Abstract
Salt mines represent an extreme environment with hypersaline conditions, complete darkness, and low nutrient availability. The diversity of filamentous fungi in such habitats is largely unknown. Eight strains of an unknown fungus were isolated from water samples of the salt mine in Berchtesgaden (Bavaria, Germany). They could be assigned to the ascomycete genus Phialosimplex, based on their common characteristics of producing conidia in chains or in heads on single phialides. Species of this genus are hitherto known to cause mycoses in dogs and have been found in mummies. Using molecular and morphological methods, the isolates are established as a new species, Phialosimplex salinarum sp. nov. Basipetospora halophila is also transferred to Phialosimplex as P. halophila comb. nov.
Keywords: Basipetospora, extremophile fungi, halophily, ITS, osmophily, salt tolerance, TSR1
INTRODUCTION
Certain fungi tolerate highly unfavourable environmental conditions such as extreme temperatures or osmotic pressures. The corresponding extreme habitats are scattered all over the world and represent attractive sites to explore for organisms with specific adaptations, so-called “extremophiles” (Madigan et al. 1997, Atlas & Bartha 1998, Gross 1998, Horikoshi & Grant 1998, Cavicchioli & Thomas 2000, Rothschild & Mancinelli 2001, Kis-Papo et al. 2014). High osmotic pressure, corresponding to low water activity (Aw), a measure based on the partial pressure of water vapour in a substance, and correlated with the ability to support microorganisms (Kis-Papo et al. 2014), is withstood by osmotolerant, and in particular, osmophilic fungi (Blomberg & Adler 1992, Hocking 1993). Fungi living in such extreme habitats may be classified as halotolerant or halophilic, i.e. salt-tolerant or salt-loving, respectively. Halotolerant species can grow without salt but withstand hypersalinity, while halophilic taxa require a certain content of salts in the substrate or water for growth. Strains which do not grow on media containing salt after 10 wk of incubation are classified as “halosensitive” (Gunde-Cimerman et al. 2006).
Hypersaline subterranean water was reported to be predominantly colonized by Archaea (Norton et al. 1993, Denner et al. 1994, Vreeland et al. 1991). Haloarchaea with a broad range of salt tolerance were found in the salt mine brines of Wieliczka (Poland), Winsford (UK), and Braunschweig (Germany) (Namyslowski 1913, Norton et al. 1993, Nehrkorn & Schwartz 1961, Vreeland & Huval 1991). Microorganisms can even be directly isolated from crystalline salt (Dombrowski 1963, Tasch 1963) and from enclosed fluids (Reiser & Tasch 1960). The examination of salt cores in Zechstein deposits, originating from the late Permian (Spötl 1988), and samples from a salt deposit near Bad Ischl (Austria) revealed extremely halophilic gram-positive coccoid bacteria (Bibo et al. 1983, Stan-Lotter et al. 1999). A study on salt sediments in the European Alps near Bad Ischl also reported halophilic, oligotrophic Halobacteriales (Archaea) (Stan-Lotter 2007).
Knowledge of fungal diversity under hypersaline conditions, in marine habitats (Buchalo et al. 1998) or salt lakes like the Dead Sea (Kis-Papo et al. 2001, Gunde-Cimerman et al. 2000), and including those of subterranean habitats (Kis-Papo et al. 2001, 2003, Grishkan et al. 2003, 2004), is relatively poor, and lacking for temperate regions. It was therefore our aim to explore the mycobiota in the abandoned salt mine galleries of the Berchtesgaden Alps (southern Germany). In the course of this study a number of fungal isolates turned out to represent an unknown species, and that is formally described here.
