Abstract
The baculovirus Anticarsia gemmatalis nucleopolyhedrovirus (AgMNPV), a member of the family Baculoviridae, has been widely applied as a biopesticide for the control of the velvetbean caterpillar, a pest of soybean crop field. Baculoviruses are considered safe and efficient agents for this purpose, because they do not infect vertebrates, being safe for the health of humans and animals, as well as to the environment. The objective of this work was to identify proteins obtained from Lonomia obliqua hemolymph with potential application in the optimization of baculovirus AgMNPV replication in Sf9 insect cell culture. In this work the improvement of the cell culture and viral replication of the AgMNPV baculovirus was observed when Grace medium was supplemented with 10 % (v/v) Fetal Bovine Serum (FBS), 1 % (v/v) hemolymph extract, or 3 % (v/v) of hemolymph fractions or hemolymph sub-fractions obtained by purifying hemolymph through High Performance Liquid Chromatography. Hemolymph presented a positive effect on the synthesis of polyhedra and enhanced baculovirus replication in Spodoptera frugiperda (Sf9) cells (TCID50/mL), and led to Sf9 cell culture improvement. Grace medium supplemented with 10 % (v/v) FBS and 1 % (v/v) hemolymph provided an increase of baculovirus replication, when the cells were infected with multiplicity of infection of 1. In this case, the baculovirus replication was 6,443.91 times greater than that obtained with the control: Grace medium supplemented with 10 % (v/v) FBS. In addition, this work suggests that hemolymph from L. obliqua could have an interesting application in biotechnology, due to an increase in the viability of the cells and virus replication.
Keywords: Baculovirus, Spodoptera frugiperda, Lonomia obliqua, Hemolymph
Introduction
Insect cells have been increasingly used for the production of recombinant proteins and biopesticide. One of the most commonly employed cell lines for the production of high-value heterologous proteins through the baculovirus expression system (BEVS) is the Spodoptera frugiperda (Sf9) cell line. In addition, to production of recombinant proteins, it can also be used for the production of the Anticarsia gemmatalis nucleopolyhedrovirus (AgMNPV) baculovirus, a member of the family Baculoviridae, which has been widely applied as a biopesticide to control the velvetbean caterpillar, a pest of soybean crops.
Cell cultures normally require Fetal Bovine Serum (FBS) when cultivated in vitro using basal medium. However, this supplement consists of an undefined mixture of components that can vary from lot to lot, and potential adventitious contaminants can be introduced into in vitro cell culture through its use. Culture medium with FBS may be used as standard medium (Vaughn and Weiss 1990).
Some studies have demonstrated the presence of pharmacologically active substances in hemolymphs from insects (Shiotsuki et al. 2000; Yamamoto et al. 1999; Guerrero et al. 1990; Jiang et al. 1999; Rosenfeld and Vanderberg 1998; Hamdaoui et al. 1998; Maranga et al. 2003; Lin et al. 1998; Zhu et al. 2000; Souza et al. 2005). The supplementation of cell cultures with hemolymph proteins had a positive effect on viral replication (Rhee and Park 2000), and recombinant protein production (Woo et al. 1997). It was shown that it is capable of increasing the activity of a recombinant protein (luciferase) by approximately 6,000 times (Kanaya and Kobayashi 2000).
Nevertheless, only few studies have succeeded in isolating and characterizing the factors involved in these effects (Ochanda et al. 1992). These factors, once identified and isolated, can be of great importance in the optimization of cell growth, in the viral replication or recombinant protein production, which contributes to more efficient cell culture and to obtain final products at lower costs. A reference reports the existence of various identified proteins able to improve cell cultures (Souza et al. 2005). However, in the case of a production process, a survey of the bioprocess costs and productivity rates must be carried out. Bioprocess costs can be reduced considering that in the same working volume (related to control culture), with the same amount of work and using the same quantities of materials, final viral titers can be increased compared to the titers obtained in the control. The objective of this work was to identify proteins obtained from Lonomia obliqua’s hemolymph with potential application for increased baculovirus AgMNPV replication and production in Sf9 insect cell culture.
Materials and methods
Cell line and culture conditions
Sf9 cells derived from S. frugiperda CRL 1711 (ATCC, Manassas, VA, USA) were grown in 100 mL Schott flasks with 13 mL working volume containing Grace medium (Gibco, Sao Paulo, Brazil) supplemented with 10 % (v/v) FBS (Gibco). The cultures were incubated at 28 °C and agitated at 100 rpm in a shaker incubator. The cultures were started with an initial inoculum of 3.0 × 105 cells/mL.
Preparation of cell inoculum
Frozen Sf9 cells were thawed and cultivated for infection assays. Cells were grown in 100 mL flasks (shaker) with 13 mL working volume. When the cell concentration reached approximately 3 × 106 cells/mL cells were subcultured in Grace medium with 10 % (v/v) FBS. The cells were infected when the cellular concentration reached 106 cells/mL (MOI of 1). Cell samples were collected on the eighth day after viral infection. Viral production evaluation was made through viral titration and quantification of the polyhedra production [polyhedrical inclusion bodies (PIBs)].
Cell viability
Cell samples were obtained daily, and cell concentration was measured using a hemacytometer (Neubauer Chamber). Cell viability was determined by trypan blue exclusion test [solution at 0.4 % (w/v)] under an Olympus light microscope.
Hemolymph collection
The urticarting bristles of L. obliqua larvae in the sixth larval stage were trimmed and its hemolymph immediately collected. The collected hemolymph was clarified by centrifugation at 1,000g for 5 min and the supernatant filtered through a 0.22 µm membrane and stored at 0 °C.
Fractionation of hemolymph by High Performance Liquid Chromatography (HPLC)
After centrifugation and filtration, 6 mL of hemolymph were further fractionated by gel filtration chromatography using HPLC (ÄKTA Purifier Chomatography system—GE Healthcare, Sao Paulo, Brazil), using gel filtration columns Hi-prep 26/60 Sephacryl 200 (GE Healthcare) at a flow rate of 1 mL/min. The elution was monitored at 280 nm and 120 fractions (4 mL each) were collected. The fractions were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) at 12.5 % and added to the cell culture in order to verify the activity on cell growth and to study viral replication. After centrifugation and filtration, 1 ml of each semi-purified fraction by initial gel filtration, Pool 1, Pool 2 and Pool 3 of proteins, were submitted to Resource-Q ion-exchange chromatography, at a flow rate of 1 mL/min. Elution was performed with a linear gradient (0–100 %) with a solution of Tris HCl 20 mM and Tris HCl 20 mM NaCl 1 M, pH 8.0. The process was monitored at 280 nm and fractions of 1 mL volume were collected. All protein fractions were analyzed by polyacrylamide gel electrophoresis (SDS-PAGE) and added to Sf9 cell cultures in order to study viral replication.
