| pO2 at Cornea (mmHg) | 22.0 ± 6.8 | 22.6 ± 7.6 | 0.792 |
| pO2 at Center (mmHg) | 10.3 ± 3.8 | 9.7 ± 5.2 | 0.660 |
| pO2 at Lens (mmHg) | 2.1 ± 2.1 | 2.6 ± 2.8 | 0.454 |
| pO2 at Angle (mmHg) | 12.0 ± 5.1 | 13.6 ± 4.9 | 0.244 |
| pO2 at PC (mmHg) | 3.7 ± 2.8 | 4.4 ± 2.7 | 0.370 |
CCT = central corneal thickness; pO2 = partial pressure of oxygen; PC = posterior chamber
For the study group, regression analysis that related CCT to age, race and oxygen levels in all five locations in the anterior segment showed that having a thinner cornea was associated increasing age (p=0.007) and increased pO2 in the anterior chamber angle (p=0.048) (Table 2). Oxygen levels at all other locations were not associated with CCT, even beneath the central cornea. As in our previous study,12 African-Americans had significantly higher pO2 in all five areas measured. These data are not shown because some patients were included in both studies. When adjusted for age and race, IOP that was not medically controllable was significantly associated with having a thinner cornea (p=0.045; Table 3, left). Glaucoma severity, as measured by visual field loss classified as none, early, moderate, or severe, was not associated with CCT (p=0.127; Table 3, right). Although African heritage has been associated with having a thinner cornea in other studies, it was not significantly associated with CCT in this patient group.
Table 2.
Association between central corneal thickness and intraocular oxygen measurements with adjustment for age and race
| β | p value | |
|---|---|---|
| Age | −1.215 | 0.007 |
| Race | 7.841 | 0.489 |
| pO2 at Cornea | 0.314 | 0.741 |
| pO2 at Center | 0.446 | 0.782 |
| pO2 at Lens | −3.622 | 0.182 |
| pO2 at Angle | −2.204 | 0.048 |
| pO2 at PC | −0.059 | 0.974 |
pO2= partial pressure of oxygen; PC = posterior chamber; β = regression coefficient
Table 3.
Association between central corneal thickness and intraocular pressure (left) or glaucoma severity (right) with adjustment for age and race
| β | p value | |
|---|---|---|
| Age | −0.919 | 0.007 |
| Race | −12.601 | 0.105 |
| IOP | −14.121 | 0.045 |
| β | p value | |
|---|---|---|
| Age | −0.896 | 0.009 |
| Race | −11.649 | 0.137 |
| Severity | −5.057 | 0.127 |
IOP = intraocular pressure; β = regression coefficient
Discussion
Central Corneal Thickness and the Risk of Open Angle Glaucoma
Following identification of the importance of CCT on the development of open angle glaucoma, the artifactual effect of CCT on Goldmann applanation tonometry has surfaced. As the instrument design was based on the assumption of an average patient’s CCT of 500um, Goldmann and Schmidt13 erroneously assumed that the Imbert-FIck law applied to the tonometer and the cornea. The variation of corneal thickness within a population may significantly interfere with the accuracy of IOP measurements with the Goldmann tonometer. Ehlers et al14 performed experiments with cannulation of normal eyes undergoing cataract extraction revealing over and underestimation of up to 7 mm Hg per 100 μm of CCT. The effect of a thicker vs. thinner-than-average cornea to over- vs. underestimate, respectively, the “true” IOP is significant. This may result in misclassification of patients as ocular hypertensives and as “normal tension” glaucoma. Correction factors for this disparity have not been validated but have been suggested by some individuals.15 However, other biomechanical properties of the cornea also impact tonometric IOP measurements,16 and alterations of IOP do not likely explain the risk factor of CCT.