MATERIAL AND METHODS
Sampling sites and sample collection
Sediment and water were sampled at three sites (numbered as “1”, “2”, and “3”) in the salt mine of Berchtesgaden (“Salzbergwerk Berchtesgaden”) located in the Haselgebirge (“Hazel Mountains”), Upper Bavaria, Germany (47°38′17.99″N, 13°1′3.21″E). The mine consists of rocks composed of evaporate minerals and clastics (Kellerbauer 1996). The temperature range is 8–10 °C and the humidity range is 10–20 %. Site 1 is a mine gallery with a large pond filled with brine, unaffected by salt mining activities since about 20 yr. Site 2 was a smaller pond of ca. 1.5 m diam with red-coloured water, probably due to iron oxides, and a wood-boarded pillar. Site 3 is a mine gallery with a large brine-filled pond, filled with abandoned mining equipment and also red coloured water.
At each site, samples were taken from: (a) brine bottom sediment (“bs”), 7 cm beneath the sediment surface, using a boring rod 22 mm diam wrapped in aluminium foil; (b) the brine free water body (“wb”) at ca. 10 cm depth using a 15 ml sterile plastic tube (Rotilabo®-centrifuge tubes Eco, Roth, Germany); and (c) the brine surface water (“sw”) with a residue film using the same type of sterile plastic tubes as in (b). The samples were transported in a cool box (8 °C) to the laboratory within 5 hr of collection, and stored for 3 d at 4 °C until fungal isolation.
Cultivation and morphological analyses
Microorganisms were isolated from two replicates per site (1–3) and substrate (bs, wb, sw). For this purpose, small amounts of sediment (ca. 0.1 cm3) and water (800 μL), respectively, were plated onto yeast malt agar plates (YMA; 1 % malt extract, 0.4 % yeast extract, 0.4 % glucose, and 1.2 % agar). Table salt (Bad Reichenhaller Markensalz, Südsalz, Germany), originating from the sampled salt-mine, was added to the medium at concentrations 4, 15, and 25 % (w/v). Media without salt addition (“0 % salt”) only contained trace amounts of salt, as included in the other ingredients. The agar plates were incubated at 15 °C and in darkness for 1 yr and examined weekly for fungal growth. Emerging mycelia were immediately transferred to fresh correspondingly composed media. Pure cultures of the strains were maintained at 4 °C and at salt concentrations where colonies exhibited maximal growth.
Cultures of P. salinarum were examined morphologically with a Zeiss Axioplan light microscope. Mycelia growing at 15 % or 25 % salinity were examined in saline water. Strains were deposited in the Leibniz-Institute DSMZ-German Collection of Microorganisms and Cell Cultures (DSM 27530 – ex-holotype culture; DSM 27526, DSM 27527, DSM 27528, DSM 27529, DSM 27531, DSM 27532, and DSM 27533, isotype cultures) and at the CBS-KNAW Fungal Biodiversity Centre, Utrecht (CBS 138583– isotype culture).
Experimental assays
Halotolerance and growth rates
For characterising the halotolerance and growth rates of the new species, cultures were incubated on YMA and potato dextrose agar (PDA; 2 % glucose, 1.5 % agar, potato extract (a filtrate from cooked potatoes) in 3 and 1 replicates, respectively, at 15 °C for up to 20 wk. In addition, submerged cultures were prepared in 100 ml Erlenmeyer flasks using the YMA medium without agar. Digital photographs of the growing colonies were taken weekly for 10 wk and fortnightly until week 20. The growth rates of DSM 27530 were determined by measuring colony diameters on YMA in three replicates and on PDA media at different incubation temperatures (0, 4, 15, 20, 25, 30, and 35 °C) and salt concentrations (0, 4, 15, and 25 %).
Substrate degradation
In order to characterize the saprotrophic capabilities of the new species, strains DSM 27526–27533 were tested for their ability to degrade chitin, starch, cellulose, proteins, lignin, and lipids using different growth media based and modified according to Bradner et al. (1999) and Peterson et al. (2009) (Table 1). Each assay was incubated at 4, 15, and 25 °C for 8 d in darkness. Clearing zones of chitin- and protein-enriched media around fungal colonies were indicative of substrate degradation. Hyaline areas after staining were caused by cellulose and starch digestion. Lignin degradation was also visualized by staining (Table 1), which induced a change in colour to greenish blue around the colonies if positive. Crystalline coagulations indicated lipid degradation. Diameters of the clearing zones and hyaline areas were measured as a proxy for intensity of degradation activity.
