Abstract
Context
Hospitals vary substantially in their end-of-life (EOL) treatment intensity. It is unknown if patterns of EOL treatment intensity are consistent across conditions.
Objective
To explore the relationship between hospitals’ cancer- and non-cancer specific EOL treatment intensity.
Methods
We conducted a retrospective cohort analysis of Pennsylvania acute care hospital admissions for either cancer or congestive heart failure (CHF) and/or chronic obstructive pulmonary disease (COPD) between 2001–2007, linked to vital statistics through 2008. We calculated Bayes’ shrunken case-mix standardized (observed-to-expected) ratios of intensive care and life-sustaining treatment use among two EOL cohorts: those ‘prospectively’ identified at high probability of dying (HPD) upon admission and those retrospectively identified as terminal admissions (decedents). We then summed these to create a hospital-specific ‘prospective’ and ‘retrospective’ overall EOL treatment intensity index for cancer versus CHF/COPD.
Results
The sample included 207,523 admissions with 15% or greater predicted probability of dying upon admission among 172,041 adults and 120,372 terminal admissions at 166 hospitals; these 2 cohorts overlapped by 52,986 admissions. There was substantial variation between hospitals in their standardized EOL treatment intensity ratios among cancer and CHF/COPD admissions. Within hospitals, cancer- and CHF/COPD-specific standardized EOL treatment intensity ratios were highly correlated for ICU admission (‘prospective’ ρ=.81; ‘retrospective’ ρ=.78), ICU length of stay (ρ=.76; .64), mechanical ventilation (ρ=.73; .73), and hemodialysis (ρ=.60; .71), and less highly correlated for tracheostomy (ρ=.43; .53), and gastrostomy (ρ=.29; .30). Hospitals’ overall EOL intensity index for cancer and CHF/admissions were correlated (ρ=.75; ρ=.75) and had equal group means (p=.631; .699).
Conclusions
Despite substantial difference between hospitals in EOL treatment intensity, within-hospital homogeneity in EOL treatment intensity for cancer- and non-cancer populations suggests the existence of condition-insensitive institutional norms of EOL treatment.
Hospitals vary substantially in their end-of-life (EOL) treatment intensity, as measured by their spending in the last 2 years of life among Medicare fee-for-service decedents with life-limiting illnesses(1) or as measured by intensive care unit (ICU) and LST use among elders at a high-probability of dying upon admission.(2)
It is unknown whether hospital-specific patterns of EOL treatment intensity are consistent across diagnosis groups. Prior studies have documented differences in treatment intensity and spending at the EOL when the patient’s death is “unexpected”.(3, 4) While cancer and prevalent non-cancer organ failures such as congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD) are all chronic, eventually fatal illnesses with similar mean survival(5–7) , cancer has a different meaning to providers, patients, and families than CHF and COPD. Specifically, there is less resistance to the acknowledgement that cancer is a terminal condition than there is for advanced CHF and COPD. Some of this resistance is cultural, but some of it is due to different functional trajectories near the end of life(8) and the greater variance in survival for organ failure than for cancer.(3) Greater confidence in the accuracy of mean prognostic estimates for cancer than other chronic, eventually fatal illnesses may result in more frequent prognostic disclosure to patients with cancer and, perhaps, greater willingness to discuss less intensive treatment options.(9) These phenomena likely contribute to the over representation of cancer patients in hospice programs(10–12), and their under representation among terminal ICU admissions.(13) It is possible that greater willingness to label certain cancer patients as “terminal,” when compared to similarly “sick” CHF and COPD patients may result in cancer patients with lower statistical probability of death being acknowledged as “dying,” while a CHF or COPD patient may require a higher statistical probability, or even be “actively” dying (i.e., failing to respond to the highest intensity medical care(9, 14–16)) when they are acknowledged to be “dying.”
The purpose of the current study was to compare hospitals’ cancer- and non-cancer EOL treatment intensity. We hypothesized that hospitals’ EOL treatment intensity for cancer and non-cancer admissions would vary substantially across hospitals, but would be highly correlated within hospitals. We additionally hypothesized that hospitals’ EOL treatment intensity would be systematically lower for cancer admissions than for non-cancer admissions.
MATERIALS AND METHODS
This is a retrospective analysis of acute care hospital admissions recorded in Pennsylvania Health Care Cost Containment Council (PHC4) hospital discharge data between April 1, 2001 and December 31, 2007, linked to Pennsylvania Department of Health Vital Statistics death records through December 31, 2008.
