Abstract
Pancreatic islet transplantation is a promising therapy for Type I Diabetes. For many years the method used worldwide for islet purification in both rodent and human islet isolation has been Ficoll-based density gradients, such as Histopaque. However, it is difficult to purify islets in laboratories with staff limitations when large scale isolations are required. We hypothesized that filtration could be a more simple and fast alternative to obtain good quality islets. Four separate islet isolations were performed per method, comparing filtration and Histopaque purification with handpicking as the gold standard method for islet purity. Different parameters of quality were assessed: yield in number of islets per pancreas, purity by dithizone staining, viability by Fluorescein Diacetate/Propidium Iodide vital staining and in vitro functionality assessed by Glucose Stimulated Insulin Secretion. Time efficiency and cost were also analyzed. The overall quality of the islets obtained both by Histopaque and filtration was good. Filtration saved almost 90 % of the time consumed by Histopaque purification, and was also cheaper. However, one-third of the islets were lost. Since human and rodent islets share similar size but different density, filtration appears as a purification method with potential interest in translation to clinic.
Keywords: Diabetes, Pancreatic islets, Mouse islet isolation, Islet purification
Introduction
Pancreatic islet transplantation is a promising therapy for type I Diabetes, supported by the success of the Edmonton Protocol and the follow-up international trial (Shapiro et al. 2000, 2006). However, one of the major obstacles to a widespread clinical use of this treatment is the islet isolation procedure itself due to its low yield [2 or more donors are needed per patient (Shapiro 2011)] and the difficulty of the islet purification methodology. Therefore, these factors limit the availability of human islets either for the clinical practice or the research field, promoting the use of animal models in the latter.
The islet isolation procedure involves three steps: enzyme perfusion, pancreas digestion and islet purification. Purification is the key step since highly purified islet preparations improve engraftment, are safer, reduce graft immunogenicity in transplants and are more suitable for immunomodulation procedures (Lakey et al. 2003).
Since the 1960s, the most common method for islet purification, both in rodents and humans, is density gradient centrifugation, based on the different densities of islets and exocrine tissue (Lacy and Kostianovsky 1967; McCall et al. 2011; Mita et al. 2010; van der Vliet et al. 1988; Lake et al. 1987). However, alternative methods based on other physical properties have been suggested in the last years, such us filtration or osmotic shock (Atwater et al. 2010; Salvalaggio et al. 2002). Recently, McCall and colleagues highlighted Histopaque in comparison to other discontinuous density gradients as the method to provide more healthy islets (McCall et al. 2011). We also speculated that a faster method is needed in large-scale isolations in terms of time optimization, especially when there is a limited staff devoted to this task. Therefore, filtration could be a good alternative, since a priori, it could be a faster, cheaper and a more simple method. However, the analysis of the quality of the islets obtained by this method remains to be performed.
In the current study, we first assessed the yield and quality of mouse islets isolated by two protocols of Histopaque and filtration purification, the latter with some modifications (Li et al. 2009), taking the handpicking as the gold standard method for islet purification. Besides, we made a balance considering also the cost and the time consumed by each procedure, which are also determinant in the election of a method for a laboratory routine.
We conclude that filtration is a feasible alternative to Histopaque, providing good quality islets, and very convenient in terms of time and cost for large-scale islet isolations and/or laboratories with limited staff. However, the yield obtained with this method is lower and each laboratory should decide which is its most suitable option.
Materials and methods
Animals
OF1 mice (8–11 weeks) were obtained from Charles River Laboratories (L’Arbresle, France) and housed under conventional conditions. Ethical approval was obtained from the Animal Welfare Committee at the University of the Basque Country (Permit number: CEBA/58/RAMIREZ DOMINGUEZ).
Mouse islet isolation
Four independent isolations (six mice each) were performed per method. OF1 mice were sacrificed by cervical dislocation and digestion of the pancreatic tissue was performed with 3 mg/mL of Collagenase P (Roche, Mannheim, Germany), and later purified according to one of the methods detailed below.
Islet purification
Handpicking
Islets were spun twice at 300 g 2 min and washed with Hank’s Balanced Salt Solution (HBSS) (Sigma, Ayrshire, UK) with 0.1 % Bovine Serum Albumin (BSA) (HyClone, South Logan, UT, USA). Then they were handpicked three times in a Petri Dish with a black background under the stereomicroscope (Zeiss, Stemi 2000-C) with side illumination.