Table 1.
Growth media and detection reagents used for substrate degradation assays.
| Test substrate | Growth medium | Detection reagent |
|---|---|---|
| Starch | 0.8 % peptone; 0.5 % NaCl; 0.6 % sol. starch; 1.2 % agar; H2O | Lugol’s solution |
| Chitin | 0.8 % peptone; 0.5 % NaCl; 0.6 % colloidal chitin; 1.2 % agar; H2O | none |
| Cellulose | 0.8 % peptone; 0.5 % NaCl; 0.6 % carboxymethyl cellulose; 1.2 % agar; H2O | Congo red; 0,5 M HCl |
| Peptides and protein | 0.8 % nutrient broth; 1.3 % powdered milk; 1.2 % agar; H2O ; pH 7.2 | none |
| Lipid | 0.8 % peptone; 0.5 % NaCl; 1 % Tween 80; 1.2 % agar; H2O | none |
| Lignin | 1 % malt extract; 0.4 % yeast extract; 0.4 % glucose ; 1.2 % agar; H2O | 0.2 % K2HPO4; 0.2 % KCl; 0.2 % MgSO4·7 H2O; 2 % agar; ABTS 1 mM; H2O; pH 5 |
Molecular analyses
DNA isolation, amplification and sequencing
Total DNA was extracted from 38 fungal cultures and the ex-type culture of Phialosimplex sclerotialis (CBS 366.77), using the Charge Switch® gDNA Plant Kit (Invitrogen, USA) as recommended by the manufacturer but using 0.2 mL tubes, and with all volumes reduced to 10 %. The ITS rRNA gene (ITS1, 5.8S, and ITS2 region) was amplified using the primer combinations fSSUh49F and rLSUh11F (Peršoh et al. 2013), ITS1F (Gardes & Bruns 1993) and ITS4 (White et al. 1990), and 1203F (GACTCAACACGGGGAAACTC) and ITS4, respectively. The TSR1 gene was amplified using the primers F1526Pc and R2434 according to Houbraken & Samson (2011). PCR was performed with the KAPA3G plant PCR kit (Kapa Biosystems, Boston, USA) according to the manufacturer’s instructions, but with all ingredients reduced to a total volume of 25 μL.
The PCR products were purified using the Charge Switch® PCR Clean-Up Kit (Invitrogen, USA) as recommended by the manufacturer. Sequencing was performed by the GATC-Biotech LightRun sequencing service (Konstanz) using the primers ITS1F or ITS4 for ITS sequences and R2434 for TSR1 sequences. ITS sequences were deposited in GenBank (www.ncbi.nlm.nih.gov) under accession numbers KF274685–KF274692; the sequence of the type strain of Phialosimplex sclerotialis (CBS 366.77) obtained accession number KF267869. TSR1 sequences are accessible under GenBank accession numbers KJ855522-KJ855529 (Table 2).
Table 2.
Accession numbers assigned to strains of Phialosimplex salinarum.
Phylogenetic analysis
The TSR1 and ITS sequences were compared with data in GenBank, using the BLAST function of NCBI (Zhang et al. 2000). The eight TSR1 sequences found to represent Phialosimplex salinarum, their closest relatives found, and representative species of Sagenomella, Aspergillus, and Penicillium were aligned using Muscle implemented in Geneious v. 6 (Biomatters, Auckland, New Zealand).