Analysis by polyacrylamide gel electrophoresis (SDS-PAGE)
Every chromatographic fraction was analyzed using SDS-PAGE at 12.5 %. A LMW-SDS Marker Kit (GE Healthcare) was used as protein mass standard. The electrophoresis was carried out at 50 mA for 90 min. Gels (SDS-PAGE) were stained by Gel Code Blue (Pierce, Rockford, IL, USA) or stained by silver nitrate (Silver Staining Kit, GE Healthcare).
Virus
The Anticarsa gemmatalis nucleopolyhedrovirus (AgMNPV) had been gratefully supplied by Dr. Ronaldo Zucatelli Mendonça. The initial viral stock was titrated and showed approximately 3–4 × 105 TCID50/mL. In the infection assays, Sf9 cells were grown in Grace medium (GIBCO) supplemented with 10 % (v/v) FBS and infected with baculovirus at the middle of the exponential phase. The experiments were performed with baculovirus at an MOI of 1. Sf9 cells were infected with baculovirus AgMNPV 48 h after start of cultivation, which corresponds to the middle of the exponential growth phase. At different times 1 % (v/v) of hemolymph (HB was added: 50 min before infection with Baculovirus (‘HB-before’), at the time of infection (‘HB-together’) or 50 min after infection with baculovirus (‘HB-after’).
Virus titration
Twelve serial dilutions of the cell culture supernatant samples were performed. Aliquots of 100µL of each diluted viral sample were placed into 96-well plates containing 105 cells/mL per well. The microplates were incubated at 28 °C for 8 days and the final viral titer was calculated using standard methodology: TCID50/mL (Rhee and Munch 1938). The infection was characterized by the formation of polyhedra (PIBs, occlusion bodies which surround the viral genetic material). At this stage, the cells undergo lysis, releasing polyhedra into the culture medium. Polyhedra concentrations/mL were determined in duplicate and counted daily using a hematocytometer (Rhee and Munch 1938).
Statistics
“Student” t test (Harris 2001) was applied in this work for establishing statistical significance (P < 0.05) between titers obtained from HB or HB-fractions tests and those obtained from the controls.
Results and discussion
Analysis of Sf9 cells supplemented with hemolymph and infected with baculovirus AgMNPV
The viral production was performed by baculovirus infection of Sf9 cells, grown in Grace medium with 10 % (v/v) of FBS, supplemented with hemolymph at 1 % (v/v). Sf9 cells were infected with baculovirus AgMNPV 48 h after start of cultivation, which corresponds to the middle of the exponential growth phase.
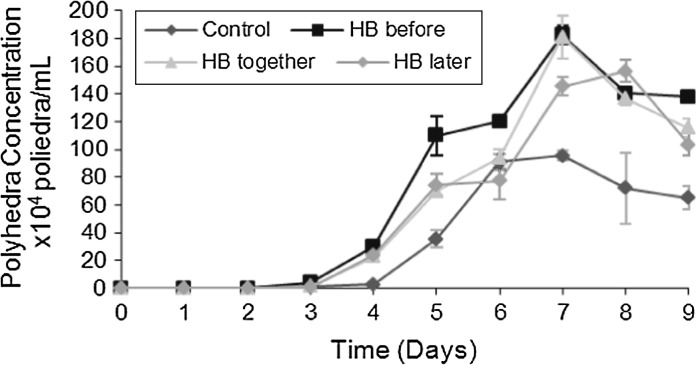
To analyze the influence of hemolymph (HB) on viral replication and production of baculovirus polyhedra, we used three different times for the supplementation of cultures with hemolymph (HB). In the first test, termed HB-before, 50 min before infection with baculovirus (MOI: 1), HB was added at 1 % (v/v) to the cultures. In the second test, referred to as HB-together, HB at 1 % (v/v) was added to the cell culture at the time of infection. In the third test, referred to as HB-after, HB at 1 % (v/v) was added to the cultures 50 min after infection with baculovirus (MOI of 1). After these different steps, the concentration of polyhedra/mL was monitored daily as shown in Fig. 1.
Fig. 1.
Polyhedra concentration/mL. HB-before stands for Sf9 cell culture supplemented with HB at 1 % (v/v) 50 min before infection with baculovirus. HB-together stands for Sf9 cell culture supplemented with HB at 1 % (v/v) at the moment of infection with baculovirus. HB-later stands for Sf9 cell culture supplemented with HB at 1 % (v/v) 50 min after infection with baculovirus. Control stands for Sf9 cell culture infected with baculovirus without supplementation with HB
It was established that when supplementing cell culture with HB 50 min before infection with baculovirus (HB-before), the concentration of polyhedra was 183 polyhedra/mL, and upon supplementing cell culture with HB at the moment of infection with baculovirus (HB-together), the concentration of polyhedra reached 181 polyhedra/mL, both at the seventh day of cultivation. When hemolymph (HB) was added to the cell culture, 50 min after the infection with baculovirus (HB-after), the maximum concentration of polyhedra obtained was 148 polyhedra/mL (at the eighth day of cultivation). In the control culture in which hemolymph (HB) was not added the maximum concentration was 95 polyhedra/mL at the seventh day of culture (Fig. 1).
The concentrations of polyhedra per mL were the following: 183 polyhedra/mL and 181 polyhedra/mL, for the cell cultures supplemented with HB-before, and for the culture supplemented with HB-together, respectively. This signifies that by using HB-before, there was an increase in the concentration of polyhedra/mL of approximately 1.92 times, and of 1.90 times, respectively, relative to the control sample. Polyhedra were mostly found occluded in the cells until the 6th day of culture. A difference in polyhedra concentration after the 7th day of culture, might signify that the Sf9 cells of the control cultures were already in the decline phase, with possible membrane rupture and consequent release of greater concentrations of polyhedra into the cell supernatant (Fig. 1). The determination of the polyhedra concentration clearly showed that the highest titer was obtained with hemolymph supplemented to cell culture 50 min before the infection with baculovirus.
Viral titers were determined according to the method developed by Rhee and Munch (1938) and the results are presented in Table 1.
Table 1.
Viral titre of baculovirus
| Assay with total extract of hemolymph (HB) | Final viral titer (TCID50/mL) |
|---|---|
| Control: Grace medium with 10 % FBS with baculovirus | 0.000419 × 109 |
| HB-before: HB supplementation 50 min before baculovirus infection | 2.7 × 109 |
| HB-together: HB supplementation at the time of baculovirus infection | 0.309 × 109 |
| HB-after: HB supplementation 50 min after baculovirus infection | 0.00020 × 109 |
Viral titre of baculovirus produced in Sf9 cell culture supplemented with 1 % (v/v) of hemolymph HB-before, HB-together, HB-after, and control culture infected with baculovirus without supplementation of hemolymph (HB)
The titration of the control sample (culture and virus production in Grace medium supplemented with 10 % (v/v) FBS) was 0.000419 × 109 (TCID50/mL) and the titre of the culture supplemented with HB at 1 % (v/v) 50 min before infection with baculovirus (HB-before) was of 2.7 × 109 (TCID50/mL), showing an improvement of the baculovirus titre by 6,443.91 fold, which could be explained by a facilitation of the adsorption of baculovirus to the receptors of the Sf9 cells due to interaction with the hemolymph proteins at the time of adsorption, thus, a host-pathogen interaction.