Sample
PHC4 data contains a predicted probably of in-hospital mortality calculated from key clinical findings (KCFs) abstracted from the medical chart during the first 48 hours of admission. For each discharge, hospital staff abstract KCFs from the medical record. These KCFs encompass over 250 data elements, including vital signs, other physical exam findings, and results of laboratory, pathology, and imaging studies. The accuracy and reliability of abstracted data are very high when compared to actual patient charts. PHC4 randomly selects 10% of all PA hospitals for audit each year. Ten patient charts are then selected at each hospital and re-abstracted. These audits confirm a 95% consistency since 1999. Incomplete record keeping, though, will result in inaccuracies, for example, if a finding is not recorded in the patient chart, it is assumed to be “not present.” It’s possible that there might be systematically less rigorous charting of past medical history on a severely ill patient with congestive heart failure who is well known to admitting staff through frequent readmissions when compared to a new patient (personal communication, Peg Richards, RN, UPMC Health System). This problem is common to even the most sophisticated physiology-based risk prediction tools, such as APACHE III, which similarly rely on the patient chart. The KCFs collected are diagnosis-specific, and so risk-prediction models are similarly diagnosis specific. The KCFs recorded is the worst measure in the first 48 hours (e.g., the lowest systolic blood pressure); additional KCFs abstracted include certain pre-admission findings recorded in the chart (e.g., EKG or imaging results in the previous 60 days). No treatment information is abstracted. These KCFs are imported into ATLAS software along with administrative fields (e.g., age, gender, race) and sent to Cardinal Health Information Companies (CHIC) to calculate an admission risk of death. CHIC-MediQual uses a validated, proprietary prediction model developed by CHIC. In a recent study of 5 conditions and 3 surgical procedures, the mean c-statistic of inpatient mortality models was 0.88 (sd 0.01).(17) Although PHC4 generally only releases categorical admission severity groups (ASG) for researchers to use in their risk-adjusted outcomes by hospital (An ASG of 0 = probability of death of 0–0.001; ASG 1 = 0.002–0.011; ASG 2 = 0.012–0.057; ASG 3 = 0.058–0.499; ASG 4 = 0.500–1), we obtained the continuous predicted probability of death at admission for use in the present study.
Following our prior work,(2, 18) we used this continuous probability of death to identify a cohort at “high probability of dying” (HPD). We defined HPD as being in the 95th percentile of predicted probability of dying (PPD), corresponding to a PPD of ≥15% among all admissions 21 years of age and older between 2001–2007. This results in an HPD cohort of similar total size as the cohort of admissions 21 years of age and older between 2001–2007 who did, in fact, die during the admission (decedents).
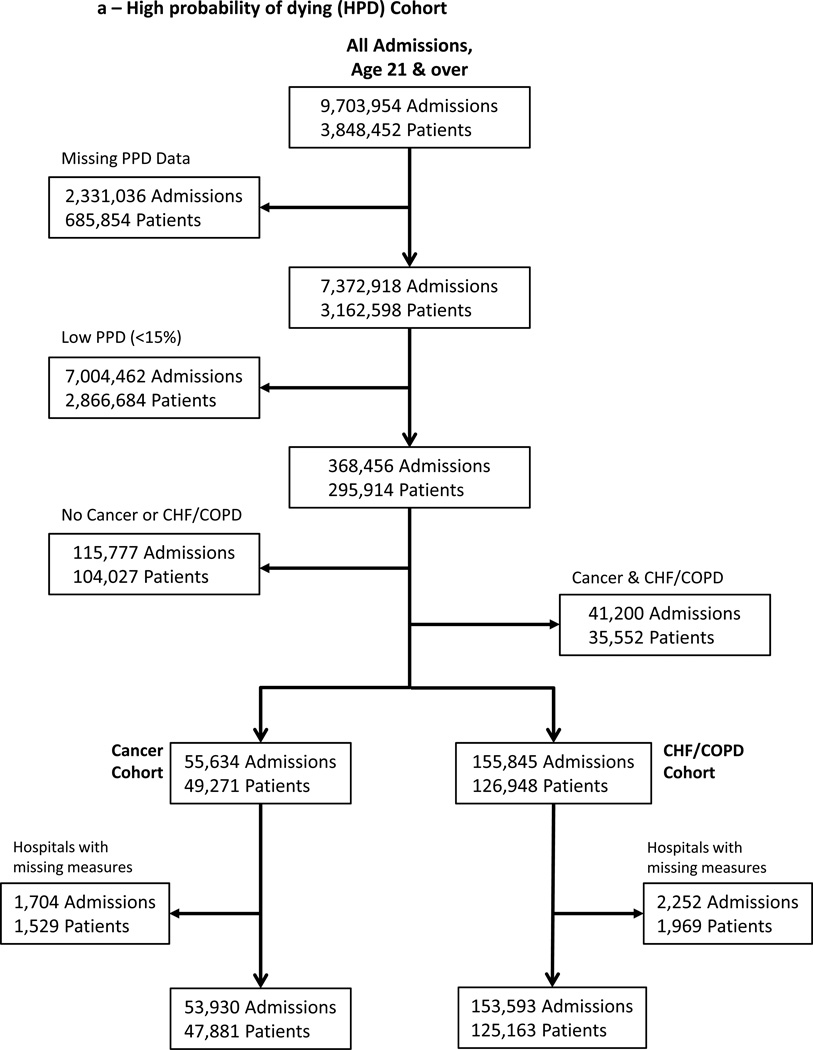
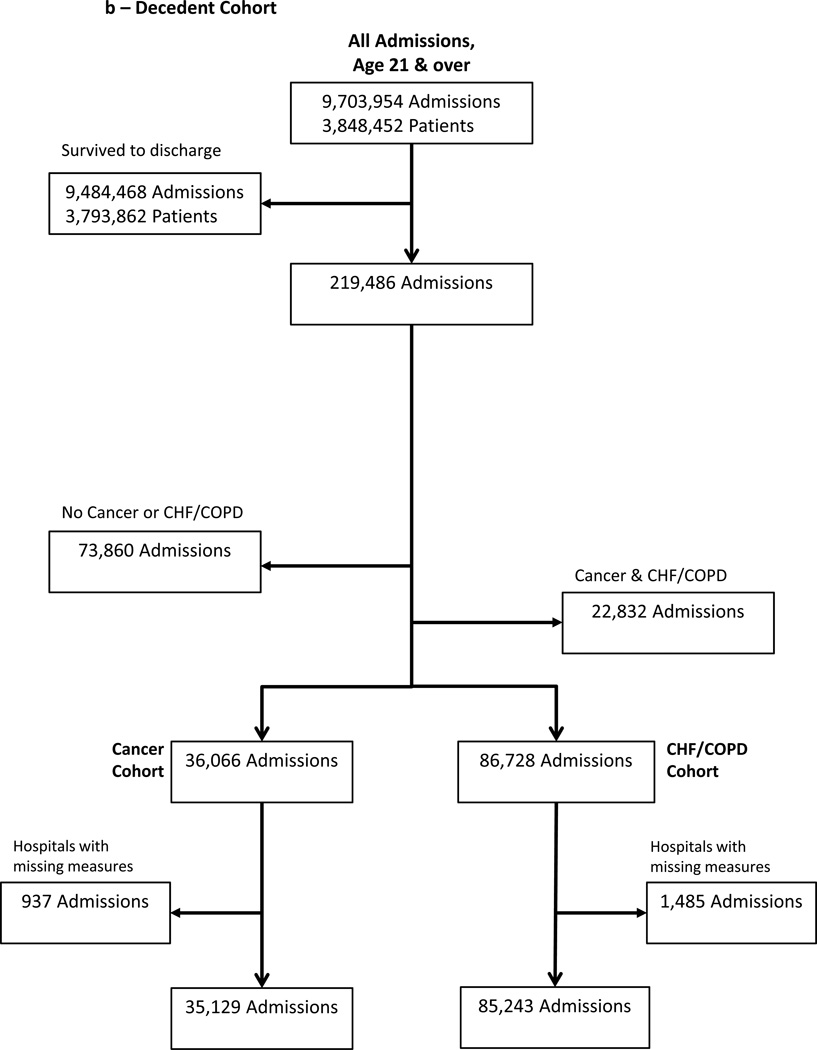
We further subset these two incompletely overlapping cohorts: those at HPD upon admission (e.g., “prospective” EOL cohort) and decedents (e.g., “retrospective” EOL cohort) into cancer and non-cancer cohorts. We selected admissions with congestive heart failure (CHF) and/or chronic obstructive pulmonary disease (COPD) for our non-cancer cohort. Cancer, CHF and COPD are highly prevalent, contribute to three of the four leading causes of death in the US, and are frequently used in studies of patients with limited life expectancy.(19–21) We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), codes in the first or second diagnosis code position on the hospital discharge record to identify patients with cancer, CHF, and/or COPD. Due to a high level of comorbidity, we created mutually-exclusive cohorts: those with cancer but without COPD/CHF and those with CHF/COPD but without cancer. (Figure 1a, 1b)