Filtration
Islets were purified according to the protocol by Li et al. 2009 with some modifications (Collagenase P instead of Collagenase XI, filtering through a 100 μm cell strainer instead of a 70 μm one).
Histopaque
Islets were purified with a discontinuous density gradient according to the protocol by Anna Novials and col. (personal communication). This gradient was generated from three layers of 10 ml each of Histopaque 1.119 (Sigma, Ayrshire, UK), Histopaque 1.089 and HBSS-0.1 % BSA by centrifugation at 800 g for 20 min. Islets were collected from the interface between Histopaque 1.089 and HBSS-BSA and washed once with ice cold HBSS-BSA.
Quality assessment
Every parameter of islet quality was assessed four times per method. After the purification step, purity was assessed (before handpicking counting) by dithizone staining under the stereomicroscope (Zeiss, Stemi 2000-C; Göttingen, Germany). Islets were then handpicked for absolute yield quantification purposes. In addition, islets were let to recover overnight at 37 °C and 5 % CO2 to test viability and in vitro functionality on the following day. Viability was assessed in triplicate by Fluorescein Diacetate/Propidium Iodide (FDA/PI) (Sigma, St.Louis, MO, USA (N = 4) staining, using a Nikon TiU fluorescence microscope (Melville, NY, USA). Functionality was assessed by Glucose Stimulated Insulin Secretion (GSIS). Aliquots of 5 islets each per quatriplicate were washed in modified KREBS buffer (120 mM·NaCl, 25 mM·NaHCO3, 5 mM·KCl, 2.5 mM·CaCl2 and 1 mM·MgCl2 with 3 % BSA and 2.8 mM glucose) and pre-incubated for 1 h in the same buffer at 37 °C and 5 % CO2. They were then incubated in alternating low (2.8 mM), high (16.7 mM) and low (2.8 mM) glucose concentration in KREBS buffer with 1 % BSA for 1 h in each solution at 37 °C and 5 % CO2. Aliquots of the supernatants were analyzed in duplicate for mouse insulin content using an enzyme linked immunosorbent assay kit (ELISA) (Mercodia, Uppsala, Sweden). The stimulation index (SI) was calculated by dividing the mean insulin secreted by the islets in high-glucose medium by the mean insulin secreted from the same islets in low-glucose medium.
Statistical analysis
Data were analyzed using GraphPad Prism 5, and P values below 0.05 were considered statistically significant. Data are shown as mean ± SEM, unless otherwise indicated in the figure legends. Statistical Analysis was performed by the Kruskal–Wallis Test or One-Way ANOVA. For statistical comparisons Dunn’s multiple comparison test or Tukey’s multiple comparison test were applied.
Time span assessment
Time devoted to each procedure was quantified in minutes (Table 2) (N = 3), considering only the time consumed by the purification step and excluding the time devoted to the preparation of reagents and the counting of the islets by handpicking (except for the handpicking method).
Table 2.
Comparison of cost and time between purification methods
| Method | Cost (€) | Time (min) |
|---|---|---|
| Handpicking | 46 | 382 ± 30 |
| Filtration | 69 | 15 ± 1* |
| Histopaque | 77 | 128 ± 6 |
Excluding mice and salary costs. Time represents only the purification step of the isolation procedure, excluding the handpicking for counting. The data showed for time are the mean ± SEM of N = 3 isolations
* p < 0.05 by Kruskal–Wallis test and Dunn’s multiple comparison test
Cost analysis
The cost of supplies was calculated in Euros (Table 2), excluding mice costs and staff wages.
Results
In this study, four independent islet isolations including six mice each were performed separately per method and the obtained tissue was assessed for islet yield and quality in vitro, in terms of purity, viability and functionality (Table 1).
Table 1.
