Abstract
Nitric oxide is an important messenger in numerous biological processes, such as angiogenesis, hypoxic vasodilation, and cardioprotection. Although nitric oxide synthases (NOS) produce the bulk of NO, there is increasing interest in NOS independent generation of NO in vivo, particularly during hypoxia or anoxia, where low oxygen tensions limit NOS activity. Interventions that can increase NO bioavailability have significant therapeutic potential. The use of far red and near infrared light (R/NIR) can reduce infarct size, protect neurons from methanol toxicity, and stimulate angiogenesis. How R/NIR modulates these processes in vivo and in vitro is unknown, but it has been suggested that increases in NO levels are involved. In this study we examined if R/NIR light could facilitate the release of NO from nitrosyl heme proteins. In addition, we examined if R/NIR light could enhance the protective effects of nitrite on ischemia and reperfusion injury in the rabbit heart. We show both in purified systems and in myocardium that R/NIR light can decay nitrosyl hemes and release NO, and that this released NO may enhance the cardioprotective effects of nitrite. Thus, the photodissociation to NO and its synergistic effect with sodium nitrite may represent a noninvasive and site specific means for increasing NO bioavailability.
Introduction
Nitric oxide (NO) is enzymatically generated during the oxygen-dependent conversion of L-arginine to L-citrulline by the nitric oxide synthases (NOS). These enzymes are present or are inducible in numerous cell types, including vascular endothelium, neuronal cells, macrophages and cardiomyocytes[1]. Although NOS are primarily responsible for the bulk of NO production, there is increasing interest in NOS independent generation of nitric oxide in vivo, particularly during hypoxia or anoxia, where low oxygen tensions limit the activity of the NOS enzymes [2]. Nitrite, and to some extent nitrate through its reduction to nitrite, have been identified as additional physiological sources of NO [3]. Identified nitrite reductases include deoxyhemoglobin [4, 5], deoxymyoglobin [6], xanthine oxidoreductase [7], cytochrome c oxidase [8, 9] and NOS itself [10]. In the case of hemoglobin, the nitrite reductase activity is related to the redox potential of the heme iron, and is significantly greater in the ‘R’ conformation than in the ‘T’ conformation allowing maximal nitrite reduction at approximately 50% oxygen saturation [11–13]. Once formed, a significant portion of the NO generated will bind to vacant deoxygenated hemes to yield the product iron-nitrosyl hemoglobin (HbNO) or iron-nitrosyl myoglobin (MbNO) [6, 14]. The extremely slow off-rate of NO from these ferrous heme complexes renders them largely inert in terms of NO storage, although it has been suggested that processes which oxidize the heme iron could facilitate NO release [6]. These observations suggest any exogenous stimulation that could enhance NO release from these stable complexes could potentiate the NO-dependent effects of nitrite.
There is tremendous interest in the use of light for clinical applications. Wavelengths in the red to near infrared (R/NIR) spectrum between 630 and 830nm have been extensively studied in vivo and in vitro. In this range there is improved tissue penetration due to the absence of melanin absorption in the skin [15]. Water exhibits minimal light absorption, thereby reducing radiant heat production in the exposed tissue to below 0.5°C [16, 17]. Near infrared light has been used to reduce infarct size, protect neurons from methanol toxicity, heal chemotherapy induced mucositis, and stimulate angiogenesis [18–21]. Increased nitric oxide is a postulated effector in the mechanism by which near infrared light exerts its actions, but how nitric oxide becomes bioavailable is not well understood. There is strong evidence that cytochrome c oxidase can act as a photoreceptor allowing the photolytic dissociation of any bound nitric oxide [22, 23]. Additionally, the nitric oxide present in the heme-Cuiia3 can be photolysed by visible light [24].
In this study we examined if R/NIR light could facilitate the release of NO from nitrosyl heme proteins. In addition, we examined if R/NIR light could enhance the protective effects of nitrite on ischemia/reperfusion injury in the isolated rabbit heart. We show in both chemical systems and in heart tissue that R/NIR light can decay nitrosyl hemes and release NO, and that this released NO may enhance the cardioprotective effects of nitrite. We suggest that R/NIR exposure may enhance the efficacy of nitrite-dependent protective strategies.
Materials and Methods
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Furthermore, all conformed to the Guiding Principles in the Care and Use of Animals of the American Physiologic Society and were in accordance with the Guide for the Care and Use of Laboratory Animals.
All experiments were carried out in 10 mM phosphate buffered saline pH 7.40 (PBS). Diethylenetriamine pentaacetic acid (DTPA) was added to all buffers at a concentration of 1mM for chelation of metal ions.
R/NIR source
The 670nm LED light source with power output up to 100mW/cm2 was obtained from Quantum Devices. Power output was measured with a light meter (X97 Irradiance meter, GigaHertz-Optik). The light sources were placed 2.5 cm from their target in all experiments.
Generation of Nitrosyl Hemoglobin
Oxyhemoglobin (OxyHb) was prepared from fresh human blood as previously described [25]. The oxyHb solution (approximately 200 µM) was placed in a long-neck quartz cuvette closed with an airtight rubber stopper. The sample was deoxygenated using gentle agitation with alternating vacuum assisted removal of oxygen and introduction of argon gas, and monitored spectrophotometrically. After deoxygenation, the NO donor, PROLI NONOate (Cayman Chemical) was added, from a deoxygenated 10 mM stock solution in 100 mM NaOH, and the UV/Vis spectrum (HP 8453) was acquired between 450 and 700 nm to verify conversion to nitrosyl hemoglobin. HbNO concentration was determined using an extinction coefficient of 12.5 mM−1cm−1 at 575 nm [26].
Generation of Nitrosyl Myoglobin
Metmyoglobin (metMb) from horse skeletal Muscle (Sigma) dissolved in PBS containing 1 mM DTPA was degassed and saturated with high purity argon, then reduced to deoxymyoglobin (deoxyMb) by titration with sodium dithionate (Sigma). Myoglobin reduction was followed spectrophotometrically. When myoglobin reached the fully deoxy state, excess of highly concentrated PROLI NONOate (Cayman Biochemicals) dissolved in 100 mM NaOH was added. Solutions were made daily, and stored under argon.
Ozone Chemiluminescence Measurement of Nitric Oxide
HbNO or MbNO (20µM) in PBS containing DTPA (1 mM) and Antifoam emulsion B were added to a purge vessel, equilibrated at 30 °C in line with a chemiluminescence NO gas analyzer (Sievers NO analyzer; GE Analytical Instruments) for the detection of NO. The vessel was purged with argon gas. The purge vessel was irradiated with the R/NIR placed 2.5 cm from the reaction vessel. NO was detected by chemiluminescence after reaction with ozone. In addition, oxyHb was deoxygenated in the purge vessel before addition of nitrite (1 mM). This mixture was then subjected to irradiation and generated NO was detected after reaction with ozone. For quantification purposes, a standard curve was constructed using PROLI NONOate.
UV-Visible Spectrum Analysis
HbNO (final concentration 20µM) was added to a quartz cuvette under aerobic conditions and irradiated with the R/NIR light source placed 2.5 cm above the cuvette. Visible absorption spectra were taken every 2.5s and deconvoluted using least squares analysis as previously described [13]. The standard reference spectra used for the least squares analysis were oxyhemoglobin, deoxyhemoglobin, iron-nitrosyl-hemoglobin, and methemoglobin. All experiments were performed in triplicate.
Electron Spin Resonance of MbNO
Rabbit myocardium from the control and ischemic zones was pulverized in liquid nitrogen and packed in an EPR sample tube. Tissue was kept frozen at all times. The tissue was examined by EPR (Bruker Elexsys) using the following conditions: temperature, 100 K; modulation amplitude: 5G; microwave power: 5 mW; scan width: 400G; scan time 41.94s, time constant: 20.48ms. The remaining control and ischemic tissue after pulverization was irradiated with red light (670 nm, 11 J total energy) before EPR analysis. All experiments were performed in triplicate.
