Abstract
Sesquiterpenes have attracted much interest with respect to their protective effect against oxidative damage that may be the cause of many diseases including several neurodegenerative disorders and cancer. Our previous unpublished work suggested that cyclosativene (CSV), a tetracyclic sesquiterpene, has antioxidant and anticarcinogenic features. However, little is known about the effects of CSV on oxidative stress induced neurotoxicity. We used hydrogen peroxide (H2O2) exposure for 6 h to model oxidative stress. Therefore, this experimental design allowed us to explore the neuroprotective potential of CSV in H2O2-induced toxicity in new-born rat cerebral cortex cell cultures for the first time. For this aim, MTT and lactate dehydrogenase release assays were carried out to evaluate cytotoxicity. Total antioxidant capacity (TAC) and total oxidative stress (TOS) parameters were used to evaluate oxidative changes. In addition to determining of 8-hydroxy-2-deoxyguanosine (8-OH-dG) levels, the single cell gel electrophoresis (or Comet assay) was also performed for measuring the resistance of neuronal DNA to H2O2-induced challenge. Our results showed that survival and TAC levels of the cells decreased, while TOS, 8-OH-dG levels and the mean values of the total scores of cells showing DNA damage (Comet assay) increased in the H2O2 alone treated cultures. But pre-treatment of CSV suppressed the cytotoxicity, genotoxicity and oxidative stress which were increased by H2O2. On the basis of these observations, it is suggested that CSV as a natural product with an antioxidant capacity in mitigating oxidative injuries in the field of neurodegenerative disorders.
Keywords: Cyclosativene, Neuroprotection, H2O2, Primary neuron, DNA damage, Oxidative stress
Introduction
Recent studies have implicated reactive oxygen species (ROS) and reactive nitrogen species (RNS) are generated by specialized plasma membrane oxidases in normal physiological signalling by growth factors and cytokines. In physiological levels, ROS and RNS may actually play important roles for survival of most living organisms including intracellular signal transduction and gene expression. However, their uncontrolled and excessive increases and exogenous exposure cause damages to lipids, proteins, and DNA (Thannickal and Fanburg 2000; Droge 2002; Turrens 2003). Under extreme oxidative conditions, or if the antioxidant protective mechanisms of cells are compromised, cellular injury and death occur (Geyikoglu and Turkez 2005a, b; Silva et al. 2010). In fact, oxidative stress has been implicated in various pathological conditions involving cardiovascular disease, cancer, neurological disorders, diabetes, ischemia/reperfusion, other diseases and ageing (Pala and Gurkan 2008; Moslehi et al. 2012; Kumar and Khanum 2013).
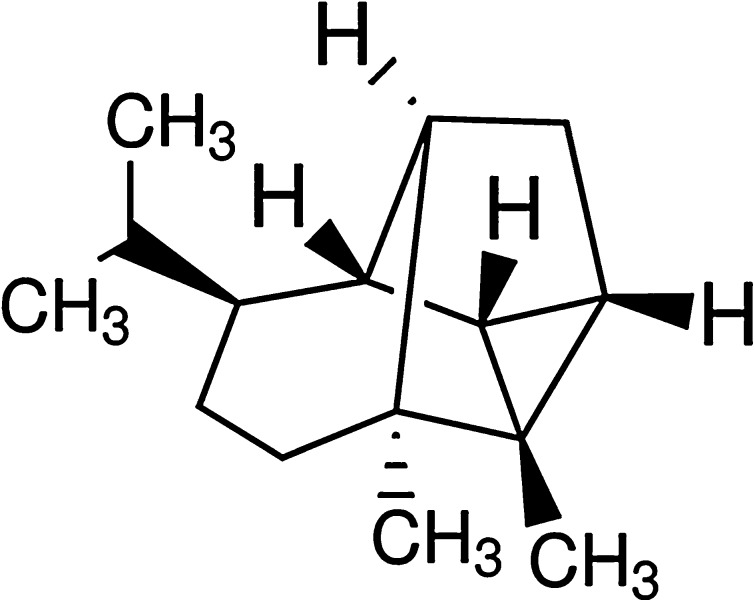
Central nervous system cells were considered to be more vulnerable to ROS and RNS toxicity due to their inherent higher oxidative metabolism and less antioxidant enzymes as catalase (CAT) and superoxide dismutase (SOD), as well as higher content of membranous fatty acids (Olmez and Ozyurt 2012; Stefanova et al. 2012). Accumulated evidences showed that the most common neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, stroke and brain trauma in which elevated levels of ROS might induce severe cell damage through oxidative stress (Nabavi et al. 2013; Si et al. 2013). Consequently, due to the efficacy of antioxidant natural compounds in preventing oxidative damage in either cultured neuronal cells or in the brains of animals treated with various neurotoxic agents, it was suggested that antioxidants have important potential therapeutic value as neuroprotective drugs in treatment of many neurodegenerative disorders. Hence, several antioxidants have been used to reduce oxidative stress or damage in human body, animal models and cell cultures (Turkez 2011; Dirican et al. 2012; Jain et al. 2012; Sumathi et al. 2012; Farah et al. 2013; Sozio et al. 2013; Cacciatore et al. 2012). Sesquiterpenes, one of the most common terpenes, are a class of natural products with a diverse range of attractive industrial properties (Scalcinati et al. 2012). They contain three isoprene units, which is fifteen carbons and twenty-four hydrogens per molecule (C15H24). There are more than 10,000 kinds of sesquiterpenes (Davis and Croteau 2000). They have long been investigated for biological activities; anticarcinogenic (Afoulous et al. 2013), antimicrobial (Wang et al. 2013), antifungal (Kundu et al. 2013), antiinflammatory (Wang et al. 2013), more recently, and antioxidant activities (Abolaji et al. 2013). Also it was reported that sesquiterpenes could provide protection against H2O2 damage in many cell lines (Tang et al. 2005; Umemura et al. 2008; Gan et al. 2009; Turkez et al. 2013). Cyclosativene (CSV) is a tetracyclic sesquiterpene (Fig. 1). The volatile organic compounds unique to Helminthosporium sativum, Helminthosporium victoriae and Centaurea cineraria from leaves included CSV (Crutwell-McFadyen 1998; Lodewyk et al. 2008; Beck et al. 2008). The recent results of our previous unpublished work have revealed that CSV exhibits many important biological activities, including antioxidant activity and anticarcinogenic activity in cultured healthy rat neurons and neuroblastoma tumour cells (Togar 2013). However, whether CSV exerts protective effects against oxidative cytotoxicity in neuronal models as a result of its antioxidant property has not been investigated. Therefore, the aim of the present study was to firstly evaluate the cytotoxic [MTT and Lactate dehydrogenase (LDH) assays], cytogenetic (Comet and 8-OH-dG assays) and oxidative effects (TAC and TOS analysis) of CSV against H2O2-induced neuronal damage using new-born rat cerebral cortex cell cultures to explore their neuroprotective potentials.