Figure 1. Sample Selection.
Seven years of hospital discharge data included nearly 10 million admissions among nearly 4 million individual patients. After restricting the sample to in-state residents over age 21 with mutually exclusive cancer or non-cancer diagnoses, there were 207,523 high probability of dying admissions (a) and 120,372 terminal admissions (b). These two cohorts overlapped by 52,986 admissions.
Hospital EOL intensity measures
Following our prior work, (2, 18) for each hospital with at least 30 cancer and 30 CHF/COPD admissions in each cohort (HPD admissions and decedents), we calculated Bayes’ shrunken case-mix standardized (observed-to-expected) treatment ratios for 6 treatment intensity measures (ICU admission, ICU length of stay, intubation/mechanical ventilation (MV), tracheostomy, hemodialysis, and gastrostomy). This involved four steps: First, we calculated the 6 observed EOL treatment intensity measures (5 rates – proportion of the cohort with an ICU admission, MV, tracheostomy, hemodialysis, gastrostomy – and 1 conditional mean – ICU length of stay among those with an ICU admission) among cancer- and non-cancer HPD admissions and decedents for each hospital. This resulted in 2×6×2 (24) total measures per hospital. Second we calculated the 24 expected treatment measures for each hospital. To do this, we estimated 10 separate multivariable logistic regression models to identify the patient-level predictors of the 5 categorical treatment intensity measures: (ICU admission, respiratory intubation and ventilation, tracheostomy, gastrostomy tube placement, hemodialysis) and 2 linear regression models to identify patient-level predictors of log-transformed ICU length of stay in two cohorts: HPD admissions and decedents. When fitting these models, we excluded cancer, CHF, and COPD as predictors so as not to adjust for the contribution of the conditions of interest to the intensity measures. We then applied these coefficients derived from these regressions to each admission to calculate a predicted probability of each of the 6 measures. If the patient had an ICU stay, we will estimate predicted ICU LOS by back-transforming to the original scale (days) by employing the smearing estimate developed by Duan (to allow for any non-normality in the error distribution). We then summed the predicted values across admissions for each hospital to calculate risk-adjusted, expected rates of ICU admission, intubation/mechanical ventilation, tracheostomy, gastrostomy, enteral/parenteral nutrition, hemodialysis and a a risk-adjusted, expected ICU LOS for each hospital for HPD admissions and decedents. Third, we divided the estimates of the hospital’s observed treatment rates and LOS by the hospital’s expected treatment rates and LOS, to obtain standardized treatment ratios for each of the individual measures for the cancer and non-cancer HPD and decedent admissions. This standardized ratio is the summary measure of hospital-level intensity for each treatment. Fourth, to address instability of estimates, particularly for smaller hospitals, we then use empiric Bayes shrinkage estimation.(22) For details, see the Technical Appendix.