Myocardial Ischemia Reperfusion Experiments
Male New Zealand white rabbits weighing between 2.5 and 3.0 kg were anesthetized with intravenous sodium pentobarbital (30 mg/kg) as previously described[27]. Briefly, a tracheostomy was performed through a midline incision, and each rabbit was ventilated with positive pressure using an air-oxygen mixture (FiO2 30%). Arterial blood gas tensions and acid-base status were maintained within a normal physiological range by adjusting the minute ventilation. A thoracotomy was performed at the left fourth intercostal space, a prominent branch of the left anterior descending coronary artery (LAD) was identified, and a silk ligature was placed around this vessel approximately halfway between the base and the apex for the production of coronary artery occlusion and reperfusion. Coronary artery occlusion was verified by the presence of epicardial cyanosis and regional dyskinesia in the ischemic zone, and reperfusion was confirmed by observing an epicardial hyperemic response. Hemodynamics were continuously recorded on a polygraph throughout each experiment.
For the in vivo generation of MbNO using nitrite in rabbit hearts, 50 mg of sodium nitrite dissolved in 0.9% saline was infused into the left atrium during 30 min LAD occlusion. After occlusion, a 5 min reperfusion was performed to ensure adequate tissue penetration of the sodium nitrite into the ischemic zone. Since MbNO generation requires nitrite in the presence of hypoxia, a 5 min reocclusion period was performed. The heart was excised at the end of reocclusion and snap frozen in liquid nitrogen.
To assess the effect of R/NIR on cardiac ischemia and reperfusion injury, all rabbits underwent a 30 min LAD occlusion followed by 3h of reperfusion. In six separate groups, rabbits (n = 6 to 7 per group) were randomly assigned (Latin square design) to receive 0.9% saline (control Group 1), or R/NIR irradiation (670 nm at 60mW/cm2) was applied to the heart for 3 min followed by a 3 min resting period. Group 2 received 3 cycles of irradiation (32.4 J) prior to the onset of ischemia. Group 3 received 5 cycles of irradiation prior to ischemia (54 J). Group 4 received 0.4mg/kg sodium nitrite (Sigma) dissolved in 0.9% normal saline i.v. 30 minutes prior to ischemia. Group 5 received 4mg/kg sodium nitrite in 0.9% normal saline as an i.v. 30 min prior to ischemia. Group 6 received a 0.4mg/kg sodium nitrite i.v. and then 3 cycles of R/NIR irradiation (32.4 J) prior to the onset of ischemia.
Cardioprotection studies were repeated using infusions of the nitric oxide scavenger carboxy-PTIO (Alexis Chemicals). Group 1 received 5 cycles of irradiation (54 J) prior to ischemia, with an infusion of c-PTIO at 0.17mg/kg/min during the entire period of irradiation. Group 2 received 0.4mg/kg sodium nitrite i.v. and then 3 cycles of irradiation (32.4 J) with a c-PTIO infusion of 0.17mg/kg/min during the irradiation period prior to the onset of ischemia.
To assess the effect of R/NIR when applied upon reperfusion, additional rabbits underwent a 30 min LAD occlusion followed by 3h of reperfusion. In four groups (n=7/group) rabbits were randomly assigned. Group 1 received 3 min of irradiation (60mW; 11 J) at the time of reperfusion, with light exposed to the myocardium just prior to the release of the ligature. Group 2 received 3 min of irradiation (3mW; 0.5 J) Group 3 received 0.4mg/kg sodium nitrite (Sigma) dissolved in 0.9% normal saline i.v. 30 minutes prior to ischemia. Group 4 received 0.4mg/kg sodium nitrite i.v. 30 min prior to ischemia and then 3 min of irradiation (3mW; 0.54 J) upon reperfusion.
To assess the role of nitric oxide scavenging on R/NIR cardioprotection, 2 additional groups were added. Group 1 received an infusion of c-PTIO (0.17mg/kg/min) over the 3 min period of irradiation (60mW/cm2) at the time of reperfusion. Group 2 received 0.4mg/kg sodium nitrite i.v. 30 min prior to ischemia and then c-PTIO (0.17mg/kg/min) over the 3 min of irradiation (0.54 J) at reperfusion.
Myocardial infarct size was determined in the following manner. The LAD was reoccluded at the completion of each experiment and 3 ml of patent blue dye was injected i.v. The left ventricular area at risk for infarction was separated from surrounding normal areas (stained blue), and the two regions were incubated at 37°C for 20 min in 1% 2,3,5-triphenyltetrazolium chloride in 0.1 M phosphate buffer adjusted to pH 7.4. Infarcted and noninfarcted myocardium within the area at risk were carefully separated and weighed after storage overnight in 10% formaldehyde. Myocardial infarct size was expressed as a percentage of the area at risk. Rabbits that developed intractable ventricular fibrillation and those with an area at risk less than 15% of total left ventricular mass were excluded from subsequent analysis.
Statistical analysis of data within and between in vivo groups was performed with analysis of variance (ANOVA) for repeated measures followed by Bonferroni's modification of Student's t test [28]. Statistical anaysis of data in vitro was with a Student’s t test. Changes were considered statistically significant when P<0.05. All data are expressed as mean ± standard deviation (SD).
Results
The Effect of 670 nm Light on the Absorption Spectra of Nitrosyl Hemoglobin
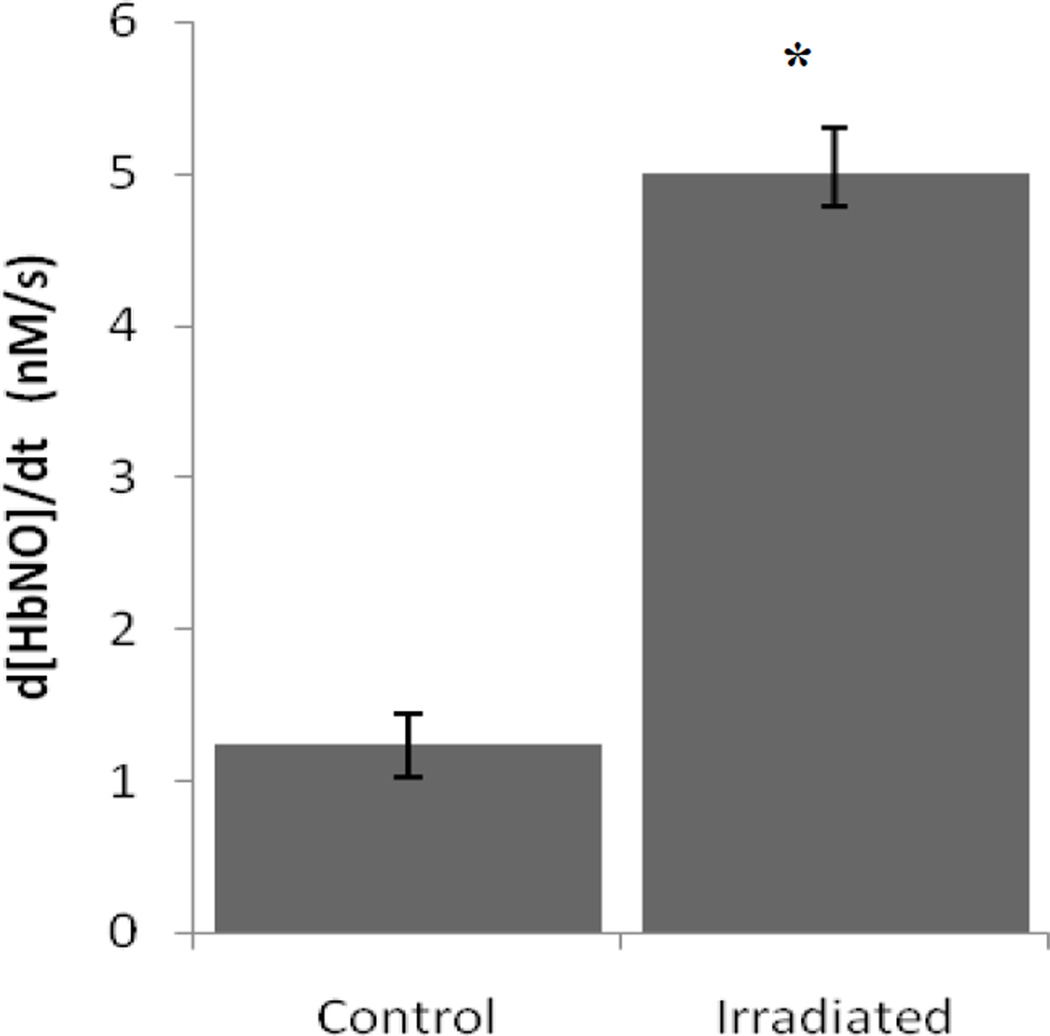
In oxygenated solution, HbNO slowly converts to metHb as a result of the release of NO. To examine if R/NIR irradiation could accelerate this processes, HbNO (20 µM) was irradiated with 670 nm light and visible spectra were obtained every second for 300 sec. Spectra were deconvoluted by multiple linear regression analysis using basis spectra for metHb and HbNO. Figure 1 shows the effect of 670 nm irradiation on the decay rate of HbNO. The background rate of HbNO conversion to metHb was increased 4 fold due to 47mW/cm2 (14 J) of irradiation. These data suggest that this light source facilitates the release of NO from nitrosyl heme proteins.
