Abstract
Background
Many breast cancer survivors experience fatigue, mood, and sleep disturbances.
Purpose
To compare a Meditative Movement practice, Qigong/Tai Chi Easy (QG/TCE), with sham Qigong (SQG), testing effects of meditation/breath aspects of QG/TCE on breast cancer survivors' persistent fatigue and other symptoms.
Methods
A double-blind, randomized controlled trial tested 12-weeks of QG/TCE versus SQG on fatigue, depression and sleep among 87 post-menopausal, fatigued breast cancer survivors, Stage 0-III, age 40–75.
Results
Fatigue decreased significantly in the QG/TCE group compared to control at post-intervention (p = 0.005) and 3 month follow-up (p = 0.024), but not depression and sleep quality. Improvement occurred over time for both interventions in depression and sleep quality (all p < 0.05).
Conclusions
QG/TCE showed significant improvement over time compared to SQG for fatigue, but not depression or sleep. Both QG/TCE and SQG showed improvement for two prevalent symptoms among breast cancer survivors, depression and sleep dysfunction.
Keywords: Fatigue, Qigong, Tai Chi, breast neoplasm, survivor symptoms
Introduction
Breast cancer survivors commonly experience symptoms, during and after treatment, that reduce quality of life, including fatigue, depression, cognitive dysfunction, sleep disorders, pain, and weight gain [1–3]. Cancer-related fatigue is one of the most consistently reported symptoms among breast cancer survivors that may persist for months, even years after treatment [4]. With the growing number of survivors, this is becoming a significant problem with an estimated 33–40% suffering from cancer-related fatigue, and a portion of these patients experiencing persistent fatigue even up to 10 years post-treatment [5,6].
The etiology of cancer-related fatigue has been suggested to be a composite of interacting responses to stress related to diagnosis and treatment, with the relationship of sleep disorders, depression, anemia, and inflammation featuring centrally for those under treatment as well as into the survivorship phase [7]. Fatigue and a number of these factors and symptoms (including sleep disruption and emotional distress) [8] have been identified as often co-occurring, with some of these associations possibly related to the underlying inflammatory biomarker changes most associated with fatigue [9]. Emotional distress (i.e., depression and anxiety) and insomnia show up as strong correlates of persistent fatigue [10,11] in breast cancer survivors, with depression persisting for 19–25% of breast cancer survivors [6] and rates of sleep disorders estimated from 60–65% [12,13]. The high prevalence and persistence of fatigue, depression and sleep disorders make these important targets for interventions in cancer supportive care.
Exercise has been shown to be an effective intervention to manage cancer-related fatigue among women being treated for and recovering from breast cancer [14–16]. Many of the studies designed to test the effects of physical activity (PA) use aerobic exercise as the intervention, and less commonly, resistance training, with generally positive associations for exercise as an effective approach for reducing fatigue and other symptoms [16,17].
Meditative Movement
Among breast cancer patients and survivors, there is a growing interest and use of mind-body practices, including Meditative Movement forms of exercise [18,19]. Meditative Movement is defined as those practices that utilize movement or posture, with a focus on the breath and a meditative state to achieve deep states of relaxation [20] and includes practices such as Tai Chi, Qigong, and Yoga. The level of exertion of these alternative types of exercise varies widely, depending on the particular practice or choice of exercise for a given session, but most have representative moves or postures that could be described as low-impact with a low-to-moderate level of aerobic exertion (e.g., Restorative Yoga, Tai Chi Easy) [20]. Many of these practices are easier to adopt than strenuous aerobic or resistance training for severely fatigued or health compromised individuals. Adherence to conventional exercise programs in studies of cancer patients have been as low as 39% of participants completing 70% of classes in multi-week interventions [16]. Notably, Tai Chi or Qigong was utilized by over 70% of breast cancer survivors in the Women's Healthy Eating and Living study [21], suggesting the importance of testing the efficacy of Meditative Movement practices as an alternative to conventional PA for recovering from symptoms.
Meditative movement practices are theorized to affect persistent symptoms of cancer survivors. In persons diagnosed, treated for, and surviving cancer, exercise in general has been shown to produce physiological adaptive changes, including reductions in fatigue, but these studies use moderate-to-vigorous levels of PA. [14, 22, 23] There is a PA element in Meditative Movement (although generally low-to-moderate intensity, depending on the specific practice) and may, therefore be used to partially explain results of many Meditative Movement interventions [20, 24–27]. Other “key components” of Meditative Movement may add to the effects, based on what might be expected with the practice of meditation, as well as slow, focused deep breathing (both included in the definition of Meditative Movement practices)[20]. For example, slow deep breathing has been associated with reductions in blood pressure, oxidative stress and balance in the autonomic nervous system and may contribute to an improved emotional state [28–33]. Further, mindfulness based interventions alone [34–37] or combined with movement [31] may reduce anxiety and depression. Mindfulness practice (e.g., mindfulness based stress reduction) that emphasizes non-active sessions (but often includes some gentle Yoga components) has been found to alleviate symptoms for breast cancer survivors [38].
Qigong and Tai Chi are two forms of Meditative Movement with similar roots and practice components. Qigong translates from Chinese to mean, roughly, “to cultivate Qi”; and Qi is considered to be the inherent functional, energetic essence of human beings in Traditional Chinese Medicine [24]. Qigong exercises consist of a series of simple, repeated practices including body posture/movement, breath practice, and meditation performed in synchrony. These exercises consist mostly of gentle movements (with some vigorous and shaking movements in addition to quiet, stillness practices) designed to attain deeply relaxed states. Tai Chi has become one of the best known forms of Qigong in the U.S., differentiated from Qigong in that traditional Tai Chi is performed as a highly choreographed, lengthy, and complex series of movements, while Qigong is typically a more repetitive practice that is simpler and easier to learn [24–26, 39]. Tai Chi Easy simplifies traditional Tai Chi by teaching a smaller set of common Tai Chi movements which are repeated [39] and also includes Qigong exercises. These adaptations to the pace, the repetition, and the ease of learning make Tai Chi Easy more like typical Qigong practices. These adaptations of Tai Chi bring the pace, the repetition, and the easy-to-learn aspects of Tai Chi Easy to be more like typical Qigong practices. In a 2010 review of randomized controlled trials testing the effects of Tai Chi and/or Qigong on various populations (including healthy elderly, chronically diseased), the physiological and psychological health outcomes most consistently supported were cardiorespiratory, physical function, balance and falls prevention, patient reported symptoms (including fatigue), quality of life, mood, and bone health [25].