We then used these 6 standardized ratios to calculate an empirically-weighted factor score, the EOL intensity index. Specifically, we used factor analysis among all HPD admissions (not just cancer- and non-cancer) and among all decedent admissions to obtain factor loadings for each of the 6 standardized ratios’ relative contribution to the underlying construct “EOL treatment intensity.” For both HPD and decedent admissions all 6 measures loaded onto a single factor (loadings ranged from .62 to .85). The factor-loadings were scaled to create weights that insured the EOL treatment intensity score for the overall HPD cohort was standardized to have a mean of 0 and a standard deviation of 1. We then multiplied the measure-specific factor loading, or weight, by the hospital’s shrunken case-mix standardized (observed-to-expected) treatment ratio for the measure, then summed the quotients from the 6 measures to obtain an empirically (factor-score) weighted EOL intensity index for the hospital. For details, see the Technical Appendix.
Statistical analysis
We compared demographic and clinical characteristics of the admissions comprising the cancer and CHF/COPD HPD cohorts using a t-test or Chi-square test, as appropriate. We used Pearson’s correlation to explore the consistency between hospitals’ cancer and CHF/COPD EOL treatment intensity measures and a two-sided paired t-test to compare the mean within-hospital cancer and CHF/COPD EOL treatment intensity measures. We used multivariable linear regression to explore the relationship between hospital characteristics (bed size, annual cancer volume, annual CHF/COPD volume, teaching status, case-mix severity [measured as the mean predicted probability of death upon admission among all admissions], and urbanicity) and condition-specific EOL treatment intensity.
Human subjects and role of the sponsor
This study was reviewed and approved by the University of Pittsburgh Institutional Review Board. The National Institute of Aging played no role in design, analysis, and interpretation or in the decision to submit for publication.
RESULTS
Sample characteristics
166/185 (90%) of Pennsylvania acute care hospitals had at least 30 admissions in each of the 4 cohorts used to profile cancer and non-cancer EOL treatment intensity over the study period (HPD cancer; HPD CHF/COPD; decedent cancer; decedent CHF/COPD), contributing 207,523 HPD admissions (Figure 1a) and 120,372 decedent admissions (Figure 1b). These admissions overlapped by 52,986. The demographics of cancer and non-cancer EOL cohorts differed, crude rates of ICU and LST use were lower for cancer admissions and, among HPD admissions, mortality was higher (Table 1). The 166 study hospitals included a heterogeneous group with respect to size, location, and teaching intensity (Table 2).
Table 1.
Characteristics of Cohorts used to Calculate Hospitals’ Cancer and Non-Cancer End-of-life Intensity Measures, Pennsylvania 2001–2008
