Abstract
Background
Destination therapy left ventricular assist devices (DT LVADs) are being increasingly implanted in older adults. Older patients are at increased risk for mortality and morbidity post LVAD, which may impact their health-related quality of life (HRQOL). We sought to examine change in HRQOL by age from before to 1 year after DT LVAD implant and identify factors associated with change.
Methods
Data were collected from 1,470 continuous flow DT LVAD patients at 108 institutions participating in INTERMACS from January 21, 2010 to March 31, 2012. Patients were divided into three cohorts: <60 years (n=457), 60-69 years (n=520), and ≥70 years (n=493). HRQOL was measured using the generic EQ-5D-3L. Data were collected pre-implant and 3, 6, and 12 months post-implant. Statistical analyses included descriptive statistics, Kaplan-Meier survival analyses, and multivariable regression analyses.
Results
HRQL improved in all patients. Generally, older patients reported better HRQOL than younger patients pre implant (≥70 yrs, mean=40; 60-69 yrs, mean=33; and < 60 yrs, mean=31, p<0.0001) and 1 year post implant (≥70 yrs, mean=77; 60-69 yrs, mean=72; and < 60 yrs, mean=70, p=0.01) using the EQ-5D visual analog scale (VAS), with 0 = worst imaginable health state and 100 = best imaginable health state. The magnitude of improvement in EQ-5D scores from pre- to 1 year post LVAD was similar in all age groups (≥70 yrs, mean change=33; 60-69 yrs, mean change=35; and < 60 yrs, mean change=35, p=0.77). Factors associated with improvement in HRQOL from before to 1 year after implant were a lower VAS score pre implant and fewer re-hospitalizations after implant (R2=61.3%, p< 0.0001).
Conclusions
Older patients reported better HRQOL than younger patients before and after LVAD implantation. The magnitude of improvement was similar for all age groups, with more than 70% of all patients evidencing clinically important increases (>10 points on the VAS). Re-hospitalization appears to reduce the magnitude of improvement.
The number of people in the U.S. ≥ 65 years is projected to be 88.5 million in 2050, more than double the population of 40.2 million in 2010.1 Because the prevalence of heart failure increases with age,2 the estimated 50,000 to 500,0003 patients with advanced heart failure is anticipated to include an increasing number of older individuals. Destination therapy (DT; intended for permanent use) mechanical circulatory support (MCS) is offered to older patients with advanced heart failure who are ineligible for heart transplantation as a consequence of advanced age and comorbidities.4-7 In 2011, 38% (620/1620) of MCS implants were DT, as reported by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS),8 and their use in older adults is increasing with more DT MCS devices used in older versus younger patients from 2006-2011 (<60 years: n=352, ≥ years: n=808).8
Importantly, outcomes after MCS have improved over time. Patient survival 2 years after MCS (70%) is approaching that of heart transplantation (~80%).4, 9 Yet, older age is a risk factor for decreased survival after MCS9, including DT MCS,8 and is associated with increased morbidity after MCS (e.g., renal failure, stroke and gastrointestinal bleeding).10, 11 Health-related quality of life (HRQOL) outcomes also improve through 2 years after MCS.12-15 However, adverse events and symptoms after MCS are risk factors for poor HRQOL and their increased incidence in older patients may diminish the HRQOL benefits in this population.16, 17 In contrast, we have previously reported that older patients have better short-term18 and long-term19 HRQOL outcomes after transplantation as compared with younger patients.
To address the gap in knowledge surrounding the HRQOL benefits of DT MCS in older patients, we examined these outcomes in INTERMACS, a prospective, multi-institutional registry of patients receiving MCS. We sought to examine change in HRQOL by age and identify factors associated with change in HRQOL from before to 1 year after DT MCS implantation. We defined HRQOL as “the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient.”20
METHODS
Sample / Sites
Data were collected retrospectively from adult (≥ 19 years) left ventricular assist device (LVAD) patients at 108 institutions that collected HRQOL data within INTERMACS. Patients who received an FDA approved continuous flow LVAD as a primary implant for DT between January 21, 2010 and March 31, 2012 were included. Patients were divided into three cohorts based on age: <60 years, 60-69 years, and ≥70 years. Our rationale for using these subgroups is derived from the gerontology literature which recognizes the diversity of old age by defining subgroups. While there is no universal definition of subgroups, a common definition, which has been used in cardiovascular research is as follows: 60-69=young-old, 70-79=middle-old, and 80+=old-old.21 We combined the middle-old and old-old DT MCS subgroups as the sample size was very small at ≥ 80 years. Thus, our choice of < 60 years, 60-69 years, and ≥ 70 years supported our desire to understand whether the young-old DT MCS patients were more similar to adult DT MCS patients < 60 years or the combined subgroups of middle- and old-old DT MCS patients regarding HRQOL outcomes. Patients were followed through March 31, 2013 to enable each patient to have 1 year of post implant follow-up.