Figure 1.
UV-visual absorbance data was collected from control HbNO (20 µM), and HbNO exposed to 14 J of light at 670nm (n=3). The decay rate of HbNO was calculated from linear regression analysis using basis spectra for metHb and HbNO. Irradiation produced a 4-fold increase in the rate of metHb conversion. p<0.05.
Effect of R/NIR Light on NO release from Nitrosyl Hemoglobin
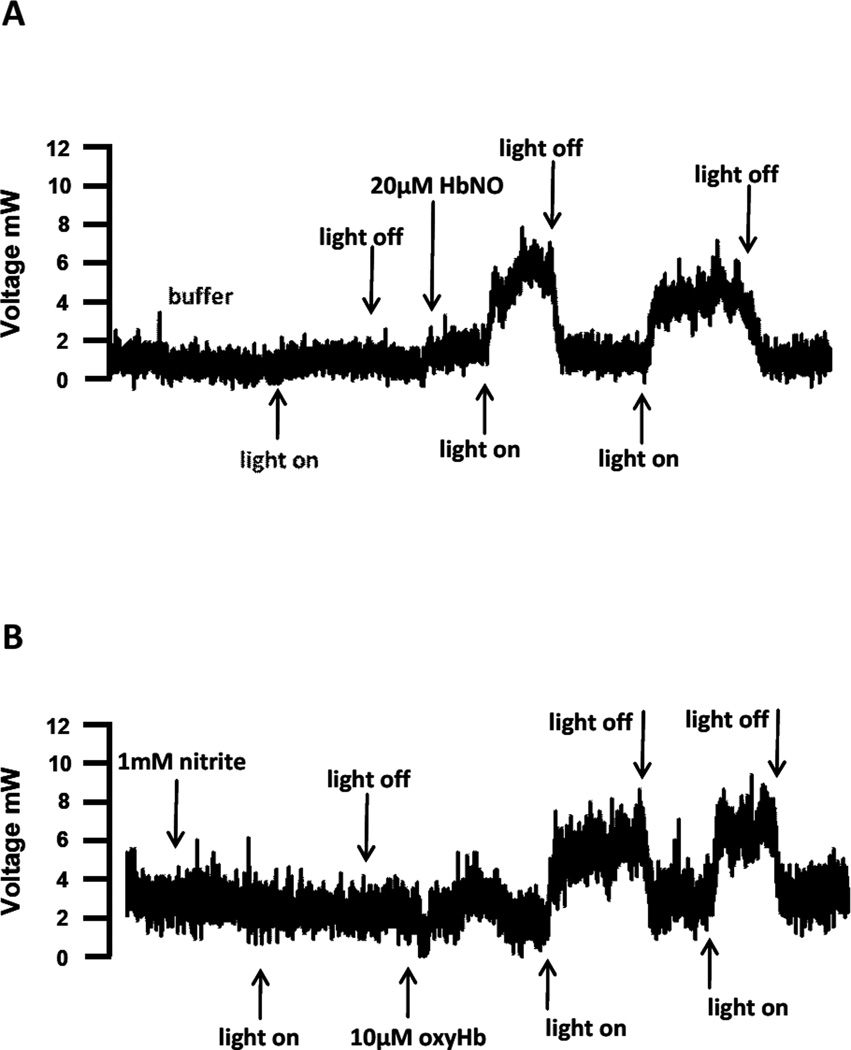
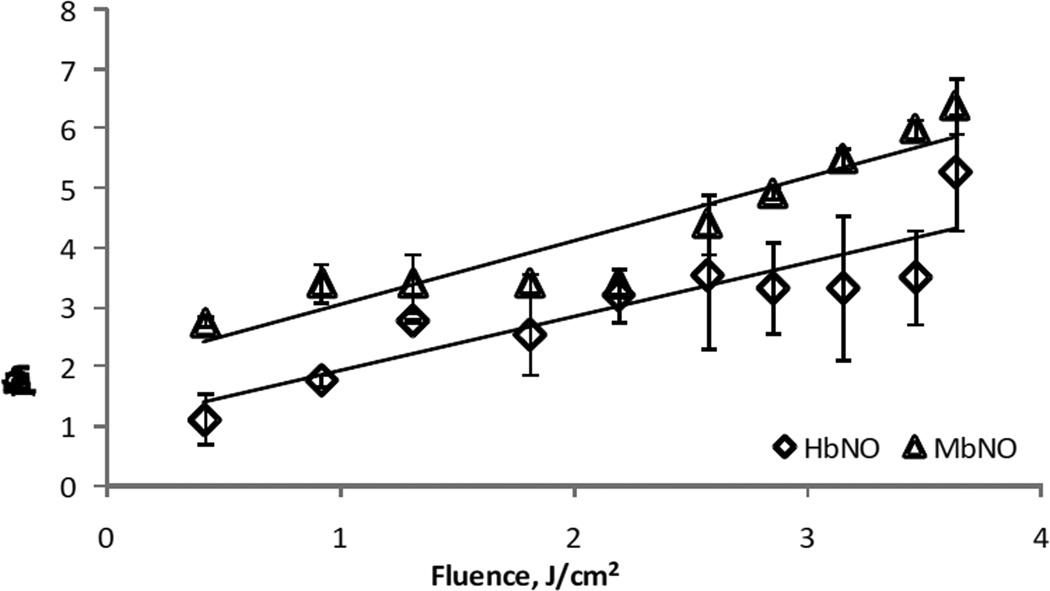
In order to examine and quantify NO release, 20µM HbNO was added to a glass purge vessel attached to a chemiluminescence analyzer and irradiated with 670nm light. As shown in Figure 2A, irradiation of buffer in the purge vessel of the NO analyzer did not generate any chemiluminescence signal. However, after injection of HbNO, a chemiluminescence signal was detected upon initiation of irradiation, and the signal returned to baseline upon cessation of irradiation in a repeatable manner. No signal was observed if either HbO2 or nitrite were added to the purge vessel and irradiated (data not shown and Figure 2B). This data strongly suggests that 670nm light is able to liberate NO from HbNO. Similar results were obtained using MbNO (data not shown). Interestingly, if nitrite was added to the purge vessel, the addition of oxyHb resulted in an increase in the chemiluminescence signal during irradiation, which returned to baseline upon cessation of irradiation. If the light was subsequently cycled on and off, a robust signal was observed only in the presence of irradiation. An interpretation of these results is that the combination of oxyHb and nitrite, in the anaerobic environment of the purge vessel generates NO via the nitrite reductase activity of deoxyHb. A significant proportion of the liberated NO is autocaptured by hemoglobin to form HbNO, and irradiation with 670 nm light liberates a portion of this autocaptured NO. Figure 3 shows a quantification of the amount of NO released from both HbNO and MbNO as a function of irradiation power. The range of NO formation rates (between 1 and 6 pmol/s) corresponds to an HbNO decay rate of between 0.5 and 3 nM/s in the 2 ml volume of the purge vessel, agreeing well with the data obtained by spectrophotometry (Figure 1).