| High probability of dying |
Decedents | |||||
|---|---|---|---|---|---|---|
| Cancer | CHF/COPD | P-value | Cancer | CHF/COPD | P-value | |
| Admissions | 53,930 | 153,593 | 35,129 | 85,243 | ||
| Unique patients | 47,881 | 125,163 | - | - | ||
| Age, mean (SD) | 71.8 (13.4) | 79.7 (10.7) | <0.001 | 69.7 (14.1) | 78.6 (11.2) | <0.001 |
| Female, N (%) | 25,871 (48) | 81,891 (53) | <0.001 | 16,933 (48) | 44,779 (53) | <0.001 |
| Race/ethnicity, N (%) | <0.001 | <0.001 | ||||
| White | 45,517 (84) | 134,186 (87) | 29,269 (83) | 74,820 (88) | ||
| Black | 5,665 (11) | 12,737 (8.3) | 3,905 (11) | 6,660 (7.8) | ||
| Hispanic | 801 (1.5) | 1,712 (1.1) | 462 (1.3) | 890 (1) | ||
| Asian/Pacific Islander | 272 (.5) | 338 (.22) | 196 (.56) | 176 (.21) | ||
| Other/unknown | 1,675 (3.1) | 4,620 (3) | 1,297 (3.7) | 2,697 (3.2) | ||
| Insurance, N (%) | <0.001 | <0.001 | ||||
| Medicare only | 13,462 (25) | 41,455 (27) | 8,213 (23) | 23,201 (27) | ||
| Medicare and Medicaid | 3,945 (7.3) | 24,034 (16) | 2,255 (6.4) | 11,379 (13) | ||
| Medicare and commercial | 22,338 (41) | 75,657 (49) | 13,077 (37) | 42,145 (49) | ||
| Medicaid only | 3,368 (6.2) | 4,969 (3.2) | 2,574 (7.3) | 3,015 (3.5) | ||
| Commercial only | 10,498 (19) | 6,981 (4.5) | 8,498 (24) | 4,915 (5.8) | ||
| Uninsured | 295 (.55) | 447 (.29) | 483 (1.4) | 531 (.62) | ||
| Admission type, N (%) | <0.001 | <0.001 | ||||
| Emergent | 37,960 (70) | 119,316 (78) | 22,666 (65) | 62,749 (74) | ||
| Urgent | 12,717 (24) | 29,497 (19) | 8,440 (24) | 17,535 (21) | ||
| Elective | 3,215 (6) | 4,644 (3) | 3,981 (11) | 4,826 (5.7) | ||
| PPD* mean (SD) | 327 (.189) | 312 (.176) | <0.001 | 259 (.251) | 225 (.231) | <0.001 |
| Charlson index, mean (SD) | 4.57 (3.16) | 2.35 (1.29) | <0.001 | 4.32 (3.14) | 2.22 (1.28) | <0.001 |
| Treatments received | ||||||
| ICU admission, N (%) | 21,705 (40) | 101,865 (66) | <0.001 | 16,045 (46) | 55,520 (65) | <0.001 |
| ICU LOS†| admission, mean (SD) | 7.68 (7.91) | 9.26 (9.97) | <0.001 | 8.44 (11.2) | 8.89 (11.6) | <0.001 |
| Mechanical ventilation, N (%) | 6,853 (13) | 40,116 (26) | <0.001 | 8,770 (25) | 30,341 (36) | <0.001 |
| Hemodialysis, N (%) | 1,379 (2.6) | 9,238 (6) | <0.001 | 1,002 (2.9) | 5,551 (6.5) | <0.001 |
| Gastrostomy tube, N (%) | 1,217 (2.3) | 5,324 (3.5) | <0.001 | 727 (2.1) | 2,299 (2.7) | <0.001 |
| Tracheostomy, N (%) | 637 (1.2) | 4,529 (2.9) | <0.001 | 689 (2) | 2,676 (3.1) | <0.001 |
| Cumulative mortality, N (%) | ||||||
| At hospital discharge | 14,842 (28) | 38,144 (25) | <0.001 | - | - | - |
| At 30 days post-admission | 28,313 (52) | 59,098 (38) | <0.001 | - | - | - |
| At 6-months post-admission | 41,862 (78) | 91,946 (60) | <0.001 | - | - | - |
PPD indicates Predicted Probability of Death on admission; HPD admission include only admissions with non-missing HPD. 11% of the decedent sample is missing PPD; 21% in the cancer group, 7% in the CHF/COPD group.