Fig. 1.
Chemical structure of CSV
Materials and methods
Chemicals and reagents
CSV (CAS No. 22469-52-9, C15H24, purity: ≥98 %), Dulbecco modified Eagles medium (DMEM), Hank’s balanced salt solution (HBSS), neurobasal medium (NBM), sodium phosphate (NaH2PO4), potassium phosphate monobasic (KH2PO4), ethylenediaminetetraacetic acid (EDTA), phosphate buffer solution (PBS), dimethylsulfoxide (DMSO), Triton-X-100, DNase type 1, Tris, low melting point agarose, normal melting point agarose, ethidium bromide were purchased from Sigma-Aldrich® Inc (St. Louis, MO, USA). Hydrogen peroxide was purchased from Merck® (Darmstadt, Germany). Fetal calf serum (FCS) and trypsin–EDTA were purchased from Biol Ind® Inc. All other chemicals were of analytical grade.
Cell culture
Primary rat cerebral cortex neuron cultures were prepared using rat foetuses as described previously (Ban et al. 2006). Briefly, a total of nine new-born Sprague–Dawley rats were used in the study. The rats were decapitated by making a cervical fracture in the cervical midline and the cerebral cortex was dissected and removed. The cerebral cortex was placed into 5 ml of HBSS, which had already been placed in a sterile Petri dish and macromerotomy was performed with two lancets. This composition was pulled into a syringe and treated at 37 °C for 25–30 min as 5 ml HBSS plus 2 ml Trypsin–EDTA (0.25 % trypsin–0.02 % EDTA) and chemical decomposition was achieved. 8 μl of DNase type 1 (120 U/ml), was added to this solution and treated for 1–2 min, and centrifuged at 800 rpm for 3 min. After having thrown away the supernatant, 31.5 ml of NBM and 3.5 ml FCS were added to the residue. The single cell which was obtained after physical and chemical decomposition was divided into 3.5 ml samples in each of 10 flasks coated with poly-d-lysine formerly dissolved in PBS. The flasks were left in the incubator including 5 % CO2 at 37 °C. The flasks were then changed with a fresh medium of half of their volumes every 3 days until the cells were branched and had reached a certain maturity and in vitro experiments were performed 8 days later. This study was conducted at the Medical Experimental Research Centres in Ataturk University (Erzurum, Turkey). The Ethical Committee of Ataturk University approved the study protocol (B.30.2.ATA.0.23.85-73).
Treatments
Cytoprotective activities of CSV on 0.5 mM H2O2-induced cell injury were investigated by MTT, LDH, TAC, TOS and Comet assays and 8-OH-dG analysis. For determining cytoprotectivity, the cells were seeded into 48-well plate at a density of 5 × 104 cells/well for 16 h and then exposed to medium in the presence of different concentrations of CSV for 0.5 h before exposure to 0.5 mM H2O2 for 6 h. The cytotoxicity and genotoxicity of CSV were also investigated. CSV was dissolved in ethanol and ethanol was evaporated to dryness at ambient temperature. CSV was applied into cultures at concentrations of 6.25, 12.5, 25, 50 and 100 μg/ml for 24 h. The doses were selected according to the works of Togar (2013) and Si et al. (2013). Cells incubated without CSV and H2O2 was considered as control group. The cell viability, oxidative alterations and DNA damage analyses were carried out in four totally independent experiments.
Evaluation of cell viability
MTT assay
Viability of cells was assessed by measuring the formation of formazan from MTT spectrophotometrically via commercial kits (Cayman Chemical®, Ann Arbor, MI, USA). At the end of the experiment, the neurons were incubated with 0.7 mg/ml MTT for 30 min at 37 °C. After washing the blue formazan was extracted from cells with isopropanol/formic acid (95:5) and was photometrically determined at 560 nm. The density of formazan formed in control cells was taken as 100 % viability.
LDH assay
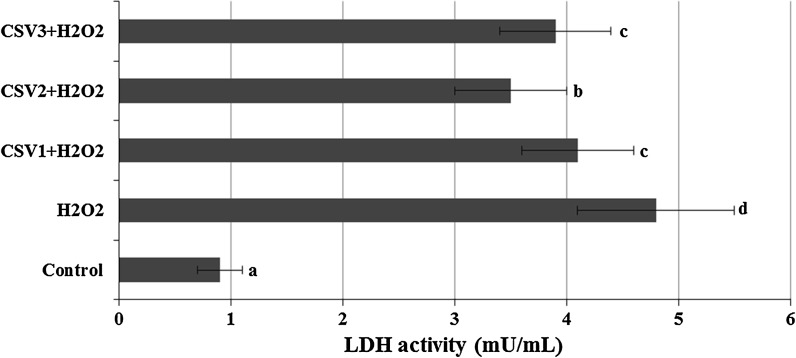
Lactate dehydrogenase released from damaged cells in culture medium was quantified by using LDH assay kit (Cayman Chemical®). A total of 100 μl of cell medium was used for LDH analysis. Released LDH catalysed the oxidation of lactate to pyruvate with simultaneous reduction of NAD+ to NADH. The rate of NAD+ reduction was measured as an increase in absorbance at 490 nm. The rate of NAD+ reduction was directly proportional to LDH activity in the cell culture.
TAC and TOS analyses
After cells were exposed to H2O2 for 6 h, the cultures were washed with ice-cold PBS and homogenized with 0.9 % normal saline. Following homogenization, intracellular levels of TAC and TOS were determined by commercially available kits (Rel Assay Diagnostics®, Gaziantep/Turkey).