Figure 2.
Ozone chemiluminescent detection of nitric oxide generated from 670nm. A) A tracing where 20uM HbNO was exposed to light (18mW/cm2). Peak height increased only with the application of light to HbNO. B) 1mM sodium nitrite could not be directly photolysed by 670nm energy (18mW/cm2), however once oxyHb was added to the sodium nitrite and the solution was deoxygenated with argon gas to generate HbNO, the same application of 670nm light energy resulted in NO detection.
Figure 3.
Quantification of NO release from HbNO by R/NIR as a function of power. 20µM HbNO and MbNO were irradiated with 670nm light at different fluences. NO release was quantified by extrapolation to a standard curve generated from Proli-NONOate. The rate of HbNO and MbNO decay increases with greater fluence.
Effect of R/NIR light on nitrosyl heme proteins in myocardium
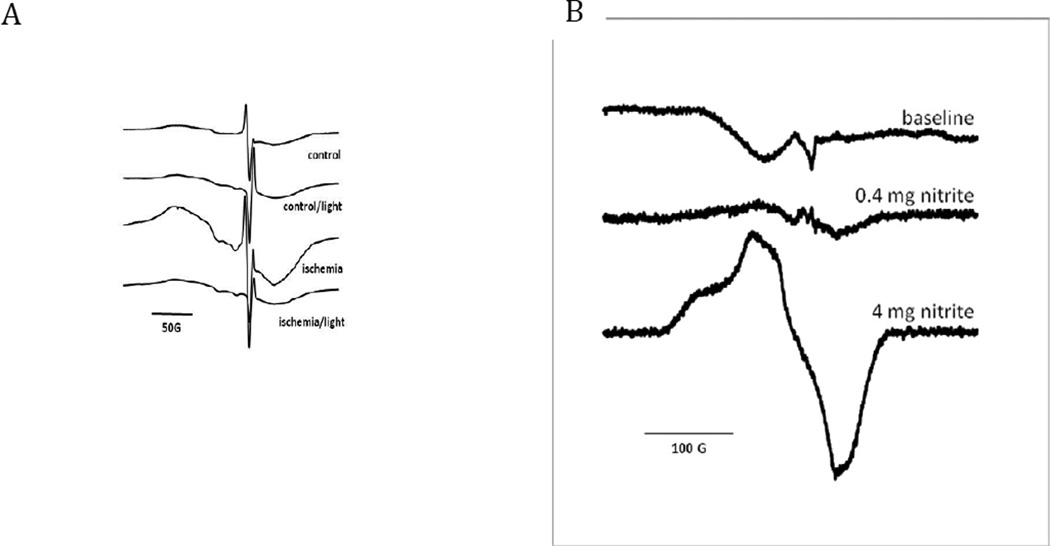
Sodium nitrite was infused into the left atrium of an anesthetized rabbit undergoing left coronary artery occlusion. Myocardium was snap-frozen and processed as described in the Methods Section. Tissue from the right, non-ischemic portion of the heart (control in Figure 4A) showed a low level of nitrosyl heme formation as measured by EPR spectroscopy. In contrast, tissue from the ischemic region exhibited a large increase in the heme nitrosyl signal. It is likely that the major origin of this heme nitrosyl signal is the reduction of nitrite to NO by cardiac nitrite reductases (including deoxymyoglobin and xanthine oxidoreductase) and the capture of nascent NO by deoxymyoglobin. When R/NIR at 670nm (18mW/cm2; 11J) was applied to the same tissue samples, the MbNO signal was reduced in the ischemic zone by ~60%, suggesting dissociation of the heme-NO bond upon irradiation. The EPR signal generated from the control region was decreased slightly with the same application of R/NIR light.
Figure 4.
A). EPR signals generated from myocardial tissue exposed to 30 minutes of ischemia while infused with sodium nitrite. The signal from the ischemic zone is highly characteristic of MbNO. This signal is dramatically reduced after exposure to 18mW/cm2 of light at 670 nm (11J). The non-ischemic control tissue has reduced MbNO signal present, with minimal reduction by light (18mW/cm2; 11J). B) EPR signals from blood collected during nitrite infusions. The characteristic HbNO signal is present at 0.4mg/kg bolus of sodium nitrite. The signal is increased with a greater dose of sodium nitrite (4mg/kg).
Effect of R/NIR light on myocardial ischemia and reperfusion injury
To examine if photolytic release of NO stores by R/NIR light is cardioprotective during ischemia and reperfusion injury, we examined the effect of R/NIR exposure in vivo with nitrite infusion. The cardioprotective effects of nitrite have been well documented in the literature [29–31]. All hemodynamic data has been provided in Table 1., with no significant hemodynamic changes between the studied groups.
Table 1.