For ‘HB-together’, the production of baculovirus was 737.47 times higher than for the control culture and for ‘HB-after’ the virus production observed was 2.09 times lower compared with the control culture.
Thus, it was found that the best moment to supplement the cell culture with HB at 1 % (v/v) was 50 min before infection with baculovirus, because this condition yielded the greatest enhancement of the final baculoviral titer.
An hemolymph protein from the Bombyx mori silkworm capable of increasing the replication of B. mori nucleopolyhedrovirus and the activity of luciferase by approximately 10,000 times has already been isolated (Kanaya and Kobayashi 2000). However, only few studies have been conducted for the isolation and characterization of factors involved in such effects (Shishikura et al. 1997; Moon et al. 1995; Ochanda et al. 1992).
In order to continue the identification of the proteins involved in the increased baculovirus production HB was fractionated by chromatography and the fractions were used to supplement the cell culture. The supplementation of Sf9 cell culture was done 50 min before infection with baculovirus.
Identification of chromatographic HB fractions with possible activity in the potentiation of baculovirus replication in Sf9 cells
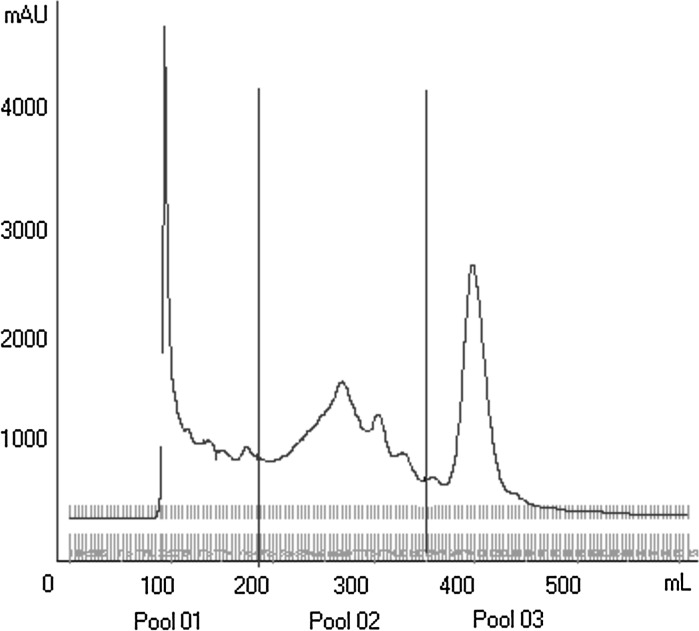
In order to isolate the protein(s) responsible for the improvement of baculovirus replication, crude hemolymph (HB) was subjected to gel filtration column chromatography on Sephacryl S200, as shown in Fig. 2. After this chromatographic step, hemolymph (HB) was subdivided into three sets of fractions called Pool 01, Pool 02, and Pool 03. These three pools were used to supplement cell cultures 50 min before infection with baculovirus.
Fig. 2.
Gel-filtration column chromatography. Gel-filtration column chromatography of HB on Sephacryl S-200. Shown are the three fractions pools obtained: Pool 01, Pool 02 and Pool 03
Pools 01, 02, and 03 were used separately and mixed together to verify the existence of an increase in viral replication depending on the isolated action or the mix of proteins contained in these pools. Based on the protein concentration and attempting to inoculate proteins contained in each one of the pools at a similar concentration, three different conditions were applied, namely: Pools 01, 02 and 03 supplemented separately at 3 % (v/v), mix of pools 01 + 02 + 03, and mix of pools 02 + 03 at 3 % (v/v). The amount of 3 % (v/v) representing a total protein concentration of 0.493 mg/mL for pool 1 (as example; for details, see Table 3) did not reach the total protein concentration present in the crude hemolymph extract (about 27.8 mg/mL), but was sufficient to show an improvement in the replication.
Table 3.
Protein concentration
| Sample analyzed | Protein concentration (mg/mL) |
|---|---|
| Hemolymph (HB) | 27.8 |
| Pool 01 | 0.493 |
| Pool 02 | 0.134 |
| Pool 03 | 0.095 |
| Q2–5 fraction | 0.146 |
| Q7–14 fraction | 0.032 |
| Q16–19 fraction | 0.047 |
Protein concentration of hemolymph and of different chromatographic fractions Pool 01, Pool 02, Pool 03, Q2–5, Q7–14 and Q16–19 (analyzed using the method of Lowry et al. (1951))
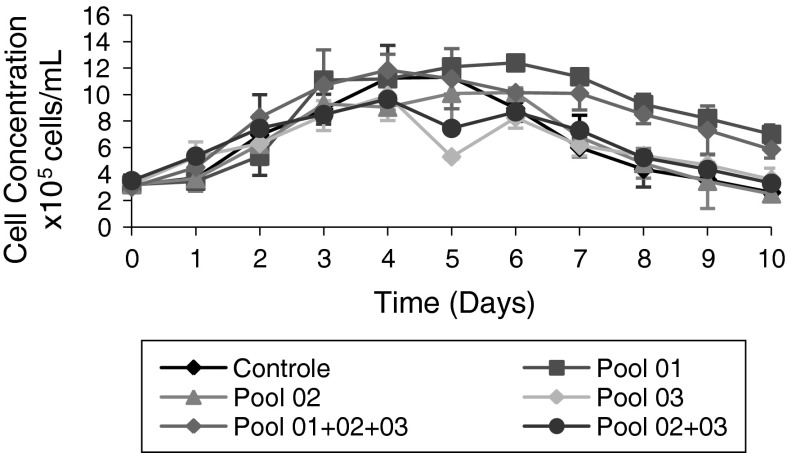
Figures 3, 4 present the effects of the supplementation of different semi-purified fractions (Pools) of HB at 3 % (v/v) on the concentration of cells/mL and polyhedra/mL. Pool 1 was able of prolonging stationary phase culture from the third to the seventh day of Sf9 cell culture. The mix of pools 01 + 02 + 03 at 3 % (v/v) was able of prolonging the stationary phase from the third to the fifth day of Sf9 cell culture. Prolongation of stationary phase of cell culture was not observed when Pools 02 and 03 were used as mix, compared to the control culture. Figure 3 shows the obtained cell concentrations using the different Pools, used mixed or separately.
Fig. 3.