Meditative Movement and Cancer-related symptoms
A growing number of studies have examined the potential of Meditative Movement practices such as Yoga and Tai Chi to help alleviate cancer survivors' symptoms. Yoga has been studied extensively as a Meditative Movement practice to address cancer patients' and survivors' symptoms; many of these publications demonstrate improvements in fatigue, psychosocial functioning, and emotional distress [40–46]. Tai Chi studies with cancer patients and survivors have produced similar findings, and more, including improvements in physical functioning, quality of life, self-esteem, improved insulin metabolism, cardiovascular health and some markers of inflammation [47–51].
Meditative Movement research utilizing Qigong for symptom relief for cancer patients and survivors is less common. A series of recent studies have indicated that there may be benefits of Qigong for cancer patients (across cancer diagnosis sites) during and after treatment, including reductions in fatigue, lowered C-reactive protein, and improved mood, cognitive function and quality of life [52–54]. A single study focused on prostate cancer survivors using Qigong showed significant improvements in fatigue and emotional distress [55]. Reviews of the current state of research on Qigong and associated changes in biomarkers with cancer patients suggest the potential for Qigong to prolong life [56–58].
Specific to breast cancer patients undergoing treatment, Qigong has been found to reduce fatigue for women with depressive symptoms at baseline [56]. To date, although there is evidence that a number of symptoms may be improved for cancer patients under treatment, or survivors other than breast cancer survivors, there are no published studies of Qigong and/or Tai Chi specifically addressing persistent fatigue, sleep and depression in breast cancer survivors.
Given these positive outcomes of the various Tai Chi and Qigong forms, and the gap in research specifically for breast cancer survivors' fatigue, the study was designed to examine effects of Qigong/Tai Chi Easy (QG/TCE) on breast cancer survivors' symptoms, with fatigue as the primary outcome to be tested.
Research with cancer patients and survivors testing effects of Qigong and Tai Chi have been criticized for not using a placebo control. Because the effects of such forms of Meditative Movement may be confounded by the physical activity aspects of practice, a “sham Qigong” (SQG) control intervention was chosen to parallel the level of physical exertion of the QG/TCE intervention, utilizing similar moves, but without the emphasis on the breathing and meditative aspects of QG/TCE. Both interventions, however, were implemented with a low level of intensity, thus the sham control group was not expected to produce the level of relief obtained in the studies incorporating more intensive (moderate-to-vigorous) PA. Rather we were testing specifically the potential of the breath and meditative components of QG/TCE to alleviate symptoms by holding the low PA levels constant across both arms of the study.
Hypothesis One (testing the primary outcome)
QG/TCE practice will reduce fatigue in breast cancer survivors more than SQG.
Hypothesis Two (testing secondary outcomes)
QG/TCE practice will reduce depression and sleep disruption in breast cancer survivors more than SQG.
Methods
Overview
This study was a double-blind randomized controlled trial (RCT) designed to examine effects of a 12-week QG/TCE intervention (compared to a sham control) on breast cancer survivors' fatigue (primary outcome) and to evaluate effects on sleep quality and depression (secondary outcomes). The study interventions included two gentle forms of exercise, QG/TCE and sham Qigong (SQG). All outcomes were measured at baseline, at the end of the 12-week intervention, and 3 months post-intervention.
Participants and Procedures
Eligibility, Recruitment and Randomization
Eligibility criteria for study inclusion required participants to be: (a) diagnosed with Stage 0-III breast cancer; (b) six months to 5 years past primary treatment (including any of the following: surgery, radiation, or chemotherapy); (c) age 40 to 75; (d) post-menopausal; (e) with no evidence of recurrence or occurrence of other cancers; and (f) reporting clinically significant fatigue, scoring ≤ 50 on the 4-item Vitality scale of the Medical Outcomes Scale short form (SF-36). [60,61,62]
Patients were excluded from the study if presenting with criteria associated with risk during physical activity [59]: hematocrit < 24; severe cachexia; frequent dizziness; bone pain; or severe nausea. Other exclusion criteria included: a score of 15 or greater on the Patient Health Questionnaire-9 (PHQ-9) indicating moderately high depression; Body Mass Index (BMI) > 32; uncontrolled diabetes; untreated hypothyroidism; chronic fatigue syndrome; auto-immune disorders; factors that could be causing fatigue other than cancer-related causes (e.g., sleep apnea, shift work, low mood preceding fatigue, fatigue preceding the cancer diagnosis, or restless leg syndrome); regularly smoke or drink more than 2 alcoholic beverages per day; having had past or current regular experience with mind-body practices that blend movement with meditative practices, such as Yoga, Tai Chi or Qigong; use of corticosteroids, cyclosporin, or regular use of sleep-aid medications. Slight changes to widen eligibility criteria (i.e., increased age from 65 to 75, increased BMI upper limit from 30 to 32, and allowed antihistamine use) were made early in the study to allow more participants into the study. A test between those admitted under the two sets of criteria showed no difference in study outcomes and were thus combined into one sample.
Researchers from a large state university partnered with a community hospital with a cancer center where the study was conducted. IRB approval was obtained from both institutions. More than half of the women screened for eligibility were contacted through letters mailed from the hospital registry. Other recruitment sources included breast cancer support groups and referring oncologists, surgeons, and internal medicine physicians. Rolling recruitment progressed over 30 months with randomized participants starting the intervention every 4–5 months for a total of 7 cohorts (i.e., initiating a QG/TCE group and a SQG group at each of the 7 cohort start times).
Randomization was stratified on two factors deemed most likely to bias results on the primary outcome of fatigue: use of estrogen suppressive therapies (yes or no) and level of physical activity at baseline (high or low). Each cohort of 6–18 participants was screened for eligibility, assessed for estrogen suppressive therapy use and level of activity, and consented. Consented individuals were randomized by the statistician to the QG/TCE intervention or SQG control condition using stratified randomization resulting in class sizes of 3–9 participants each. (Further details on measures for eligibility criteria and stratification factors are in Electronic Supplementary Material A).