Instrument
The EQ-5D-3L, a generic instrument, was used to measure HRQOL, via patient self-report.22, 23 The EQ-5D-3L consists of five questions that assess the HRQOL dimensions of mobility, self-care, usual activities, pain / discomfort, and anxiety / depression.22, 23 Patients were asked to rate the extent to which they had problems for each dimension using a 3-level response format (no problems, some or moderate problems, extreme problems). This 3-level response format has been used since initiation of the registry. A 5-level response version of the EQ-5D became available after the launch of INTERMACS, but the 3-level format has been used to maintain consistency. The EQ-5D-3L also includes an overall health status rating, using a vertical visual analog scale (VAS), with 0 = worst imaginable health state and 100 = best imaginable health state. Psychometric support for this instrument has been reported, including for patients with cardiovascular disease.24, 25
Procedures
Institutional Review Board approval was received from all sites prior to participation in INTERMACS. After providing informed consent, patients were enrolled in the registry. HRQOL and medical records were abstracted by research coordinators pre-implant (up to 30 days prior) and at 3, 6, and 12 months post-implant or until device removal, re-implantation, transplant, or death. When the EQ-5D-3L instrument was not completed, reasons for missing data were documented. Data were entered electronically into the INTERMACS database and analyzed by the data coordinating center at the University of Alabama, Birmingham, AL.
Statistical Analyses
Data were analyzed using SAS, version 9.1 (Carey, NC). Statistical analyses included Kaplan-Meier survival analyses, chi square tests to compare frequencies, one way ANOVA to compare means (using all available data for each time period), paired t-tests to compare means (when complete data were available at the pre and 1 year post implant time periods), and multiple linear stepwise (forward) regression analyses. Distribution of change in the VAS score was also examined over time and a priori, a change of > 10 units was considered to be clinically important. This decision was based on the cancer literature, which estimates a change of 8-12 in VAS scores as a “minimally important difference (MID)” for self-rated health status among cancer patients.26 We did not find MIDs for VAS scores in other disease states in the literature.
The EQ-5D VAS score is reported as a mean ± standard deviation, and dimension scores are reported as frequencies. A VAS rating of 0 was assigned to patients who were too sick to respond, based on the spread of the scores for those patients who responded. Data from the five dimensions were organized into two groups: (group 1) physical function / activities of daily living (dimensions=mobility, self-care, and usual activities) and (group 2) pain / emotions (dimensions=pain / discomfort and anxiety / depression). This grouping was used because a response level of “Extreme problems” was assigned (post hoc) to patients who were too sick to respond for the physical function / activities of daily living group to reduce the potential for overestimation of HRQOL in patients who were most severely ill. No assignment of responses was made for too sick patients in the pain / emotions group, as being too sick does not necessarily indicate extreme problems regarding pain or negative emotions. We performed sensitivity analyses to assess the influence of assignment of VAS scores for patients too sick to respond.
Multivariable regression analyses were conducted, combining the three age groups and including some basic transformations (age2 and log age), using change in the VAS score from pre to 1 year post implant as the dependent variable. Independent variables included the institution at which the surgery was performed, pre implant VAS score, demographic characteristics (age, gender, race, education, and marital status), pre implant clinical variables (e.g., diagnosis, INTERMACS patient profile, New York Heart Association class, left ventricular ejection fraction < 20%, severely decreased right ventricular function, cardiac index, pre-albumin, pre implant interventions [i.e., inotropes, intra aortic balloon pump, ventilator, dialysis, extracorporeal membrane oxygenation, and implantable cardioverter defibrillator], and presence of co-morbidities [i.e., previous cancer, diabetes, cerebrovascular accident, peripheral vascular disease, chronic obstructive pulmonary disease, rheumatologic disease, current smoker, abuse of alcohol, and abuse of drugs), concomitant surgery, and post-operative adverse events within 1 year post implant [i.e., bleeding, infection, neurological dysfunction, psychiatric episode, device malfunction, and re-hospitalization]). These demographic and pre- and post-operative clinical variables were selected based on their potential to influence HRQOL. For example, co-morbidities, such as diabetes; pre implant interventions, including use of inotropes; and adverse events after implant, such as device malfunction, can all influence change in HRQOL from before to after implant. Pre-implant interventions, comorbidities, concomitant surgery, and adverse events were operationalized as dichotomous variables (i.e., present / not present). There were no outliers, and multicollinearity was minimal. Level of significance was p < 0.05 for all analyses.