Evaluation of cell growth of Sf9 cells (cell concentration/mL) in cultures supplemented with different fractions of HB 50 min before infection with baculovirus (MOI 1) 48 h after start of the cultures. Control Sf9 cells in Grace medium supplemented with 10 % (v/v) FBS and infected with Baculovirus. Pool 01 Control supplemented with Pool 01 at 3 % (v/v). Pool 02 Control supplemented with Pool 02 at 3 % (v/v). Pool 03 Control supplemented with Pool 03 at 3 % (v/v). Pool 1 + 2 + 3 Control supplemented with a mix of Pools 01, 02, and 03 at 3 % (v/v). Pool 2 + 3 Control supplemented with a mix of Pools 02 and 03 at 3 % (v/v)
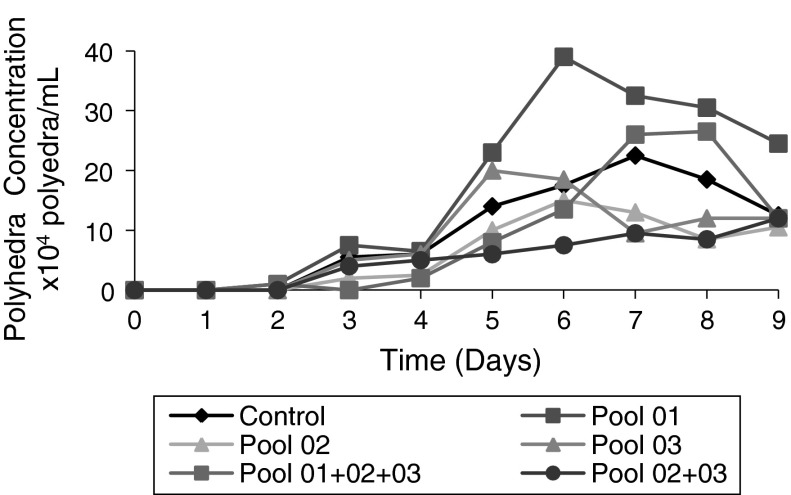
Fig. 4.
Evaluation of polyhedra production (polyhedra concentration/mL) by Sf9 cells cultivated in presence/absence of different supplementations of HB-fractions. Control Sf9 cells infected with baculovirus. Pool 01 Control supplemented at 3 % (v/v) with Pool 01, 50 min before infection with baculovirus. Pool 02 Control supplemented at 3 % (v/v) with Pool 02, 50 min before infection with baculovirus. Pool 03 Control supplemented at 3 % (v/v) with Pool 03, 50 min before infection with baculovirus. Mix of Pools 01 + 02 + 03 Control supplemented at 3 % (v/v) with a mix of Pools 01, 02, and 03, 50 min before infection with baculovirus. Mix of Pool 02 + 03 Control supplemented at 3 % (v/v) with a mix of Pools 02 and 03, 50 min before infection with baculovirus. The infections were done with an MOI of 1, 48 h after start of cultivation
At the end of the cell cultures, a final viral titre was determined to confirm the action of each of these fractions with respect to the improvement of viral replication (Table 2). With respect to virus replication, it was found that only Pool 01 used alone was responsible for the improvement of baculovirus replication. When Pool 1 was used separately, an increase of 611.9 was observed. The titer of baculovirus in Pool 01 was 2,570.0 × 102 TCID50/mL, whereas the titer of baculovirus in the control was 4.2 × 102 TCID50/mL. The mix of Pools 01, 02, and 03, containing Pool 01, showed an increase of approximately 833.33 times in the final baculoviral titer with respect to the control.
Table 2.
Baculovirus titers (TCID50/mL) obtained from Sf9 cultures supplemented with different HB fractions: Control, Pool 01, Pool 02, Pool 03, mix of Pools 02 and 03, and mix of Pools 01, 02, and 03 (for Pool 01, Pool 02, Pool 03, see Fig. 2)
| Assay with fractions Pool 01, Pool 02, Pool 03, mix of Pools 02 and 03, and mix of Pools 01, 02, and 03 | Final viral titer (TCID50/mL) |
|---|---|
| Control: Grace medium with 10 % (v/v) FBS and baculovirus | 4.2 × 102 |
| Pool 01: 50 min before baculovirus | 2,570.0 × 102 |
| Pool 02: 50 min before baculovirus | 68.0 × 102 |
| Pool 03: 50 min before baculovirus | 4.2 × 102 |
| Mix of Pools 02 and 03: 50 min before baculovirus | 3.09 × 102 |
| Mix of Pools 01, 02, and 03: 50 min before baculovirus | 3,500.0 × 102 |
The titer of Baculovirus for the mix of Pools 01 + 02 + 03 was 3,500.0 × 102 TCID50/mL, for Pool 01 2,570.0 × 102 TCID50/mL and for the control 4.2 × 102 TCID50/mL. The mix of Pools 01 + 02 + 03 reached a final viral titer of approximately 1.36 times greater than Pool 01 used separately. These results indicate that one or several proteins contained in pool 01 are responsible for the augmentation of the virus-cell interaction and/or virus replication by the infected cells leading to greater concentrations of polyhedra/mL, thus improving baculovirus replication in Sf9 cells. When Pool 01 was used separately, the polyhedra concentration was 39.0 × 104 polyhedra/mL (Fig. 4), showing higher concentration of polyhedra/mL. As polyhedra containing multiple viral particles are ingested by the soybean caterpillar, and since the higher the polyhedra concentration is on the crop, the greater will be the concentration which can be ingested and which will act as biopesticide for soybean plants. In the case that the polyhedra contain several baculovirus virions, the caterpillar pests of soybean will die more rapidly after ingestion of the polyhedra. In addition, due to the higher concentration of polyhedra/mL, there will be a higher probability of ingestion by the soy caterpillar causing them to die.
Identification of chromatographic fractions derived from Pool 01 with possible activity in the potentiation of baculovirus replication
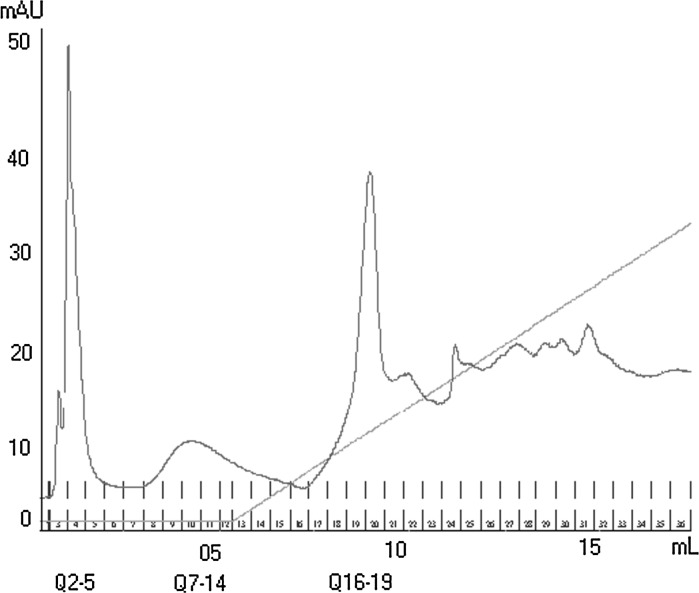
Pool 01 was responsible for the prolonged stationary phase, higher concentration of polyhedra/mL, and higher final viral titer. Pool 01 was subjected to further chromatographic processes, thereby trying to isolate proteins involved in the improvement of virus replication. For that, Pool 01 was subjected to ion-exchange column chromatography (Resource-Q type), resulting in three protein fractions named: Q2–5, Q7–14, and Q16–19 as shown in Fig. 5. Three fractions were identified, taking into consideration the volume and concentration of proteins in the injected Pool 01. Based on the baseline determined via the preliminary washings it could be established that there was no protein fraction to be analyzed after fraction Q16–19.