After full consent, completion of eligibility checks, agreement to join the study and randomization, patients were assigned to the next set of class start dates, and were scheduled for baseline data collection within one week of the first class session. Post intervention data were collected within a week of completion of the 12 weeks of classes, and again 3 months after completion of the intervention.
Study Outcome Measures
The primary outcome, fatigue, was assessed using the Fatigue Symptom Inventory (FSI), a 16-item self-report measure shown to be sensitive to assessing change in fatigue among breast cancer survivors and with strong internal consistency [63]. The scale index provides a score between 0 and 10, with higher scores representing greater fatigue [63]. A mean score of 3 or more is considered a clinically meaningful level of fatigue [64].
Secondary outcomes included sleep quality and depression. The Pittsburgh Sleep Quality Index (PSQI) uses 19 items to assess sleep quality and has shown internal consistency and validity, with a global PSQI score >5 distinguishing good from poor sleepers with 89.6 % sensitivity and 89.5% [65]. The 20-item Beck Depression Inventory (BDI) has shown internal consistency and validity in a population of non-psychiatric patients [66]. Internal consistency of these measures for the current study participants at baseline (computed as Cronbach's α) was .94, .85, and .71 for the FSI, PSQI and BDI respectively.
Not surprisingly, there were highly significant correlations among the outcomes. The Fatigue Symptom Inventory was highly correlated with both the Beck Depression Inventory (r = 0.47, p < 0.0001) and the Pittsburgh Sleep Quality Index (r = 0.40, p < 0.0001). The Beck Depression Inventory and Pittsburgh Sleep Quality Index also were highly correlated (r = 0.37, p = 0.005). These outcomes were analyzed separately as they represent distinct facets of breast cancer survivor symptoms.
Intervention, Process Control and Fidelity
The QG/TCE classes were taught by a nurse and SQG was taught by an exercise physiologist, both experienced in leading exercise with cancer patients. Sessions were sixty minutes long, delivered over 12-weeks, meeting twice a week for the first two weeks to intensify the opportunity to learn the practices well, then once a week for the remainder of the period. Participants were blinded to study intervention and randomized to one of two classes (both called “Rejuvenating Movement”). Study staff involved in data collection or analysis were blinded to assignment.
Participants were asked to practice at home at least 30 minutes a day, 5 days per week and to keep a log of the frequency, minutes of practice and level of exertion. Level of exertion, dose and frequency were equivalent across the two arms of the study. Perceived equivalence of the two interventions was assessed, finding that the participants' expected benefit, perceived value of complementary/alternative medicine practices, and perception that the intervention was “like Tai Chi or Qigong” were similar across the arms of the study, while significantly different for perception of meditative focus (as intended). Additional details regarding intervention development, description, instructor qualifications and experience, and process control and fidelity measures are in the Electronic Supplementary Material A.
Statistical Analysis Plan
This study was initially powered to detect a 0.75 effect size at power 85% for fatigue, assuming 30 participants in each of two groups after an expected attrition of 15% (and not designed to be an intention-to-treat analysis). Only individuals who attended some portion of the classes are included in the statistical analysis (irrespective of whether they had measurements at all three time points). Baseline characteristics of the randomized participants were compared for the QG/TCE and SQG groups using two-sample independent t tests for continuous variables, and Fisher's Exact tests for categorical variables. Normality was assessed using histograms and box plots.
Hierarchical linear models (linear mixed effects models) were used to assess the independent effects of intervention group (QG/TCE versus SQG) and time (baseline, post-intervention and 3-month follow-up) for the primary and secondary outcomes. Analyses were not adjusted for baseline values. Hierarchical linear models account for the correlation observed among measurements within the same individual, and also allow inclusion of any individuals with missing data at one or more of the time points. For all models, the SQG intervention group was considered the reference category. The baseline measurement was the reference value for time, with fixed effects for the post-intervention and 3-month follow-up. Missing values were not imputed due to the relatively small number of missing measurements across study participants (< 10%).
The interaction between time and intervention was tested to assess the statistical significance of the effect of QG/TCE versus SQG. This test was performed by comparing the log-likelihood of the model with the interaction terms to a model without the interaction terms, using a likelihood ratio test. Since there were two fixed effects for time (post-intervention and 3-month follow-up), there were two time × intervention interaction terms. The likelihood ratio test assessed the null hypothesis that both interaction terms were 0 versus the alternative that at least one was not 0. If the overall interaction test was statistically significant, then the significance of the individual interaction terms was assessed.
The results from the hierarchical linear model include the parameter estimates of the intercept (mean value of the SQG group at baseline), intervention (QG/TCE versus SQG), post-intervention (versus baseline), 3 months follow-up (versus baseline), and the interactions between the intervention × post-intervention and the intervention × 3 month follow-up. The estimated coefficients, their z (Wald) statistics and p-values are reported. Since there was one primary (fatigue) and two secondary outcomes (depression and sleep quality), the alpha level for the assessment of interaction using the likelihood ratio tests was adjusted for multiple comparisons using the Bonferroni procedure (0.05/3 = 0.0167). The significance of the interaction at the individual time points was interpreted only if the overall interaction was significant at the adjusted alpha level. All p-values reported are two-sided. The effect size for fatigue was computed as the mean change from baseline to post-intervention (3 month follow-up) for the QG/TCE group minus the mean change for the SQG group divided by the pooled standard deviation. The effect size for improvement (i.e., reduction in fatigue) was reported as positive in sign, consistent with convention. STATA 12.1 was used for all analyses.
Results
Two hundred and forty-five women were assessed for eligibility, with 88 ineligible (with the most common reasons including inadequate levels of fatigue, use of an exclusionary drug, or presenting outside the range of diagnosis, age, or time since treatment ended), and 56 declining to participate, resulting in 101 (41%) randomized participants. The CONSORT diagram of the study participants is shown in Figure 1, displaying the reasons for failure to proceed to randomization. There were 14 individuals who failed to attend the classes (6 in the QG/TCE and 2 in SQG arms of study prior to the start of the intervention, and 6 more after class commencement but prior to final data collection), leaving 87 women (13.9% attrition). Class attendance ranged from 4 to 14 sessions, with a mean of 10.2 classes attended per participant, and no difference in attendance between arms of the study. There were no significant differences between those who were included in the analysis in the study (i.e., those who attended the classes) and those who were not included on any of the baseline characteristics.
