Abstract
Matrine is a bioactive component of the traditional Chinese medical herb Sophora flavescens that has been used in China to treat various kinds of diseases including virus hepatitis. However, the molecular mechanisms underlying its hepatoprotective effects remains elusive. In the present study, primary human hepatocytes were employed to elucidate the protective effects and molecular mechanisms of matrine. We observed that low concentrations of matrine had no significant impact on albumin secretion, but high concentrations (>140 mg/L) of matrine decreased the albumin secretion in hepatocytes. Western blot data indicated that matrine at 140 mg/L at 72 h induced protein expression of CYP2A6, CYP2B6 and CYP3A4. Furthermore, high concentrations of matrine reduced LDH and AST levels and were cytotoxic to hepatocytes, leading to a decreased cell viability and total protein amount. Moreover, low concentrations of matrine, enhanced the ECOD activity and decreased the level of NO2− induced by cytokines in human hepatocytes. Taken together, the present study sheds novel light on the molecular mechanisms of matrine and potential application of matrine in hepatic diseases.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-013-9680-1) contains supplementary material, which is available to authorized users.
Keywords: Primary human hepatocytes, Matrine, ECOD, CYP450, Apoptosis
Introduction
Hepatitis B virus (HBV) infection presents a global public health problem. It is estimated that more than 350 million people worldwide, among which approximately 120 million people in China, suffer from chronic HBV infection (Wright 2006). It is the primary cause of cirrhosis and hepatocellular carcinoma (HCC) as well as one of the ten leading causes of death due to hepatitis B associated liver diseases (Lok and McMahon 2001). However, searching for effective therapeutic strategies for HBV infection remains an unmet need. Interferon alpha (IFN-α) and lamivudine (a nucleotide analogue) are routine antiviral drugs that can inhibit HBV replication. IFN-α has antiviral and immunomodulatory effects and lamivudine is a well-tolerated, orally administered drug that suppresses HBV replication (Lau et al. 2000; Manns 2002). However, the clinical application of both drugs is very limited due to the risks of relapse after the termination of treatment and the risk of viral mutation (Akuta et al. 2003), high cost and frequently-occurring adverse effects (Fontaine and Pol 2001).
Matrine, one of the major bioactive components extracted from the traditional Chinese herbal medicine Sophora flavescens, has been recommended for treating chronic hepatitis B and C. The chemical structure of matrine is shown in Fig. 1a. Matrine was reported to have potential anti-bacterial, anti-parasitic, anti-virus, anti-fibrosis, anti-cancer and anti-inflammatory effects (Li et al. 2005; Wan et al. 2009; Zhang et al. 2011; Zhao et al. 2011; Wang et al. 2012). A systematic review of randomized clinical trials was conducted to evaluate the effects of matrine on chronic hepatitis B (Liu et al. 2003). These results lend support to the conclusion that matrine possesses antiviral activity, by affecting liver biochemical parameters and by improving disease symptoms and signs. Although matrine is widely used in clinic to treat HBV infection, the mechanisms remain largely unknown. Therefore, the present study was designed to investigate the pharmacological effects and molecular mechanisms of matrine in human hepatocytes.
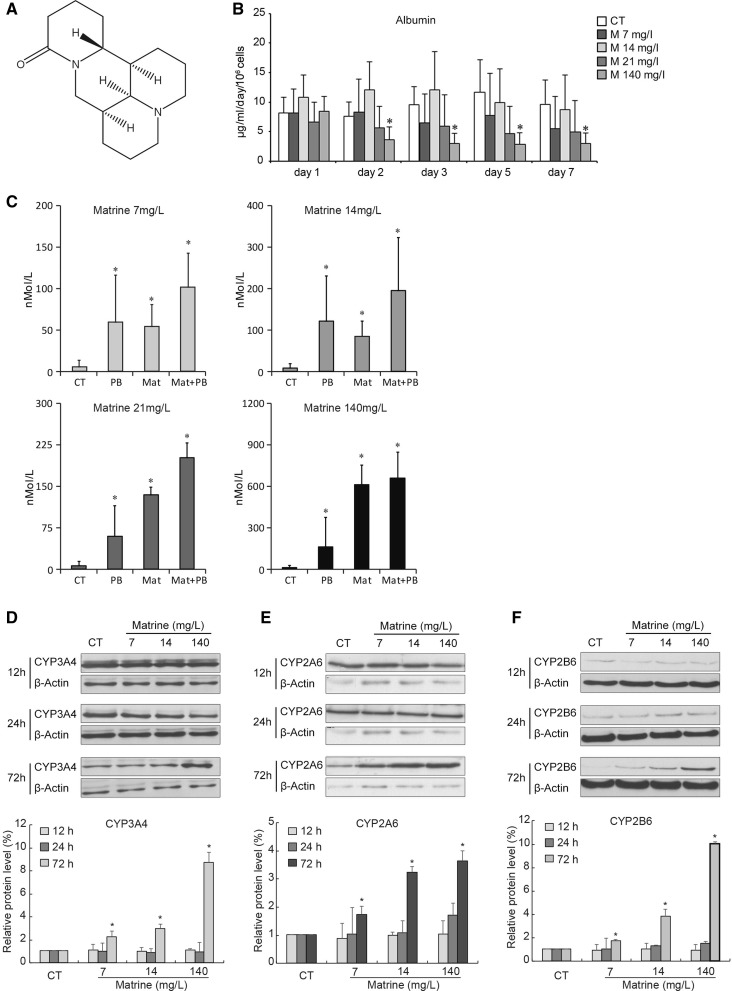
Fig. 1.