Fig. 5.
Ion-exchange column chromatogram. Pool 1 was fractioned using ion-exchange column chromatography. The chromatogram presents the three fractions obtained: Q2–5, Q7–14, and Q16–19
The three obtained fractions, Q2–5, Q7–14, and Q16–19 were used to supplement Sf9 cell cultures at 3 % (v/v) 50 min before infection with baculovirus at an MOI of 1. The concentration of the hemolymph in the crude extract was 27.8 mg/mL. Experiments with protein fractions, such as Pool 1, were all performed with a protein concentration of 1.5 mg/mL, sufficient to check biological activity. To achieve a concentration of 1.5 mg/mL it was necessary to supplement each purified fraction to the cell culture at a concentration of 3 % (v/v). A protein concentration of 1.5 mg/mL was enough to indicate a possible improvement of replication (Table 3). Figure 6 shows the effects of supplementation of fractions Q2–5, Q7–14, and Q16–19 derived from ion-exchange column chromatography, of fraction Pool 01, and of buffer (Tris 20 mM NaCl 1 M), all at a concentration of 3 % (v/v).
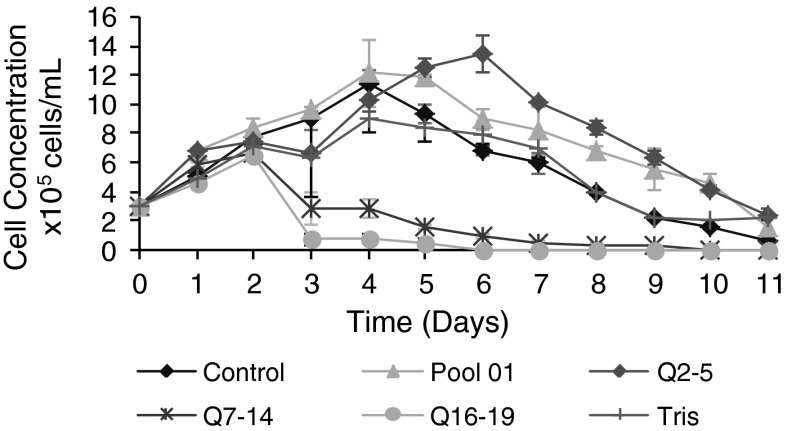
Fig. 6.
Cell growth kinetics. Supplementation of different hemolymph fractions to Sf9 cell cultures. Control Grace medium with 10 % (v/v) FBS, infection with baculovirus. Pool 01 Control supplemented with 3 % (v/v) Pool 01. Q2–5 Control supplemented with 3 % (v/v) fraction Q2–5. Q7–14 Control supplemented with 3 % (v/v) fraction Q7–14. Q16–19 Control supplemented with 3 % (v/v) fraction Q16–19. Tris Control supplemented with 3 % (v/v) Tris 20 mM and NaCl 1 M. Time of supplementation with the respective fractions was 50 min before infection with baculovirus. All cultures had been infected with baculovirus 48 h after the start of cell culture at an MOI of 1
As seen in Fig. 6, fraction Q2–5 used at 3 % (v/v) for supplementing the Sf9 cell culture (HB-before) was capable of prolonging the stationary phase from the fourth to the sixth day of cell culture. Fractions Q7–14 and Q16–19 were toxic for the cell culture. Buffer (Tris 20 mM NaCl 1 M) used in ion-exchange chromatography did not influence cell growth when supplemented at 3 %.
The culture supplemented with Pool 01 showed a more prolonged stationary phase than the control culture. Fraction Q2–5 showed a stationary phase prolonged for 2–3 days relative to the control culture (Fig. 6).
The protein concentration in the fractions used was approximately 1.5 mg/mL and the highest purified fraction Q2–5 having a comparable protein concentration to Pool 01, enabled the improvement of the Sf9 cell culture, suggesting that one or several components were responsible for prolonging the stationary phase of the cell culture. On the sixth day the viable cell concentration was 1.35 × 106 cells/mL when using fraction Q2–5. On the fourth day the maximum concentration of viable cell was 1.21 × 106 cells/mL when using Pool 1, and 1.14 × 106 cells/mL in the control culture. The increase in the cell number using fraction Q2–5 was 1.18 times greater than the maximum concentration of cells obtained in the control sample, and 1.11 times greater regarding the maximum concentration of viable cells observed for sample Pool 01. In this case there was no statistical difference.
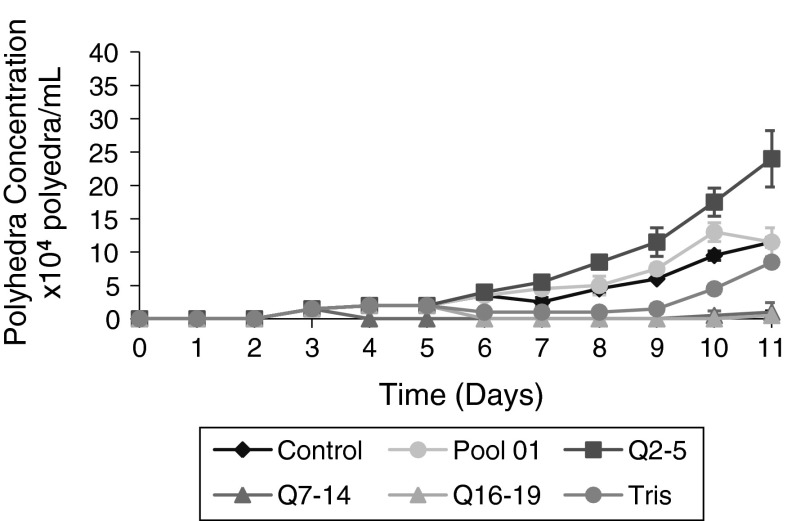
As seen in Fig. 7, there was a greater amount of polyhedra/mL in the cultures supplemented with fraction Q2–5 at 3 % (v/v). On the ninth day of culture, the concentrations of polyhedra in the control samples, in the cultures supplemented with Pool 01, and in the cultures supplemented with fraction Q2–5 were 6 × 104, 7.5 × 104, and 11.5 × 104 polyhedra/mL, respectively.