Fig. 1.
CONSORT diagram of participant recruitment and flow
Table 1 presents the descriptive information on demographics, medical variables and study outcomes for the total sample and the two intervention groups separately. There were no baseline differences between the two intervention groups.
Table 1.
Baseline Characteristics of Participants by Arm of Study
| Combined (N = 87) | SQG (N = 45) | QG/TCE (N = 42) | |||
|---|---|---|---|---|---|
| Variable | Mean(SD) | Mean(SD) | Mean(SD) | t (df) | P-valuea |
| Age | 58.8 (8.94) | 59.8 (8.93) | 57.7 (8.94) | 1.06 (84) | 0.292 |
| Body Mass Index | 26.8 (4.49) | 26.6 (3.62) | 27.1 (5.33) | −0.43 (82) | 0.665 |
| Metabolic Equivalent of Task (METs) hrs./week | 11.7 (11.40) | 10.2 (10.58) | 13.3 (12.16) | −1.29 (84) | 0.201 |
| Patient Health Questionnaire-9 | 7.9 (3.59) | 8.5 (3.69) | 7.4 (3.43) | 1.44 (83) | 0.155 |
| Fatigue Score Index | 4.2 (1.80) | 3.8 (1.78) | 4.6 (1.77) | −1.76 (84) | 0.078 |
| Time from Last Treatment (years) | 2.0 (1.38) | 2.1 (1.40) | 1.9 (1.36) | −0.61 (84) | 0.543 |
| n (%) | n (%) | n (%) | P-valueb | ||
|---|---|---|---|---|---|
| Estrogen Suppressive Therapy | 60 (68.97) | 30 (66.67) | 30 (71.43) | 0.639 | |
| Cancer Stage | |||||
| Stage 1 | 34 (39.08) | 20 (44.44) | 14 (33.33) | 0.722 | |
| Stage 2 | 40 (45.98) | 22 (48.89) | 18 (42.86) | ||
| Stage 3 | 3 (3.45) | 1 (2.22) | 2 (4.76) | ||
| Ethnicity | |||||
| Latino | 2 (2.30) | 1 (2.22) | 1 (2.38) | 1.000 | |
| Non Latino | 75 (86.21) | 40 (88.89) | 35 (83.33) | ||
| Race | |||||
| White | 79 (90.80) | 42 (93.33) | 37 (88.10) | 1.000 | |
| Other | 5 (5.75) | 3 (6.67) | 2 (4.76) | ||
| Household Income | |||||
| < $ 34,999 | 12 (13.79) | 10 (22.22) | 2 (4.76) | 0.121 | |
| $ 35,000 to $49,999 | 11 (12.64) | 8 (17.78) | 3 (7.14) | ||
| $ 50,000 to $74,999 | 14 (16.09) | 6 (13.33) | 8 (19.05) | ||
| $ 75,000 to $99,999 | 11 (12.64) | 5 (11.11) | 6 (14.29) | ||
| > $100,000 | 18 (20.69) | 8 (17.78) | 10 (23.81) | ||
| Highest Grade of Education | |||||
| High School or Less | 13 (14.94) | 7 (15.56) | 6 (14.29) | 0.746 | |
| Some College | 51 (58.62) | 25 (55.56) | 26 (61.90) | ||
| Post-graduate | 20 (22.99) | 12 (26.67) | 8 (19.05) |
p-value from two sample independent t test
p-value from Fisher's Exact Test
Comparisons of the Two Intervention Conditions
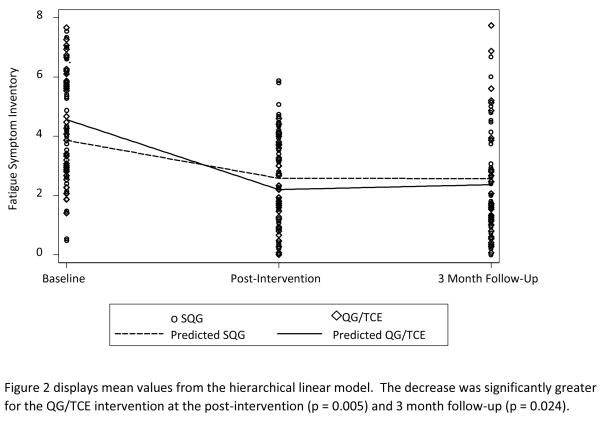
Table 2 summarizes the results from the comparison of SQG versus QG/TCE across the three measurement times (baseline, post-intervention, and 3-month follow-up) in the Fatigue Symptom Inventory (primary outcome– upper panel), the Beck Depression Inventory, and Pittsburgh Sleep Quality Index (secondary outcomes – lower panel). For the Fatigue Symptom Inventory, there was a statistically significant interaction between intervention group and time (p = 0.0116). The decrease in the Fatigue Symptom Inventory was significantly greater for the QG/TCE intervention at both the post-intervention (p = 0.005) and 3 month follow-up (p = 0.024). Figure 2 displays the mean values for the Fatigue Symptom Inventory, depicting interaction and change across time for both groups. Using common guidelines for interpreting effect sizes, the post-intervention and 3 month follow-up were considered “medium” effect sizes (0.56 and 0.43, respectively). There were no statistically significant interactions for either the Beck Depression Inventory or the Pittsburgh Sleep Quality Index (p = 0.94 and 0.27, respectively), suggesting no significant differences between the QG/TCE versus SQG groups' responses to the interventions across time. However, both secondary endpoints showed significant decreases across time for both the QG/TCE and SQG groups (all p < 0.05). Figures A and B in Electronic Supplemental Material B display the mean values across time for the Beck Depression Inventory and the Pittsburgh Sleep Quality Index, respectively.
Table 2.