Quality assessment of pancreatic islets obtained by each method of purification
| Method | Purity (%) | Yield (islets/pancreas) | Viability (%) | Stimulation index |
|---|---|---|---|---|
| Handpicking | 98.50 ± 0.23 | 170 ± 9 | 84.99 ± 2.64 | 3.76 ± 0.85 |
| Filtration | 98.79 ± 0.12 | 101 ± 7* | 94.60 ± 1.55† | 2.12 ± 0.34 |
| Histopaque | 99.70 ± 0.12‡ | 155 ± 8 | 85.40 ± 2.58 | 4.82 ± 1.13 |
Data displayed are islet purity (%), assessed by dithizone staining before handpicking counting; yield, expressed as the absolute number of islets obtained per pancreas after handpicking; viability, assessed by FDA/IP staining by fluorescence microscopy; and SI calculated by dividing the mean insulin released by the islets in the high-glucose medium by the mean insulin released from the initial low-glucose medium. The numbers shown are the mean ± SEM of four independent experiments each involving six mice
*,†,‡ p < 0.05 by Kruskal–Wallis test and Dunn’s multiple comparison test
Purity was assessed (before handpicking counting) by dithizone staining (Fig. 1). A significant advantage in islet purity was observed using Histopaque (Histopaque: 99.70 % purity, p = 0.0007 versus filtration and handpicking).
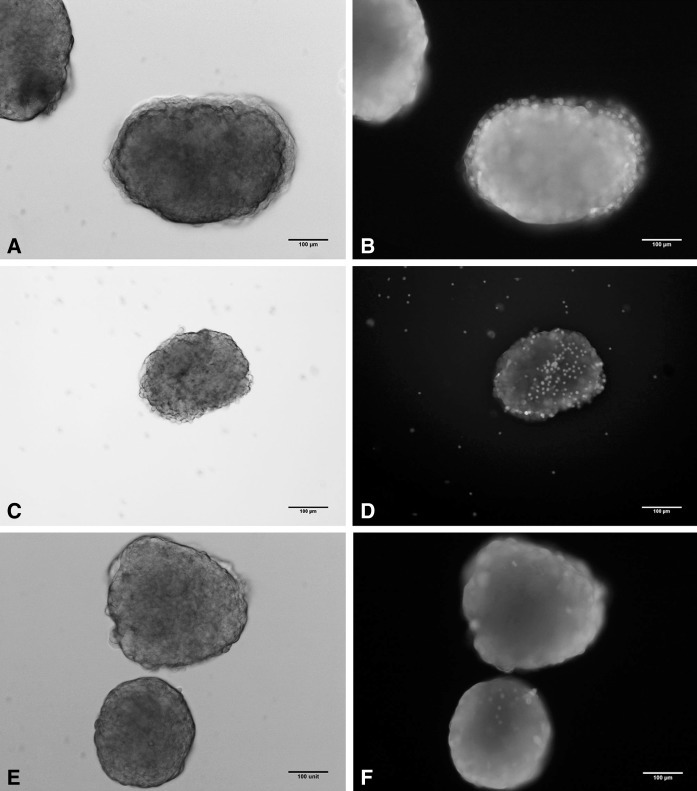
Fig. 1.

Purity assessment by dithizone staining. Dark field micrographs show purified islets by handpicking (a), Histopaque (b) and filtration (c). (Scale bar 400 μm)
Yield was assessed by handpicking counting of the islets resulting from each purification method and averaged over the four isolations. There were significant differences (p < 0.0001) in the number of islets obtained per pancreas between filtration with a cell strainer of 100 μm and handpicking (101 vs. 155 vs. 170 islets for filtration, Histopaque and handpicking).
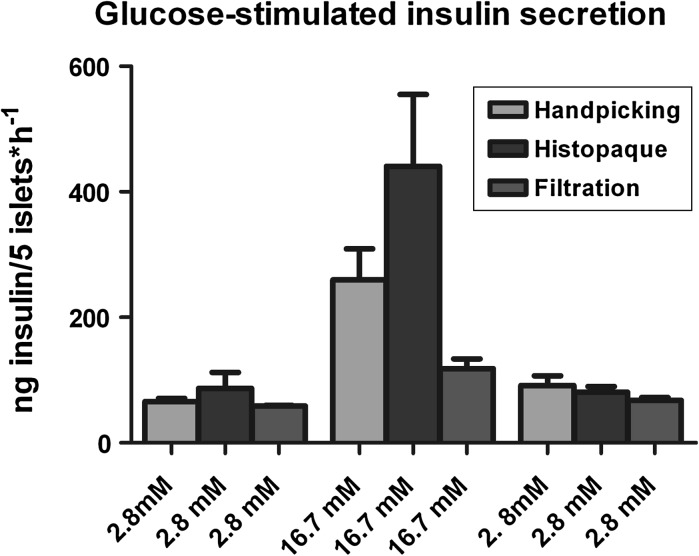
Viability was tested by FDA/PI staining after overnight culture of the islets (Table 1; Fig. 2). Islets obtained by the filtration method had the highest viability with statistical significance (94.60 % viability, p = 0.0123 versus Histopaque and handpicking). Immediately afterwards islets were challenged by GSIS with sequential concentrations of glucose (low, high and low) (Fig. 3). The resulting insulin released was measured by ELISA averaged and compared. No significant differences were detected between the same conditions. The SI was also calculated in order to quantify the potential of insulin release for each method, showing no significant differences (3.76 ± 0.85 vs. 2.12 ± 0.34 vs. 4.82 ± 1.13 for handpicking, filtration and Histopaque, p = 0.1672).