Effect of low concentrations of matrine on the metabolic functions of primary human hepatocytes. a The chemical structure of matrine. b Albumin secretion in human hepatocyte cultures treated with different concentrations of matrine (0, 7, 14, 21, and 140 mg/L) was measured in the supernatant at days 1, 2, 3, 5 and 7 after the cells were seeded. Each value represents the mean of triplicates of 3 individually performed experiments ± SD. (*, P < 0.05 vs control group at the same day) c The activity of ECOD-associated CYP enzymes induced by PB (2 mM), matrine (7, 14, 21 and 140 mg/L) or a combination of matrine and PB. The values represent the mean of triplicates ± SD of five individually performed experiments and are expressed as nMol/L (*, P < 0.05 vs control (CT)). Time and dose dependent protein expressions of CYP3A4 (d), CYP2A6 (e) and CYP2B6 (f) in human hepatocytes cultured with different concentrations of matrine (upper panels). Lower panels of d, e and f present of the statistical analysis for expression of CYP3A4, CYP2A6 and CYP2B6. (*, P < 0.05 vs control)
Materials and methods
Isolation, culture and treatment of primary human hepatocytes
Human hepatocytes were isolated from biopsies (n = 38) obtained from patients undergoing partial liver resections as therapy due to primary or secondary liver tumors. Liver tissues were collected according to institutional guidelines with the approval of the local ethics committee and with informed consent of the donors. After resection for medical reasons, a small piece of liver tissue (10–40 g) was immediately taken from the operation room and transferred to the laboratory in a sterile ice-cold culture medium. Only samples of macroscopically normal tissue were used for the isolation of hepatocytes. Classification for liver resection was: hepatocyte carcinoma (n = 14), metastasis tumor (n = 13) and other liver diseases (n = 11). A two-step collagenase perfusion of the liver tissue was performed (Dorko et al. 1994). The viability of the isolated hepatocytes was determined by trypan blue exclusion test. A viability of 80–90 % was achieved. Freshly isolated hepatocytes were seeded into collagen (1 %, Nycomed GmbH, Munich, Germany) pre-coated 12-well culture plates at a density of 0.5 × 106 cells/well. Hepatocytes were left untreated for at least 18 h at 37 °C, and 5 % CO2 in an incubator, allowing cell attachment. The medium was changed on the following day to remove unattached cells. The cells were routinely examined by phase contrast microscopy and the culture medium (Human Hepatocyte Maintenance Medium, Primacyt GmbH, Schwerin, Germany) was replenished every 24 h (Nussler et al. 1994). The supernatants and cell pellets were collected at the indicated time points and stored at −20 °C for further assays.
Matrine injection solution, 35 mg/2 mL/ampoule, was produced by Kangpu Pharmacy (Hunan, China). In the clinic, patients received matrine injection of 35–70 mg per day per patient. The volume of the blood in the body is about 5,000 mL, so the initial concentration of matrine in the blood is about 7–14 mg/L. In this study, we used the following concentrations of matrine: 7, 14, 21 mg/L (three times to clinical dose) and 140 mg/L (ten times to clinical dose). We chose also very high concentrations of matrine (250, 500 and 1,000 mg/L) for the toxicity experiment of matrine. When we used the low concentrations of matrine (7, 14, 21 and 140 mg/L), the hepatocytes were cultured with them immediately after isolation. High concentrations of matrine were added 18–24 h after isolation. The hepatocytes of the control group were cultured only with William’s E medium (Invitrogen, Karlsruhe, Germany).
Measurement of LDH and AST
To evaluate the protective effects of matrine on primary human hepatocyte cultures, lactate dehydrogenase (LDH), and aspartate transaminases (AST) as signs for membrane leakages were measured. LDH (kit: NobiFlow LDH-UV, #Fa Nobis 034570, HITADO Diagnostic Systems, Mohnesee-Delecke, Germany) and AST (kit: NobiFlow AST-UV, #Fa Nobis 034545, HITADO Diagnostic Systems) were measured using kinetic UV-method test kits according to the manufacturer’s instructions.
Albumin measurement
The continuous secretion of albumin from hepatocytes cultured with or without matrine was measured by an enzyme-linked immunosorbent assay (ELISA) using a Urinary Albumin kit (WAK-Chemie Medical GmbH, Steinbach, Germany). The ELISA was performed according to the manufacturer’s instructions and to a previous report (Katenz et al. 2007).
MTT assay
MTT assay was used to examine the effect of matrine on cell viability of hepatocytes. In brief, freshly isolated primary human hepatocytes were plated into collagen-coated 96-well culture plates at a density of 0.05 × 106 hepatocytes/well. Cells in control wells were killed with 2 μL 10 % Triton X-100, 15 min before pipetting the reagent. 20 μL of the CellTiter 96® AQueous One Solution Reagent (Promega, Madison, WI, USA) was added into each well of the 96-well plate containing the samples, controls and blanks in 100 μL of culture medium. Then the plates were incubated for 4 h at 37 °C in a humidified incubator (95 % air and 5 % CO2). The absorbance was then detected at 490 nm using a 96-well plate reader (Anthos htII, Labtec, Salzburg, Austria).