Comparison of Sham Qigong (SQG) versus Qigong/Tai Chi Easy (QG/TCE) from Baseline to Post-Intervention to 3 Months Follow-Up
| Mean (SD) n | Coefficient from hierarchical linear modela (z statistic)b p-valueb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | Time by Intervention Interaction | ||||||||
|
| |||||||||
| Baseline | Post-Intervention | 3 Month Follow up | Intercept | Intervention | Post-Intervention | 3 Month Follow Up | Post-Intervention | 3 Month Follow up | |
|
| |||||||||
| Primary Outcome – Fatigue Symptom Inventoryc | |||||||||
|
| |||||||||
| SQG | 3.8 (1.78) | 2.6 (1.65) | 2.5 (1.67) | 3.86 (15.24) | 0.70 (1.92) | −1.28 (−4.85) | −1.29 (−4.66) | −1.08 (−2.83) | −0.90 (−2.26) |
| 44 | 44 | 38 | < 0.001 | 0.054 | < 0.001 | < 0.001 | 0.005 | 0.024 | |
| QG/TCE | 4.6 (1.77) | 2.1 (1.34) | 2.3 (1.97) | ||||||
| 42 | 40 | 35 | |||||||
|
| |||||||||
| Secondary Outcomes | |||||||||
|
| |||||||||
| Beck Depression Inventoryd | |||||||||
|
| |||||||||
| SQG | 11.3 (6.82) | 6.3 (5.06) | 6.6 (6.25) | 11.2 (12.24) | 1.23 (0.93) | −4.79 (−5.78) | −4.81 (−5.57) | 0.42 (0.35) | 0.15 (0.12) |
| 44 | 43 | 38 | < 0.001 | 0.353 | < 0.001 | < 0.001 | 0.725 | 0.902 | |
| QG/TCE | 12.3 (6.89) | 7.9 (5.97) | 7.3 (6.09) | ||||||
| 40 | 38 | 34 | |||||||
|
| |||||||||
| Pittsburgh Sleep Quality Indexe | |||||||||
|
| |||||||||
| SQG | 9.9 (4.77) | 7.3 (4.06) | 7.7 (4.94) | 9.64 (14.34) | 0.44 (0.45) | −2.17 (−4.16) | −1.76 (−2.47) | −1.14 (−1.49) | −1.29 (−1.18) |
| 30 | 37 | 15 | < 0.001 | 0.653 | < 0.001 | 0.014 | 0.136 | 0.239 | |
| QG/TCE | 10.0 (3.85) | 6.6 (3.27) | 6.3 (3.00) | ||||||
| 28 | 31 | 11 | |||||||
Hierarchical linear model parameter estimates of the intercept (mean value of the SQG group at baseline), intervention (QG/TCE versus SQG), post-intervention (versus baseline), 3 months follow-up (versus baseline), and the interactions between the intervention × post-intervention and the intervention × 3 month follow-up
z (Wald statistics) and p-value from the hierarchical linear model for each term
Likelihood ratio p-value for interaction = 0.0116
Likelihood ratio p-value for interaction = 0.94
Likelihood ratio p-value for interaction = 0.27
Fig. 2.
Fatigue Symptom Inventory Mean Values for Participants in Sham Qigong (SQG) and Qigong/Tai Chi Easy (QG/TCE)
Discussion
The strength of this study is the double blind RCT design with a “placebo” control group that controls for the usual attention and time, but also allows a test of the effects of the novel components of QG/TCE, the meditative state and breath focus, beyond the PA component associated with these practices.
With the preponderance of persistent symptoms in this population, it is important to explore a variety of PA options that may appeal to different women's motivational profiles. The intention of our study was to test more specifically the meditative and breath focus characteristics of Meditative Movement practices, so as to examine if there is an effect of these key components over and above the effects that might be seen with just gentle exercise. Our QG/TCE intervention with the accompanying focus on breath and meditative states to create a deep sense of relaxation, showed an advantage over gentle physical activity for overcoming fatigue among breast cancer survivors. The mean score for fatigue improved from a moderate level of 4.6 (with a score of 3 considered to be clinically significant fatigue), to 2.1 (with less than 3 considered not fatigued) for women in the QG/TCE intervention. This effect persisted through to the 3-month follow-up, suggesting the potential for lasting change, at least for the short term.
Comparing the two interventions, however, did not indicate a significant effect for the depression or sleep outcomes. The QG/TCE intervention was designed to achieve results on fatigue (with the selection of exercises drawn from the “Vitality Method” to boost specific meridian flows and Qi balance often associated in Traditional Chinese Medicine with overall wellness and energy). It is possible that this alignment with overall principles of Qi balance and focus on vitality produced a specific result for fatigue, but not as much for depression and sleep. These secondary outcomes were not significantly changed by practicing QG/TCE in comparison to the SQG intervention, suggesting that the intervention was targeted most effectively for the primary outcome, fatigue.
The study was designed specifically to examine effects on the primary outcome (and powered accordingly), because of the prior literature on various Meditative Movement practices' achievements on fatigue outcomes in other populations [25, 67, 68]. At the time of the study design, there was less research showing effects on sleep and mood, although more recently a number of publications have indicated that these outcomes both may be responsive to Meditative Movement (especially mood) [41–46,69,70] as well as fatigue [19,41,45,46,51]. Given these more recent results for effects of Meditative Movement on sleep and depression in a wide range of populations, it would be important to examine these studies' effect sizes and consider powering future studies of QG/TCE with breast cancer survivors to evaluate results with more informed power calculations.
Both of our “Rejuvenating Movement” interventions (QG/TCE and SQG) involving gentle, low-intensity exercise, showed significant impacts over the time of the interventions and into the 3-month follow-up period on breast cancer survivors' symptoms of fatigue, depression and sleep quality. It appears that even low-intensity PA programs may be helpful to get women moving and that this movement may help with physical and psychological symptoms that continue past the time of treatment. Beyond the gentle exercise, there may be additional benefit to the mind-body aspects of a Meditative Movement practice such as Qigong or Tai Chi.
These initial findings are a meaningful contribution to our understanding of what QG/TCE and, possibly other Meditative Movement practices, may bring to the care of breast cancer survivors' recovery from fatigue. The results on low-intensity exercise effects across outcomes widens the opportunity for recommendations for a number of exercise options for women working toward symptom reduction and exercise adoption.