Oxygen Distribution in the Anterior Segment of the Eye
Previous reports of the stable pO2 gradients within the anterior and posterior segments of the rabbit17 and human eye18 in vivo demonstrated higher pO2 at the corneal endothelial surface and lower pO2 around the lens. The higher oxygen levels beneath the cornea reflect the diffusion of oxygen across the corneal surface and oxygen consumption by corneal cells. The gradient across the anterior chamber indicated oxygen consumption by the lens. However, our studies did not detect a correlation between CCT and pO2 beneath the central cornea. This is probably because corneal thickness is determined primarily by stromal thickness. Therefore, thinner corneas would not necessarily have many fewer oxygen-consuming cells, since cell density in the stroma is significantly lower than in the epithelium or endothelium.
In contrast to pO2 beneath the central cornea, oxygen near the AC angle appears to be regulated by blood oxygen saturation in the ciliary body vasculature. Increasing or decreasing blood oxygen saturation selectively increased or decreased oxygen levels in the rabbit anterior chamber angle.17 Similarly, increased oxygen in the posterior chamber following vitrectomy and cataract surgery nearly doubled oxygen levels in the human anterior chamber angle.18 We postulated that the increased oxygen on the aqueous side of the ciliary epithelium would decrease the extraction of oxygen from the blood by the ciliary epithelium, thereby increasing oxygen saturation in the ciliary body circulation. The ciliary body vasculature is composed of fenestrated capillaries, meaning that plasma proteins and oxygen can readily diffuse into the ciliary body stroma. We proposed that some of the excess oxygen in the ciliary body stroma would diffuse, along with extravascular plasma proteins, into the AC angle from the ciliary body stroma through the iris stroma, via the pathway described by Freddo et al.19 Thus, the correlation of CCT with pO2 in the AC angle may be related to factors that alter both corneal thickness and oxygen diffusion from the ciliary body vasculature into the anterior chamber angle.
As a potential explanation for this association, Maul and colleagues20 found that CCT was inversely related to choroidal thickness in the macular region. If this relationship extended into the anterior uveal tract, thinner corneas would be associated with greater volume in the ciliary body stroma, which could increase oxygen diffusion into the anterior chamber angle. Similarly, variants in the expression or function of collagens VIII and V, which have been associated with CCT, could alter the density of the ciliary body stroma, affecting diffusion into the anterior chamber angle.
In agreement with the potential importance of oxygen as a cause of damage to the outflow pathway as a source of reactive oxygen species, we identified statistically significant increases in pO2 in the anterior chamber angle in patients who had undergone prior vitrectomy and cataract surgery18 as well as in African-American patients.12 Since both characteristics are associated with increased risk of OAG, elevated oxygen in the anterior chamber angle may explain these increased risks.21,22
Racial characteristics, Heritability and Central Corneal Thickness variation
Examination of different racial groups23 revealed thinner CCT in Africans (sub-Saharan, Afro-Caribbean and African-Americans;24 and Asians, but no correlation with IOP. The Los Angeles Latino Eye Study noted that Hispanics had intermediate CCT compared to African Americans and Caucasians.25 Understanding the genetic determinants of CCT may aid in uncovering the underlying connection between CCT, OAG and pO2 in the AC angle. CCT has been shown to be one of the most heritable of the risk factors for glaucoma. Alsbirk et al26 found heritability estimates of 0.6–0.7 for CCT in Eskimo families in Greenland. Toh et al27 conducted a CCT heritability study on mono- and dizygotic twins in Australia and the United Kingdom and calculated a heritability factor of 0.95. Another twin study performed in China28 by Zheng et al estimated heritability for CCT to be 0.88 for males and 0.91 for females.
Mapping of CCT-associated loci through candidate gene and genome-wide association studies (GWAS) identified the ZNF469 gene, which encodes a zinc finger protein associated with “brittle cornea syndrome” 29 and FOXO1, a transcription factor involved in regulating metabolism in many tissues.30 Other genetic associations with CCT include variants in collagen genes (COL5A1 and COL8A2).11 However, in the large GLAUGEN and NEIGHBOR cohorts, all 20 loci previously associated with CCT were not significantly associated with open angle glaucoma.31 However, this study identified several other single nucleotide polymorphisms that had suggestive genome-wide association with CCT (p<10−5) and significant association with OAG. The genes linked to these loci (CNTNAP4, NTM) were expressed in the brain and optic nerve, but at minimal levels in the sclera and cornea. However, this study did not examine their expression in the tissues of the uveal tract, which could affect exposure of the trabecular meshwork to oxygen.