Only unambiguously alignable positions were included in the phylogenetic analyses of TSR1 sequences, which correspond to base pairs 1–516 and 554–610 of sequence JN121732 (Phialosimplex chlamydosporus). A subsequent alignment of ITS sequences comprised only sequences of taxa with closer relationship to Phialosimplex as revealed by preliminary analysis (data not shown). It included the base pair positions 1761–1767, 1772–1803, 1806–1826, 1848–1859, 1865–1895, 1900–1914, 1916–1935, 1940–2149, 2159–2230, 2232–2252 of sequence GQ169326 (P. chlamydosporus). A maximum likelihood tree was calculated using PhyML v. 2.2.0 (Guindon & Gascuel 2003). Node support was evaluated based on 500 bootstrap replicates and a GTR (general-time-reversible) substitution model, as suggested by modeltest v. 3.7. In accordance with Sigler et al. (2010), the phylogenetic tree including only the genera Phialosimplex and Sagenomella was rooted with Eremascus albus (U18359.1). Penicillium olsonii (JN121771) was used as the outgroup in the initial Trichocomaceae tree.
RESULTS
Forty filamentous and 25 yeast-like fungi, along with a few colonies of Archaea, were isolated from the salt mine. The filamentous fungi belonged to Trichocomaceae, with Penicillium spp. most frequently isolated. Eight isolates of a hitherto unknown fungus were isolated from three water bodies (“wb”) and five surface water (“sw”) samples at sampling sites 2 and 3. It is described here as Phialosimplex salinarum. DSM 27530 is designated as the holotype, while DSM 27526–27529 and DSM 27531–27533 are deposited as additional strains.
Morphology
Morphologically, all strains of Phialosimplex salinarum conformed with the traits considered to be diagnostic for the genus Phialosimplex (Sigler et al. 2010).
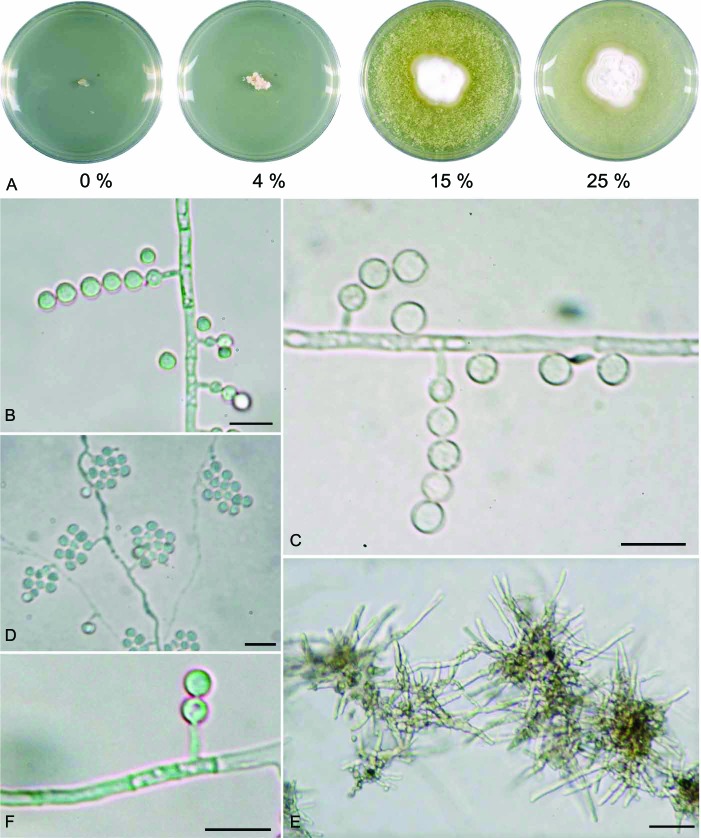
Colonies on YMA containing 15 % salt had a velvety to powdery appearance, mostly with white, rarely with a yellowish, surface. On media with 0 % or 4 % salt, the colonies were yellowish and exhibited a granular, occasionally yeast-like, structure. Some strains formed granular conglomerates of the surface. Growth on YMA was slow, with colonies attaining diameters of 20–28 mm on 15 % salt YMA and 15–23 mm on 25 % salt YMA within 70 d at 15 °C. On YMA containing ≥15 % salt, a cotton-like aerial mycelium developed after 28 d. All hyphae grew straight and were mostly unbranched, except for media with ≤4 % salt. At low-salt conditions, the colonies formed much shorter, knobbly hyphae with thicker cell walls. Conidiogenous cells were phialides laterally borne on short, unbranched conidiophores. Conidia were globose to subglobose, and produced in long chains or occasionally heads (Fig. 3, Table 3).