Phase I enzyme activities
To evaluate the influence of matrine on phase I enzyme activity of the isolated human hepatocytes, specific enzymological assays, such as deethylation of ethoxyresorufin (EROD) and ethoxycoumarin-O-deethylation (ECOD), were determined. ECOD was measured in monolayer culture over the culture period according to a protocol published by Fry et al. (1980). Briefly, the hepatocytes were induced by addition of 2 mM Phenobarbital (Sigma, Taufkirchen, Germany) or/and matrine (7, 14, 21 and 140 mg/L). The medium was replaced daily for three consecutive days. At the indicated time point the cultured hepatocytes were washed once with 0.01 M PBS and supplied with fresh culture medium without the inducer and incubated for 30 min to allow equilibration. Then hepatocytes were incubated for 2 h at 37 °C with 25 μM ethoxycoumarin (Sigma, Taufkirchen, Germany) and 1.5 mM salicylamide (Sigma, Taufkirchen, Germany), which was added to the medium to prevent conjugation of 7-hydroxy metabolites of 7-ethoxycoumarin (Burke and Orrenius 1978). The culture medium served as control and the standard curve was generated by culture medium spiked with 7-hydroxycoumarin ranging from 15.625 to 1,000 nM. After incubation the 7-hydroxycoumarin formation was measured using a Fluorescent Galaxy plate reader (MTX Lab Systems, Vienna, VA, USA) at an excitation of 390 nm and an emission wavelength of 460 nm. ECOD activity was calculated from the standard curve and expressed as formed 7-hydroxycoumarin in nMol/L (Burke and Mayer 1974). For EROD activity, 3-methylcholanthrene (3-MC,2.5 μM; Fluka Chemie GmbH, Buchs, Switzerland) was used instead of Phenobarbital and data were collected by the reader at an excitation wave length of 544 nm and an emission wavelength of 590 nm.
Urea measurement
The ammonia produced through amino acid deamination can be detoxified by the reaction with CO2 forming urea. Therefore, the rate of urea synthesis is a good prognostic indicator of liver function (Watts et al. 1995). The urea synthesis rate was determined using a urea-Glutamatdehydrogenase (GLDH)-method kit (HITADO Diagnostic Systems) according to the manufacturer’s instructions.
Western blot analysis
Primary human hepatocytes were isolated and 10 mL of the cell (cells number about: 7 × 106) suspension were seeded into 100 mm Petri dishes and treated with or without matrine. At each time point, cultured hepatocytes were harvested. Then lysates were separated using SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane (Bio-Rad, Munich Germany). Membranes were blocked in Tris-buffered saline with 5 % milk and 0.05 % Tween 20 and probed with primary antibodies at 4 °C overnight. The following antibodies were used at a dilution of 1:1,000 unless otherwise indicated: anti-CYP1A1 and anti-CYP1A2 (Chemicon, Temecula, CA, USA); CYP2A6 and CYP3A4 (Affinity Bioreagents, Weinheim, Germany); CYP2B6 (Diagnostica Vertrieb GmbH, Eching, Germany); CYP2E1 (Calbiocom—Merck KGaA, Darmstadt, Germany); β-Actin (Sigma, Deisenhofen, Germany). Appropriate horseradish peroxidase-conjugated secondary antibodies (Amersham, Freiburg, Germany) were used for detection with the ECL regents (Forevergen Biosciences, Guangzhou, China). For quantification, intensity of individual bands was quantified using the ImageJ densitometry software, and expressed relative to Actin signals, as a measure of protein relative abundance in the different samples. Control groups were set to 1 for relative ratio analysis. Statistical data were shown as mean ± SEM.
Nitrite measurement
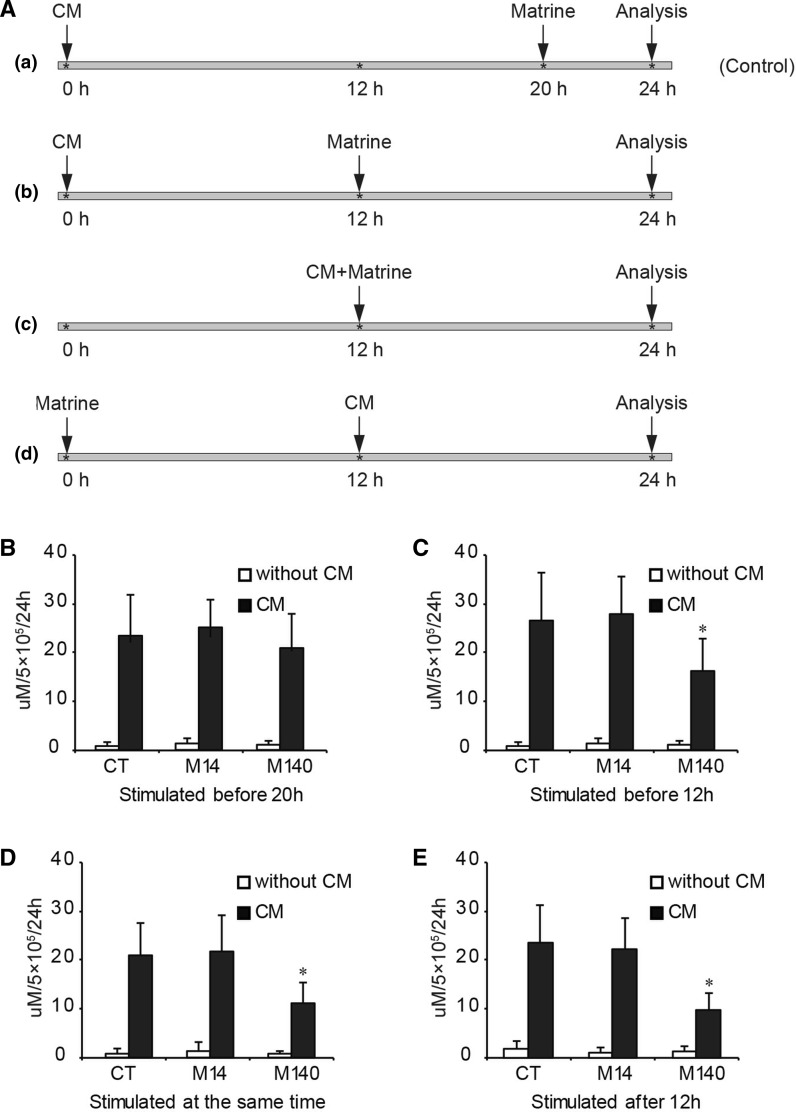
Human hepatocytes were stimulated with 1.6 U/mL recombinant human IL-1β (Cistron, Pine Brook, NJ, USA), 166 U/mL recombinant human TNF-α (Genzyme, Cambridge, MA, USA) and 33 U/mL recombinant human IFN-γ (Amgen, Thousand Oaks, CA, USA), along with 3.3 mg/mL LPS (E. coli 0111:B4, Sigma) with or without matrine added at different time points as shown in Fig. 3a. Briefly, there were four different groups as follows: (a) cells were stimulated by CM for 20 h and later by matrine for 4 h (this group was designed as control); (b) 12 h before exposure to matrine, the cells were stimulated by CM for 12 h; (c) CM and matrine were added to the cultured cells at the same time and incubated for 12 h; (d) hepatocytes were stimulated by CM for 12 h after the cells were exposed to matrine for 12 h. After incubation at 37 °C and 5 % CO2 for 24 h, the supernatants were collected for determination of nitrite/nitrate levels. The content of nitrite was assessed as described elsewhere (Marzinzig et al. 1997). Samples (150 μL) were mixed with 75 μL ice-cold 4,4′-diamino-diphenylsulfone; 14 mM in 2 N HCl (dapsone; from Merck, Darmstadt, Germany), then 75 μL NED [N-(1-Naphthyl)ethylenediamine dihydrochloride; from Merck, Darmstadt, Germany; 4 mM in H2O] was added and the mixture was incubated at room temperature for 5 min. Light absorption was measured at 550 nm. Nitrite levels were calculated from NO2− standard curves (0.2–100.0 mM). Results were expressed as μM/5 × 105 cells/24 h.