RESULTS
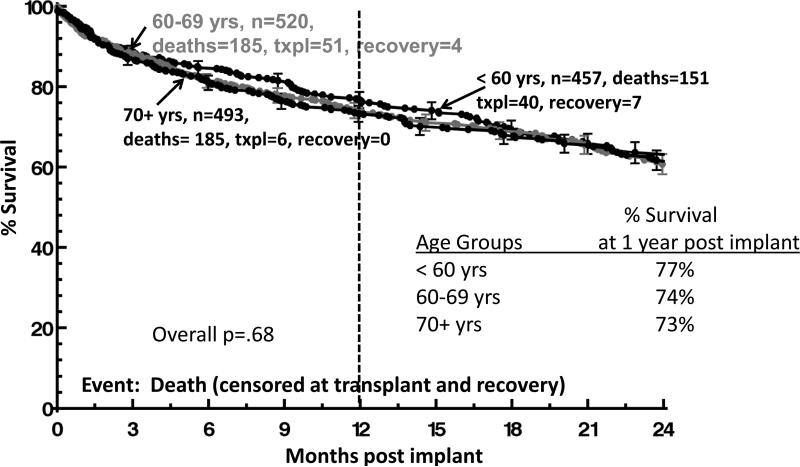
Between January 21, 2010 and March 31 2012, 1470 patients (mean age=63.4 years, 82% male, and 75% white) enrolled in INTERMACS received primary continuous flow DT LVADs. Sample size by age group was: <60 yrs, n=457 (31%); 60-69 yrs, n=520 (35%); and ≥70 yrs, n=493 (34%). Significant differences were detected among the age groups for demographic characteristics and pre and post implant variables (Table 1). The youngest age group included the highest proportion of unmarried respondents, more nonwhite respondents, more women, more individuals with less than a high school education, and more individuals who reported substance abuse than the two older age groups. Notably, fewer older patients were INTERMACS profile 1, as compared to the two younger age groups. Survival did not differ among age groups (figure 1).
Table 1.
Baseline (pre implant) characteristics of MCS recipients by age
| Pre-Implant Characteristics | Total n= 1470 | Age < 60 years n=457 | Age=60-69 years n=520 | Age≥ 70 years n=493 | p-value |
|---|---|---|---|---|---|
| Demographic and behavioral characteristics | |||||
| Age at implant (mean years) | 63.4±11.8 | 49.0±9.4 | 65.7±2.9 | 74.1±3.0 | < .0001 |
| Male (%) | 82 | 75 | 83 | 88 | < .0001 |
| Race (% white) | 75 | 55 | 83 | 86 | < .0001 |
| Married at time of implant (%) | 73 | 53 | 79 | 85 | < .0001 |
| > high school education (%) | 50 | 42 | 50 | 58 | < .0001 |
| Currently smoking (%) | 11 | 22 | 10 | 4 | < .0001 |
| Current alcohol abuse (%) | 15 | 25 | 13 | 8 | < .0001 |
| Current drug abuse (%) | 2 | 7 | 1 | 0 | < .0001 |
| Clinical characteristics | |||||
| Primary cardiac diagnosis (%) | |||||
| Ischemic cardiomyopathy | 58 | 40 | 67 | 38 | < .0001 |
| Dilated cardiomyopathy | 38 | 56 | 29 | 32 | < .0001 |
| Other | 4 | 4 | 4 | 4 | .76 |
| Co-morbidities (%) | |||||
| Diabetes | 44 | 38 | 51 | 43 | .0004 |
| CVA | 8 | 7 | 10 | 7 | .16 |
| Right heart failure (RVEF severe) | 19 | 23 | 17 | 18 | .27 |
| Pre COPD | 18 | 18 | 17 | 19 | .82 |
| Cancer | 13 | 12 | 15 | 12 | .22 |
| NYHA class IV (%) | 76 | 75 | 79 | 75 | .21 |
| Intra aortic balloon pump (%) | 26 | 27 | 26 | 25 | .79 |
| Ventilator (%) | 5 | 7 | 4 | 3 | .01 |
| ECMO (%) | 1 | 3 | 1 | 0 | .003 |
| Dialysis (%) | 2 | 2 | 1 | 1 | .20 |
| INTERMACS profile at implant (%) | |||||
| 1 | 11 | 15 | 11 | 7 | .0001 |
| 2 | 36 | 37 | 36 | 35 | .76 |
| 3 | 32 | 32 | 32 | 32 | .95 |
| 4 | 15 | 10 | 17 | 19 | .0003 |
| 5 | 3 | 2 | 3 | 4 | .26 |
| 6 | 2 | 2 | 1 | 2 | .44 |
| 7 | 1 | 1 | 1 | 1 | .49 |
| Inotrope therapy (%) | 78 | 82 | 78 | 75 | .03 |
| Implantable cardioverter defibrillator (%) | 85 | 82 | 87 | 87 | .02 |
| Temporary circulatory support (%) | 15 | 18 | 16 | 13 | .07 |
MCS = mechanical circulatory support, CVA = cerebrovascular accident, COPD = chronic obstructive pulmonary disease, ECMO = extracorporeal membrane oxygenation
*Those patients who were ‘too sick’ have been included and assigned VAS=0 and physical dimensions as ‘extreme problems’
Figure 1.
Survival Analyses by Age Group
EQ-5D-3L instrument completion rates overall and by age group
Rates of instrument completion were identified for all three age groups (table 2). Before implant, 70%, 73%, and 76% of data were available for patients < 60 years, 60-69 years, and ≥ 70 years, respectively, which included patients who were too sick to respond for whom the VAS score was assigned a 0. At 12 months after implant, EQ-5D completion rates for the 3 age groups ranged from 52% to 64%. After implant, very few patients were too sick to respond. Reasons for post implant lack of survey completion were primarily administrative (e.g., patient not consented, no contact with the patient during the window of time that a survey was due) and patient refusal to participate.
Table 2.