| Reperfusion | ||||||
|---|---|---|---|---|---|---|
| Baseline | Occlusion | 3 min | 1 h | 2 h | 3 h | |
| HR (min−1) | ||||||
| control | 253 ± 35 | 246 ± 40 | 229 ± 26 | 225 ± 28 | 221 ± 29 | 212 ± 31 |
| NIR5x | 264 ± 37 | 245 ± 10 | 240 ± 23 | 235 ± 21 | 230 ± 25 | 221 ± 21 |
| NIR3x | 244 ± 31 | 204 ± 15 | 209 ± 15 | 208 ± 20 | 204 ± 22 | 189 ± 10 |
| .4Nitr | 253 ± 29 | 250 ± 24 | 234 ± 20 | 237 ± 19 | 222 ± 19 | 227 ± 23 |
| 4.0Nitr | 260 ± 25 | 245 ± 20 | 238 ± 17 | 233 ± 16 | 222 ± 12 | 218 ± 16 |
| .4Nitr+NIR3x | 248 ± 29 | 217 ± 29 | 219 ± 28 | 212 ± 32 | 203 ± 22 | 198 ± 22 |
| 3mWRad-Post | 253 ± 21 | 233 ± 21 | 220 ± 12 | 221 ± 15 | 209 ± 12 | 201 ± 11 |
| 60mWRad-Post | 241 ± 13 | 222 ± 18 | 210 ± 18 | 206 ± 21 | 199 ± 17 | 196 ± 18 |
| 3mWRad-Post+.4Nitr | 244 ± 27 | 219 ± 23 | 214 ± 25 | 214 ± 26 | 206 ± 30 | 201 ± 31 |
| MAP (mmHg) | ||||||
| control | 74 ± 10 | 58 ± 9 | 57 ± 6 | 60 ± 9 | 61 ± 11 | 59 ± 10 |
| NIR5x | 74 ± 14 | 55 ± 8 | 67 ± 10 | 68 ± 12 | 66 ± 7 | 66 ± 7 |
| NIR3x | 76 ± 8 | 56 ± 11 | 59 ± 11 | 63 ± 7 | 58 ± 7 | 64 ± 9 |
| .4Nitr | 69 ± 15 | 61 ± 12 | 60 ± 9 | 59 ± 9 | 60 ± 9 | 60 ± 9 |
| 4.0Nitr | 77 ± 13 | 60 ± 10 | 59 ± 11 | 55 ± 7 | 59 ± 12 | 62 ± 12 |
| .4Nitr+NIR3x | 73 ± 9 | 55 ± 9 | 58 ± 9 | 57 ± 8 | 58 ± 6 | 59 ± 4 |
| 3mWRad-Post | 67 ± 6 | 64 ± 2 | 64 ± 5 | 67 ± 4 | 66 ± 5 | 67 ± 6 |
| 60mWRad-Post | 71 ± 9 | 64 ± 10 | 65 ± 11 | 63 ± 9 | 61 ± 10 | 63 ± 11 |
| 3mWRad-Post+.4Nitr | 69 ± 6 | 55 ± 7 | 56 ± 9 | 59 ± 7 | 58 ± 9 | 59 ± 5 |
| RPP (min−1•mmHg•10−3) | ||||||
| control | 21.2 ± 4.3 | 16.7 ± 3.9 | 15.4 ± 2.3 | 15.7 ± 2.4 | 16.0 ± 3.6 | 14.6 ± 3.5 |
| NIR5x | 22.1 ± 4.3 | 15.7 ± 2.1 | 18.4 ± 3.2 | 18.5 ± 3.8 | 17.6 ± 2.9 | 16.9 ± 2.5 |
| NIR3x | 21.0 ± 3.5 | 13.4 ± 1.8 | 14.3 ± 2.7 | 15.1 ± 1.5 | 13.6 ± 1.4 | 13.8 ± 1.9 |
| .4Nitr | 20.4 ± 6.1 | 17.7 ± 4.8 | 16.4 ± 2.4 | 16.2 ± 2.8 | 15.6 ± 2.7 | 15.7 ± 2.7 |
| 4.0Nitr | 22.5 ± 3.8 | 17.2 ± 3.4 | 16.5 ± 3.1 | 15.1 ± 0.9 | 15.3 ± 2.6 | 15.5 ± 2.3 |
| .4Nitr+NIR3x | 20.6 ± 3.2 | 14.3 ± 2.8 | 15.0 ± 2.7 | 14.4 ± 3.1 | 13.7 ± 1.4 | 13.6 ± 1.1 |
| 3mWRad-Post | 19.5 ± 2.4 | 16.9 ± 1.6 | 16.2 ± 1.8 | 17.0 ± 1.6 | 15.9 ± 1.7 | 15.5 ± 1.6 |
| 60mWRad-Post | 19.5 ± 2.8 | 16.5 ± 3.0 | 15.8 ± 3.2 | 15.3 ± 3.3 | 14.3 ± 2.9 | 14.2 ± 3.0 |
| 3mWRad-Post+.4Nitr | 19.2 ± 2.8 | 14.3 ± 2.5 | 14.1 ± 2.7 | 14.7 ± 2.7 | 14.1 ± 3.3 | 13.9 ± 2.9 |
Data are mean ±SD
Abbreviations: HR = heart rate; MAP = mean arterial pressure; RPP = rate pressure product;
Figure 4B shows the level of HbNO formed in blood after infusion of the rabbit with 0, 0.4 and 4 mg/kg nitrite. In the absence of nitrite the signal largely consisted of a copper (II) signal (likely derived from plasma ceruloplasmin and erythrocyte superoxide dismutase). Upon infusion of 0.4 mg/kg nitrite, after subtraction of the copper background signal, the formation of a nitrosyl heme spectrum was observed. This signal was markedly enhanced after administration of 4 mg/kg nitrite.
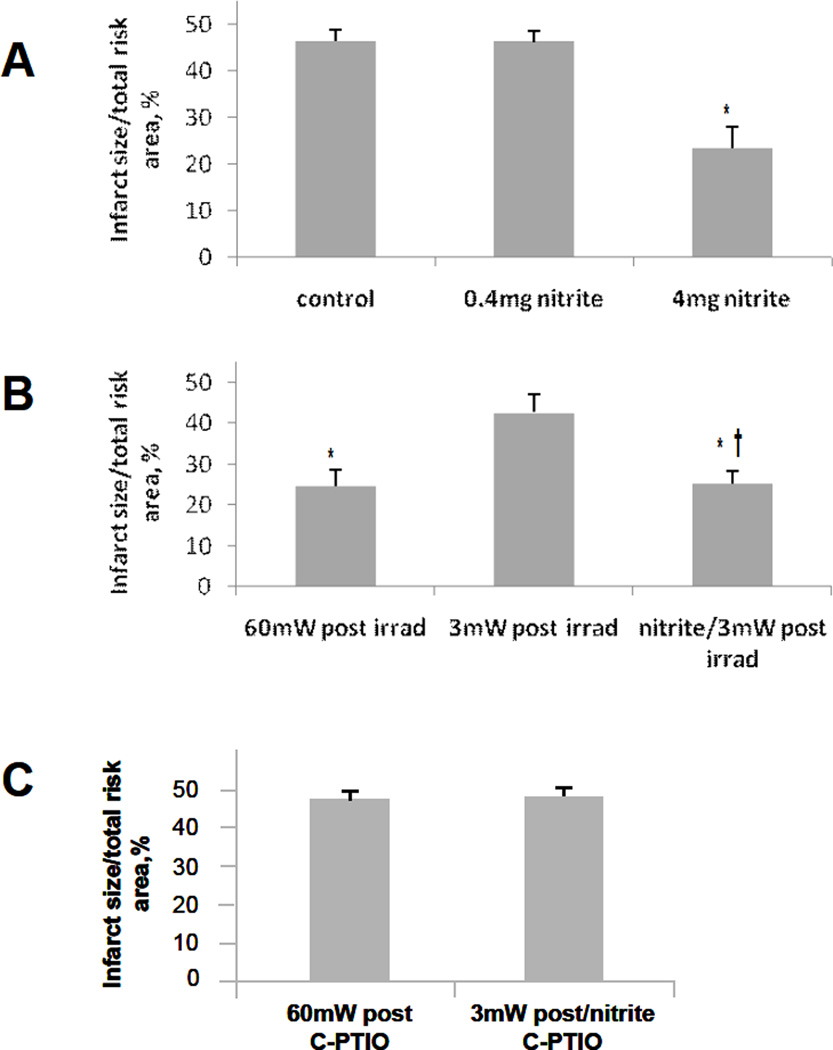
Coronary artery ischemia and reperfusion in control rabbits receiving saline (Figure 5A), caused infarction in 46% of the area of risk. If the rabbit was given a i.v. bolus of 4 mg/kg sodium nitrite before the onset of ischemia, cardioprotection was observed after reperfusion (Figure 5A). A lower dose of sodium nitrite (0.4 mg/kg) was found to be ineffective (Figure 5A), again in agreement with published studies [31].
Figure 5.