Fig. 7.
Polyhedra production (per ml) by Sf9 cultures supplemented with chromatographic fractions 50 min before baculovirus infection. All cultures were infected with baculovirus 48 h after the start of the cultures at an MOI of 1. Kinetics of the concentration of polyhedra after infection of Sf9 cells grown in Grace medium with 10 % (v/v) FBS with baculovirus in the presence or absence of different HB fractions. Control Grace medium with 10 % (v/v) FBS. Fraction Q2–5 Control supplemented with 3 % (v/v) of fraction Q2–5. Fraction Q7–14 Control supplemented with 3 % (v/v) of fraction Q7–14. Fraction Q16–19 Control supplemented with 3 % (v/v) of fraction Q16–19. Pool 01 Control supplemented with 3 % (v/v) of fraction Pool 01. Tris Control supplemented with 3 % (v/v) of buffer Tris 20 mM, NaCl 1 M. For all conditions, Sf9 cells were supplemented 50 min before infection with baculovirus
On the 11th day at the end of the culture, the control culture and the culture supplemented with Pool 01 have produced 11.5 × 104 polyhedra/mL, whereas the culture supplemented with fraction of Q2–5 have produced 24.0 × 104 polyhedra/mL (Table 4). There was an increase in the amount of polyhedral/mL of the order of 2.08 times greater relative to the control culture and Pool 01 using fraction Q2–5. Table 4 shows also that on days 9 and 10, the cultures supplemented with Pool 01 showed higher Polyhedra levels per mL than the control cultures confirming previous results (Fig. 4). However, as mentioned, these values were lower than those obtained with fraction Q2–5 supplementation. On the ninth day, the concentration of polyhedral/mL using Pool 01 was 1.25 times greater than the concentration of polyhedral/mL of the control culture. Using fraction Q2–5, the value was 1.91 times greater than that obtained in the control culture. These results indicated that fraction Q2–5 derived from Pool 01, retained biological activity favoring a higher concentration of cells/mL and polyhedra/mL (Figs. 6, 7). Among the three fractions derived from Pool 01, fraction Q2–5 was the only one that showed a potential improvement of viral replication and seems to concentrate the biological activity of Pool 01. Final baculoviral titers obtained by cultures supplemented with the three fractions Q2–5, Q7–14, and Q16–19 are presented in Table 5.
Table 4.
Polyhedra concentration/mL. Control, Pool 01, fraction Q2–5
| Sample and day of cultivation | Polyhedra/mL |
|---|---|
| Control 9th day | 6.0 × 104 |
| Control 10th day | 9.6 × 104 |
| Control 11th day | 11.5 × 104 |
| Pool 01 9th day | 7.5 × 104 |
| Pool 01 10th day | 13.0 × 104 |
| Pool 01 11th day | 11.5 × 104 |
| Q2–5 9th day | 11.5 × 104 |
| Q2–5 10th day | 17.5 × 104 |
| Q2–5 11th day | 24.0 × 104 |
Referring to Fig. 7
Table 5.
Final baculovirus titers obtained in cultures supplemented with the following fractions: fractions Q2–5, Q7–14, Q16–19, Pool 01, and Tris buffer, and the non-supplemented control culture (ref. Fig. 6)
| Tests | Viral final titer (TCID50/mL) |
|---|---|
| Control (Grace medium at 10 % FBS with baculovirus) | 3.16 × 105 |
| Q2–5 (50 min before of infection with baculovirus) | 178.0 × 105 |
| Q7–14 (50 min before of infection with baculovirus) | 0.00231 × 105 |
| Q16–19 (50 min before of infection with baculovirus) | 0.0316 × 105 |
| Pool 01 (50 min before of infection with baculovirus) | 2,310.0 × 105 |
| Tris (50 min before of infection with baculovirus) | 3.98 × 105 |
Figures 1, 7 depict polyhedra production per mL. Figure 7 shows that the concentration of polyhedra/mL of the control reached 11 × 104 polyhedra/mL, whereas Fig. 1 depicts the concentration of polyhedra/mL of the control culture reaching 100 × 104 of polyhedra/mL. The difference in concentration of polyhedra of the control samples at different passages, indicate how subsequent passages of baculovirus may decrease polyhedra concentration per mL.
Over succeeding cell culture passages, there was a likelihood of reducing the baculovirus titers (Rodas et al. 2005). Thus we had chosen to use a viral stock with less cell culture passages for the evaluation of three different fractions (Q2–5, Q7–14, and Q16–19) on baculovirus productions presented in Table 5.
The use of Pool 1 led to a titer of 2,310 × 105 TCID50/mL corresponding to a 731 times increase of the final viral titer, and when fraction Q2–5 was used, the titer was 178 × 105 TCID50/mL, corresponding to an increase of 56.33 times of the final baculoviral titer, both with respect to the control (3.16 × 105 TCID50/mL, Table 5). This indicates that a more highly purified fraction, Q2–5, was able to show activity in enhancing the baculovirus titer, despite of the loss of overall protein concentration due to purification; however, leading to a concentration of the active compounds. Using the Pool 1, it was possible to obtain higher titer than when using the Q2–5 fraction. It has to be recalled here that Pool 1 has a higher protein concentration as an earlier semi-purified fraction than fraction Q2–5. Still, it could be shown that the Q2–5 fraction was capable of causing a beneficial impact on production of polyhedra.
Using fractions Q7–14 and Q16–19, a decrease in the final baculoviral titer was observed in relation to the control, suggesting a possible toxic effect when cultures were supplemented with both fractions (Fig. 6).
Among the three fractions derived from ion-exchange chromatography, fraction Q2–5 has shown an enhancing effect on cell culture with a higher concentration of cells/mL, polyhedral/mL and higher final baculoviral titer, suggesting that Q2–5 fraction is responsible for enhancing cell and polyhedra concentration per mL.
As already mentioned, the number of passages of the baculovirus has an impact on virus production and on the titers which can be obtained (Rodas et al. 2005). In this context, the differences in the baculovirus titers obtained when using Pool 1 shown in Table 2 (2,570.0 × 102 TCID50/mL) and Table 5 (2,310.0 × 105TCID50/mL) can be explained by the number of subsequent baculovirus passages, which were lower for the data shown in Table 5 than for those in Table 2. The titers presented in Table 2 were in the order of magnitude of ×102 TCID50/mL, whereas the titers shown in Table 5 were in the order of magnitude of ×105 TCID50/mL. However, despite of this fact, it was possible to establish that the titers obtained when using Pool 1 were higher than those obtained for the control conditions (Tables 2, 5).