Oxidative stress and glaucoma
Increased outflow resistance as a result of damage to the trabecular meshwork (TM) cells has been a major focus for studies of glaucoma pathogenesis. The age-related loss of TM cells as first noted by Alvarado et al,32 is likely to contribute to increased outflow resistance. Numerous subsequent studies provide evidence of increased oxidative damage and reduced resistance to oxidative stress in the outflow pathway33 and in the degeneration of retinal ganglion cells.34 Oxidative stress may be the result of IOP induced neuronal damage in an in vitro study of cultured retinal ganglion cells.35 Increased oxidative DNA damage, as quantified by 8-hydroxyl-2′-deoxyguanosine (8-OHdG) measured within trabecular meshwork cells of glaucoma patients,36 provides evidence for oxidative stress as a factor in the etiology of glaucomatous optic neuropathy. Additionally, Sacca et al37 demonstrated that the quantity of oxidative DNA damage measured within the TM cells correlates with increased IOP and visual field loss in human patients. These studies suggest a decrease in both “local” and, perhaps, systemic antioxidant capacity in patients with glaucoma as a result of chronic oxidative insult. Superoxide dismutase activity, an important enzymatic antioxidant factor, decreases with age in normal cadaveric TM,32 suggesting an age-related decrease in anti-oxidant protection. Human TM has been shown to be more sensitive to oxidative stress as compared to the adjacent tissues of the iris and cornea.38 Mitochondrial DNA deletion was significantly increased in the TM of glaucoma patients as compared to controls and was associated with a decreased number of mitochondria per cell.39 Mitochondrial dysfunction and decreased quantities per cell could result in decreased oxygen consumption, higher local pO2 and a potential source of reactive oxygen species. A recent study40 demonstrated that oxidative stress stabilizes selected mRNAs (IL-6 and IL-8) in human TM cells for cytokines associated with glaucoma progression.
Our study is limited by the invasive nature of our measurements, requiring the inclusion of only patients undergoing intraocular surgery, thereby without an ideal control. As we did not identify any statistically significant differences in pO2 at any location in patients with a diagnosis of glaucoma versus cataract, or with glaucoma severity/IOP control, it is admittedly difficult to determine a cause/effect relationship with CCT and glaucoma. However, our findings are important, as this local increase in pO2 may represent the source of the oxidative stress that has been measured in other studies.36–38
In summary, the source of the oxidative stress and reactive oxygen species in open angle glaucoma has yet to be identified. We propose that alterations of oxygen distribution in susceptible patients may result in increased exposure to molecular oxygen and formation of reactive oxygen species in the local environment or in the trabecular meshwork cells themselves. The fact that oxygen increased in the angle of patients with thinner corneas provides the first potential physiologic explanation for the increased risk of OAG in these patients. Studies are underway in a primate model to evaluate the effects of local elevation of pO2 on trabecular meshwork structure, oxidative damage, cell survival and outflow facility.
Acknowledgments
Funding/Support:
American Health Assistance Foundation – National Glaucoma Research Grant, Clarksburg, MD (CJS)
Grace Nelson Lacy Glaucoma Research Grant, St. Louis, MO
National Eye Institute grants EY021515 (CJS) and EY015863 (DCB)
Unrestricted grant from Research to Prevent Blindness (New York, NY) to the Washington University Department of Ophthalmology and Visual Sciences. The funding organizations had no role in the design or conduct of this research.
Biography

Carla J. Siegfried, MD is a professor of Ophthalmology and Visual Sciences at Washington University School of Medicine in St. Louis. She received her MD from the University of Missouri-Kansas City, completed residency training at the University of Illinois Eye and Ear Infirmary and glaucoma fellowship at Northwestern University. Her research interests include ocular oxygen metabolism and the etiology of glaucoma. She received the Achievement and the Secretariat Awards from the American Academy of Ophthalmology.