HRQOL data availability by age group
| < 60 years |
60 – 69 years |
70+ years |
Totals |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Doint | exp | *n | too sick to respond to survey: VAS = 0 | exp | *n | too sick to respond to survey: VAS = 0 | exp | *n | too sick to respond to survey: VAS = 0 | exp | *n | too sick to respond to survey: VAS = 0 |
| Pre-implant | 457 | 318 (70%) | 85 (27%) | 520 | 377 (73%) | 71 (19%) | 493 | 377(76%) | 47 (12%) | 1470 | 1072 (73%) | 203 (19%) |
| 3 month | 406 | 205 (50%) | 16 (8%) | 463 | 234 (51%) | 21 (9%) | 440 | 242(55%) | 25 (10%) | 1309 | 681 (52%) | 62 (9%) |
| 6 month | 381 | 194 (51%) | 4 (2%) | 429 | 237 (55%) | 10 (4%) | 411 | 249(61%) | 8 (3%) | 1221 | 680 (56%) | 22 (3%) |
| 12 month | 323 | 169 (52%) | 2 (1%) | 359 | 207 (58%) | 3 (1%) | 351 | 223 (64%1 | 3 (1%) | 1033 | 599 (58%) | 8 (1%) |
exp=expected
n includes patients who completed the survey and patients who were too sick to respond and were assigned a VAS score = 0
Differences in HRQOL by age group before and after DT LVAD implantation
Differences in overall HRQOL (using the VAS score) among age groups were examined cross-sectionally within each time period and longitudinally from before to 1 year after DT LVAD implant (table 3). Using all available data, differences were detected among the three age groups, with the older age group demonstrating the best overall HRQOL (≥70 yrs, mean=40; 60-69 yrs, mean=33; and < 60 yrs, mean=31, p<0.0001) before implant. Similarly, differences were detected among age groups at 1 year after implant, showing the best overall HRQOL in the oldest patients (≥70 yrs, mean=77; 60-69 yrs, mean=72; and < 60 yrs, mean=70, p=0.01). Overall HRQOL improved significantly in all three age groups from before to 1 year after DT LVAD implant, as per analyses with paired data.
Table 3.
Mean VAS scores for adult primary DT LVAD patients
| < 60 years |
60 – 69 years |
70+ years |
|||||
|---|---|---|---|---|---|---|---|
| Time point | n | mean VAS* | n | mean VAS | n | mean VAS | p-value |
| All available data | |||||||
| Pre-implant | 311 | 31.3 | 371 | 33.3 | 371 | 40.2 | < .0001 |
| 1 Year Follow-up | 158 | 70.1 | 199 | 72.0 | 217 | 76.5 | .01 |
| Paired data | |||||||
| Pre-implant | 124 | 35.8 | 144 | 37.7 | 167 | 44.4 | .02 |
| 1 Year Follow-up | 124 | 70.7 | 144 | 72.7 | 167 | 77.0 | .03 |
| Difference (1 yr-pre) | 124 | 34.9 | 144 | 35.0 | 167 | 32.6 | .77 |
| p-value | < .0001 | < .0001 | < .0001 | ||||
VAS=visual analog scale
Importantly, the amount of change in the VAS score across time did not differ by age group (≥70 yrs, mean change=33; 60-69 yrs, mean change=35; and < 60 yrs, mean change=35, p=0.77) (table 3). Sensitivity analyses, without assignment of 0 for the VAS (when patients were too sick to respond) resulted in similar findings (i.e., similar improvement in mean VAS scores and similar amounts of change for the three age groups across time). The vast majority of patients in each age group demonstrated clinically meaningful improvement (i.e., > 10 points) in their VAS scores from before to 1 year after DT LVAD implant (≥70 yrs, 74.3%; 60-69 yrs,74.9%; and < 60 yrs, 73.4%) (table 4).
Table 4.