Fig. 2.
Viability assessment of islets obtained with the three purification methods by FDA/PI vital staining. Fluorescence micrographs show bright field islets (a, c, e), obtained by Handpicking (a), Histopaque (c) and Filtration (e) and overlaid pictures (b, d, f) show the overall viability of the islets (FDA plus PI staining). (Scale bar 100 μm)
Fig. 3.
Glucose-stimulated insulin secretion from purified islets. Five islets from N = 4 isolations were handpicked and assessed in quatriplicate for each method. Islets were washed and preincubated for 60 min in modified KREBS Buffer with 3 % BSA followed by sequential incubations with 2.8, 16.7 and 2.8 glucose in the same buffer with 1 % BSA. N = 4 supernatants were collected and analyzed on an ELISA kit in duplicate. (p > 0.05 between the same conditions, by Kruskal–Wallis test and Dunn’s multiple comparison test)
With regard to time consumption, the fastest method to isolate the islets was filtration (15 ± 1 vs. 128 ± 6 vs. 382 ± 30 min for filtration, Histopaque, and handpicking, respectively), and differences were statistically significant compared to handpicking and Histopaque (p = 0.0265 for a p < 0.05). Regarding to cost, Histopaque is the most expensive method, a 10.19 % more expensive than filtration (46 vs. 69 vs. 77 Є, for handpicking, filtration and Histopaque) (Table 2).
Discussion
The purification of pancreatic islets is a key step in the isolation process, intimately linked to the quality of the obtained islets. Contamination of islets by acinar cells may lead to a high level of proteases that will hamper islet integrity and functionality (O’Dowd 2009) and could cause serious postsurgical complications following intraportal transplantation (Lakey et al. 2003; Gray et al. 1988). In this study we aimed to compare the quality of islets obtained by an established method of islet purification, Histopaque, with an appealing alternative one, filtration, although some modifications of the original protocol were needed.
Our data show that the overall quality of both methods is good, in particular in terms of purity and viability. The values obtained in the quality assessment were always in the expected range, except for the yield obtained by filtration, which is lower than usual. Overall, the only significant differences between the two methods were related to purity, yield and viability.
The islets of highest purity were obtained by the Histopaque method, although purity achieved by the other methods was also above 98.5 %, which is indeed an acceptable level of purity. In this sense, in our hands the filtration protocol described by Li et al. (2009) achieved only 71.96 ± 3.60 % of purity (unpublished results) in contrast to their claim of almost 100 % purity in most cases, making this protocol unfeasible for the purification of islets by filtration alone with a 70 μm cell strainer. Therefore, we decided to filter preparations through a 100 μm cell strainer, as reported by Salvalaggio et al. (2002). Other reports included an extra step of purification by handpicking after applying this protocol (Daniel et al. 2011; Dhanesha et al. 2012; Mosedale et al. 2012; Pang et al. 2010) and our results further confirm the improvement of islet purity after adding this extra step.