Fig. 3.
Effect of different concentrations of matrine on the nitrite levels in human hepatocytes induced by a combination of cytokines (CM). a Schematic diagram of the setup of the experiments. b CM stimulation of hepatocytes for 20 h before matrine treatment; c CM stimulation of hepatocytes for 12 h before matrine treatment; d CM and matrine given at the same time; e CM was given after matrine treatment for 12 h. CT means control; M14 means with matrine at 14 mg/L; M140 means with matrine at 140 mg/L. Each value represents the mean ± SD of triplicates (*, P < 0.05 vs CM control group)
Statistical analysis
All experiments were performed in triplicates and repeated separately for at least three times. Significance of differences was determined by using the non-parametric Mann–Whitney Test (SPSS statistics program, version 10.0). Values are expressed as mean ± standard deviation (SD). Statistical significance was established at a P value < 0.05.
Results
Effect of low concentrations of matrine on the metabolic functions of primary human hepatocytes
Albumin is a major plasma protein secreted by hepatocytes (Dufour et al. 2000). Hepatocytes cultured without matrine showed a continuous albumin secretion over 7 days (Fig. 1). Hepatocytes cultured with 7 days). Hepatocytes cultured with 7, 14, 21 mg/L matrine also secreted albumin at a stable rate. Hepatocyte cultured with 140 mg/L of matrine secreted the same amount of albumin at day 1, but a rapid decline was observed over the next days, which persisted until the end of the observation period (Fig. 1b).
The result of the ECOD assay in human hepatocytes is shown in Fig. 1c. The hepatocytes were exposed to the ECOD inducer, phenobarbital (PB), for 72 h as positive control. We observed that the presence of PB caused a robust increase in ECOD-associated CYP enzymatic activity. Matrine induced ECOD-associated CYP enzymatic activity in a dose-dependent manner. The combination of matrine and PB act in synergy to increase ECOD activity compared with the single use of either agent. These findings were observed for the four low concentrations of matrine used.
Furthermore, the EROD assay mainly reflects the activity of CYP1A in hepatocytes. The basal and induced CYP1A activity of human hepatocytes were measured in the presence or absence of 3-methylcholantren (3-MC) and matrine. No differences of EROD were observed between the control group and the groups stimulated by the single use of matrine (data not shown). In addition, no significant difference was also observed in the LDH level and secretion of AST between these groups. Using the MTT assay, we determined that the number of viable cells was not statistically different among the groups at the same day. Also, the total amount of cell protein and the formation of urea presented no significant differences among the groups.
Next, we examined the CYP protein expression by Western blot from 3 different donors. As shown in Fig. 1d, intense protein expression of CYP3A4 was observed in the control group at 12 and 24 h. At 72 h the CYP3A4 intensity tended to decrease. Matrine treated groups expressed the same level compared to the control group at 12 h and comparable levels at 24 h. At 72 h, the intensity of the bands in the matrine groups were stronger, concurrent with the increase of dose of matrine used. Highest expression was achieved with 140 mg/L of matrine. Similarly, matrine (at 140 mg/L) markedly induced CYP2A6 (Fig. 1e) and CYP2B6 (Fig. 1f) expression at 72 h. Statistical analysis of the expression of CYP3A4, CYP2A6 and CYP2B6 are shown in the lower panels of Fig. 1d, e and f. These results suggested that CYP3A4, CYP2A6 and CYP2B6 were significantly induced after treatment with matrine (140 mg/L) for 72 h, suggesting the role of matrine in regulating CYP450 enzyme systems.