LOS indicates Length of Stay
Table 2.
Characteristics of Study Hospitals (N=166 hospitals)
| Bed Size, median (IQR) | 217 (125–317) |
| Teaching Status*, N (%) | |
| Non-teaching | 88 (53%) |
| Minor Teaching | 52 (32%) |
| Major Teaching | 25 (15%) |
| Proportion of admissions which are cancer, mean (sd) | 13.2 (2.99) |
| Proportion of admissions which are CHF/COPD, mean (sd) | 33 (8.36) |
| Mean predicted probability of death on admission, mean (sd) | 3.18 (.562) |
| Proportion of admissions among Medicaid, mean (sd) | 12.5 (7.68) |
| Proportion of admissions among Black, mean (sd) | 9.47 (16.2) |
Teaching status is based upon the resident-to-bed ratio: major teaching=resident-to-bed ratio >0.25; minor teaching=resident-to-bed ratio>0 but<0.25; and non-teaching=resident-to-bed ratio = 0
Cancer- and Non-cancer EOL treatment intensity
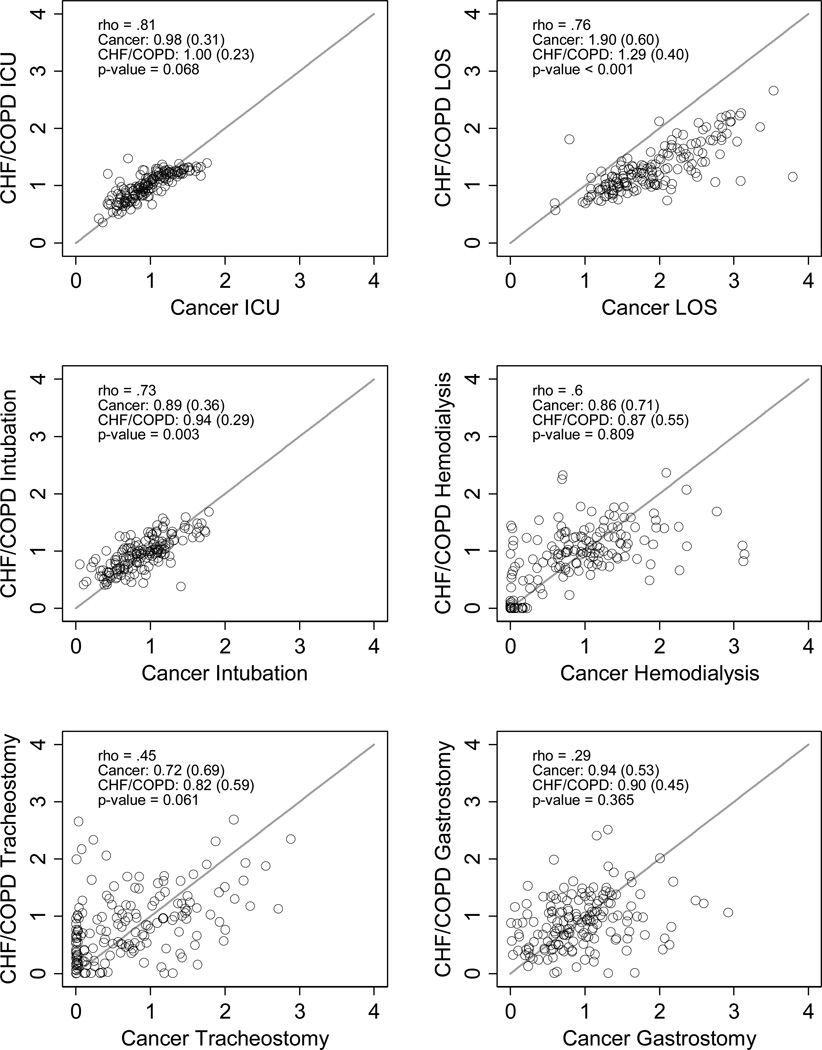
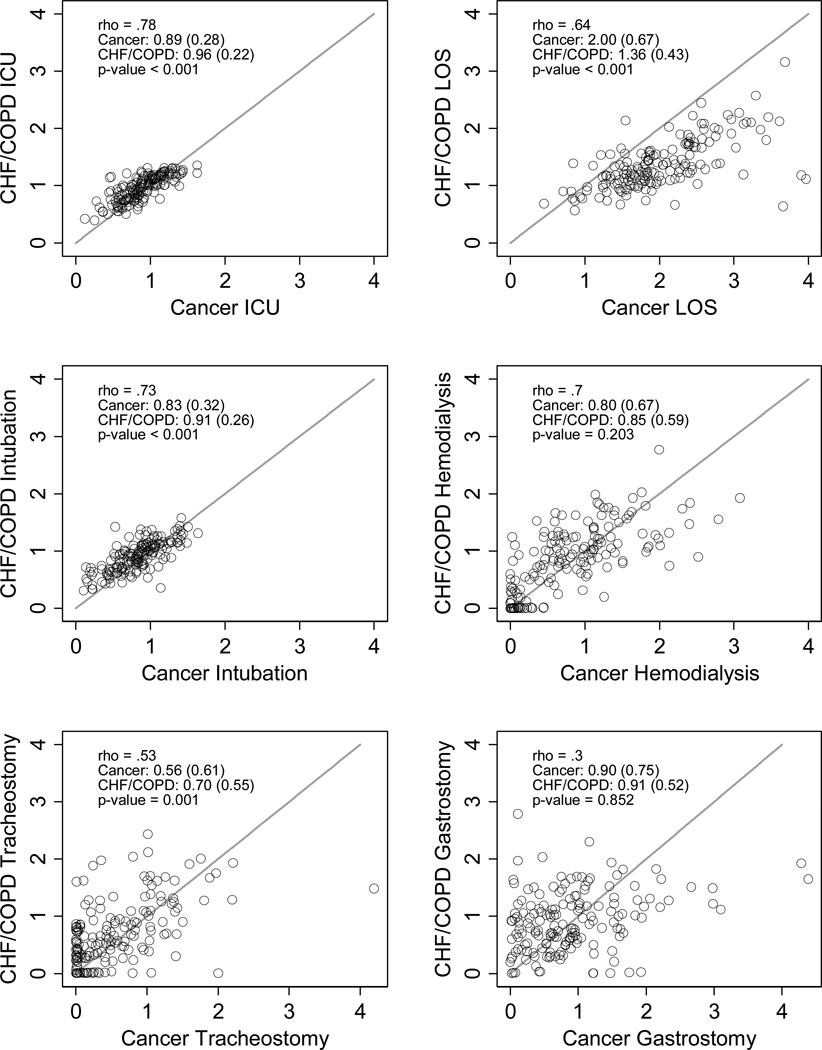
There was wide variation between hospitals in cancer and non-cancer standardized ratios of ICU and LST use (Figure 2, panels a-f and g-l), with larger, urban, teaching hospitals having greater EOL treatment intensity (Appendix Table A1). Within hospitals, however, the cancer and non-cancer measures were highly correlated for ICU admission, ICU length of stay, intubation/mechanical ventilation, and hemodialysis, and moderately correlated for tracheostomy, and gastrostomy (Figure 2, Table 3). Cancer-specific ICU admission (decedent only), mechanical ventilation (HPD and decedent), and tracheostomy (decedent only) measures were systematically lower than the non-cancer measures, but higher for ICU LOS (Figure 2, Table 3). The cancer and non-cancer hemodialysis and gastrostomy did not differ systematically (e.g., had equal sample means). Hospitals’ cancer and non-cancer overall EOL intensity indices were highly correlated and had equal group means (Table 3)
Figure 2. Condition-specific Standardized End-of-Life Treatment Ratios.
Scatter plots and density plots of cancer and CHF/COPD standardized (observed to expected treatment) intensity ratios among admissions ‘prospectively’ identified as being at the ‘end of life’ (e.g., at high probability of dying upon admission; panels a-f) and among admissions ‘retrospectively’ identified as being at the ‘end of life’ (e.g., terminal admissions; panels g-l). In the scatter plots, each circle represents a single hospital. Hospitals closer to the 45 degree line treat patient with cancer and CHF/COPD more similarly. Circles above the line treat CHF/COPD patients more intensely than cancer patients, while circles below the line treat cancer patients more intensely than CHF/COPD patients. (Note: one hospital’s data point in Figure 2, panel e, falls outside the axis with a cancer-specific standardized ratio for tracheostomy of ~ 8).
Table 3.