Fig. 3.
Phialosimplex salinarum (DSM 27530) on yeast malt agar. A. Colony growing at different NaCl concentrations. B, C. Phialides producing conidia in chains. D. Phialides producing conidia in heads. E. Vegetative hyphae on YMA submerse culture containing 4 % salt. F. Single phialide. Bars = 10 μm.
Table 3.
Phenotypic characteristics of Phialosimplex spp. [data for P. caninus, P. chlamydosporus, and P. sclerotialis from Sigler et al. (2010), for Phialosimplex sp. by Ravindran et al. (2012).] *Description of the synonymous taxa Scopulariopsis halophilica and Oospora halophila included; lack of data in the compiled descriptions indicated as “n/a”.
| Taxon | Phialides lenght [μm] | Conidia in heads presence | Chlamydospores presence | Sclerotia presence | Growth at 35°C | Conidial length [μm] | Conidial width [μm] | Conidial shape | Soluble pigment presence | Habitat and associations | Halotolerance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. caninus | 4.5–16 | + | – | – | + | 2.2–4.0 | 1.8–3.7 | subglobose | + | Bone marrow aspirate, Canidae-associated (Canis lupus familiaris – zoopathogenous) | n/a |
| P. chlamydosporus | 3–14 | + | + | – | + | 2.3–5.8 | 1.7–3.0 | ovoid, pyriform | + | Canidae-associated (Canis lupus familiaris – zoopathogenous) | n/a |
| B. halophila* | 3–20 | – | n/a | – | n/a | 3.5–9.0 | 3.5–8.0 | spherical, pyriform, subglobose, ovoid | – | Phaeophyta-associated (Undaria pinatifida) | halophilic |
| P. salinarum | 1.5–8.7 | + | – | – | + | 2.8–4.0 | 2.8–4.0 | mainly globose, subglobose | – | Water in salt mine | halophilic |
| P. sclerotialis | 5–15 | – | – | + | + | 3.0–4.5 | 1.5–2.0 | ovoid, with truncate base | – | Poaceae-associated (Lolium perenne – as fodder) | n/a |
| P. sp. | n/a | n/a | + | n/a | n/a | n/a | n/a | n/a | n/a | Deep sea water | halophilic |
Experimental assays
Salt and temperature preferences
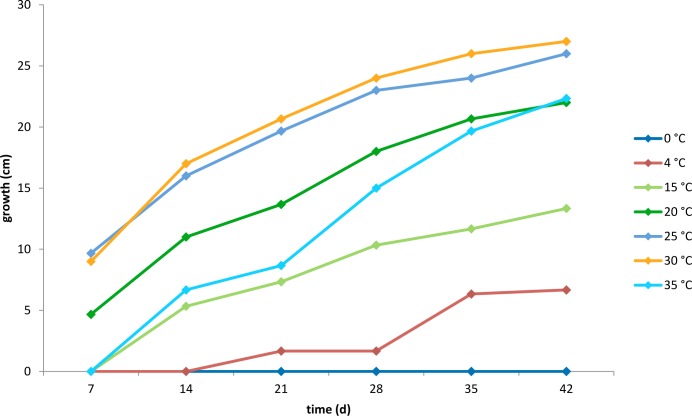
All isolated strains of Phialosimplex salinarum exhibited optimal growth on agar amended with salt concentrations of 15 % and 25 %. Strains DSM 27528–27533 did not grow on YMA without additional salt and were categorised as being halophilic sensu Gunde-Cimerman et al. (2006). Strains DSM 27526 and DSM 27527 grew slowly on media lacking salt and were therefore classified as being halotolerant. All strains grew poorly at 4 % salinity. The best growth rates among the tested conditions were in yeast malt submerged cultures with 15 % and 25 % salt concentration. The topotype strain (DSM 27530) cannot grow at 0 °C (Fig. 1), but showed slow growth at 15–25 °C on YMA with 4 % salt, reaching 4 mm diam after 21 d. On YMA with 15 % and 25 % salt, the strain had an increasing growth rate with increasing temperatures up to 30 °C, at which the colony grew to 21 mm diam after 21 d at 15 % salinity, and to 17 mm at 25 % salinity. After 21 d at 35 °C it reached 9 mm at 15 % salinity and 12 mm at 25 % salinity. DSM 27530 grew slightly slower on PDA, but temperature preferences were similar.