Evaluation of DNA damage
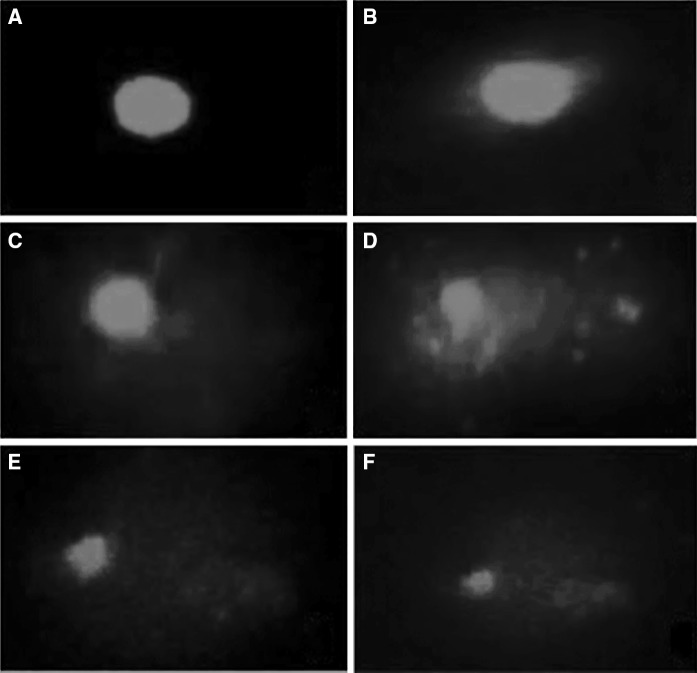
The DNA damage evaluation was performed by single cell gel electrophoresis (SCGE also known as Comet test) assay. After the application of coverslips, the slides were allowed to gel at 4 °C for 30–60 min. The slides were immersed in freshly prepared cold lysing solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1 % sodium sarcosinate, pH 10.0) with 1 % Triton X-100 and 10 % DMSO added just before use for a minimum of 1 h at 4 °C) and refrigerated overnight followed by alkali treatment, electrophoresis (at 1.6 V/cm for 20 min, 300 mA) and neutralization (0.4 M Tris, pH 7.5). The dried slides were then stained using ethidium bromide (20 μg/ml) after appropriate fixing for 10 min (Singh et al. 1988). The whole procedure was carried out in dim light to minimize artefact. DNA damage analysis was performed at a magnification of 100× using a fluorescence microscope (Nicon Eclips E6600, Tokyo, Japan) after coding the slides by one observer (Togar B). A total of 100 cells were screened per slide. A total damage score for each slide was derived by multiplying the number of cells assigned to each grade of damage by the numeric value of the grade and summing over all grades (giving a maximum possible score of 500, corresponding to 100 cells at grade 5). The scoring criteria for determining damage levels in cultured neurons treated with the compounds in our Comet analysis are shown in Fig. 2.
Fig. 2.
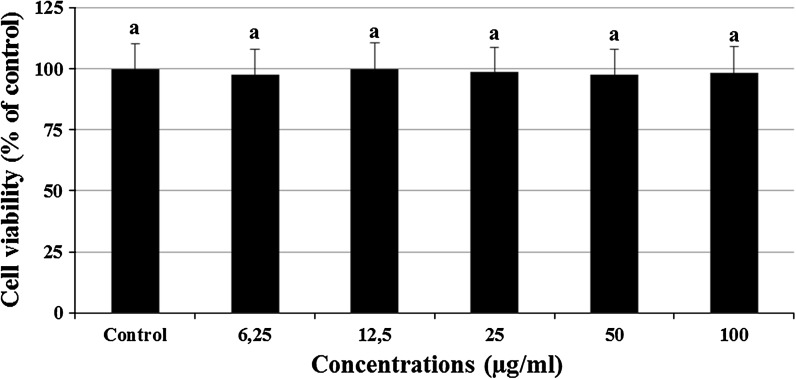
Viability of rat primary cortical neurons after 24 h exposure to (0–100 μg/ml) CSV. The results are presented as percentage of the control group (n = 4). Data are expressed as mean ± SD. Values with the same superscript letters are not significantly different from each other at the level of p < 0.05
Nucleic acid oxidation
8-hydroxy-2′-deoxyguanosine assay kits were purchased from Cayman Chemical® for determining 8- OH-dG levels in the cultures. Since it is a competitive assay that can be used for the quantification of 8-OHdG in homogenates and recognizes both free 8-OHdG and DNA-incorporated 8-OH-dG, many researches have been performed using this protocol. This assay depends on the competition between 8-OHdG and 8 OHdGacetylcholinesterase (AChE) conjugate for a limited amount of 8-OHdG monoclonal antibody (Abdel-Wahab and Metwally 2011). All procedures were carried out in accordance with the provider’s manual.
Data analysis
The data are expressed as the mean ± standard derivation (SD) of four repetitions. One-way analysis of variance (ANOVA) was used to determine the significant differences between the groups followed by a Dunnett’s t test for multiple comparisons. A probability <0.05 was considered as significant. All analyses were performed using SPSS version 15.0 (SPSS Inc®).