Footnotes
Contributions of Authors:
All authors (CS, YBS, FB, DCB) participated in the design and conduct of study; collection, management, analysis and interpretation of data and in the preparation, review and approval of manuscript. Each author is responsible for the integrity of the entire study and manuscript.
Financial Disclosures: Dr. Siegfried receives research support from Alcon, Inc. and is a consultant, lecturer, and developer of educational presentations for Allergan, Inc. (Irvine, CA). Dr. Beebe is a consultant for PanOptica (Bernardsville, NJ) and has received royalties from Elsevier, Inc. (Philadelphia, PA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 2.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 3.The accuracy and clinical application of predictive models for primary open-angle glaucoma in ocular hypertensive individuals. Ophthalmology. 2008;115(11):2030–2036. doi: 10.1016/j.ophtha.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S, Kim CY, Seong GJ, Hong YJ. Central corneal thickness and visual field progression in patients with chronic primary angle-closure glaucoma with low intraocular pressure. Am J Ophthalmol. 2007;143(2):362–363. doi: 10.1016/j.ajo.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros FA, Sample PA, Zangwill LM, Bowd C, Aihara M, Weinreb RN. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136(5):805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 6.Brandt JD, Gordon MO, Gao F, Beiser JA, Miller JP, Kass MA. Adjusting intraocular pressure for central corneal thickness does not improve prediction models for primary open-angle glaucoma. Ophthalmology. 2012;119(3):437–442. doi: 10.1016/j.ophtha.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas JB, Stroux A, Velten I, Juenemann A, Martus P, Budde WM. Central corneal thickness correlated with glaucoma damage and rate of progression. Invest Ophthalmol Vis Sci. 2005;46(4):1269–1274. doi: 10.1167/iovs.04-0265. [DOI] [PubMed] [Google Scholar]
- 8.Jonas JB, Holbach L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Invest Ophthalmol Vis Sci. 2005;46(4):1275–1279. doi: 10.1167/iovs.04-0851. [DOI] [PubMed] [Google Scholar]
- 9.Jonas JB, Stroux A, Oberacher-Velten IM, Kitnarong N, Juenemann A. Central corneal thickness and development of glaucomatous optic disk hemorrhages. Am J Ophthalmol. 2005;140(6):1139–1141. doi: 10.1016/j.ajo.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed-Noor J, Bochmann F, Siddiqui MA, et al. Correlation between corneal and scleral thickness in glaucoma. J Glaucoma. 2009;18(1):32–36. doi: 10.1097/IJG.0b013e31816b2fd1. [DOI] [PubMed] [Google Scholar]
- 11.Vithana EN, Aung T, Khor CC, et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Genet. 2011;20(4):649–658. doi: 10.1093/hmg/ddq511. [DOI] [PubMed] [Google Scholar]
- 12.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Racial differences in ocular oxidative metabolism: implications for ocular disease. Arch Ophthalmol. 2011;129(7):849–854. doi: 10.1001/archophthalmol.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldmann HST. Uber applanationstonometrie. Ophthalmologica. 1957;134:221–242. doi: 10.1159/000303213. [DOI] [PubMed] [Google Scholar]
- 14.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 1975;53(1):34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 15.Herndon LW. Measuring intraocular pressure-adjustments for corneal thickness and new technologies. Curr Opin Ophthalmol. 2006;17(2):115–119. doi: 10.1097/01.icu.0000193093.05927.a1. [DOI] [PubMed] [Google Scholar]
- 16.Herndon LW, Weizer JS, Stinnett SS. Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol. 2004;122(1):17–21. doi: 10.1001/archopht.122.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Shui Y-B, Fu J-J, Garcia C, et al. Oxygen Distribution in the Rabbit Eye and Oxygen Consumption by the Lens. Invest Ophthalmol Vis Sci 2006. 2006;47(4):1571–1580. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- 18.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51(11):5731–5738. doi: 10.1167/iovs.10-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res. 2001;73(5):581–592. doi: 10.1006/exer.2001.1056. [DOI] [PubMed] [Google Scholar]