Effect of high concentrations of matrine on primary human hepatocytes
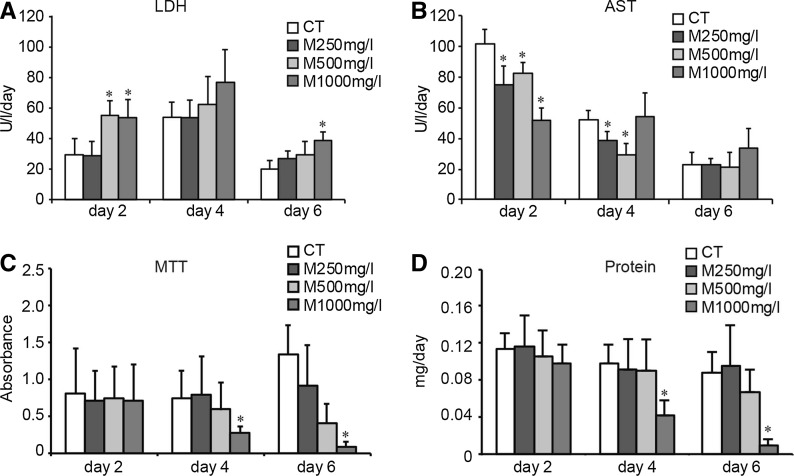
To examine whether high concentrations of matrine have cytotoxicity on human hepatocytes, we incubated hepatocytes with three different concentrations of matrine (250, 500 and 1,000 mg/L). In Fig. 2, we observed that high concentrations of matrine damaged the membrane system of hepatocytes and led to elevation of LDH release at day 2 and day 6. Next, at day 2, levels of AST were lowered for all treatment groups of matrine, compared to the control group. At day 4, the level of AST was lowered only in the groups of 250 and 500 mg/L matrine. But at day 6, AST levels of all treatment groups did not show significant differences (Fig. 2b). MTT test was used to determine the cytotoxicity of high concentrations of matrine on hepatocytes. No statistically significant differences were observed at the onset of cell culture. However, from day 4 on, 1,000 mg/L of matrine visibly led to a significant loss of viability observed by phase-contrast microscopy (Fig. 2c). Higher concentrations of matrine influenced the total protein amount of hepatocytes. In the 1,000 mg/L matrine group, the amount of protein on the 4th and 6th day was lower than in the control group on the same day (P < 0.05 vs control group on the same day, Fig. 2d). These data indicated that high concentrations of matrine (>1,000 mg/L) are toxic to hepatocytes.
Fig. 2.
Effect of high concentrations of matrine on primary human hepatocytes. Aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) enzyme activities were measured in culture supernatants with commercially available test kits. a The levels of LDH of hepatocytes treated with different concentrations of matrine over 6 days. Each value represents the mean of triplicates of 3 individually performed experiments ± SD. (*, P < 0.05 vs control group at the same day). b The levels of AST of hepatocytes treated with high concentrations of matrine for 6 days. Each value represents the mean of triplicates of 3 individually performed experiments ± SD and is expressed as U/l/day. (*, P < 0.05 vs control group at the same day). c Results of the MTT test performed in human hepatocytes, which were treated with high concentrations matrine for 6 days. Each value represents the mean of triplicates of 3 individually performed experiments ± SD. (*, P < 0.05 vs control cultures at the same day) d The amount of total cell protein was determined. The hepatocytes were cultured with high concentrations of matrine. Each value represents the mean ± SD of triplicates. (*, P < 0.05 vs control cultures at the same day)
Effect of matrine on the nitrite levels in human hepatocytes induced by a combination of cytokines
Nitric oxide (NO) plays an important role in liver homeostasis and diseases, and its production is paradoxically implicated in both cytoprotection and cytotoxicity (Taylor et al. 1998). In this study, we investigated the effect of matrine on the nitrite levels induced by a mixture of cytokines (CM). The mixture contained TNF- α (166 U/mL), IFN-γ (33 U/mL), IL-1β (1.66 U/mL) and LPS (3.3 mg/mL). Four types of stimulations were outlined as below: (a) cells were stimulated by CM for 20 h and later by matrine for 4 h (this group was designed as control); (b) 12 h before exposure to matrine, the cells were stimulated by CM for 12 h; (c) CM and matrine were added to the cultured cells at the same time and incubated for 12 h; (d) hepatocytes were stimulated by CM for 12 h after the cells were exposed to matrine for 12 h (Fig. 3a). At the indicated time points, the culture supernatants were collected and the nitrite levels were determined based on the Griess reaction (Marzinzig et al. 1997). The level of nitrite in the CM group was significantly higher than that in the unstimulated group. As shown in Fig. 3b, if cells were exposed to matrine for only 4 h, the level of nitrite after CM stimulation would not be suppressed, excluding some unknown possibility of chemical interference by matrine. When the cells were cultured with matrine at 140 mg/L in the other three groups, the level of nitrite was suppressed (Fig. 3c, d, e); especially, when matrine was added at the same time as the CM for 12 h (c treatment group indicated in Fig. 3a) or added before addition of CM for 12 h (d treatment group indicated in Fig. 3a), the levels of nitrite were significantly lower than for the CM control group (Fig. 3d, e). These results indicate that matrine could decrease the inflammatory responses caused by CM in hepatocytes.
Morphological presentation of human hepatocytes cultured with different concentrations of matrine
As depicted in Fig. 4a, we observed that hepatocytes showed a typically hexagonal shape after being cultured for 6 days with low concentrations of matrine (14, 21 and 140 mg/L). We could clearly observe cell–cell contacts, evidenced by white lines between the cells. No obvious pathological findings were expressed. In Fig. 4b, hepatocytes showed normal morphological characteristics only in the control group, when cultured for 6 days. After treating the cells with high concentrations of matrine (250, 500 and 1,000 mg/L), we observed that the cell morphology obviously changed during the culture period, with concomitant cell detachment. In addition, there were many bright and transparent vesicles in the cytoplasm. These phenomena were more obvious with the increase of the matrine concentration. These data suggest that low concentration of matrine is protective and high concentration is toxic for hepatocytes in culture.
Fig. 4.