Results
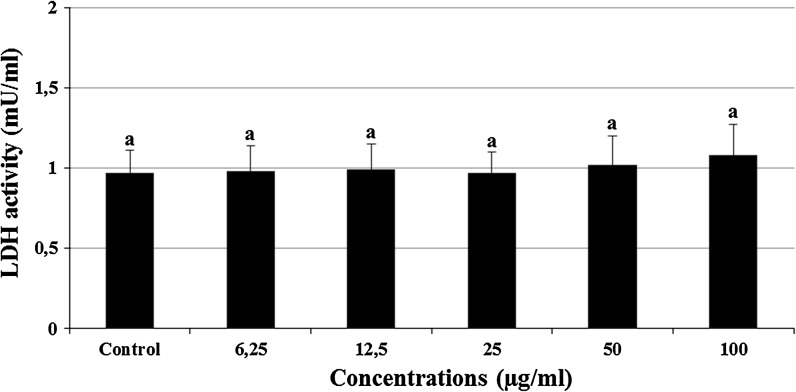
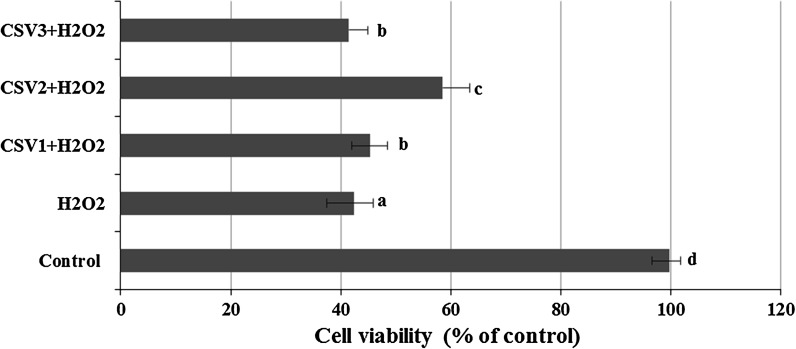
The cultured rat primary cerebral cortical neurons exposed to 6.25, 12.5, 25, 50 and 100 μg/ml concentrations of CSV did not show any significant alterations in cell viability during 24 h as determined by MTT and LDH assays (Figs. 2, 3). When cerebral cortical neurons were exposed to 0.5 mM H2O2, MTT absorbance was 42.4 ± 1.9 % of that of untreated controls, indicating that H2O2 caused neuronal cell death. CSV applications significantly inhibited the decrease of MTT reduction by 0.5 mM H2O2. In fact, in cultures treated with CSV (12.5, 25 and 50 μg/ml), H2O2-induced neuronal death was significantly reduced (Fig. 4). H2O2-induced neuronal cell death was clearly evidenced by five folds increases (from 0.98 to 4.76 mU/ml) in the activity of LDH compared with the observations of untreated controls. In contrast, 12.5, 25 and 50 μg/ml of CSV significantly blocked the H2O2-induced elevation of intracellular LDH release (Fig. 5).
Fig. 3.
Extracellular level of LDH in cultured rat cortical neurons maintained in the presence of CSV for 24 h. The abbreviations are as in Fig. 4
Fig. 4.
The effects of CSV applications on cell viability against H2O2-induced cell death. The cells were pre-treated with different CSV concentrations for 30 min before exposure to 0.5 mM H2O2 for 6 h. Then, cell viability of CSV treated cultures was determined via MTT assay. CSV1 12.5 μg/ml CSV, CSV2 25 μg/ml CSV, CSV3 50 μg/ml CSV, H 2 O 2 0.5 mM hydrogen peroxide. The results are presented as percentage of the control group (n = 4). Data are expressed as mean ± SD. Values with the same superscript letters are not significantly different from each other at the level of p < 0.05
Fig. 5.
The effects of CSV applications on H2O2-induced LDH release. The cells were pretreated with different CSV concentrations for 30 min before exposure to 0.5 mM H2O2 for 6 h. After, intracellular levels of LDH were measured in CSV treated cultures. The abbreviations are as in Fig. 4
Table 1 reflects the oxidant-antioxidant profile of CSV on cultured primary rat neurons. As seen from the table: the applications with the lowest concentrations (6.25 μg/ml) of CSV compounds did not lead to any alterations in TAC levels while higher concentrations of CSV below 100 μg/ml (12.5, 25 and 50 μg/ml) significantly (p < 0.05) increased TAC levels compared to control value. In fact, after CSV applications at 25 μg/ml for 24 h, the TAC levels reached to a maximum of the observed level as 20.97 %. Whereas CSV treatments at concentration of 100 μg/ml did not lead to increases of TAC levels. Therefore we have determined the most suitable/applicable concentrations as 12.5, 25 and 50 μg/ml for investigations dealing with their in vitro protective effects in this study.
Table 1.
In vitro levels of TAC (as mmol Trolox Equiv./L) and TOS (as mmol H2O2 Equiv./L) in cultured rat cortical neurons maintained in the presence of CSV for 24 h. The abbreviations are as in Fig. 4
| Concentrations (μg/ml) | CSV | |
|---|---|---|
| TAC level | TOS level | |
| Control | 28.6 ± 3.0a | 1.7 ± 0.1a |
| 6.25 | 29.4 ± 3.2a | 1.7 ± 0.2a |
| 12.5 | 32.0 ± 2.9b | 1.5 ± 0.2a |
| 25 | 34.6 ± 3.1c | 1.7 ± 0.2a |
| 50 | 30.4 ± 3.4b | 1.6 ± 0.3a |
| 100 | 28.0 ± 3.4a | 1.7 ± 0.3a |
The same column followed by the different superscript letters present significant differences at the p < 0.05 level
Results revealed that exposure to H2O2 for 6 h resulted in significant decreases in the TAC levels as compared to those of the untreated group. Also, the addition of H2O2 for 6 h led to a significant elevation in the level of TOS as compared to that of the control group (Table 2). Results of the present study demonstrated that level of TAC was significantly increased in cortical neurons pre-treated with CSV as compared to those of H2O2-intoxicated cultures. Also, pre-exposure to CSV led to a significant augmentation in the levels of TOS as compared to those of H2O2-intoxicated cultures (Table 2).
Table 2.
The effects of CSV applications on oxidative alterations by H2O2 in vitro. The cells were pre-treated with different CSV concentrations for 30 min before exposure to 0.5 mM H2O2 for 6 h. Then, oxidative effects of CSV treated cultures were determined via TAC and TOS assays. The abbreviations are as in Fig. 4
| Treatments | CSV | |
|---|---|---|
| TAC level | TOS level | |
| Control | 28.6 ± 3.0d | 1.7 ± 0.1a |
| H2O2 (0.5 mM) | 13.6 ± 2.5a | 4.8 ± 0.4d |
| CSV1 + H2O2 | 15.5 ± 2.7b | 3.0 ± 0.3b |
| CSV2 + H2O2 | 17.8 ± 2.4c | 2.2 ± 0.3c |
| CSV3 + H2O2 | 15.2 ± 2.8b | 3.3 ± 0.3b |
The same column followed by the different superscript letters present significant differences at the p < 0.05 level
In the Comet assay, no significant difference in the induction of DNA damage was observed between the groups treated with CSV concentrations of 6.25, 12.5, 25, 50 and 100 μg/ml and the negative control for 24 h (Table 3). However, DNA damage was significantly increased by H2O2-intoxication at 0.5 mM for 6 h when compared to the untreated group. Furthermore, CSV pre-treatments significantly reduced DNA damage in cultures treated with the three concentrations of the compound (12.5, 25 and 50 μg/ml) plus H2O2 when compared to treatment with H2O2 alone. The highest percent reductions in DNA damage for CSV treatment was 27.05 % (Table 4). The scoring criteria for determining DNA damage levels using comet formations in cultured neurons treated with the compounds are shown in Fig. 6. The levels of 8-OH-dG, a hallmark of oxidative stress-DNA base damage, were measured using an 8-OH-dG detection kit. There were no significant differences between the intracellular levels of 8-OH-dG in the control and all CSV treated groups. On the contrary, the intracellular level of 8-OH-dG was significantly higher in H2O2-treated cultures in comparison with untreated cultures. But pre-treatment of CSV decreased the 8-OH-dG formations which were increased by H2O2. The percent decreases ranged from 4.65 to 16.27 % (Table 4). The highest percent reductions in the adduct formation for CSV treatment (at 25 μg/ml) was 34.88 % (Table 4).