- 20.Maul EA, Friedman DS, Chang DS, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118(8):1571–1579. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 22.Koreen L, Yoshida N, Escariao P, et al. Incidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomy. Retina. 2012;32(1):160–167. doi: 10.1097/IAE.0b013e318217fffb. [DOI] [PubMed] [Google Scholar]
- 23.Nemesure B, Wu SY, Hennis A, Leske MC. Corneal thickness and intraocular pressure in the Barbados eye studies. Arch Ophthalmol. 2003;121(2):240–244. doi: 10.1001/archopht.121.2.240. [DOI] [PubMed] [Google Scholar]
- 24.Mercieca K, Odogu V, Fiebai B, Arowolo O, Chukwuka F. Comparing central corneal thickness in a sub-Saharan cohort to African Americans and Afro-Caribbeans. Cornea. 2007;26(5):557–560. doi: 10.1097/ICO.0b013e3180415d90. [DOI] [PubMed] [Google Scholar]
- 25.Hahn S, Azen S, Ying-Lai M, Varma R Los Angeles Latino Eye Study G. Central corneal thickness in Latinos. Invest Ophthalmol Vis Sci. 2003;44(4):1508–1512. doi: 10.1167/iovs.02-0641. [DOI] [PubMed] [Google Scholar]
- 26.Alsbirk PH. Corneal thickness. I. Age variation, sex difference and oculometric correlations. Acta Ophthalmol (Copenh) 1978;56(1):95–104. doi: 10.1111/j.1755-3768.1978.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 27.Toh T, Liew SH, MacKinnon JR, et al. Central corneal thickness is highly heritable: the twin eye studies. Invest Ophthalmol Vis Sci. 2005;46(10):3718–3722. doi: 10.1167/iovs.04-1497. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Ge J, Huang G, et al. Heritability of central corneal thickness in Chinese: the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci. 2008;49(10):4303–4307. doi: 10.1167/iovs.08-1934. [DOI] [PubMed] [Google Scholar]
- 29.Christensen AE, Knappskog PM, Midtbo M, et al. Brittle cornea syndrome associated with a missense mutation in the zinc-finger 469 gene. Invest Ophthalmol Vis Sci. 2010;51(1):47–52. doi: 10.1167/iovs.09-4251. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Dimasi DP, Hysi PG, et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010;6(5):e1000947. doi: 10.1371/journal.pgen.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulmer M, Li J, Yaspan BL, et al. Genome-wide analysis of central corneal thickness in primary open-angle glaucoma cases in the NEIGHBOR and GLAUGEN consortia. Invest Ophthalmol Vis Sci. 2012;53(8):4468–4474. doi: 10.1167/iovs.12-9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21(5):714–727. [PubMed] [Google Scholar]
- 33.Green K. Free radicals and aging of anterior segment tissues of the eye: a hypothesis. Ophthalmic Res. 1995;27 (Suppl 1):143–149. doi: 10.1159/000267860. [DOI] [PubMed] [Google Scholar]
- 34.Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18(2):93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Ju WK, Crowston JG, et al. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 2007;48(10):4580–4589. doi: 10.1167/iovs.07-0170. [DOI] [PubMed] [Google Scholar]
- 36.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612(2):105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84(3):389–399. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009;50(11):5251–5258. doi: 10.1167/iovs.09-3871. [DOI] [PubMed] [Google Scholar]
- 39.Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch Ophthalmol. 2010;128(6):724–730. doi: 10.1001/archophthalmol.2010.87. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki H, Murphy CJ, Brandt JD, Kiuchi Y, Russell P. Altered stability of mRNAs associated with glaucoma progression in human trabecular meshwork cells following oxidative stress. Invest Ophthalmol Vis Sci. 2012;53(4):1734–1741. doi: 10.1167/iovs.12-7938. [DOI] [PMC free article] [PubMed] [Google Scholar]