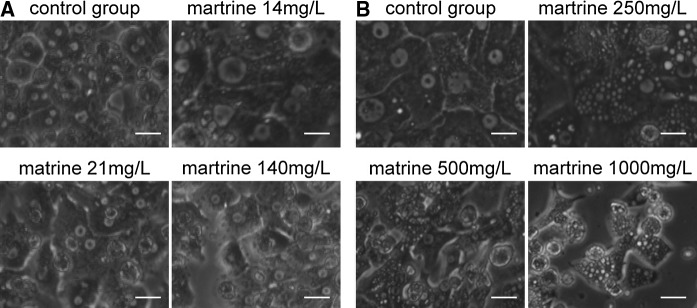
Morphological changes of human hepatocytes cultured with different concentrations of matrine. a Light microscopic morphology of human hepatocytes treated with low concentrations of matrine (14, 21 and 140 mg/L, the selection of dosages was not even, usually 5 or 10 times of change) at the 6th culture day. b Human hepatocytes on the 6th culture day treated with high concentrations of matrine (250, 500 and 1,000 mg/L), shown under the light microscope. 320 × times magnified. Scale bar 20 μm
Discussion
In this study, primary human hepatocytes was used to elucidate the protective role of a Traditional Chinese Medicine—matrine. We found that high concentrations of matrine decreased the albumin secretion in hepatocytes, upregulated the protein level of CYP2A6, CYP2B6 and CYP3A4 and reduced LDH and AST levels. Furthermore, we demonstrated that low concentrations of matrine enhanced the ECOD activity and decreased the level of NO2− induced by cytokines in human hepatocytes.
In the last decades matrine has been one of the most important drugs to treat viral hepatitis in China. Although many experiments were made with animals, the mechanisms of its effects are not yet elucidated. Isolated human hepatocytes are the ideal source for bio-artificial liver support devices, hepatocyte transplants and pharmacological and toxicological studies (Morsiani et al. 2002; Horslen and Fox 2004; Lloyd et al. 2004). Unlike immortalized cell lines, the specific functions are retained in freshly-isolated primary human hepatocytes in vitro, including albumin secretion and cytochrome P450 inducibility.
Cultured hepatocytes are capable of secreting a large number of different proteins into the culture medium. Among these proteins, albumin is one of the most abundant. Isolated hepatocytes can synthesize and secret albumin for a few days in vitro (Weigand and Otto 1974; Miranda et al. 2009). According to the results of this study, the isolated human hepatocytes retained the property of secreting albumin during seven culture days and the quantity of albumin in culture supernatants was stable. Low concentrations of matrine (7–21 mg/L) did not influence the albumin secretion, but the quantity of albumin declined rapidly in the group cultured with 140 mg/L matrine. That means matrine decreased the ability of hepatocytes to secret albumin when its concentration was higher than 140 mg/L.
CYP is the principal enzyme system for the metabolism of exogenous compounds. These enzymes are mainly located in the liver (Nebert and Russell 2002). The expression of the major CYP proteins is a qualitative marker to determine whether or not the isolated and cultured cells can be used to study various aspects of liver related diseases or for the analyzing of drug metabolism (Frye et al. 2006). Today more than 30 human isoenzymes are identified, the major human CYP isoforms involved in drug metabolism are CYP1A2, CYP2A6, CYP2E1 and CYP3A4. Among these, CYP3A4 is the most abundant: it catalyzes the metabolism of approximately 50 percent of therapeutic agents (Tang and Stearns 2001). Actually, we have investigated six types of CYP protein using western blot: CYP1A1, CYP1A2, CYP2A6, CYP3A4, CYP2E1, and CYP2B6. The results show that matrine induced human hepatocytes to express CYP1A2 (Supplemental Figure 1), CYP2A6, CYP2B6 and CYP3A4 (Fig. 1d, e, f), while the expression of CYP2E1 (Supplemental Figure 2) and CYP1A1 (Supplemental Figure 3) was not induced. In general, matrine could induce CYP450 in hepatocytes. Previous reports show that down regulation of CYP (like CYP2A6) may be related to inflammation caused by HBV and HCV infection (Gripon et al. 2002; Hara and Adachi 2002; Jover et al. 2002). Our results showed that low concentration of matrine (140 mg/L) would increase the protein level of some CYP proteins (like CYP2A6), indicating the protective effect of matrine on cultured hepatocytes. Further study is needed to clarify if this function is of benefit or harmful to patients.
AST and LDH are cytosolic enzymes, which are present in little quantities in serum under normal conditions (Skibba and Gwartney 1997). The degree of cell injury caused by toxic substances or by the long culture time can be estimated through the leakage of enzymes, like LDH and AST (Takahashi et al. 2009). In the present study we found that LDH increased on culture day two along with the concentration of matrine and showed significantly higher levels than in the control group at the same day (Fig. 2, P < 0.05). The result contradicts other publications, which stated that matrine used at the same concentrations did not damage the membrane system of rat hepatocytes (Si et al. 2001). The result of AST level after the cells treated with high concentrations of matrine do not accord with the result of LDH except for the group treated with 1,000 mg/L matrine. LDH and AST are markers of cell injury (Baur et al. 1975). LDH enzyme release is a reliable indicator for the degree of the cell membrane damage while AST is an index for mitochondrial damage since 80 % of them are AST is located in the mitochondria (Zimmerman et al. 1965). From the result shown in Fig. 2b, AST level only increased on day 6 under the treatment of matrine of 1,000 mg/L, indicating that only higher concentrations of matrine present for a longer period can matrine damage the mitochondrial membrane. We suggest that AST and LDH may come from different parts of hepatocyte, which resulted in the different increase patterns of AST and LDH.