In order to adapt the protocol for the comparison of the methods we changed the type of Collagenase (Collagenase P instead of Collagenase XI). Despite there were significant differences in the initial purity between the two types of collagenase when the original protocol was reproduced (71.96 ± 3.60 vs. 60.92 ± 3.75, for Collagenase XI and Collagenase P, p = 0.0409. Unpublished data) no significant differences were observed in the final purity once handpicked the islets (98.45 ± 0.25 vs. 99.08 ± 0.14 for Collagenase XI and Collagenase P with a 70 μm filter, p = 0.0661, and 98.45 ± 0.25 vs. 98.79 ± 0.12 for Collagenase XI with a 70 μm filter and Collagenase P with a 100 μm filter, p = 0.5965. Unpublished data). Regarding to the yield, although there was no effect on the collagenase type (134 ± 10 vs. 160 ± 9 for Collagenase XI and Collagenase P with a 70 μm filter, p = 0.0601. Unpublished data) there were statistically significant differences when the original protocol was compared with the adapted here with Collagenase P and a 100 μm cell strainer (134 ± 10 vs. 101 ± 7 for Collagenase XI and Collagenase P, p = 0.049. Unpublished data).
Regarding yield, the values obtained were in the expected range (O’Dowd 2009) for Histopaque and handpicking, with higher values for handpicking. However, since islets range from 40 to 400 μm in diameter, filtration is unable to recover islets with less % than 100 μm, which account for 40.59 % and 34.84 % of the islets recovered with handpicking and Histopaque, respectively.
When assessing the viability of islets, the best values are obtained by filtration, with significant differences, which can be due to the less traumatic nature of this procedure.
Islet function is defined by its ability to regulate the release of insulin and other hormones in response to changes in extracellular glucose concentration (Carter et al. 2009). Therefore, GSIS provides an accepted measure of islet function and, consequently, of the quality of the preparation. The purpose of alternating “basal” or “unstimulated” conditions with stimulation with high glucose was to confirm that islets in the preparation were able to respond properly when the glucose was lowered again by decreasing insulin secretion. This also discarded the possibility of sustained high values of insulin secretion due to cell death processes.
On the other hand, a number of studies have reported toxic effects of Ficoll to islets during the isolation process (Lake et al. 1987; Salvalaggio et al. 2002; Scharp et al. 1973). However, islets in this work were healthy, with a SI between 2 and 20 (Carter et al. 2009) not showing any harmful effect of Histopaque on the islets, as supported also by more recent studies performed with Ficoll-based gradients (McCall et al. 2011; Mita et al. 2010; Lamb et al. 2011), nor were any statistical differences between methods observed. However, although GSIS is an important predictor of islet function it does not have a correlation with clinical transplantation outcomes (Ricordi et al. 2001; Street et al. 2004). Therefore, islets obtained by filtration (with the lowest GSIS and the highest viability) may recover once transplanted and improve their functionality in vivo (Papas et al. 2009).
Another issue to take into account regarding functionality is the size of the islets. Some articles have reported the superiority of rodent and human islets under 100-125 μm against large islets in in vitro and in vivo function (MacGregor et al. 2006; Lehman et al. 2007; Farhat et al. 2013). In this regard, Fujita et al. (2011) reported that large islets secreted less insulin than small islets per islet equivalent. This is consistent with the fact that, in human pancreata, there is a higher proportion of beta cells in small islets (39 %) than in large islets (63 %), and its closer contact with blood vessels may affect insulin secretion (Farhat et al. 2013). However, in a more recent study by Nam and colleagues (2010) the islets with the highest SI value had between 100 and 150 μm diameter in a heterogeneous population, which are in the size range of all methods. Additionally, we studied if 100 μm filtration could lead to loss of some islets with higher SI value, but we did not find statistical differences with islets over 100 μm (2.12 ± 0.22 vs. 1.77 ± 0.20 for islets under 100 μm and over 100 μm by filtration, p = 0.2615. Unpublished results).
In terms of time, handpicking demands almost threefold and 25-fold the time employed to purify islets by Histopaque and filtration, and is therefore not feasible as a routine procedure. Besides, comparing Histopaque and filtration, the latter saves almost 90 % of the time devoted to purify islets by Histopaque density gradient. With only 2–3 min to purify islets from one pancreas, filtration is clearly the fastest method.
Reagent costs should be balanced together with time and quality data. Histopaque is the most expensive method, almost twofold compared to handpicking and 10.19 % more expensive than filtration (excluding mice cost and staff wages) while filtration has an intermediate price. If staff wages are taken into account, which are proportional to the time consumed by each procedure, filtration is the cheapest option.