Change in VAS scores by age: pre vs 12 months post implant
| < 60 years |
60–69 years |
70+ years |
||||||
|---|---|---|---|---|---|---|---|---|
| Unit Change | n | % | n | % | n | % | ||
| No Change or Decline | >10 units worse | 10 | 8.1% | 8 | 5.6% | 8 | 4.8% | |
| >1-10 units worse | 5 | 4.0% | 9 | 6.3% | 8 | 4.8% | ||
| Change | 5 | 4.0% | 9 | 6.3% | 5 | 3.0% | ||
| Improvement | 1-10 units better | 13 | 10.5% | 10 | 6.9% | 22 | 13.1% | |
| *MID | 11-20 units better | 14 | 11.3% | 18 | 12.5% | 27 | 16.1% | |
| 21-30 units better | 14 | 11.3% | 13 | 9.0% | 18 | 10.8% | ||
| 31-40 units better | 14 | 11.3% | 16 | 11.1% | 17 | 10.2% | ||
| 41-50 units better | 6 | 4.8% | 17 | 11.8% | 17 | 10.2% | ||
| 51-60 units better | 12 | 9.7% | 10 | 6.9% | 14 | 8.4% | ||
| 61-70 units better | 10 | 8.1% | 14 | 9.7% | 11 | 6.6% | ||
| 71+ units better | ?1 | 16.9% | 20 | 13.9% | 20 | 12.0% | ||
| Totals | 124 | 100.0% | 144 | 100.0% | 167 | 100.0% | ||
| p(overall)=.76 | ||||||||
MID=minimally important difference
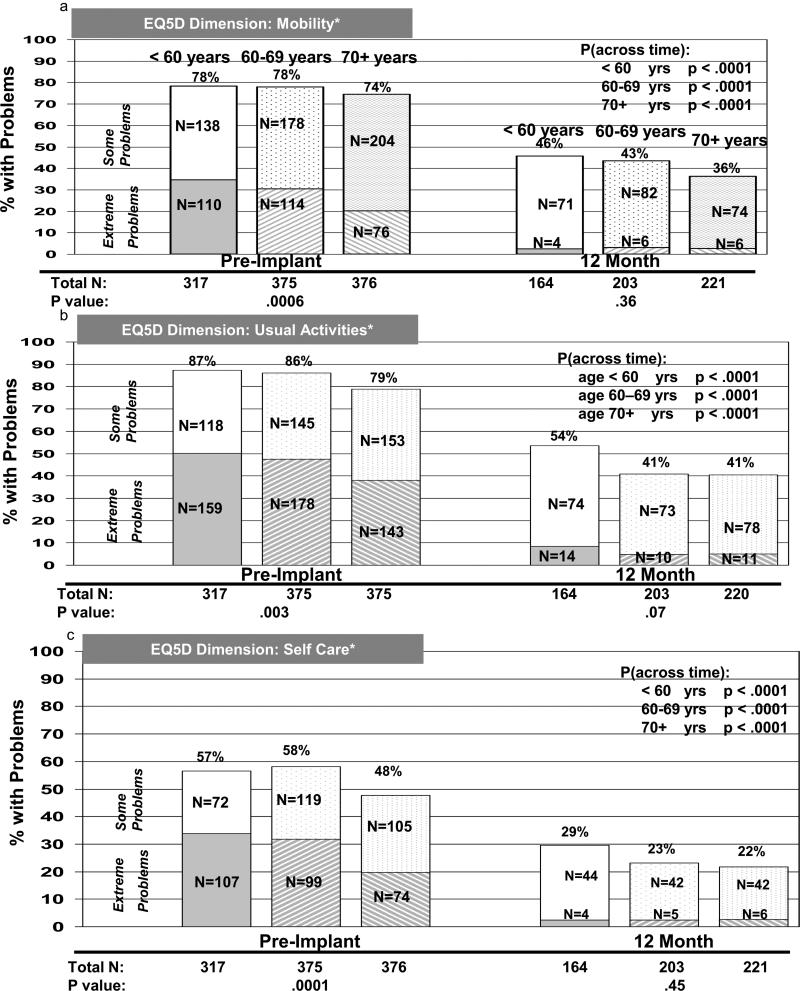
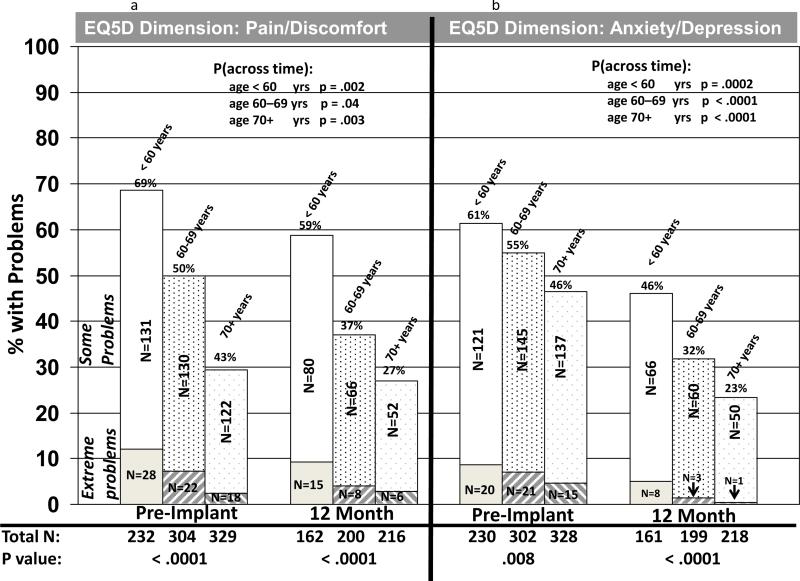
EQ-5D dimensions were also examined by age group cross-sectionally (within each time period) and longitudinally. Before DT LVAD implant, there were significant differences among the three age groups regarding reporting of problems for all five dimensions, with older patients reporting fewer problems than younger cohorts (figures 2 and 3). Differences were not significant among the age groups at 12 months after implant for mobility, self-care, and usual activities, although there was a trend toward younger patients having more problems with usual activities than the two older age groups (figure 2a-c). However, the oldest patients reported fewer problems at 12 months after implant, as compared to the two younger groups regarding pain / discomfort and anxiety / depression (figure 3a-b). For both physical function / activities of daily living (dimensions=mobility, self-care, and usual activities) and pain / emotions (dimensions=pain / discomfort and anxiety / depression), patients in all three age groups reported significantly fewer problems from before to 1 year after implant (figures 2 and 3).