Table 3.
Total DNA damage score (Comet assay) and the levels of 8-OH-dG adducts (as pg/ml) in cultured rat cortical neurons maintained in the presence of different CSV concentrations for 24 h. The abbreviations are as in Fig. 4
| Concentrations (μg/ml) | CSV | |
|---|---|---|
| Total DNA damage score | 8-OH-dG level | |
| Control | 35.4 ± 5.3a | 0.9 ± 0.1a |
| 6.25 | 36.3 ± 5.1a | 0.9 ± 0.2a |
| 12.5 | 36.0 ± 5.4a | 1.0 ± 0.1a |
| 25 | 35.9 ± 5.5a | 1.1 ± 0.3a |
| 50 | 37.3 ± 4.6a | 1.0 ± 0.1a |
| 100 | 37.8 ± 5.1a | 1.0 ± 0.2 |
The same column followed by the different superscript letters present significant differences at the p < 0.05 level
Table 4.
The effect of CSV pre-treatments on DNA damage and 8-OH-dG levels (as pg/ml) generated by H2O2 treatment. The abbreviations are as in Fig. 4
| Treatments | CSV | |
|---|---|---|
| Total DNA damage score | 8-OH-dG level | |
| Control | 35.4 ± 5.3a | 0.9 ± 0.1a |
| H2O2 (0.5 mM) | 196.3 ± 21.4d | 4.3 ± 0.3c |
| CSV1 + H2O2 | 174.5 ± 24.5c | 3.5 ± 0.3bc |
| CSV2 + H2O2 | 143.2 ± 26.8b | 2.8 ± 0.3b |
| CSV3 + H2O2 | 180.1 ± 24.9c | 4.0 ± 0.3c |
The same column followed by the different superscript letters present significant differences at the p < 0.05 level
Fig. 6.
The scoring criteria for determining damage levels in cultured neurons (A Class 0 (undamaged); B class 1 (slightly damaged); C class 3 (damaged); D class 4 (highly damaged); E class 5 (very highly damaged); F class 6 (extremely damaged)
Discussion
Neurons have been known to be more susceptible to oxidative damage than other cells due to their high oxygen consumption, low activity of antioxidant enzymes, elevated concentration of polyunsaturated fatty acids in the cell membrane, high number of mitochondria, unfavourable space/volume ratio and vicinity of microglia cells which are likely to produce increased amounts of superoxide radical (Karpinska and Gromadzka 2013). Based on this, searching for neuroprotective drugs of natural origin against oxidative stress-induced neuronal death has thereby attracted increasing research interests. In particular our investigation has been focused on cortex cells since they were evaluated as vulnerable to Alzheimer’s and Parkinson’s pathologies via oxidative stress (Di Stefano et al. 2010; Sozio et al. 2010; McCarthy et al. 2012; Suntrup et al. 2013). In comparison to more mature neurons in culture, immature neurons are particularly susceptible to cell damage induced by oxidative stress, for example, by H2O2 (Chen et al. 2009). Thus, primary cultured cortical neurons are commonly used as a suitable in vitro model system for protection against H2O2-induced cellular damages.
Cyclosativene was recently shown to have antioxidant properties (Togar 2013). However, perhaps for the first time, here different CSV applications were shown to have protective effects against H2O2-induced cell death in primary cultures of cortical neurons. H2O2, the freely diffusible form of ROS, is generated by different intracellular reactions. Extracellular H2O2 might cross membranes, thereby directly altering their intracellular concentrations. Hence, neuronal overload with ROS initiated a chain of deleterious cellular responses via forming oxidative stress (Kang et al. 2012). LDH leakage and MTT assays were performed for in vitro cytotoxicity testing since they were considered as sensitive, accurate and rapid methods (Avalos Funez et al. 2013). In this study, the cytotoxic effects of H2O2 on cultured rat cortical neuronal cells were demonstrated by its strong inhibition on cell viability (Fig. 4) and elevated LDH leakage (Fig. 5). Our findings also revealed that CSV pre-treatments at lower concentrations than 100 µg/ml reduced the cytotoxicity by H2O2. These results suggest that CSV is capable of reducing H2O2-induced cytotoxicity and lipid peroxidation. Our findings were in line with previous reports. Gan et al. (2009) reported that linderagalactone E (5), linderane, hydroxylindestenolide, and linderalactone showed hepatoprotective activity against H2O2-induced oxidative damages on HepG2 cells. In addition, huperzine A showed protective activity against H2O2-induced damage in SHSY5Y cells (Tang et al. 2005). Moreover, Turkez et al. (2013) found that α-FNS and β-FNS, naturel sesquiterpenic compounds, were capable of protecting against H2O2-induced cytotoxicity and oxidative DNA damage in cultured rat primary cortical neuronal cells. Since, high levels of exogenously generated H2O2 induced cell death via extensive DNA damage (Panieri et al. 2013) and Comet formations with almost all DNA in the tail were often referred to as ‘hedgehog’ Comets and are widely assumed to represent apoptotic cells (Lorenzo et al. 2013): we further determined high rates of total damage scores indicating increases of DNA strand breaks, alkali-labile sites and incomplete excision repair in cells after H2O2 intoxication. Here, we also presented evidence that CSV pretreatment possessed strong protective effect against H2O2-induced DNA damage in cultured cortical neurons.