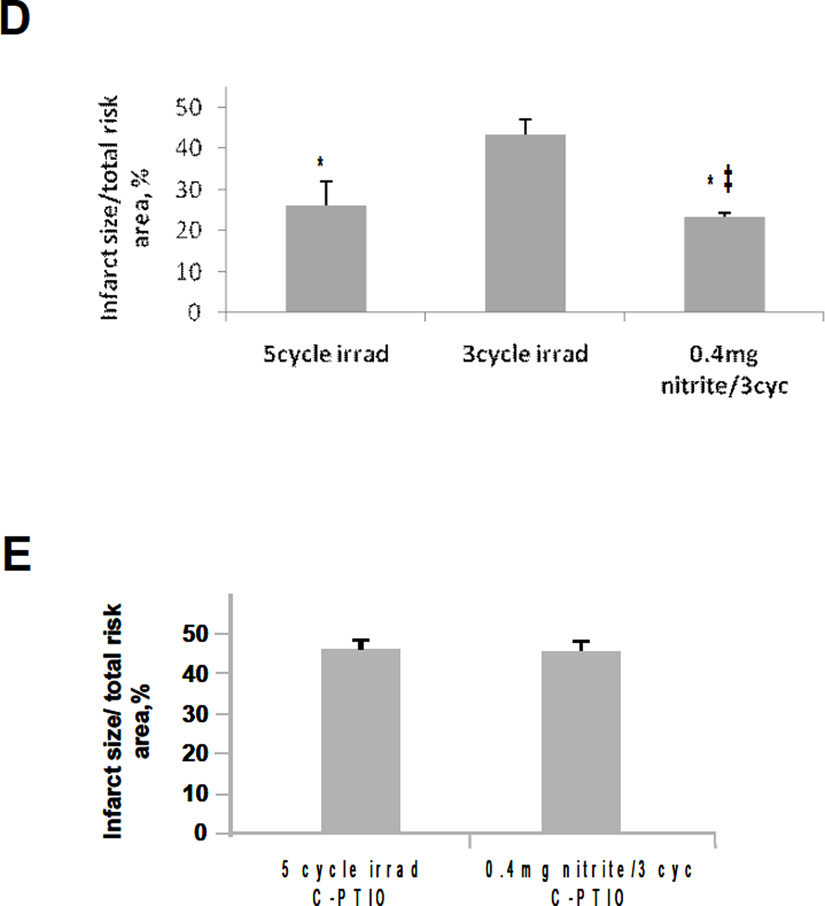
Effect of R/NIR on myocardial ischemia and reperfusion injury. A) The control group received normal saline prior to ischemia. Infarct size was significantly reduced compared to the control in the 4mg/kg sodium nitrite. There was no significant reduction in infarct size compared to the control in the group receiving 0.4mg/kg sodium nitrite. B) Infarct size as a % of area at risk in rabbits undergoing LAD occlusion was significantly reduced compared to control when exposed to high dose (60mW/cm2) R/NIR at reperfusion. Low dose (3mW/cm2) R/NIR exposure on reperfusion did not decrease infarct size. When 0.4mg/kg of sodium nitrite is administered prior to ischemia, and low dose R/NIR light is given at reperfusion, infarct reduction is similar to high dose R/NIR. C) Infusion of C-PTIO abolished R/NIR cardioprotection at reperfusion in both the high dose R/NIR and the low dose R/NIR with 0.4mg/kg sodium nitrite. D) 5 cycles of R/NIR at 670nm (11 J/cycle) prior to ischemia can significantly reduce infarct size compared to saline control. There was no significant reduction in infarct size compared to the control when 3 cycles (11 J/cycle) of R/NIR light was administered prior to ischemia. However a significant reduction in infarct size could be obtained when 0.4mg/kg sodium nitrite was combined with 3 cycles of light exposure. This suggests the effect of near infrared light on infarct size is enhanced by addition of nitrite. E) Infusion of c-PTIO abolished the preconditioning effects of 5 cycles of R/NIR and 3 cycles of R/NIR with 0.4mg/kg sodium nitrite. *p<0.01 compared to control; †p<0.01 compared to 60mW post irradiation; ‡p<0.01 compared to 5 cycles R/NIR.
As shown in Figure 5B, exposure of the rabbit cardiac preparation to 60 mW/cm2 (11 J) of 670 nm light, in the absence of nitrite, at the time of reperfusion resulted in a significant reduction in infarct size whereas a lower power radiation (3 mW/cm2, 0.5J) was ineffective. Interestingly, when low dose radiation (3 mW/cm2) was combined with low-dose nitrite (0.4 mg/kg) a synergistic effect was observed suggesting that R/NIR is able to potentiate the cardioprotective effects of nitrite. The infusion of carboxy PTIO (c-PTIO), a nitric oxide scavenger, throughout the irradiation period abolished the protective effects of R/NIR and R/NIR with low-dose nitrite (Figure 5C). To verify thermal effects were not contributing to our observations, temperature at the heart surface was measured, with a temperature elevation < 0.5°C after light exposure.
To examine if R/NIR light can precondition the myocardium from ischemia and reperfusion injury, a similar protocol was performed with irradiation occurring prior to ischemia (Figure 5D). In this protocol the irradiation was administered in short bursts in a similar manner to ischemic and anesthetic pre-conditioning protocols [27, 32]. It was found that 3 cycles of irradiation (see methods section) of 670 nm light (60 mW/cm2) exposure were not cardioprotective, where as 5 cycles significantly preconditioned myocardium, markedly reducing infarct size. Again, low-dose nitrite (0.4 mg/Kg) enhanced the ability of low-dose irradiation to condition the heart against injury. When c-PTIO was infused during the irradiation period, the effect of R/NIR and combined R/NIR with low-dose nitrite (0.4 mg/Kg) on infarct size reduction was abolished (Figure 5E).
In combination these data suggest that the ability of R/NIR light to liberate nitric NO from tissue stores contributes to cardioprotection, and that nitrite, by potentially increasing the size of these NO stores, in the form of increases in MbNO and HbNO, enhances cardioprotection at lower doses of R/NIR.
Discussion
This investigation provides evidence that electromagnetic energy at a wavelength of 670nm can release NO from the nitrosylated forms of hemoglobin and myoglobin, and that this augmented NO release can be cardioprotective. R/NIR light accelerated the conversion of HbNO to metHb by a factor of about 4. HbNO slowly auto-oxidizes to metHb and nitrate due to the slow release of NO from the heme iron, followed by oxygen binding to the vacant heme and rapid reaction of NO with the newly formed oxyHb. This latter reaction generates metHb. The increased rate of conversion of HbNO to metHb in the presence of R/NIR suggests an increased rate of release of NO from this complex. We directly measured NO after irradiation of HbNO and MbNO using ozone-dependent chemiluminescence. This method utilizes the reaction between NO and ozone to generate light and can detect as little as 1 pmol of NO [33]. HbNO is injected into a sealed vessel, is purged with argon, and the gas stream is fed to the reaction cell of a chemiluminescence analyzer. Any NO that is liberated will either re-bind to deoxyhemoglobin or be swept out of solution in the gas phase. Importantly, no signals were observed upon irradiation after injection of oxyHb or nitrite alone. However, the combination of oxyHb (which is rapidly deoxygenated under the anaerobic conditions of the purge vessel) and nitrite, resulted in a measurable release of NO, consistent with the earlier observation of Gladwin et al [5]. It is expected under these conditions that the nitrite reductase activity of hemoglobin will release NO and that a significant proportion of this NO will be autocaptured by deoxyHb to form HbNO [5]. Irradiation of this HbNO solution resulted in a robust formation of NO. This experiment raises the possibility that R/NIR light could be used to augment the effects of nitrite as an NO-releasing agent.