Figure 2.
EQ-5D Physical Function / Activities of Daily Living Dimensions by Age Group
Figure 3.
EQ-5E Pain / Emotions Dimensions by Age Group
Multivariable analyses of factors related to HRQOL by age group
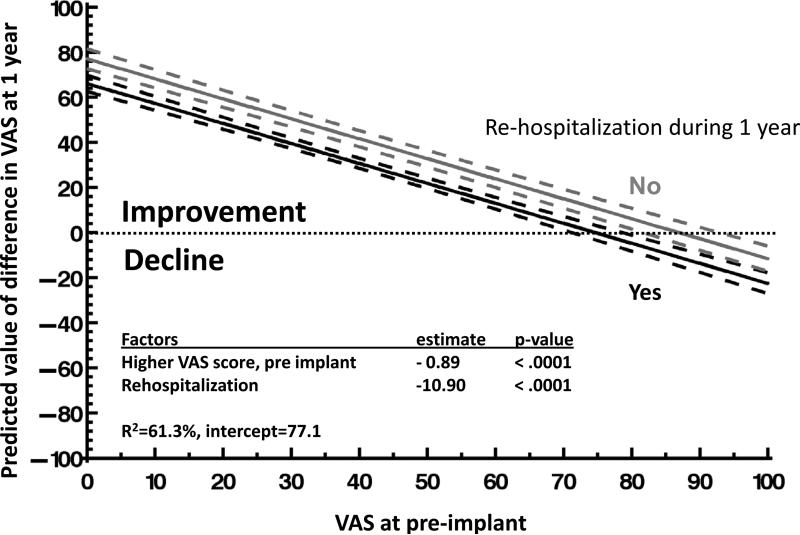
Factors associated with change in overall HRQOL from before to 1 year after DT implant were examined, using change in the VAS score from before to 1 year after implant as the dependent variable. Factors associated with improvement in HRQOL from before to 1 year after implant were a lower VAS score pre implant and fewer re-hospitalizations after implant (R2=61.3%, p< 0.0001) (figure 4). Age was not a significant variable both before and after adjustment for re-hospitalization.
Figure 4.
Factors Associated with Change in HRQOL (VAS score), n=435
DISCUSSION
Older patients reported better HRQOL than younger patients before and 1 year after DT LVAD implantation; yet overall HRQOL improved similarly, independent of age. The vast majority of patients in all three age groups improved overall HRQOL by more than 10 units from before to 1 year after LVAD implant, and the amount of change in HRQOL was similar for all three age groups across time. Rehospitalization was associated with less improvement in HRQOL from before to 1 year after implant. Notably, the youngest age group (< 60 years) differed from the older age groups socio-demographically and behaviorally. Since younger patients are more commonly bridged to transplant with an LVAD, factors related to HRQOL in this younger DT cohort require further study. In other studies, older age is related to lower VAS scores and lower EQ-5D index scores (which correlate highly with VAS scores), including in patients with chronic conditions.27, 28 These findings are in contradistinction to our findings. The higher pre implant VAS scores of older patients may have been due to being less sick (i.e., fewer older patients having INTERMACS profile 1 at time of implant) than younger cohorts.
Our findings provide important new information about HRQOL with which to inform patients who are considering DT LVAD implant and help guide more tailored care after implant by age group. For example, patients can be informed that HRQOL improves after DT LVAD implant, irrespective of age. Also, it is important to inform patients about the risk of re-hospitalization and its potential effect on HRQOL after implant.
The LVAD and heart transplant literature partially support our findings. Adamson et al.,29 compared HRQOL between younger (< 70 years [n=25]) and older (≥ 70 years [n=30]) patients from a single site whose implant strategies were bridge to transplant or DT. Both groups experienced improved HRQOL from baseline to 6 months after implant, with no difference between groups, perhaps because their sample sizes were small. We reported that older heart transplant recipients were more satisfied with HRQOL, had less negative affect and depression, and had better overall functioning than younger and middle-aged heart transplant recipients at 5 years after surgery.19 Similarly, in this report, we found that older DT LVAD patients had less depression than younger patients after implant. Older age was also related to enhanced (emotional) quality of life in heart transplant candidates.30
Reports from other chronic illness populations, after invasive device therapy, also provide support for some of our findings. Older heart failure patients who undergo cardiac resynchronization therapy derive similar HRQOL benefits as younger patients, for as long as 2 years.31, 32 Older patients with end-stage renal disease who undergo peritoneal dialysis or hemodialysis, also report HRQOL that is better than or similar to younger age groups.33-35 When domains were examined, older dialysis patients were more challenged by physical problems, but mental health was similar to or better than reported by younger patients.33, 34 The finding of more physical problems in older dialysis patients is different than our findings, wherein the frequency of physical problems was similar among the age groups after implant, which may be explained by elderly DT MCS patients having a lower risk profile than younger patients.8
The relationship between hospital readmission and HRQOL deserves comment. Our finding that re-hospitalization was associated with less improvement in HRQOL pre to post DT LVAD implant may be due to frequency of readmissions. Rates of hospital readmission during the first year after LVAD implant are 65% (e.g., due to gastrointestinal bleeding, cardiac-related causes, infection, stroke, and renal failure).36, 37
We also found that less improvement in the VAS score from before to 1 year after implant was related to having a higher VAS score before implant. This may be a ceiling effect (i.e., inability to discriminate between comparatively good health states), 25 but is also logical and supported by our previous report from INTERMACS wherein we found that patients with higher INTERMACS profiles (and higher HRQOL) had less change in HRQOL than patients with lower INTERMACS profiles (and lower HRQOL) before implant.38 Similar findings have been reported in the cardiac surgical literature.39
Our study has limitations. We collected HRQOL data using a brief, generic HRQOL survey, which may have been less responsive in this population of patients, since it is not disease and / or treatment specific. Reduced instrument completion before and after LVAD implantation may have limited generalizability of our findings. To address this issue, we used a strategy of post hoc assignment of scores for patients who were too sick to respond. Also, only variables available in the registry were used to build models; other variables (e.g., socio-economic factors and family support), not collected in the registry, may have explained variance in HRQOL. Notably, educational level, which is a commonly used proxy for socio-economic status, was included in our modeling. Finally, survivorship bias in our cohort may have contributed to overly optimistic findings. However, there was no difference in survival by age group.