Consistent with the protective effect on cytotoxicity caused by H2O2, CSV displayed a significant protective capability against H2O2-induced DNA damage (Table 4, Fig. 6). ROS-induced oxidative DNA damage has been implicated in mutagenesis and carcinogenesis and has attracted much attention in the last years since ROS might attack DNA readily, generating a variety of DNA lesions, such as oxidized bases and strand breaks (Turkez and Togar 2010; Hassan et al. 2013). Then, ROS-induced DNA damage is believed to contribute to carcinogenesis, aging and neurodegeneration (Maynard et al. 2009; Mehri et al. 2012). Recent reports revealed that both mitochondrial oxidative damage and oxidative DNA damage played important roles in the pathogenesis of many neurodegenerative diseases like Alzheimer’s and Parkinson’s (Isobe et al. 2010). In addition to Comet test, oxidative DNA damage was also evaluated in cell cultures by measuring the 8-OH-dG level, since, in particular, this marker is most frequently measured as an indicator of oxidative DNA damage in neuronal degeneration models both in vitro and in vivo (Kikuchi et al. 2011). We observed significant elevation in 8-OH-dG release from neurons was observed after treatment with H2O2, in contrast, low-dose of CSV pre-treatments led to significant decreases in 8-OH-dG release (Table 4). Here, we also presented evidence that CSV pre-treatment possessed protective effect against H2O2-induced DNA damage in cultured cortical neurons.
The neuroprotection of CSV against H2O2-induced oxidative cell death in cultured cortical neurons was further thought to be likely associated with alleviation of free radical production, although little is known on the antioxidative property of CSV and its mechanisms have not been elucidated yet. To further explore the mechanism of the in vitro protective effect of CSV on H2O2-induced oxidative damage in cortical neurons, TAS and TOS levels were also analysed. The results in Tables 1 and 2 show that CSV applications increased TAC levels of the cells without any alterations in TOS levels as compared to untreated cultures. Hence, our results suggest that CSV is capable of suppressing intracellular H2O2 formation. Although other mechanisms are possible, the above observations involving neuroprotective actions might be explained by suppressing intracellular H2O2 formation via pre-treatment with CSV. However recent studies suggested several molecular mechanisms for natural antioxidants-mediated neuroprotection against H2O2 intoxication. In fact, these mechanisms were found to be related with activation of the PI3K/Akt and alpha 2-adrenergic signalling pathways, tyrosine kinase of Trk receptors, inhibition of n-methyl-d-aspartic acid (NMDA) receptors, and regulation of apoptosis (Hou et al. 2003; Shin et al. 2003; Jiang et al. 2003; Liu et al. 2010; Iizuka et al. 2010; Zhang et al. 2012; Vlasova et al. 2013).
In summary, the present findings suggest that CSV, a natural sesquiterpenic compound, is capable of protecting against H2O2-induced cytotoxicity and oxidative DNA damage in cultured rat primary cortical neuronal cells. Taken together, present data suggest that CSV compound may prevent neurodegeneration via the antioxidative and antigenotoxicity mechanisms and have potential therapeutic values for Alzheimer’s and Parkinson’s like neurodegenerative disorders of central nervous system. Further studies will be needed to clarify the mechanisms involved.
Acknowledgments
We are grateful to our lab specialists for their help and efforts in experiments done in the pharmacology laboratories and animal housing.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abdel-Wahab BA, Metwally ME. Ginkgo biloba enhances the anticonvulsant and neuroprotective effects of sodium valproate against kainic acid-induced seizures in mice. J Pharmacol Toxicol. 2011;6:679–690. doi: 10.3923/jpt.2011.679.690. [DOI] [Google Scholar]
- Abolaji AO, Eteng MU, Omonua O, Adenrele Y. Influence of coadministration of artemether and lumefantrine on selected plasma biochemical and erythrocyte oxidative stress indices in female Wistar rats. Hum Exp Toxicol. 2013;32:206–215. doi: 10.1177/0960327112464666. [DOI] [PubMed] [Google Scholar]
- Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, Bouajila J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem Toxicol. 2013;56:352–362. doi: 10.1016/j.fct.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Avalos Funez A, Isabel Haza A, Mateo D, Morales P. In vitro evaluation of silver nanoparticles on human tumoral and normal cells. Toxicol Mech Methods. 2013;23:153–160. doi: 10.3109/15376516.2012.762081. [DOI] [PubMed] [Google Scholar]
- Ban JY, Jeon SY, Nguyen TT, Bae K, Song KS, Seong YH. Neuroprotective effect of oxyresveratrol from smilacis chinae rhizome on amyloid Beta protein (25–35)-induced neurotoxicity in cultured rat cortical neurons. Biol Pharm Bull. 2006;29:2419–2424. doi: 10.1248/bpb.29.2419. [DOI] [PubMed] [Google Scholar]
- Beck JJ, Smith L, Merrill GB. In situ volatile collection, analysis, and comparison of three Centaurea species and their relationship to biocontrol with herbivorous insects. J Agric Food Chem. 2008;56:2759–2764. doi: 10.1021/jf073383u. [DOI] [PubMed] [Google Scholar]
- Cacciatore I, Baldassarre L, Fornasari E, Cornacchia C, Di Stefano A, Sozio P, Cerasa LS, Fontana A, Fulle S, Di Filippo ES, La Rovere RM, Pinnen F. (R)-α-lipoyl-glycyl-l-prolyl-l-glutamyl dimethyl ester codrug as a multifunctional agent with potential neuroprotective activities. Chem Med Chem. 2012;7:2021–2029. doi: 10.1002/cmdc.201200320. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang Q, Cheng Q, Ding F. Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol Cell Biochem. 2009;32:85–93. doi: 10.1007/s11010-009-0177-3. [DOI] [PubMed] [Google Scholar]