Oxygenated derivatives of hemoglobin and myoglobin react with NO with a rate constant of approximately 4–5 × 107 M−1s1[34]. This reaction converts NO into nitrate and has been suggested to a be major route of NO destruction in mammalian systems [34]. In contrast, the reaction of NO with deoxyHb and deoxyMb forms a quasi-stable nitrosyl derivative where the NO is directly bound to the ferrous heme [5, 6, 35]. While the rate constant of formation of these derivatives from NO is similar to the rate of reaction of NO with oxyHb, their stability is derived from an extremely slow off rate that is measured in tens of minutes to hours [36]. The half time of HbNO in humans in vivo has recently been measured to be about 20 min [37]. In addition, MbNO has been detected in myocardium as a result of iNOS induction [38, 39]. Of interest is the fact that HbNO and MbNO are major products of the nitrite reductase activities of these heme proteins. Both deoxyHb and deoxyMb can reduce nitrite to NO via a process that is strongly pH dependent, occurring more rapidly under acidic conditions [13]. The initial product of this reaction is NO, and this reaction has been shown to be involved vasodilatory responses and cardioprotection, giving a plausible mechanism for hypoxia, or ischemia, driven NO generation [5, 6]. However, a proportion of the nascent NO is captured by a local deoxygenated heme, and in the test-tube, the final end products of this reaction are an equal mixture of HbNO and metHb [35]. It is worth noting that the relative rates of nitrite reduction are T-state Hb <<R-State Hb < Mb [6]. Consistent with other metalloporphyrin-ligand interactions, MbNO and HbNO were found to be photodissociable at wavelengths up to 608 nm, however their quantum yield is extremely low (ϕ=0.001)[40, 41].
We observed the generation of measurable levels of MbNO in ischemic myocardium during infusion of nitrite, as determined by EPR spectrometry. After 670 nm light exposure there was a large reduction in the MbNO EPR signal, strongly suggesting the photolysis of MbNO by 670 nm light in this tissue.
Results of the present investigation demonstrate that 670 nm light and nitrite can act synergistically to precondition and postcondition myocardium against ischemia and reperfusion. We hypothesize that the mechanism of synergy involves the photolysis of NO stores generated from the administration of nitrite. Based on experiment with purified HbNO, MbNO and ex vivo cardiac MbNO it is conceivable that these proteins represent examples of such stores. The mechanism of cardioprotection by NO both during the preconditioning window and during reperfusion has been extensively examined and discussed in the literature and is an area of on-going debate. It is likely that many components of the cardiomyocyte response to ischemia/reperfusion are modulated by NO, including mitochondrial and sarcolemmal ATP-sensitive potassium channels, mitochondrial Ca2+ uptake upon reperfusion, and apoptotic signaling pathways [42]. Interestingly NO generated by nitrite reductase activity of deoxymyoglobin has been demonstrated to regulate mitochondrial respiration through modulation cytochrome c oxidase activity [6]. Regardless of the mechanistic intricacies it has become clear that increasing the steady state NO level as a result of pharmacological intervention is a cardioprotective strategy and it appears as though R/NIR irradiation may contribute to this process. It must be acknowledged that other pathways of action of R/NIR cannot be discounted.
The clinical promise for the combined use of R/NIR light and nitrite is of considerable interest. R/NIR light facilitates localized NO bioavailability in a site-specific manner secondary to increased concentrations of HbNO and MbNO in ischemic regions. The combination has the advantage over well utilized nitric oxide donors sodium nitroprusside and nitroglycerin, because nitrite does not induce tolerance [3]. Nitroglycerin requires conversion to nitrite by the P450 mitochondria aldehyde dehydrogenase, which leads to the development of tolerance [3].
The far red/near infrared region of the electromagnetic spectrum (between 630 nm and 1000 nm) has a number of properties that are attractive to possible physiological and clinical use. The potential for tissue damage is limited because the photon energy in this region is non-ionizing. The thermal effect in this range is also minimized by the low absorption of light energy by water and melanin in this range and this improves overall penetration of the light into tissues. There has been intense clinical interest in the use of irradiation therapy in tissue healing. It has been shown that R/NIR light heals mucositis in pediatric patients undergoing chemotherapy, decreases infarct size, and has a neuroregenerative effect in methanol induced retinal toxicity [18, 19, 21].
An alternative or parallel mechanism to explain the biological activity R/NIR is the release of NO from nitrosylated cytochrome c oxidase. Cytochrome c oxidase has been identified as a mediator of some biological effects of R/NIR [18]. It has been suggested that the mechanism by which cytochrome c oxidase exerts its action is via NO. Nitric oxide binds to the fully and partially reduced heme a3 CuB binuclear center and will decrease mitochondrial oxygen consumption[43]. This cyt a32+-NO species is photosensitive and application of light at 500nm was able to photolyse NO from the complex, which led to the reversal of mitochondrial respiratory inhibition [24]. This observation led to speculation that application of red light or near infrared light could release NO and make it available for other biological applications e.g. altering gene expression, vasodilation [22, 23]. Although it is plausible this reaction could occur, the relative amounts of NO which would be bound to the enzyme and available for release would be quite limited [44]. Additional complexity to the role of NO and cytochrome c oxidase inhibition, is that the oxidized heme a3 CuB center can form a complex with nitrite, which is insensitive to light. Nitrite bound to the cyt a33+ dissociates from the binuclear site and appears not to undergo further reduction to NO [45–47]. Thus, conditions which favor formation of the photosensitive nitrosyl species are low O2 tensions, and high concentrations of reduced cytochrome c, which are present after ischemia [26]. Conversely, high O2 and low concentrations of reduced cytochrome c favor nitrite formation, which occurs under physiological conditions [45]. It might be speculated that the direct actions of light-dependent reversal of NO mediated cytochrome c oxidase inhibition, and thus mitochondrial respiration, could be effective in modulating reperfusion injury, however it is not clear if this would be protective or damaging [6]. NO induced inhibition of cytochrome c oxidase during reperfusion has been suggested to be beneficial for preventing cardiac injury[6, 48, 49].
The photodissociation of HbNO and MbNO by R/NIR light has clinical importance. Increasing evidence of the clinical benefits to R/NIR light in a variety of tissues may have a common mechanism that includes generation of significant amounts of NO. The possibility of exerting biological effects on remote organ systems in a noninvasive manner through excitation of NO stores in the blood or muscle is feasible, and site specificity for ischemic regions is highly likely.
Table 2.