Thus, to verify the reliability of the results obtained with the control and fraction Q2–5 samples (Table 5) baculovirus production assays have been performed using five times subsequently passaged baculovirus. It could be shown statistically that fraction Q2–5 was able to actually increase the concentration of cells and polyhedra per mL even when later passage baculovirus stocks were used for infection.
The obtained differences in the values of the final baculoviral titres between the control and the fraction Q2–5 samples (Table 6) were statistically different (t test, n = 5). Table 7 shows how many times the final baculoviral titers obtained with sample Q2–5 were higher in comparison to the titers obtained with the control samples.
Table 6.
Final baculovirus titers at the end of five succeeding baculovirus passages comparing the control conditions and fraction Q2–5 supplementations, relative to cell cultures shown in Fig. 6
| Control sample Grace medium with baculovirus at 10 % FBS |
Viral final title (TCID50/mL) | Sample fraction Q2–5 Control with fraction Q2–5 |
Viral final title (TCID50/mL) |
|---|---|---|---|
| Control (1st titration) | 3.16 × 105 | Q2–5 (1st titration) | 178.0 × 105 |
| Control (2nd titration) | 3.98 × 105 | Q2–5 (2nd titration) | 251.0 × 105 |
| Control (3rd titration) | 5.62 × 105 | Q2–5 (3rd titration) | 178.0 × 105 |
| Control (4th titration) | 1.77 × 105 | Q2–5 (4th titration) | 31.6 × 105 |
| Control (5th titration) | 3.16 × 105 | Q2–5 (5th titration) | 56.0 × 105 |
Table 7.
Final viral titres obtained with fraction Q2–5 indicating how many times the final viral titres obtained with fraction Q2–5 were higher than those obtained with the control sample for five succeeding baculovirus passages
| Samples of fraction Q2–5 (Control with fraction Q2–5) | How much titration with Q2–5 fraction was greater than the control sample |
|---|---|
| Q2–5 fraction (1st titration) | 56.33 times greater than 1st titration of Control sample |
| Q2–5 fraction (2nd titration) | 63.06 times greater than 2nd titration of Control sample |
| Q2–5 fraction (3rd titration) | 31.67 times greater than 3rd titration of Control sample |
| Q2–5 fraction (4th titration) | 17.85 times greater than 4th titration of Control sample |
| Q2–5 fraction (5th titration) | 17.71 times greater than 5th titration of Control sample |
Considering the results in Figs 1, 4, 7 the concentration of polyhedra/mL during cell culture in the control samples were, respectively, 8.0 × 105, 2.2 × 105, and 1.15 × 105 polyhedra/mL. Thus, there was a decrease in the concentration of polyhedra during cell subculture, whereas the viral titers of baculovirus in these three control samples were 4.19 × 105 TCID50/mL (Table 1), 4.2 × 102 TCID50/mL (Table 2), and 3.16 × 105 TCID50/mL (Table 5), respectively. Despite the decrease in the amount of polyhedra/mL, no significant decrease was observed in the final baculoviral titre between the first and third passages of the control sample. There was a decrease in the concentration of polyhedra/mL, whereas the viral titers were similar in the control sample, suggesting no alteration of viral replication of baculovirus in the control samples. Rodas et al. (2005) reported a significant loss in the amount of polyhedra (PIBs) in successive passages of baculovirus on Sf9 cell cultures. After the fourth passage in cell culture, there was a decrease of virulence by 50 %, suggesting the formation of defective viral PIBs without biological activity during successive passages in Sf9 cell culture. At high passages polyhedra without viruses have been observed (Rodas et al. 2005).
A decreased virulence of baculovirus, as reported by Rodas et al. (2005), was not observed in the Sf9 cell culture infected with Baculovirus AgMNPV supplemented with Pool 01 at 3 % (v/v), as shown in Tables 2 and 5. The titer of Baculovirus in cell culture supplemented with Pool 01 ranged from 2.57 × 105 TCID50/mL (Table 2) to 2,310.0 × 105 TCID50/mL (Table 5), indicating a significant improvement in the baculovirus replication with respect to the control culture.
The main objective of the present study was to identify a protein fraction from L. obliqua’s hemolymph able to enhance the replication of baculovirus AgMNPV after long-term studies. Besides the use of bioactive proteins to improve baculovirus replication, other approaches can be studied in order to optimize upstream–downstream bioprocess and enable large-scale production of baculovirus for its application as biopesticide. Formulation of culture means favoring viral replication, higher-density cell cultures using anti-apoptotic proteins and growth factors, and the use of a suitable host cell for the specific baculovirus are further directions to work on (Sf9, Sf21, Hive-Five) considering the type of crop as well as its specific pests (pest of soybean, corn pest). Besides those, the integrated crop management of biopesticides and chemical herbicides, aiming at a better result in crop pest control, are paramount for the required gradual reduction in the use of chemical herbicides in agricultural biotechnology scenario which is objective of the present study.
Conclusions
In conclusion, a semi-purified protein fraction termed Q2–5 which was able to increase baculovirus replication (AgMNPV) was identified. Steps for the purification of L.obliqua’s hemolymph were standardized and consist of: size exclusion chromatography followed by ion-exchange chromatography (type “Resource-Q”), using High Performance Liquid Chromatography.
Acknowledgments
The authors are grateful and appreciated financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, Brazil), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Butantan Foundation (São Paulo, Brazil).