- Crutwell-McFadyen RE. Biological control of weeds. Annu Rev Entmol. 1998;43:369–393. doi: 10.1146/annurev.ento.43.1.369. [DOI] [PubMed] [Google Scholar]
- Davis ME, Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes topics in current. Top Curr Chem. 2000;209:54–92. [Google Scholar]
- Di Stefano A, Sozio P, Cerasa LS, Iannitelli A, Cataldi A, Zara S, Giorgioni G, Nasuti C. Ibuprofen and lipoic acid diamide as co-drug with neuroprotective activity: pharmacological properties and effects in β-amyloid (1–40) infused Alzheimer’s disease rat model. Int J Immunopath Ph. 2010;23:589–599. doi: 10.1177/039463201002300221. [DOI] [PubMed] [Google Scholar]
- Dirican E, Turkez H, Togar B. Modulatory effects of Thymbra spicata L. different extracts against the mercury induced genotoxicity in human lymphocytes in vitro. Cytotechnology. 2012;64:181–186. doi: 10.1007/s10616-011-9406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Farah R, Khamisy-Farah R, Amit T, Youdim MB, Arraf Z. Lithium’s gene expression profile, relevance to neuroprotection A cDNA microarray study. Cell Mol Neurobiol. 2013;33:411–420. doi: 10.1007/s10571-013-9907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan LS, Zheng YL, Mo JX, Liu X, Li XH, Zhou CX. Sesquiterpene lactones from the root tubers of Lindera aggregata. J Nat Prod. 2009;72:1497–1501. doi: 10.1021/np900354q. [DOI] [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H. Protective effect of sodium selenite on genotoxicity to human whole blood cultures induced by aflatoxin B-1. Brazil Arch Biol Technol. 2005;48:905–910. doi: 10.1590/S1516-89132005000800006. [DOI] [Google Scholar]
- Geyikoglu F, Turkez H. Genotoxicity and oxidative stress induced by some bismuth compounds in human blood cells in vitro. Fresenius Environ Bull. 2005;14:854–860. [Google Scholar]
- Hassan HA, Hafez HS, Goda MS (2013) Mentha piperita as a pivotal neuro-protective agent against gamma irradiation induced DNA fragmentation and apoptosis: mentha extract as a neuroprotective against gamma irradiation. Cytotechnology 65:145–156 [DOI] [PMC free article] [PubMed]
- Hou RC, Huang HM, Tzen JT, Jeng KC. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res. 2003;74:123–133. doi: 10.1002/jnr.10749. [DOI] [PubMed] [Google Scholar]
- Iizuka Y, Hong S, Kim CY, Yang WI, Lee JE, Seong GJ. Protective mechanism of agmatine pre-treatment on RGC-5 cells injured by oxidative stress. Braz J Med Biol Res. 2010;43:356–358. doi: 10.1590/S0100-879X2010007500018. [DOI] [PubMed] [Google Scholar]
- Isobe C, Abe T, Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2′- deoxyguanosine in the CSF of patients with Alzheimer’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. J Neurol. 2010;257:399–404. doi: 10.1007/s00415-009-5333-x. [DOI] [PubMed] [Google Scholar]
- Jain V, Baitharu I, Barhwal K, Prasad D, Singh SB, Ilavazhagan G. Enriched environment prevents hypobaric hypoxia induced neurodegeneration and is independent of antioxidant signaling. Cell Mol Neurobiol. 2012;32:599–611. doi: 10.1007/s10571-012-9807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Liu JH, Bao Y, An LJ. Hydrogen peroxide-induced apoptosis in PC12 cells and the protective effect of puerarin. Cell Biol Int. 2003;27:1025–1031. doi: 10.1016/j.cellbi.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Kang SM, Cha SH, Ko JY, Kang MC, Kim D, Heo SJ, Kim JS, Heu MS, Kim YT, Jung WK, Jeon YJ. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2- induced oxidative stress in murine hippocampal HT22 cells. Environ Toxicol Pharmacol. 2012;34:96–105. doi: 10.1016/j.etap.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Karpińska A, Gromadzka G. Oxidative stress and natural antioxidant mechanisms: the role in neurodegeneration. From molecular mechanisms to therapeutic strategies. Postepy Hig Med Dosw. 2013;67:43–53. doi: 10.5604/17322693.1029530. [DOI] [PubMed] [Google Scholar]
- Khan R, Sultana S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of Wistar rats. Chem Biol Interact. 2011;192:193–200. doi: 10.1016/j.cbi.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Yasuhara T, Agari T, Kondo A, Kuramoto S, Kameda M, Kadota T, Baba T, Tajiri N, Wang F, Tayra JT, Liang H, Miyoshi Y, Borlongan CV, Date I. Urinary 8-OHdG elevations in a partial lesion rat model of Parkinson’s disease correlate with behavioral symptoms and nigrostriatal dopaminergic depletion. J Cell Physiol. 2011;226:1390–1398. doi: 10.1002/jcp.22467. [DOI] [PubMed] [Google Scholar]
- Kumar KH, Khanum F (2013) Hydroalcoholic extract of Cyperus rotundus ameliorates H2O2-induced human neuronal cell damage via its anti-oxidative and anti-apoptotic machinery. Cell Mol Neurobiol 33:5–17 [DOI] [PMC free article] [PubMed]
- Kundu A, Saha S, Walia S, Shakil NA, Kumar J, Annapurna K. Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J Environ Sci Health B. 2013;48:516–522. doi: 10.1080/03601234.2013.761921. [DOI] [PubMed] [Google Scholar]
- Liu T, Hu HT, Sun QR. Neuroprotective effects of emodin on primary rat cortical neurons apoptosis induced by hydrogen peroxide. Zhong Yao Cai. 2010;33:1116–1119. [PubMed] [Google Scholar]
- Lodewyk MW, Gutta P, Tantillo DJ. Computational studies on biosynthetic carbocation rearrangements leading to sativene, cyclosativene, alpha-ylangene, and beta-ylangene. J Org Chem. 2008;73:6570–6579. doi: 10.1021/jo800868r. [DOI] [PubMed] [Google Scholar]
- Lorenzo Y, Costa S, Collins AR, Azqueta A. The comet assay, DNA damage, DNA repair and cytotoxicity: hedgehogs are not always dead. Mutagenesis. 2013;28:427–432. doi: 10.1093/mutage/get018. [DOI] [PubMed] [Google Scholar]
- Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Saith S, Linnertz C, Burke JR, Hulette CM, Welsh-Bohmer KA, Chiba-Falek O (2012) The Alzheimer’s associated 5′ region of the SORL1 gene cis regulates SORL1 transcripts expression. Neurobiol Aging 33: 1485.e1-8 [DOI] [PMC free article] [PubMed]
- Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslehi M, Meshkini A, Yazdanparast R. Flavonoid baicalein modulates H2O2-induced mitogenactivated protein kinases activation and cell death in SK-N-MC cells. Cell Mol Neurobiol. 2012;32:549–560. doi: 10.1007/s10571-011-9795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi SF, Nabavi SM, Habtemariam S, Moghaddam AH, Sureda A, Mirzaei M. Neuroprotective effects of methyl-3-O-methyl gallate against sodium fluoride-induced oxidative stress in the brain of rats. Cell Mol Neurobiol. 2013;33:261–267. doi: 10.1007/s10571-012-9893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60:208–212. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Pala S, Gurkan H. The role of free radicals in ethiopathogenesis of diseases. Adv Mol Biol. 2008;1:1–9. [Google Scholar]
- Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B. Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med. 2013;57:176–187. doi: 10.1016/j.freeradbiomed.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Scalcinati G, Partow S, Siewers V, Schalk M, Daviet L, Nielsen J. Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb Cell Fact. 2012;11:117. doi: 10.1186/1475-2859-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HT, Chung SH, Lee JS, Kim SS, Shin HD, Jang MH, Shin MC, Bahn GH, Paik EK, Park JH, Kim CJ. Protective effect of shenqi-wan against H2O2-induced apoptosis in hippocampal neuronal cells. Am J Chin Med. 2003;31:675–686. doi: 10.1142/S0192415X03001454. [DOI] [PubMed] [Google Scholar]
- Si CL, Shen T, Jiang YY, Wu L, Yu GJ, Ren XD, Xu GH, Hu WC (2013). Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food Chem Toxicol 59:145–152 [DOI] [PubMed]
- Silva FM, Marques A, Chaveiro A. Reactive oxygen species: a double-edged sword in reproduction. Open Vet Sci J. 2010;4:127–133. doi: 10.2174/1874318801004010127. [DOI] [Google Scholar]
- Sinclair D, Donegan S, Isbar, Lalloo DG (2012) Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev 6:CD005967 [DOI] [PMC free article] [PubMed]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Sozio P, D’Aurizio E, Iannitelli A, Cataldi A, Zara S, Cantalamessa F, Nasuti C, Di Stefano A. Ibuprofen and lipoic acid diamides as potential codrugs with neuroprotective activity. Arch Pharm. 2010;343:133–142. doi: 10.1002/ardp.200900152. [DOI] [PubMed] [Google Scholar]
- Sozio P, Cerasa LS, Laserra S, Cacciatore I, Cornacchia C, Di Filippo ES, Fulle S, Fontana A, Di Crescenzo A, Grilli M, Marchi M, Di Stefano A. Memantine-sulfur containing antioxidant conjugates as potential prodrugs to improve the treatment of Alzheimer’s disease. Eur J Pharm Sci. 2013;49:187–198. doi: 10.1016/j.ejps.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012;21:393–404. doi: 10.1007/s12640-011-9294-3. [DOI] [PubMed] [Google Scholar]
- Sumathi T, Shobana C, Christinal J, Anusha C. Protective effect of Bacopa monniera on methyl mercuryinduced oxidative stress in cerebellum of rats. Cell Mol Neurobiol. 2012;32:979–987. doi: 10.1007/s10571-012-9813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntrup S, Teismann I, Bejer J, Suttrup I, Winkels M, Mehler D, Pantev C, Dziewas R, Warnecke T. Evidence for adaptive cortical changes in swallowing in Parkinson’s disease. Brain. 2013;36:726–738. doi: 10.1093/brain/awt004. [DOI] [PubMed] [Google Scholar]
- Tang LL, Wang R, Tang XC. Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur J Pharmacol. 2005;519:9–15. doi: 10.1016/j.ejphar.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:1005–1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Togar B (2013) The cytological, biochemical and genetic effects of selected sesquiterpenes on healthy neuron and N2a neuroblastoma cell cultures. PhD’s thesis, Atatürk University, Gradute School of Natural and Applied Sciences, Department of Biology, Erzurum, Turkey
- Turkez H. The role of ascorbic acid on titanium dioxide-induced genetic damage assessed by the comet assay and cytogenetic tests. Exp Toxicol Pathol. 2011;63:453–457. doi: 10.1016/j.etp.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Turkez H, Togar B. The genotoxic and oxidative damage potential of olanzapine in vitro. Toxicol Ind Health. 2010;26:583–588. doi: 10.1177/0748233710373090. [DOI] [PubMed] [Google Scholar]
- Turkez H, Sozio P, Geyikoglu F, Tatar A, Hacimuftuoglu A, Di Stefano A. Neuroprotective effects of farnesene against hydrogen peroxide-induced neurotoxicity in vitro. Cell Mol Neurobiol. 2013 doi: 10.1007/s10571-013-9991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura K, Itoh T, Hamada N, Fujita Y, Akao Y, Nozawa Y, Matsuura N, Iinuma M, Ito M. Preconditioning by sesquiterpene lactone enhances H2O2-induced Nrf2/ARE activation. Biochem Biophys Res Commun. 2008;368:948–954. doi: 10.1016/j.bbrc.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Vlasova IA, Zakharova IO, Sokolova TV, Avrova NF. Metabolic effects of ganglioside GM1 on PC12 cells at oxidative stress depend on modulation of activity of tyrosine kinase of trk receptor. Zh Evol Biokhim Fiziol. 2013;49:15–23. [PubMed] [Google Scholar]
- Wang H, Wang Y, Liu P, Wang W, Fan Y, Zhu W. Purpurides B and C, two new sesquiterpene esters from the aciduric fungus Penicillium purpurogenum JS03-21. Chem Biodivers. 2013;10:1185–1192. doi: 10.1002/cbdv.201200175. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Huang WD, Lv XY, Yang YM. Puerarin protects differentiated PC12 cells from H2O2-induced apoptosis through the PI3 K/Akt signalling pathway. Cell Biol Int. 2012;36:419–426. doi: 10.1042/CBI20100900. [DOI] [PubMed] [Google Scholar]