| Reperfusion | ||||||
|---|---|---|---|---|---|---|
| Baseline | Occlusion | 3 min | 1 h | 2 h | 3 h | |
| HR (min−1) | ||||||
| cPT&5xPre | 258 ± 22 | 218 ± 17 | 213 ± 10 | 215 ± 19 | 201 ± 26 | 204 ± 31 |
| cPnit&3xPre | 245 ± 30 | 219 ± 20 | 219 ± 23 | 221 ± 37 | 220 ± 31 | 213 ± 27 |
| cPT&60mWPo | 245 ± 24 | 236 ± 29 | 226 ± 35 | 224 ± 26 | 221 ± 23 | 214 ± 26 |
| cPnit&3mWPo | 253 ± 25 | 235 ± 42 | 245 ± 21 | 238 ± 28 | 237 ± 32 | 215 ± 28 |
| MAP (mmHg) | ||||||
| cPT&5xPre | 68 ± 15 | 60 ± 11 | 60 ± 9 | 65 ± 11 | 69 ± 9 | 57 ± 16 |
| cPnit&3xPre | 69 ± 5 | 53 ± 3 | 51 ± 6 | 49 ± 4 | 50 ± 3 | 53 ± 3 |
| cPT&60mWPo | 64 ± 12 | 53 ± 12 | 53 ± 6 | 54 ± 9 | 53 ± 6 | 59 ± 13 |
| cPnit&3mWPo | 67 ± 11 | 59 ± 9 | 61 ± 5 | 67 ± 6 | 60 ± 5 | 57 ± 3 |
| RPP (min−1•mmHg•10−3) | ||||||
| cPT&5xPre | 20.2 ± 5.3 | 15.3 ± 3.5 | 14.9 ± 2.5 | 16.3 ± 3.6 | 16.0 ± 3.8 | 14.1 ± 5.6 |
| cPnit&3xPre | 21.8 ± 1.0 | 13.7 ± 1.6 | 13.5 ± 2.9 | 13.4 ± 3.0 | 13.4 ± 1.7 | 13.3 ± 2.3 |
| cPT&60mWPo | 20.1 ± 3.0 | 14.8 ± 3.6 | 14.2 ± 2.7 | 14.4 ± 3.3 | 13.9 ± 2.1 | 14.8 ± 3.8 |
| cPnit&3mWPo | 19.7 ± 4.1 | 16.7 ± 4.4 | 17.4 ± 1.6 | 18.2 ± 1.2 | 16.6 ± 2.8 | 14.3 ± 1.3 |
Data are mean ± SD
Abbreviations: HR = heart rate; MAP = mean arterial pressure; RPP = rate pressure product;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Massion PB, et al. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93(5):388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 2.Dweik RA, et al. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J Clin Invest. 1998;101(3):660–666. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews. Drug Discovery. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 4.Nagababu E, et al. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278(47):46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 5.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9(12):1498–1505. doi: 10.1038/nm954. [see comment]. [DOI] [PubMed] [Google Scholar]
- 6.Shiva S, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 2007;100(5):654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 7.Schwengel RH, et al. Characterization of pulsed-dye laser-mediated vasodilatation in a rabbit femoral artery model of vasoconstriction. Lasers in Surgery & Medicine. 1993;13(3):284–295. doi: 10.1002/lsm.1900130305. [DOI] [PubMed] [Google Scholar]
- 8.Castello PR, et al. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3(4):277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Castello PR, et al. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proc Natl Acad Sci U S A. 2008;105(24):8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautier C, et al. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem Biophys Res Commun. 2006;341(3):816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Huang KT, et al. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280(35):31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 12.Crawford JH, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107(2):566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Z, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radical Biology & Medicine. 2004;36(6):707–717. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Owen-Reece H, et al. Near infrared spectroscopy. Br J Anaesth. 1999;82(3):418–426. doi: 10.1093/bja/82.3.418. [DOI] [PubMed] [Google Scholar]
- 16.Bozkurt A, Onaral B. Safety assessment of near infrared light emitting diodes for diffuse optical measurements. Biomed Eng Online. 2004;3(1):9. doi: 10.1186/1475-925X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadler I, et al. Alteration of skin temperature during low-level laser irradiation at 830 nm in a mouse model. Photomed Laser Surg. 2004;22(3):227–231. doi: 10.1089/1549541041438560. [DOI] [PubMed] [Google Scholar]
- 18.Wong-Riley MT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 19.Whelan HT, et al. NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg. 2002;20(6):319–324. doi: 10.1089/104454702320901107. [DOI] [PubMed] [Google Scholar]
- 20.Bibikova A, Belkin V, Oron U. Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low-energy laser irradiation. Anat Embryol (Berl) 1994;190(6):597–602. doi: 10.1007/BF00190110. [DOI] [PubMed] [Google Scholar]
- 21.Ad N, Oron U. Impact of low level laser irradiation on infarct size in the rat following myocardial infarction. Int J Cardiol. 2001;80(2–3):109–116. doi: 10.1016/s0167-5273(01)00503-4. [DOI] [PubMed] [Google Scholar]
- 22.Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3(2):211–216. doi: 10.1039/b306126d. [DOI] [PubMed] [Google Scholar]
- 23.Karu TI, et al. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Sarti P, et al. Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochem Biophys Res Commun. 2000;274(1):183–187. doi: 10.1006/bbrc.2000.3117. [DOI] [PubMed] [Google Scholar]
- 25.Rossi-Fanelli A, Antonini E, Caputo A. Studies on the relations between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J Biol Chem. 1961;236:391–396. [PubMed] [Google Scholar]
- 26.Antonini E, Brunori M. In: Hemoglobin and myoglobin in their reactions with ligands. Antonini Eraldo, Brunori Maurizio., editors. Amsterdam: North-Holland Pub. Co.; 1971. p. xx.p. 436. [Google Scholar]
- 27.Tanaka K, et al. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology. 2002;97(6):1485–1490. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Duranski MR, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. Journal of Clinical Investigation. 2005;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez FM, et al. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117(23):2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker JE, et al. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K (ATP) channels. J Mol Cell Cardiol. 2007;43(4):437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MV, Liu GS, Downey JM. Preconditioning causes improved wall motion as well as smaller infarcts after transient coronary occlusion in rabbits. Circulation. 1991;84(1):341–349. doi: 10.1161/01.cir.84.1.341. [DOI] [PubMed] [Google Scholar]
- 33.Chou HJ, Yates RL. A rapid and selective method for determining potential nitrosating agents in cosmetic products by chemiluminescence detection of nitric oxide. J AOAC Int. 1998;81(2):368–372. [PubMed] [Google Scholar]
- 34.Keszler A, et al. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008;283(15):9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle MP, et al. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256(23):12393–12398. [PubMed] [Google Scholar]
- 36.Huang TH. NMR studies of the quaternary structure and heterogeneity of nitrosyl- and methemoglobin. J Biol Chem. 1979;254(22):11467–11474. [PubMed] [Google Scholar]
- 37.Piknova B, et al. Electron paramagnetic resonance analysis of nitrosylhemoglobin in humans during NO inhalation. J Biol Chem. 2005;280(49):40583–40588. doi: 10.1074/jbc.M506292200. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi AL, et al. Electron spin resonance analysis of heme-nitrosyl and reduced iron-sulfur centered complexes in allogeneic, heterotopic cardiac transplants: effects of treatment with pyrrolidine dithiocarbamate. Free Radic Biol Med. 1998;25(2):201–207. doi: 10.1016/s0891-5849(98)00051-3. [DOI] [PubMed] [Google Scholar]
- 39.Tiravanti E, Samouilov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem. 2004;279(12):11065–11073. doi: 10.1074/jbc.M311908200. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman BM, Gibson QH. On the photosensitivity of liganded hemoproteins and their metal-substituted analogues. Proc Natl Acad Sci U S A. 1978;75(1):21–25. doi: 10.1073/pnas.75.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibson QH, Ainsworth S. Photosensitivity of haem compounds. Nature. 1957;180(4599):1416–1417. doi: 10.1038/1801416b0. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res. 2006;70(2):231–239. doi: 10.1016/j.cardiores.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Cooper CE, et al. Nitric oxide ejects electrons from the binuclear centre of cytochrome c oxidase by reacting with oxidised copper: a general mechanism for the interaction of copper proteins with nitric oxide? FEBS Lett. 1997;414(2):281–284. doi: 10.1016/s0014-5793(97)01009-0. [DOI] [PubMed] [Google Scholar]
- 44.Osipov AN, Borisenko GG, Vladimirov YA. Biological activity of hemoprotein nitrosyl complexes. Biochemistry (Mosc) 2007;72(13):1491–1504. doi: 10.1134/s0006297907130068. [DOI] [PubMed] [Google Scholar]
- 45.Sarti P, et al. Nitric oxide and cytochrome oxidase: reaction mechanisms from the enzyme to the cell. Free Radic Biol Med. 2003;34(5):509–520. doi: 10.1016/s0891-5849(02)01326-6. [DOI] [PubMed] [Google Scholar]
- 46.Mason MG, et al. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc Natl Acad Sci U S A. 2006;103(3):708–713. doi: 10.1073/pnas.0506562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25(4–5):434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 48.Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic Biol Med. 2002;33(11):1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, et al. Endothelial nitric oxide synthase (NOS3) knockout decreases NOS2 induction, limiting hyperoxygenation and conferring protection in the postischemic heart. Am J Physiol Heart Circ Physiol. 2007;292(3):H1541–H1550. doi: 10.1152/ajpheart.00264.2006. [DOI] [PubMed] [Google Scholar]