CONCLUSION
Overall HRQOL improves similarly over time for all age groups after DT LVAD implantation, although older patients experience better HRQOL than younger patients before and after implant. Hospitalization after implant is an important factor associated with less improvement in HRQOL from before to 1 year after implant. These findings add to the body of evidence by which clinicians can educate and inform patients considering VADS as a treatment option, as well as tailor care to patients of all ages who are living with VADs.
Acknowledgments
FUNDING SOURCES
“This project has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Kathleen Grady, PhD – AHA grant-in-aid (PI)
David Naftel, PhD – no disclosures
Susan Myers, BA – no disclosures
Mary Amanda Dew, PhD – no disclosures
Gerdi Weidner, PhD – supported by grants from the Alexander-von-Humboldt Foundation
John Spertus, MD – Grant support from ACCF, NIH, PCORI, Amorcyte, Lilly, Genentech and Gilead.
Owns the copyright to the KCCQ. Serves as a consultant to the Scientific Advisory Board of United Healthcare, Amgen and Jannsen.
Katharine Idrissi, RN, MSN – no disclosures
Hochang B. Lee, MD – no disclosures
Edwin C. McGee, MD – consultant and speaker HeartWare, Inc.
James K Kirklin, MD – no disclosures
Contributor Information
Kathleen L. Grady, Northwestern University Chicago, IL.
David C. Naftel, University of Alabama, Birmingham Birmingham, AL.
Susan Myers, University of Alabama, Birmingham Birmingham, AL.
Mary Amanda Dew, University of Pittsburgh Pittsburgh, PA.
Gerdi Weidner, San Francisco State University San Francisco, CA.
John A. Spertus, St. Luke's Mid America Heart Institute and UMKC Kansas City, MO.
Katharine Idrissi, Columbia University, New York, NY.
Hochang B. Lee, Yale University New Haven, CT.
Edwin C. McGee, Northwestern University Chicago, IL.
James K. Kirklin, University of Alabama, Birmingham Birmingham, AL.
REFERENCES
- 1.Vincent G, Velkoff V. The older population in the United States: 2010 to 2050. U.S. Census Bureau, U.S, Department of Commerce; May, 2010. The next four decades. pp. P25–1138. [Google Scholar]
- 2.Go A, Mozaffarian D, Roger V, et al. Heart Disease and Stroke Statistics-2013 Update A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller L. Is left ventricular assist device therapy underutilized in the treatment of heart failure? Circulation. 2011;123:1552–1558. doi: 10.1161/CIRCULATIONAHA.110.958991. [DOI] [PubMed] [Google Scholar]
- 4.Stehlik J, Edwards L, Kucheryavaya A, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th Official adult heart transplant report-2012. J Heart Lung Transplant. 2012;31(10):1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Jessup M, Abraham W, Casey D, et al. Focused Update: ACCF/AHA Guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 6.Feldman D, Pamboukian S, Teuteberg J, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support: Executive Summary. 2013;32(2):157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin J, Naftel D, Kormos R, et al. Third INTERMACS annual report: The evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin J, Naftel D, Pagani F, et al. Long-term mechanical circulatory support (destination therapy): On track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584–603. doi: 10.1016/j.jtcvs.2012.05.044. PMC3443856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirklin J, Naftel D, Kormos R, et al. Fifth INTERMACS annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Sandner S, Zimpfer D, Zrunek P, et al. Age and outcome after continuous-flow left ventricular assist device implantation as bridge to transplant. J Heart Lung Transplant. 2009;28:367–372. doi: 10.1016/j.healun.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal A, Pant R, Kumar S, et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012;93:1534–1540. doi: 10.1016/j.athoracsur.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 12.Grady KL, Meyer PM, Dressler D, Mattea A, Chillcott S, Loo A, et al. Longitudinal change in quality of life and impact on survival after left ventricular assist device implantation. Ann Thorac Surg. 2004;77(4):1321–1327. doi: 10.1016/j.athoracsur.2003.09.089. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 14.Rogers J, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: Combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–683. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Wissman S, Naftel D, Myers S, et al. Predictors of health-related quality of life at 6 months after left ventricular assist device implantation: Findings from INTERMACS. J Heart Lung Transplant. 2012;31:S18. doi: 10.1016/j.healun.2016.01.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grady K, Meyer P, Mattea A, et al. Predictors of quality of life at 1 month after implantation of a left ventricular assist device. Am J Crit Care. 2002;11(4):345–352. [PubMed] [Google Scholar]