Pennsylvania Hospitals’ Mean Cancer and Non-Cancer End-of-life Treatment Intensity, by Cohort (N=166 hospitals)*
| Measure | Cancer | CHF/COPD | P-value | Pearson’s Correlation |
|---|---|---|---|---|
| HPD-based EOL intensity measures | ||||
| Standardized treatment ratios, mean (SD) | ||||
| ICU admission | .98 (.31) | 1.0 (.23) | 0.068 | 0.81 |
| ICU LOS | ICU admission | 1.9 (.60) | 1.3 (.41) | <0.001 | 0.76 |
| Mechanical ventilation | .89 (.36) | .94 (.29) | 0.003 | 0.73 |
| Hemodialysis | .86 (.71) | .87 (.55) | 0.809 | 0.60 |
| Tracheostomy | .76 (.88) | .82 (.60) | 0.334 | 0.43 |
| Gastrostomy tube placement | .94 (.53) | .90 (.45) | 0.365 | 0.29 |
| EOL intensity index, mean (SD)† | .029 (.82) | .053 (.97) | 0.631 | 0.75 |
| Decedent-based EOL Intensity Measures | ||||
| Standardized treatment ratios, mean (SD) | ||||
| ICU admission | .89 (.28) | .96 (.22) | <0.001 | 0.78 |
| ICU LOS | ICU admission | 2 (.67) | 1.4 (.43) | <0.001 | 0.64 |
| Mechanical ventilation | .84 (.32) | .91 (.26) | <0.001 | 0.73 |
| Hemodialysis | .80 (.67) | .85 (.59) | 0.203 | 0.71 |
| Tracheostomy | .56 (.61) | .71 (.55) | 0.001 | 0.53 |
| Gastrostomy tube placement | .90 (.75) | .91 (.52) | 0.852 | 0.30 |
| EOL intensity index, mean (SD)† | .035 (.87) | .055 (.98) | 0.699 | 0.75 |
Values provided are the mean treatment intensity scores for the standardized observed to expected treatment intensity ratio for cancer and CHF/COPD. Scores <1 indicate the intensity score for that treatment was less than would expected, while scores >1 indicated the intensity score for that treatment was greater than would be expected.
The end-of-life intensity index is an empirically-weighted factor score based upon observed-to-expected ratios of each of the individual treatment ratios (ICU use, ICU length of stay, intubation/mechanical ventilation, tracheostomy, hemodialysis, and gastrostomy tube placement) for patients at a high probability of dying during the hospitalization.
LOS indicates Length of Stay
DISCUSSION
In this retrospective analysis of discharge data from Pennsylvania acute care hospitals over 8 years, we observed heterogeneity between hospitals in EOL treatment intensity, but considerable homogeneity within hospitals in their EOL treatment intensity among cancer and non-cancer cohorts.
This study extends our prior work that demonstrated substantial between-hospital variation in EOL treatment intensity and suggested hospitals have consistent practice patterns, or “fingerprints,” of EOL treatment.(2, 18) In the current study we provide additional evidence of these “fingerprints” by demonstrating consistency in hospitals’ EOL treatment intensity for distinct populations of seriously ill patients – those with cancer and those with the non-cancer life-limiting organ failures CHF and/or COPD. This condition-specific consistency of EOL treatment was most robust for measures of ICU use, intubation/mechanical ventilation, and tracheostomy, but somewhat weaker for the other measured life-sustaining treatments – hemodialysis and gastrostomy. The lack of consistency in these measures may be explained by the relatively low frequencies of these treatments, introducing noise from sampling variability.
Counter to our hypothesis, cancer- and non-cancer treatment ratios and overall EOL intensity indices did not reveal universally lower EOL treatment intensity for cancer than for CHF/COPD. There was systematically lower cancer-specific ICU admission, intubation/mechanical ventilation, and tracheostomy among decedents within hospitals, compared to CHF/COPD, which may reflect greater use of advance care planning in cancer populations compared to non-cancer organ failure populations(5, 9) or in ICU admission and intubation decision making by physicians.(13, 23) However, current guidelines promulgated by the Society of Critical Care Medicine recommend that the severity of the acute disease process, rather than the underlying chronic disease diagnosis, be used for ICU triage criteria.(24)
Our study is subject to several limitations. Between 2001 and 2007, PHC4 admissions with missing data for predicted probability of dying increased from 14% to 34% overall. This increase is due to the PA legislature’s reduction of mandated clinical data abstraction to 35 DRGs (see Technical Appendix) in order to decrease burden of public reporting on hospitals. PPD missingness was systematically greater for cancer admissions over this time period, increasing from 4.2% in 2001 to 43% in 2007, than for either CHF (2.5% to 1.8%) or COPD (2.4% to 2%), and missingness was correlated with EOL intensity. Nonetheless, analyses restricted to earlier years with less missing data produced qualitatively similar results. Parallel analyses using terminal admissions (e.g., decedents) rather than HPD admissions, to measure EOL intensity produced similar results, although the within-hospital differences in some of the individual cancer vs. non-cancer EOL treatment intensity measures (ICU admission, tracheostomy) were more pronounced in the decedent than the HPD cohort. Another limitation is that we restricted the cancer and CHF/COPD cohorts to patients without co-morbid CHF/COPD and cancer, respectively. This may have inadvertently selected a healthier population of patients for calculating condition-specific EOL treatment intensity. Finally, we do not have any data regarding indications for treatment or about do not resuscitate orders or other treatment limitations chosen by patients or their families.
In conclusion, despite substantial difference between hospitals in EOL treatment intensity, within-hospital homogeneity in EOL treatment intensity for cancer- and non-cancer populations suggests the existence of condition-insensitive institutional norms of EOL treatment. To the extent that these differences reflect institutional norms and not patient preferences, this may reflect non patient-centered EOL treatment.
Supplementary Material
Acknowledgments
DISCLOSURES AND ACKNOWLEDGMENTS
This work was funded by a research grant awarded to Amber Barnato from the National Institute of Aging (R01 AG035112).