Taken together, our results show that the quality of the islets obtained by both methods is good, while the speed of filtration using a 100 μm cell is particularly convenient for large scale islet isolation and/or laboratories with limited staff. However, one-third of the islets are lost with filtration compared to Histopaque, and more animals are needed, so each laboratory should decide which method is more suitable according to their aims or economical situation. It is currently accepted that mouse and human islets differ in their anatomy and functionality (Cabrera et al. 2006) and that the human pancreas is more fibrous and dense than in rodents (Lakey et al. 2003; O’Dowd 2009). But both human and rodent islets are in the same range of size (Kim et al. 2009), thus making size-based purification like filtration a method of interest also for human islet purification.
Acknowledgments
M. R. D. conceived the experimental design, researched data, performed experiments, analyzed results and wrote the manuscript. L. C. contributed to discussion and edited the manuscript. The authors acknowledge Dr. Anna Novials (Institut d’Investigacions Biomédiques August Pi i Sunyer, Barcelona, Spain), Dr. Franz Martín (Centro Andaluz de Biología Molecular y Medicina Regenerativa, Sevilla, Spain), Dr. Eduard Montanya (Hospital de Bellvitge-IDIBELL, Barcelona, Spain) and Dr. Simona Marzorati (Ospedale San Raffaele, Milán, Spain) for scientific discussion. We thank Dr. José Ramón Bilbao, Dr. David Fogarty (University of the Basque Country, Leioa, Spain) and Dr. Raquel Malumbres (Centro de Investigación Médica Aplicada, Pamplona, Spain) for editorial assistance. Technical and human support provided by SGIker (UPV/EHU, MICINN, GV/EJ, ERDF and ESF) is gratefully acknowledged. This work was supported by a grant of the Basque Government for Groups of Excellence (IT-472-07) and a Grant of the Department of Industry of the Basque Government (SAIO09-PE09BF01). The authors have no duality of interest to declare.
References
- Atwater I, Guajardo M, Caviedes P, Jeffs S, Parrau D, Valencia M, Romero C, Arriagada C, Caamaño E, Salas A, Olguin F, Atlagich M, Maas R, Mears D, Rojas E (2010) Isolation of viable porcine islets by selective osmotic shock without enzymatic digestion. Transplant Proc 42:381–386 [DOI] [PubMed]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A (2006) The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103:2334–2339 [DOI] [PMC free article] [PubMed]
- Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208:1501–1510. doi: 10.1084/jem.20110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanesha N, Joharapurkar A, Shah G, Kshirsagar S, Dhote V, Sharma A, Jain M (2012) Inhibition of 11 β-hydroxysteroid dehydrogenase 1 by carbenoxolone affects glucose homeostasis and obesity in db/db mice. Clin Exp Pharmacol Physiol 39:69–77 [DOI] [PubMed]
- Farhat B, Almelkar A, Ramachandran K, Williams SJ, Huang HH, Zamierowksi D, Novikova L, StehnoBittel L (2013) Small human islets comprised of more β cells with higher insulin content than large islets. Islets 5:87–94 [DOI] [PMC free article] [PubMed]
- Fujita Y, Takita M, Shimoda M, Itoh T, Sugimoto K, et al. Large human islets secrete less insulin per islet equivalent than smaller islets in vitro. Islets. 2011;3:1–5. doi: 10.4161/isl.3.1.14131. [DOI] [PubMed] [Google Scholar]
- Gray DW, Sutton R, McShane P, Peters M, Morris PJ. Exocrine contamination impairs implantation of pancreatic islets transplanted beneath the kidney capsule. J Surg Res. 1988;45:432–442. doi: 10.1016/0022-4804(88)90193-X. [DOI] [PubMed] [Google Scholar]
- Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M (2009) Islet architecture: a comparative study. Islets 1:129–136 [DOI] [PMC free article] [PubMed]
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lake SP, Anderson J, Chamberlain J, Gardner SJ, Bell PR, James RF (1987) Bovine serum albumin density gradient isolation of rat pancreatic islets. Transplantation 43:805–808 [PubMed]