Limitations
Although these results are positive and promising, there remain some limitations to the study design and methodological approach that should be taken into account when interpreting results. The fact that both interventions produced positive results on the primary and secondary outcomes from pre- to post-intervention could be explained by maturation effects, but the selection of participants whose fatigue was persistent 6 months to 5 years past treatment reduces that likelihood for this central outcome. There is a concern that the contrast between the two interventions may have not been enough. With similar movements and similar levels of physical exertion, the only differences between the two groups' practice was the focus on the breath and meditative state. Even with this nominal distinction between the intervention groups on this component, the fatigue outcome was significantly improved for QG/TCE. A general comparison to a control group matched for time and attention, but not including physical activity (known to help alleviate sleep and mood disorders), might serve as a general test for QG/TCE effects on depression and sleep.
Intention-to-treat analyses were not used, potentially biasing the study results if study participants who dropped out systematically responded differently than those who completed. Analysis of baseline data indicate no significant differences between dropouts and those who continued in the study, allaying some concerns about bias. This does not, however, assure that response to the intervention might not have differed for those who did not complete.
Blinding in this study was a challenge. Beyond the successful blinding of participants to the preferred intervention, and blinding of study staff, it was impossible to blind the instructors. The instructors could have unintentionally biased the results or the differences in their initial training and experience (a nurse and an exercise physiologist) could have made a difference in the delivery of the interventions and outcomes achieved. A further limitation is the distal completion rate. Approximately 1 in 7 randomized participants did not complete the 12 week intervention, limiting interpretability of the effectiveness of the results to a broader population of breast cancer survivors.
Conclusions
A general conclusion can be drawn that gentle exercise—that is, just getting breast cancer survivors moving--may be beneficial in reducing a number of symptoms. Our findings suggest that gentle exercise, even if not at the moderate-to-vigorous levels of activity usually recommended, may provide initial and short-term continuing benefits for overcoming numerous symptoms and well-being of breast cancer survivors. Low-intensity exercises, with or without meditative focus, may be suggested as a way to begin to recover from symptoms that persist for breast cancer survivors well past the time of treatment, and may be more acceptable for those who find it difficult to get started with physical activity.
When that low-intensity exercise is QG/TCE, that is, a Meditative Movement practice that includes a focus on the breath and a meditative state, there appears to be an advantage for improving one of the most recalcitrant symptoms for these survivors, persistent fatigue. Given the potential for meditative and breath-focused practices to calm the autonomic nervous system, as well as Meditative Movement practices such as Yoga demonstrating effects on cancer survivors' fatigue [19], it is possible that the novel components of QG/TCE made the difference for achieving this significant result for fatigue immediately following the intervention as well as 3 months later. The components of meditative practice with a focus on breathing suggests an intriguing set of potential mechanisms to explore, including discovering how such a practice may directly affect neurohormonal balance associated with stress and emotional distress (e.g. cortisol, C-reactive protein, insulin) [47,52].
Given the non-significant differences between the SQG and QG/TCE interventions on the secondary outcomes examined, it would be important to re-examine sample size and power, as well as comparison group choices for future studies. Of course, it is possible that there simply is not enough of a difference for the meditative state and breath focus of the QG/TCE intervention to influence sleep and depression beyond what gentle exercise may do. Conversely, these factors may take longer to demonstrate change to the degree that statistical significance may be seen. Confirming the specific effects of QG/TCE may require a longer intervention time for testing, or a no-intervention/usual care (e.g., a wait list) control group. The usual care control group is an essential study design feature recommended in recent complementary and alternative medicine research guidelines in which three-arm designs (intervention arm, placebo control arm and usual care control arm) are suggested rather than the two arms used in conventional medicine studies (intervention arm, placebo control arm) [71,72].
It would further be important to investigate whether benefits are sustained in the long term with participants who continue to practice QG/TCE or other gentle exercises. Thus, the current positive findings suggest the need for further evaluation of QG/TCE, and the mechanisms by which it may reduce fatigue (as well as other symptoms), and impacts of QG/TCE on biological changes, such as full blood count [73] and CRP [53]. It would also be important to begin building the evidence base for Meditative Movement practice effects, discover more about how Meditative Movement works (across practices or only for specific practices), and determine what components make a difference for improving symptoms for cancer survivors in both the short and long term.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (National Center for Complementary and Alternative Medicine and Office of Women's Health) grant number 5 U01 AT002706-03 and the Arizona Cancer Center Support Grant (P50 CA023074). Scottsdale Healthcare Shea Medical Center and the associated Virginia G. Piper Cancer Center provided additional support.