References
- Batista FRX, Pereira CA, Mendonça RZ, Moraes AM. Enhancement of Sf9 cells and baculovirus production employing Grace’s medium supplemented with milk whey ultrafiltrate. Cytotechnology. 2005;49:1–9. doi: 10.1007/s10616-005-4206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista FRX, Pereira CA, Mendonça RZ, Moraes AM. Evaluation of concentrated milk whey as a supplement for Sf9 Spodoptera frugiperda cells in culture. Electron JBiotechnol. 2006;9:522–532. [Google Scholar]
- Choi SS, Rhee WJ, Park TH. Inhibition of human cells apoptosis by silkworm hemolymph. Biotechnol Prog. 2002;18:874–878. doi: 10.1021/bp020001q. [DOI] [PubMed] [Google Scholar]
- Guerrero B, Perales J, Gil A, Arocha-Pinango CL. Effect on platelet FXIII and partial characterization of Lonomin V, a proteolytic enzyme from Lonomia achelous caterpillars. Thromb Res. 1990;5:243–252. doi: 10.1016/s0049-3848(98)00169-8. [DOI] [PubMed] [Google Scholar]
- Hamdaoui A, Wataleb S, Devreese B, Chiou SJ, Vanden Broeck J, Van Beeumen J, De Loof A, Schoofs L. Purification and characterization of a group of five novel peptide serine protease inhibitors from ovaries of the desert locust, Schistocerca gregaria. FEBS Lett. 1998;422:74–78. doi: 10.1016/S0014-5793(97)01585-8. [DOI] [PubMed] [Google Scholar]
- Harris DC. Análise química quantitativa. 5a. Rio de Janeiro: Editora LTC; 2001. [Google Scholar]
- Jiang H, Wang Y, Kanost MR. Four serine proteinases expressed in Manduca sexta haemocytes. Insect Mol Biol. 1999;8:39–53. doi: 10.1046/j.1365-2583.1999.810039.x. [DOI] [PubMed] [Google Scholar]
- Kanaya T, Kobayashi J. Purification and characterization of an insect hemolymph protein promoting in vitro replication of the Bombyx mori nucleopolyhedrovirus. J Gen Virol. 2000;81:1135–1141. doi: 10.1099/0022-1317-81-4-1135. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Rhee WJ, Park TH. Isolation and characterization of an apoptosis-inhibiting component from the hemolymph of Bombyx mori. Biochem Biophys Res Commum. 2001;285:224–228. doi: 10.1006/bbrc.2001.5148. [DOI] [PubMed] [Google Scholar]
- Kim EJ, et al. Inhibition of apoptosis by recombinant 30 K protein originating from silkworm hemolymph. Biochem Biophys Res Commum. 2003;308:523–528. doi: 10.1016/S0006-291X(03)01425-6. [DOI] [PubMed] [Google Scholar]
- Kurata K, Nakamura M, Okuda T, Hirano H, Shinbo H. Purification and characterization of a juvenile hormone binding protein from hemolymph of the silkworm, Bombyx mori. Comp Biochem Physiol Biochem Mol Biol. 1994;109:105–114. doi: 10.1016/0305-0491(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Lee SH, Park TH. Growth limiting factors influencing high density culture of insect cells in Grace´s medium. Biotechnol Lett. 1994;16:327–332. [Google Scholar]
- Lin CY, Chen SH, Kou GH, Kuo CM. Identification and characterization of a hyperglycemic hormone from freshwater giant prawn, Macrobrachium rosenbergii. Comp Biochem Physiol A: Mol Integr Physiol. 1998;121:315–321. doi: 10.1016/S1095-6433(98)10139-3. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maranga L, Mendonça RZ, Bengala A, Peixoto CC, Moraes RHP, Pereira CA, Carrondo MJT. Enhancement of Sf9 cells growth and longevity through supplementation of culture medium with hemolymph. Biotechnol Prog. 2003;19:58–63. doi: 10.1021/bp025583q. [DOI] [PubMed] [Google Scholar]
- Moon HJ, Kurata S, Natori S, Lee BL. Purification and cDNA cloning of an antifungal protein from the hemolymph of Holotrichia diomphalia larvae. Biol Pharm Bull. 1995;18:1049–1052. doi: 10.1248/bpb.18.1049. [DOI] [PubMed] [Google Scholar]
- Ochanda JO, Osir EO, Nguu EK, Olembo NK. Isolation and properties of 600-kDa and 23-kDa hemolymph proteins from the tsetse fly, Glossina morsitans: their possible role as biological insecticides. Scand J Immunol Suppl. 1992;11:41–47. doi: 10.1111/j.1365-3083.1992.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Peters ID, Rancourt DE, Davies PL, Walker VK. Isolation and characterization of an antifreeze protein precursor from transgenic Drosophila: evidence for partial processing. Biochim Biophys Acta. 1993;1171:247–254. doi: 10.1016/0167-4781(93)90062-I. [DOI] [PubMed] [Google Scholar]
- Rhee LJ, Munch H. A simple method of estimating fifty per cent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Rhee WJ, Park TH. Silkworm hemolymph inhibits baculovirus-induced insect cell apoptosis. Biochem Biophys Res Commun. 2000;271:186–190. doi: 10.1006/bbrc.2000.2592. [DOI] [PubMed] [Google Scholar]
- Rodas VM, Marques FH, Honda MT, Soares DM, Jorge SAC, Antoniazzi MM, Medugno C, Castro MEB, Ribeiro BM, Souza ML, Tonso A, Pereira CA. Cell culture derived AgMNPV bioinsecticide: biological constraints and bioprocess issues. Cytotechnology. 2005;48:27–39. doi: 10.1007/s10616-005-3175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld A, Vanderberg JP. Identification of electrophoretically separated proteases from midgut and hemolymph of adult Anopheles stephensi mosquitoes. J Parasitol. 1998;84:361–365. doi: 10.2307/3284496. [DOI] [PubMed] [Google Scholar]
- Shiotsuki T, Bonning BC, Hirai M, Kikuchi K, Hammock BD. Characterization and affinity purification of juvenile hormone esterase from Bombyx mori. Biosci Biotechnol Biochem. 2000;64:1681–1687. doi: 10.1271/bbb.64.1681. [DOI] [PubMed] [Google Scholar]
- Shishikura F, Abe T, Ohtake S, Tanaka K. Purification and characterization of a 39,000-Da serine proteinase from the hemolymph of a solitary ascidian, Halocynthia roretzi. Comp Biochem Physiol B: Biochem Mol Biol. 1997;118:131–141. doi: 10.1016/S0305-0491(97)00217-4. [DOI] [PubMed] [Google Scholar]
- Souza APB, Peixoto CC, Maranga L, Carvalhal AV, Moraes RHP, Mendonça RMZ, Pereira CA, Carrondo MJT, Mendonça RZ. Purification and characterization of an anti-apoptotic protein isolated from Lonomia obliqua hemolymph. Biotechnol Prog. 2005;21:99–105. doi: 10.1021/bp049831p. [DOI] [PubMed] [Google Scholar]
- Vaughn JL, Weiss SA. Large-scale propagation of insect cells. Bioprocess Technol. 1990;10:597–618. [PubMed] [Google Scholar]
- Wang MY, Kmong S, Bentley WE. Effects of oxygen/glucose/glutamine feeding on insect cells baculovirus protein expression: a study on epoxide hydrolase production. Biotechnol Prog. 1993;9:355–361. doi: 10.1021/bp00022a002. [DOI] [PubMed] [Google Scholar]
- Woo SD, Kim WJ, Kim HS, Choi JY, Jin BR, Kang SK. Effect of silkworm hemolymph on the expression of E. coli beta-galactosidase in insect cells lines infected with recombinant baculoviruses. Mol Cells. 1997;31:572–574. [PubMed] [Google Scholar]
- Yamamoto Y, Watabe S, Kageyama T, Takahashi SY. Purification and characterization of cysteine proteinase specific inhibitors from the hemolymph of Bombyx mori. Arch Insect Biochem Physiol. 1999;42:119–129. doi: 10.1002/(SICI)1520-6327(199910)42:2<119::AID-ARCH2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zhu S, Li W, Jiang D, Zeng X. Evidence for the existence of insect defensin-like peptide in scorpion venom. IUBMB Life. 2000;50:57–61. doi: 10.1080/15216540050176601. [DOI] [PubMed] [Google Scholar]