- Lakey JR, Burridge PW, Shapiro AM. Technical aspects of islet preparation and transplantation. Transplant Int. 2003;16:613–632. doi: 10.1111/j.1432-2277.2003.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Lamb M, Storrs R, Li S, Liang O, Laugenour K, Dorian R, Chapman D, Ichii H, Imagawa D, Foster C 3rd, King S, Lakey JR (2011) Function and viability of human islets encapsulated in alginate sheets. In vitro and in vivo culture. Transplant Proc 43:3265–3266 [DOI] [PubMed]
- Lehman R, Zuellig RA, Kugelmeier P, Baenninger PB, Moritz W, Perren A, Clavien PA, Weber M, Spinas GA (2007) Superiority of small islets in human islet transplantation. Diabetes 56:594–603 [DOI] [PubMed]
- Li DS, Yuan YH, Tu HJ, Liang QL, Dai LJ. A protocol for islet isolation from mouse pancreas. Nat Protoc. 2009;4:1649–1652. doi: 10.1038/nprot.2009.150. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Williams SJ, Tong PY, Kover K, Moore WV, StehnoBittel L (2006) Small islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab 290:E771–E779 [DOI] [PubMed]
- McCall MD, Maciver AH, Pawlick R, Edgar R, Shapiro AM. Histopaque provides optimal mouse islet purification kinetics: comparison study with Ficoll, iodixanol and dextran. Islets. 2011;3:144–149. doi: 10.4161/isl.3.4.15729. [DOI] [PubMed] [Google Scholar]
- Mita A, Ricordi C, Messinger S, Miki A, Misawa R, Barker S, Molano RD, Haertter R, Khan A, Miyagawa S, Pileggi A, Inverardi L, Alejandro R, Hering BJ, Ichii H (2010) Antiproinflammatory effects of iodixanol (OptiPrep)-based density gradient purification on human islet preparations. Cell Transplant 19:1537–1546 [DOI] [PMC free article] [PubMed]
- Mosedale M, Egodage S, Calma RC, Chi NW, Chessler SD. Neurexin-1α contributes to insulin-containing secretory granule docking. J Biol Chem. 2012;287:6350–6361. doi: 10.1074/jbc.M111.299081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Yong W, Harvat T, Adewola A, Wang S, Oberholzer J, Eddington DT (2010) Size-based separation and collection of mouse pancreatic islets for functional analysis. Biomed Microdevices 12:865–874 [DOI] [PubMed]
- O’Dowd JF. The isolation and purification of rodent pancreatic islets of Langerhans. Methods Mol Biol. 2009;560:37–42. doi: 10.1007/978-1-59745-448-3_3. [DOI] [PubMed] [Google Scholar]
- Pang Z, Wu N, Zhang X, Avallone R, Croci T, Dressler H, Palejwala V, Ferrara P, Tocci MJ, Polites HG (2010) GPR40 is partially required for insulin secretion following activation of beta3-adrenergic receptors. Mol Cell Endocrinol 325:18–25 [DOI] [PubMed]
- Papas KK, Suzynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14:674–682. doi: 10.1097/MOT.0b013e328332a489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricordi C, Lakey JR, Hering BJ. Challenges towards standardization of islet isolation technology. Transplant Proc. 2001;33:1709. doi: 10.1016/S0041-1345(00)02651-8. [DOI] [PubMed] [Google Scholar]
- Salvalaggio PR, Deng S, Ariyan CE, Millet I, Zawalich WS, Basadonna GP, Rothstein DM (2002) Islet filtration: a simple and rapid new purification procedure that avoids Ficoll and improves islet mass and function. Transplantation 74:877–879 [DOI] [PubMed]
- Scharp DW, Kemp CB, Knight MJ, Ballinger WF, Lacy PE. The use of ficoll in the preparation of viable islets of langerhans from the rat pancreas. Transplantation. 1973;16:686–689. doi: 10.1097/00007890-197312000-00028. [DOI] [PubMed] [Google Scholar]
- Shapiro AM. Strategies toward single-donor islets of Langerhans transplantation. Curr Opin Organ Transplant. 2011;16:627–631. doi: 10.1097/MOT.0b013e32834cfb84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed]
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, SeyfertMargolis V, Bluestone J, Lakey JR (2006) International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330 [DOI] [PubMed]
- Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T, Korbutt GS (2004) Islet graft assessment in the Edmonton protocol implications for predicting long-term clinical outcome. Diabetes 53:3107–3114 [DOI] [PubMed]
- van der Vliet JA, Meloche RM, Field MJ, Chen DJ, Kaufman DB, Sutherland DE (1988) Pancreatic islet isolation in rats with ductal collagenase distention, stationary digestion, and dextran separation. Transplantation 45:493–495 [DOI] [PubMed]