Footnotes
Statement of Conflict of Interest and Adherence to Ethical Standards Linda K. Larkey, PhD; Denise J. Roe, DrPH; Karen L. Weihs, MD; Roger Jahnke, OMD; Ana Maria Lopez, MD, MPH, FACP; Carol E. Rogers, PhD, RN; Byeongsang Oh, OMD, PhD; and Jose Guillen-Rodriguez declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1.Schmidt ME, Chang-Claude J, Vrieling A, Heinz J, Flesch-Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. Journal of Cancer Survivorship. 2012;6(1):11–19. doi: 10.1007/s11764-011-0197-3. [DOI] [PubMed] [Google Scholar]
- 2.Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It's not over when it's over: Long-term symptoms in cancer survivors—a systematic review. The International Journal of Psychiatry in Medicine. 2010;40(2):163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 3.Epplein M, Zheng Y, Zheng W, et al. Quality of life after breast cancer diagnosis and survival. Journal of Clinical Oncology. 2011;29(4):406–412. doi: 10.1200/JCO.2010.30.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli Kluthcovsky A, Urbanetz A, Carvalho D, Pereira Maluf E, Schlickmann Sylvestre G, Bonatto Hatschbach S. Fatigue after treatment in breast cancer survivors: Prevalence, determinants and impact on health-related quality of life. Supportive care in cancer. 2012;20(8):1901. doi: 10.1007/s00520-011-1293-7. [DOI] [PubMed] [Google Scholar]
- 5.Noal S, Levy C, Hardouin A, et al. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. International Journal of Radiation Oncology* Biology* Physics. 2011;81(3):795–803. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fosså SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. Journal of Cancer Survivorship. 2010:1–10. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan KKA. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28(4):373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Supplement 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbances in patients with cancer, patients with clinical depression, and community-dwelling adults. Journal of Pain and Symptom Management. 2003;25(4):307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 11.Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist. 2013;18(10):1135–1143. doi: 10.1634/theoncologist.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF. Sleep disruption in breast cancer patients and survivors. Journal of the National Comprehensive Cancer Network. 2013;11(12):1523–1530. doi: 10.6004/jnccn.2013.0179. [DOI] [PubMed] [Google Scholar]
- 13.Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage. 2010;39(3):535–547. doi: 10.1016/j.jpainsymman.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008;2(2) doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Sprod L, Hsieh C, Hayward R, Schneider C. Three versus six months of exercise training in breast cancer survivors. Breast Cancer Res Treat. 2010;121(2):413–419. doi: 10.1007/s10549-010-0913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: A meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 2010;22(3):208–221. doi: 10.1016/j.clon.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Brown JC, Huedo-Medina TB, Pescatello SM, Ferrer RA, Johnson BT, Pescatello LS. The efficacy of exercise interventions on fatigue among cancer survivors: A meta-analysis. Medicine & Science in Sports & Exercise. 2010;42(5):264–265. [Google Scholar]
- 18.Elkins G, Fisher W, Johnson A. Mind-body therapies in integrative oncology. Current treatment options in oncology. 2010;11(3–4):128–140. doi: 10.1007/s11864-010-0129-x. [DOI] [PubMed] [Google Scholar]
- 19.Carlson LE. Meditation and yoga. In: Holland JC, Breitbart WS, Jacobsen PB, Lederberg MS, Loscalzo MJ, McCorkle R, editors. Psycho-oncology. 2nd ed. Oxford Univ Pr; USA: 2010. p. 429. [Google Scholar]
- 20.Larkey LK, Jahnke R, Etnier J, Gonzalez J. Meditative movement as a category of exercise: Implications for research. Journal of Physical Activity & Health. 2009;6(2):230–238. doi: 10.1123/jpah.6.2.230. [DOI] [PubMed] [Google Scholar]
- 21.Saquib J, Madlensky L, Kealey S, et al. Classification of CAM use and its correlates in patients with early-stage breast cancer. Integrative cancer therapies. 2011;10(2):138–147. doi: 10.1177/1534735410392578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craft LL, VanIterson EH, Helenowski I, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: A systematic review and meta-analysis. Cancer epidemiology, biomarkers & prevention. 2012;21(1):3–19. doi: 10.1158/1055-9965.EPI-11-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz AAL. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract. 2000;8(1):16–24. doi: 10.1046/j.1523-5394.2000.81003.x. [DOI] [PubMed] [Google Scholar]
- 24.Jahnke R. The healing promise of qi: Creating extraordinary wellness through qigong and tai chi. McGraw-Hill; 2002. [Google Scholar]
- 25.Jahnke R, Larkey LK, Rogers C, Etnier J, Lin F. A comprehensive review of health benefits of qigong and tai chi. American Journal of Health Promotion. 2010;24(6):1–25. doi: 10.4278/ajhp.081013-LIT-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chodzko-Zaiko W, Jahnke RA, Working Group [Accessibility verified July 16, 2014];National expert meeting on qigong and tai chi: Consensus report. 2005 Oct 24; Available at http://www.wisdomtaichi.com/Consensus_Article.pdf.
- 27.Rogers CE, Larkey LK, Keller C. A review of clinical trials of tai chi and qigong in older adults. West J Nurs Res. 2009;31(2):245–279. doi: 10.1177/0193945908327529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharya S, Pandey US, Verma NS. Improvement in oxidative status with yogic breathing in young healthy males. Indian J Physiol Pharmacol. 2002;46(3):349–354. [PubMed] [Google Scholar]
- 29.Joseph C, Porta C, Casucci G, et al. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46(4):714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- 30.Martarelli D, Cocchioni M, Scuri S, Pompei P. Diaphragmatic breathing reduces exercise-induced oxidative stress. eCAM. 2009:nep169. doi: 10.1093/ecam/nep169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsang HWH, Fung KMT. A review on neurobiological and psychological mechanisms underlying the anti-depressive effect of qigong exercise. Journal of Health Psychology. 2008;13(7):857–863. doi: 10.1177/1359105308095057. [DOI] [PubMed] [Google Scholar]
- 32.Kamei T, Toriumi Y, Kimura H, Ohno S, Kumano H, Kimura K. Decrease in serum cortisol during yoga exercise is correlated with alpha wave activation. Percept Mot Skills. 2000;90(3 pt. 1):1027–1032. doi: 10.2466/pms.2000.90.3.1027. [DOI] [PubMed] [Google Scholar]
- 33.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29(4):448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 34.Kristeller JL, Hallett CB. An exploratory study of a meditation-based intervention for binge eating disorder. Journal of Health Psychology. 1999;4(3):357–363. doi: 10.1177/135910539900400305. [DOI] [PubMed] [Google Scholar]
- 35.Lillis J, Hayes S, Bunting K, Masuda A. Teaching acceptance and mindfulness to improve the lives of the obese: A preliminary test of a theoretical model. Annals of Behavioral Medicine. 2009;37(1):58–69. doi: 10.1007/s12160-009-9083-x. [DOI] [PubMed] [Google Scholar]