The increasing evidence proves that nitric oxide (NO) is one of the most versatile mediators in the control of viral infections, being the earliest antiviral response of the host (Torre et al. 2002). It also takes part in the pathogenesis of many human infectious and inflammatory diseases, acting as pro-apoptotic inducers in some cell types or as an anti-apoptotic modulator in other cell types including hepatocytes (Zamora et al. 2000). NO also led to liver necrosis, immunomediated liver damage, and DNA fragmentation by blocking mitochondrial function and depleting cellular pyridine nucleotides (Carnovale et al. 2000). Excessive NO production and increased iNOS induction in the liver are reported in chronic liver diseases (Sen et al. 2002). The present study shows that 140 mg/L matrine can lead to a decrease of the level of NO in isolated human hepatocytes, which are induced by a mixture of cytokines TNF-α, IFN-γ, IL-1 and LPS, suggesting that matrine might alleviate inflammatory reactions and possibly protect hepatocytes. This could perhaps be one of the mechanisms of matrine acting against hepatitis and as such is of great interest for further investigations at a larger scale.
Taken together, the present study demonstrates that low concentrations of matrine (7–140 mg/L) have no obvious negative effects on primary human hepatocytes in culture, whereas high concentrations of matrine (250–1,000 mg/L) exert cytotoxicity against the cells. Our findings provide a meaningful basis for evaluating the clinical applications of matrine in liver diseases.
Electronic supplementary material
Acknowledgments
The authors declare no conflict of interest. This work was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Ministry of Education (Grant No. 890[2008]) and the Medical Scientific Research Foundation of Guangdong Province (Grant No. A2009357).
Abbreviations
- MA
Matrine
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
- CYP
Cytochrome P
- ECOD
Ethoxycoumarin-O-deethylation
- EROD
Deethylation of ethoxyresorufin
- GST
Glutathione-S-transferase
- LDH
Lactate dehydrogenase
Footnotes
Xiaobing Gong and Yuan Gao have contributed equally to this work.
References
- Akuta N, Suzuki F, Kobayashi M, Matsuda M, Sato J, Takagi K, Tsubota A, Suzuki Y, Hosaka T, Someya T, Saitoh S, Arase Y, Ikeda K, Kumada H. Virological and biochemical relapse according to YMDD motif mutant type during long-term lamivudine monotherapy. J Med Virol. 2003;71:504–510. doi: 10.1002/jmv.10519. [DOI] [PubMed] [Google Scholar]
- Baur H, Kasperek S, Pfaff E. Criteria of viability of isolated liver cells. Hoppe Seylers Z Physiol Chem. 1975;356:827–838. doi: 10.1515/bchm2.1975.356.s1.827. [DOI] [PubMed] [Google Scholar]
- Burke MD, Mayer RT. Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos. 1974;2:583–588. [PubMed] [Google Scholar]
- Burke MD, Orrenius S. The effect of albumin on the metabolism of ethoxyresorufin through O-deethylation and sulphate-conjugation using isolated rat hepatocytes. Biochem Pharmacol. 1978;27:1533–1538. doi: 10.1016/0006-2952(78)90481-1. [DOI] [PubMed] [Google Scholar]
- Carnovale CE, Scapini C, Alvarez ML, Favre C, Monti J, Carrillo MC. Nitric oxide release and enhancement of lipid peroxidation in regenerating rat liver. J Hepatol. 2000;32:798–804. doi: 10.1016/S0168-8278(00)80249-4. [DOI] [PubMed] [Google Scholar]
- Dorko K, Freeswick PD, Bartoli F, Cicalese L, Bardsley BA, Tzakis A, Nussler AK. A new technique for isolating and culturing human hepatocytes from whole or split livers not used for transplantation. Cell Transplant. 1994;3:387–395. doi: 10.1177/096368979400300505. [DOI] [PubMed] [Google Scholar]
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027–2049. [PubMed] [Google Scholar]
- Fontaine H, Pol S. Side effects of interferon-alpha in treating hepatitis C virus infection. Transplant Proc. 2001;33:2327–2329. doi: 10.1016/S0041-1345(01)02010-3. [DOI] [PubMed] [Google Scholar]
- Fry JR, Wiebkin P, Bridges JW. 7-Ethoxycoumarin O-deethylase induction by phenobarbitone and 1,2-benzanthracene in primary maintenance cultures of adult rat hepatocytes. Biochem Pharmacol. 1980;29:577–581. doi: 10.1016/0006-2952(80)90379-2. [DOI] [PubMed] [Google Scholar]
- Frye RF, Zgheib NK, Matzke GR, Chaves-Gnecco D, Rabinovitz M, Shaikh OS, Branch RA. Liver disease selectively modulates cytochrome P450–mediated metabolism. Clin Pharmacol Ther. 2006;80:235–245. doi: 10.1016/j.clpt.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Adachi T. Contribution of hepatocyte nuclear factor-4 to down-regulation of CYP2D6 gene expression by nitric oxide. Mol Pharmacol. 2002;61:194–200. doi: 10.1124/mol.61.1.194. [DOI] [PubMed] [Google Scholar]
- Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004;77:1481–1486. doi: 10.1097/01.TP.0000113809.53415.C2. [DOI] [PubMed] [Google Scholar]
- Jover R, Bort R, Gomez-Lechon MJ, Castell JV. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: molecular mechanism and transcription factors involved. FASEB J. 2002;16:1799–1801. doi: 10.1096/fj.02-0195fje. [DOI] [PubMed] [Google Scholar]
- Katenz E, Vondran FW, Schwartlander R, Pless G, Gong X, Cheng X, Neuhaus P, Sauer IM. Cryopreservation of primary human hepatocytes: the benefit of trehalose as an additional cryoprotective agent. Liver Transpl. 2007;13:38–45. doi: 10.1002/lt.20921. [DOI] [PubMed] [Google Scholar]
- Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J, Kuhns MC, Liang TJ, Hoofnagle JH. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828–834. doi: 10.1053/jhep.2000.17912. [DOI] [PubMed] [Google Scholar]
- Li CQ, Zhu YT, Zhang FX, Fu LC, Li XH, Cheng Y, Li XY. Anti-HBV effect of liposome-encapsulated matrine in vitro and in vivo. World J Gastroenterol. 2005;11:426–428. doi: 10.3748/wjg.v11.i3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu M, Shi R, Yang M. Radix Sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. Am J Chin Med. 2003;31:337–354. doi: 10.1142/S0192415X03001107. [DOI] [PubMed] [Google Scholar]
- Lloyd TD, Orr S, Patel R, Crees G, Chavda S, Vadyar H, Berry DP, Sherlock D, Dennison AR. Effect of patient, operative and isolation factors on subsequent yield and viability of human hepatocytes for research use. Cell Tissue Bank. 2004;5:81–87. doi: 10.1023/B:CATB.0000034079.10985.bd. [DOI] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225–1241. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- Manns MP. Current state of interferon therapy in the treatment of chronic hepatitis B. Semin Liver Dis. 2002;22(Suppl 1):7–13. doi: 10.1055/s-2002-35695. [DOI] [PubMed] [Google Scholar]
- Marzinzig M, Nussler AK, Stadler J, Marzinzig E, Barthlen W, Nussler NC, Beger HG, Morris SM, Jr, Bruckner UB. Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide. 1997;1:177–189. doi: 10.1006/niox.1997.0116. [DOI] [PubMed] [Google Scholar]
- Miranda JP, Leite SB, Muller-Vieira U, Rodrigues A, Carrondo MJ, Alves PM. Towards an extended functional hepatocyte in vitro culture. Tissue Eng Part C Methods. 2009;15:157–167. doi: 10.1089/ten.tec.2008.0352. [DOI] [PubMed] [Google Scholar]
- Morsiani E, Brogli M, Galavotti D, Pazzi P, Puviani AC, Azzena GF. Biologic liver support: optimal cell source and mass. Int J Artif Organs. 2002;25:985–993. doi: 10.1177/039139880202501013. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- Nussler AK, Liu ZZ, Di Silvio M, Sweetland MA, Geller DA, Lancaster JR, Jr, Billiar TR, Freeswick PD, Lowenstein CL, Simmons RL. Hepatocyte inducible nitric oxide synthesis is influenced in vitro by cell density. Am J Physiol. 1994;267:C394–C401. doi: 10.1152/ajpcell.1994.267.2.C394. [DOI] [PubMed] [Google Scholar]
- Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22(Suppl 2):5–13. doi: 10.1034/j.1600-0676.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- Si W-K, Pan J, Lu H, Li Z-Q. Study on matrine inhibiting proliferation of HepG2 cell and the relation between its dosage and inhibiting style. World J Gastroenterol. 2001;9:5. [Google Scholar]
- Skibba JL, Gwartney EA. Liver hyperthermia and oxidative stress: role of iron and aldehyde production. Int J Hyperthermia. 1997;13:215–226. doi: 10.3109/02656739709012384. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yamazoe H, Sassa F, Suzuki H, Fukuda J. Preparation of coculture system with three extracellular matrices using capillary force lithography and layer-by-layer deposition. J Biosci Bioeng. 2009;108:544–550. doi: 10.1016/j.jbiosc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Tang W, Stearns RA. Heterotropic cooperativity of cytochrome P450 3A4 and potential drug-drug interactions. Curr Drug Metab. 2001;2:185–198. doi: 10.2174/1389200013338658. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc) 1998;63:766–781. [PubMed] [Google Scholar]
- Torre D, Pugliese A, Speranza F. Role of nitric oxide in HIV-1 infection: friend or foe? Lancet Infect Dis. 2002;2:273–280. doi: 10.1016/S1473-3099(02)00262-1. [DOI] [PubMed] [Google Scholar]
- Wan XY, Luo M, Li XD, He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact. 2009;181:15–19. doi: 10.1016/j.cbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wang L, You Y, Wang S, Liu X, Liu B, Wang J, Lin X, Chen M, Liang G, Yang H. Synthesis, characterization and in vitro anti-tumor activities of matrine derivatives. Bioorg Med Chem Lett. 2012;22:4100–4102. doi: 10.1016/j.bmcl.2012.04.069. [DOI] [PubMed] [Google Scholar]
- Watts P, Smith MD, Edwards I, Zammit V, Brown V, Grant H. The influence of medium composition on the maintenance of cytochrome P-450, glutathione content and urea synthesis: a comparison of rat and sheep primary hepatocyte cultures. J Hepatol. 1995;23:605–612. doi: 10.1016/0168-8278(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Weigand K, Otto I. Secretion of serum albumin by enzymatically isolated rat liver cells. FEBS Lett. 1974;46:127–129. doi: 10.1016/0014-5793(74)80350-9. [DOI] [PubMed] [Google Scholar]
- Wright TL. Introduction to chronic hepatitis B infection. Am J Gastroenterol. 2006;101(Suppl 1):S1–S6. doi: 10.1111/j.1572-0241.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–373. [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Liu ZY, Li YY, Luo Y, Liu ML, Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG, Li ZC. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011;44:573–579. doi: 10.1016/j.ejps.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Zhao X, Kan Q, Zhu L, Zhang GX. Matrine suppresses production of IL-23/IL-17 and ameliorates experimental autoimmune encephalomyelitis. Am J Chin Med. 2011;39:933–941. doi: 10.1142/S0192415X11009317. [DOI] [PubMed] [Google Scholar]
- Zimmerman HJ, Kodera Y, West M. Rate of increase in plasma levels of cytoplasmic and mitochondrial enzymes in experimental carbon tetrachloride hepatotoxicity. J Lab Clin Med. 1965;66:315–323. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.