- 18.Grady K, Jalowiec A, White-Williams C. Predictors of Quality of Life in Patients at 1 Year After Heart Transplantation. The Journal of Heart and Lung Transplantation. 1999;18(3):202–210. doi: 10.1016/s1053-2498(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 19.Shamaskin A, Rybarczyk B, Wang E, White-Williams C, Cotts W, McGee E, Grady K. Older patients have better quality of life, adjustment and adherence than younger patients 5 years post heart transplantation. J Heart Lung Transplant. May. 2012;31(5):478–484. doi: 10.1016/j.healun.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Spilker B. Quality of life and pharmacoeconomics in clinical trials. Lippincott Williams & Williams; New York: 1996. [Google Scholar]
- 21.Forman DE, Berman AD, McCabe3 CH, Baim DS, Wei JY. PTCA in the elderly: the “young-old” versus the “old-old. J Am Geriatr Soc. 1992;40(1):19–22. doi: 10.1111/j.1532-5415.1992.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 22.EuroQol group EuroQol a new facility for the measurement of health related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 23.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 24.van Agt H, Essink-Bot ML, Krabbe P, Bonsel G. Test-retest reliability of health state valuations collected with the EuroQoL questionnaire. Soc Sci Med. 1994;39:1537–44. doi: 10.1016/0277-9536(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 25.Dyer M, Goldsmith K, Sharples L, Buxton M. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health and Quality of Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szende A, Janssen B. Socio-demographic indicators based on EQ-5D. In Self-reported Population Health: An International Perspective based on EQ-5D. 2014 Springer Open. [PubMed] [Google Scholar]
- 28.Sullivan P, Lawrence W, Ghushchyan V. A National Catalog of Preference-Based Scores for Chronic Conditions in the U.S. Medical Care. 2005;43(7):736–749. doi: 10.1097/01.mlr.0000172050.67085.4f. [DOI] [PubMed] [Google Scholar]
- 29.Adamson RM, Stahovich M, Chillcott S, et al. Clinical strategies and outcomes in advanced heart failure patients older than 70 years of age receiving the HeartMate II left ventricular assist device. J Am Coll Cardiol. 2011;57:2487–95. doi: 10.1016/j.jacc.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Bunyamin V, Spaderna H, Weidner G, for the Waiting for a New Heart Study Group Health behaviors contribute to quality of life in patients with advanced heart failure independent of psychological and medical patient characteristics. Quality of Life Research. 2013;22(7):1603–11. doi: 10.1007/s11136-012-0312-6. [DOI] [PubMed] [Google Scholar]
- 31.Foley PW, Chalil S, Khadjooi K, et al. Long-term effects of cardiac resynchronization therapy in octogenarians: A comparative study with a younger population. Europace. 2008;10:1302–7. doi: 10.1093/europace/eun263. [DOI] [PubMed] [Google Scholar]
- 32.Delnoy PP, Ottervanger JP, Luttikhuis HO, et al. Clinical response of cardiac resynchronization therapy in the elderly. Am Heart J. 2008;155:746–51. doi: 10.1016/j.ahj.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Taveras Ae, Bekui AM, Gorban-Brennan N, Raducu R, Finkelstein FO. Peritoneal dialysis in patients 75 years of age and older – a 22-year experience. Adv Perit Dial. 2012;28:84–8. [PubMed] [Google Scholar]
- 34.Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis study of elderly people on dialysis: a prospective cohort study. The Lancet. 2000;356:1543–50. doi: 10.1016/S0140-6736(00)03123-8. [DOI] [PubMed] [Google Scholar]
- 35.Griva K, Yu Z, Chan S, et al. Age is not a contraindication to home-based dialysis – Quality of life outcomes favour older patients on peritoneal dialysis regimes relative to younger patients. J Adv Nurs. 2014 Feb 4; doi: 10.1111/jan.12355. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Hasin T, Marmor Y, Kremers W, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 37.Forest S, Bello R, Friedmann P, et al. Readmissions after ventricular assist device: Etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95:1276–1281. doi: 10.1016/j.athoracsur.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 38.Grady KL, Naftel DC, Stevenson L, et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J Heart Lung Transplant. 2013 Oct 23; doi: 10.1016/j.healun.2013.10.017. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noyez L, Markou A, van Breugel F. Quality of life one year after myocardial revascularization. Is preoperative quality of life important? Inter Cardiovasc Thorac Surg. 2006;5:115–20. doi: 10.1510/icvts.2005.120113. [DOI] [PubMed] [Google Scholar]