- 36.Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: The conceptual foundation. Eat Disord. 2011;19(1):49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- 37.Carlson L, Speca M, Faris P, Patel K. One year pre-post intervention follow up of, immune, endocrine and blood pressure outcomes of mindfulness based stress reduction (MBSR) in breast and prostate cancer patients. Brain Behav Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-Oncology. 2009;18(12):1261–1272. doi: 10.1002/pon.1529. [DOI] [PubMed] [Google Scholar]
- 39.Jahnke RA, Larkey LK, Rogers C. Dissemination and benefits of a replicable tai chi and qigong program for older adults. Geriatr Nurs. 2010;31(4):272–280. doi: 10.1016/j.gerinurse.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: Physical and psychological benefits. Psycho-Oncology. 2006;15(10):891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 41.Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of awareness program for menopausal symptoms in breast cancer survivors: Results from a randomized trial. Supportive care in cancer. 2009;17(10):1301–1309. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 42.Ulger O, Yagli NV. Effects of yoga on the quality of life in cancer patients. Complement Ther Clin Pract. 2010;16(2):60–63. doi: 10.1016/j.ctcp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: Effects on quality of life. J Clin Oncol. 2007;25(28):4387–4395. doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 44.Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: Findings from a randomized pilot study. Psychooncology. 2009;18(4):360–368. doi: 10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speed-Andrews AE, Stevinson C, Belanger LJ, Mirus JJ, Courneya KS. Pilot evaluation of an iyengar yoga program for breast cancer survivors. Cancer Nurs. 2010;33(5):369–381. doi: 10.1097/NCC.0b013e3181cfb55a. [DOI] [PubMed] [Google Scholar]
- 46.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors. Cancer. 2012;118(15):3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janelsins MC, Davis PG, Wideman L, et al. Effects of tai chi chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clinical Breast Cancer. 2011;11(3):161–170. doi: 10.1016/j.clbc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mustian KM, Palesh OG, Flecksteiner SA. Tai chi chuan for breast cancer survivors. Med Sport Sci. 2008;52:209–217. doi: 10.1159/000134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rausch S M, Robins Jolynne, Walter J M, McCain N L. Tai chi as biobehavioral intervention for women with breast cancer. Brain Behav Immun. 2006;20(e58-e59) [Google Scholar]
- 50.Galantino ML, Capito L, Kane RJ, Ottey N, Switzer S, Packel L. The effects of tai chi and walking on fatigue and body mass index in women living with breast cancer: A pilot study. Rehabilitation Oncology. 2003;21(1):17–22. [Google Scholar]
- 51.Mustian KM, Katula JA, Gill DL, Roscoe JA, Lang D, Murphy K. Tai chi chuan, health-related quality of life and self-esteem: A randomized trial with breast cancer survivors. Support Care Cancer. 2004;12(12):871–876. doi: 10.1007/s00520-004-0682-6. [DOI] [PubMed] [Google Scholar]
- 52.Oh B, Butow PN, Mullan BA, et al. Effect of medical qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: A randomized controlled trial. Supportive Care in Cancer. 2011:1–8. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 53.Oh B, Butow P, Mullan B, et al. Impact of medical qigong on quality of life, fatigue, mood and inflammation in cancer patients: A randomized controlled trial. Annals of Oncology. 2010;21(3):608–614. doi: 10.1093/annonc/mdp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh B, Butow PN, Mullan BA, et al. Effect of medical qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: A randomized controlled trial. Supportive Care in Cancer. 2012;20(6):1235–1242. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 55.Campo RA, Agarwal N, LaStayo PC, et al. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of qigong. Journal of Cancer Survivorship. 2013:1–10. doi: 10.1007/s11764-013-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z, Meng Z, Milbury K, Bei W, Zhang Y, Thornton B. Qigong improves quality of life in women undergoing radiotherapy for breast cancer. Cancer. 2013 doi: 10.1002/cncr.27904. n-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee MS, Chen KW, Ernst E. Supportive Cancer Care with Chinese Medicine. 2010. Supportive cancer care with qigong; pp. 77–94. [Google Scholar]
- 58.Oh B, Butow P, Mullan B, et al. A critical review of the effects of medical qigong on quality of life, immune function, and survival in cancer patients. Integrative cancer therapies. 2012;11(2):101–110. doi: 10.1177/1534735411413268. [DOI] [PubMed] [Google Scholar]
- 59.Courneya KS, Mackey JR, Jones LW. Coping with cancer: Can exercise help? The Physician and sportsmedicine. 2000;28(5):49–58. doi: 10.3810/psm.2000.05.896. [DOI] [PubMed] [Google Scholar]
- 60.Brown LF, Kroenke K, Theobald DE, Wu J. Comparison of SF-36 vitality scale and Fatigue Symptom Inventory in assessing cancer-related fatigue. Support Care Cancer. 2011 Aug;19(8):1255–9. doi: 10.1007/s00520-011-1148-2. [DOI] [PubMed] [Google Scholar]
- 61.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992:473–483. [PubMed] [Google Scholar]
- 62.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology. 2000;18(4):743–743. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 63.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: Further validation of the fatigue symptom inventory. Quality of Life Research. 2000;9(7):847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 64.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the fatigue symptom inventory. J Pain Symptom Manage. 2008;36(5):480. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buysse D, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 66.Beck AT, Steer RA, Carbin MG. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 67.Galantino Mary Lou, et al. The effects of Tai Chi and walking on fatigue and body mass index in women living with breast cancer: a pilot study. Rehabil Oncol. 2003;21:17–22. [Google Scholar]
- 68.Taylor-Piliae Ruth E., et al. Change in perceived psychosocial status following a 12-week Tai Chi exercise programme. Journal of Advanced Nursing. 2006;54(3):313–329. doi: 10.1111/j.1365-2648.2006.03809.x. [DOI] [PubMed] [Google Scholar]
- 69.Irwin Michael R., Olmstead Richard, Motivala Sarosh J. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. SLEEP-NEW YORK THEN WESTCHESTER- 2008;31(7):1001. [PMC free article] [PubMed] [Google Scholar]
- 70.Yeh Gloria Y., et al. Tai Chi Exercise in Patients With Chronic Heart FailureA Randomized Clinical TrialTai Chi in Patients With Chronic Heart Failure. Archives of Internal Medicine. 2011;171(8):750–757. doi: 10.1001/archinternmed.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linde K, Streng A, Jürgens S, et al. Acupuncture for patients with migraine: A randomized controlled trial. JAMA. 2005;293(17):2118–2125. doi: 10.1001/jama.293.17.2118. [DOI] [PubMed] [Google Scholar]
- 72.Yeh GY, Kaptchuk TJ, Shmerling RH. Prescribing tai chi for fibromyalgia--are we there yet? N Engl J Med. 2010;363(8):783–784. doi: 10.1056/NEJMe1006315. [DOI] [PubMed] [Google Scholar]
- 73.Yeh ML, Lee TI, Chen HH, Chao TY. The influences of chan-chuang qi-gong therapy on complete blood cell counts in breast cancer patients treated with chemotherapy. Cancer Nurs. 2006;29(2):149–155. doi: 10.1097/00002820-200603000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.