The authors acknowledge the intellectual contributions of Max Farrell to protocols for data management and statistical analysis and of Derek Angus, Judy Lave, and Mark Roberts to obtaining funding.
Footnotes
DISCLAIMER
This research was conducted under a data use agreement with Pennsylvania Health Care Cost Containment Council (PHC4). PHC4 provided this data to further PHC4’s mission of educating the public and containing health care costs in Pennsylvania. PHC4, its agents and staff, have made no representation, guarantee, or warranty, express or implied, that the data are error-free, or that the use of the data will avoid differences of opinion or interpretation, or disputes with those who use published reports or purchased data. PHC4, its agents and staff, will bear no responsibility or liability for the results of the analysis, or consequences of its use.
None of the authors has a conflict of interest in the research reported.
REFERENCES
- Wennberg JE, Fisher ES, Stukel TA, Skinner JS, Sharp SM, Bronner KK. Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States. Bmj. 2004;328(7440):607. doi: 10.1136/bmj.328.7440.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnato AE, Farrell MH, Chang CC, Lave JR, Roberts MS, Angus DC. Development and validation of hospital "end-of-life" treatment intensity measures. Med Care. 2009;47(10):1098–1105. doi: 10.1097/MLR.0b013e3181993191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn J, Harrell F, Jr, Cohn F, Wagner D, Connors AF., Jr Prognoses of seriously ill hospitalized patients on the days before death: implications for patient care and public policy. New Horiz. 1997;5(1):56–61. [PubMed] [Google Scholar]
- Detsky AS, Stricker SC, Mulley AG, Thibault GE. Prognosis, survival, and the expenditure of hospital resources for patients in an intensive-care unit. N Engl J Med. 1981;305(12):667–672. doi: 10.1056/NEJM198109173051204. [DOI] [PubMed] [Google Scholar]
- Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. Bmj. 2002;325(7370):929. doi: 10.1136/bmj.325.7370.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More 'malignant' than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Christakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. Chicago, IL: The University of Chicago Press; 1999. [Google Scholar]
- Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. Jama. 2003;289(18):2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- Claessens MT, Lynn J, Zhong Z, Desbiens NA, Phillips RS, Wu AW, et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(5 Suppl):S146–S153. doi: 10.1111/j.1532-5415.2000.tb03124.x. [DOI] [PubMed] [Google Scholar]
- Gibbs LM, Addington-Hall J, Gibbs JS. Dying from heart failure: lessons from palliative care. Many patients would benefit from palliative care at the end of their lives. Bmj. 1998;317(7164):961–962. doi: 10.1136/bmj.317.7164.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DE, Lynn J, Louis TA, Shugarman LR. Medicare program expenditures associated with hospice use. Ann Intern Med. 2004;140(4):269–277. doi: 10.7326/0003-4819-140-4-200402170-00009. [DOI] [PubMed] [Google Scholar]
- Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. New England Journal of Medicine. 1996;335(3):172–178. doi: 10.1056/NEJM199607183350306. [DOI] [PubMed] [Google Scholar]
- Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- Hofmann JC, Wenger NS, Davis RB, Teno J, Connors AF, Jr, Desbiens N, et al. Patient preferences for communication with physicians about end-of-life decisions. SUPPORT Investigators. Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment. Ann Intern Med. 1997;127(1):1–12. doi: 10.7326/0003-4819-127-1-199707010-00001. [DOI] [PubMed] [Google Scholar]
- Weeks JC, Cook EF, O'Day SJ, Peterson LM, Wenger N, Reding D, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. Jama. 1998;279(21):1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- Krumholz HM, Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, et al. Resuscitation preferences among patients with severe congestive heart failure: results from the SUPPORT project. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Circulation. 1998;98(7):648–655. doi: 10.1161/01.cir.98.7.648. [DOI] [PubMed] [Google Scholar]
- Pine M, Jordan HS, Elixhauser A, Fry DE, Hoaglin DC, Jones B, et al. Enhancement of claims data to improve risk adjustment of hospital mortality. Jama. 2007;297(1):71–76. doi: 10.1001/jama.297.1.71. [DOI] [PubMed] [Google Scholar]
- Barnato AE, Chang CC, Farrell MH, Lave JR, Roberts MS, Angus DC. Is survival better at hospitals with higher "end-of-life" treatment intensity? Med Care. 2010;48(2):125–132. doi: 10.1097/MLR.0b013e3181c161e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. Jama. 1995;274(20):1591–1598. [PubMed] [Google Scholar]
- Christakis NA, Iwashyna TJ, Zhang JX. Care after the onset of serious illness: a novel claims-based dataset exploiting substantial cross-set linkages to study end-of-life care. J Palliat Med. 2002;5(4):515–529. doi: 10.1089/109662102760269751. [DOI] [PubMed] [Google Scholar]
- Ash AS, Shwartz M, Pekoz EA. Chapter 12: Comparing oucomes across providers. In: Iezzoni LI, editor. Risk Adjustment for Measuring Health Oucomest. Chicago, IL: Academy Health; 2005. pp. 297–333. [Google Scholar]
- Joynt GM, Gomersall CD, Tan P, Lee A, Cheng CA, Wong EL. Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome. Intensive Care Med. 2001;27(9):1459–1465. doi: 10.1007/s001340101041. [DOI] [PubMed] [Google Scholar]
- Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):633–638. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.