Fig. 1.
Growth of Phialosimplex salinarum (DSM 27530) at different temperatures on YMA containing 15 % salt.
Substrate degradation
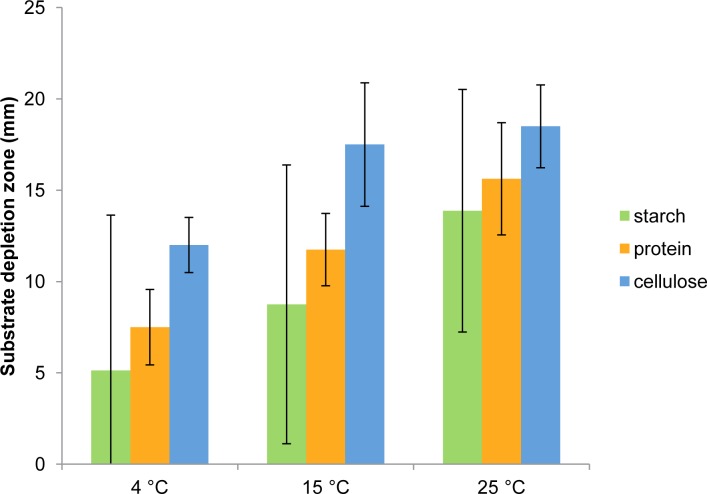
All strains increasingly degraded cellulose and proteins with rising incubation temperatures (Fig. 2). Decomposition of starch was more heterogeneous. While DSM 27526, DSM 27532 and DSM 27533 decomposed starch at all temperatures, DSM 27527, DSM 27528, and DSM 27531 did not at 4 °C. DSM 27529 and DSM 27530 hardly showed any decomposition of starch. Lipids, lignin, and chitin were not degraded by any strain of Phialosimplex salinarum.
Fig. 2.
Temperature-dependence of starch, protein, and cellulose degradation by Phialosimplex salinarum. Average (bars), maximal and minimal (whiskers) depletion zones observed for strains DSM 27526–27533 are indicated.
Molecular analysis
The ITS regions of strains DSM 27526–27529, DSM 27531 and DSM 27533 are identical. Strain DSM 27530 differs in two nucleotide positions in the ITS1 region and DSM 27532 differs in one position in the ITS2 region.
BLAST search
ITS sequences of strains DSM 27526–27533 (Phialosimplex salinarum) did not exactly match any sequences in the international sequence databases (INSDC; www.insdc.org). The next similar ITS sequence assigned at species level originated from P. chlamydosporus (GenBank Acc. no. GQ169326, grade 97.9 %, coverage 100 %, similarity 95.7 %). The next similar TSR1 sequence originated from Basipetospora halophila (JN121815, grade 88.8 %, coverage 83.6 %, similarity 94 %).
Phylogeny
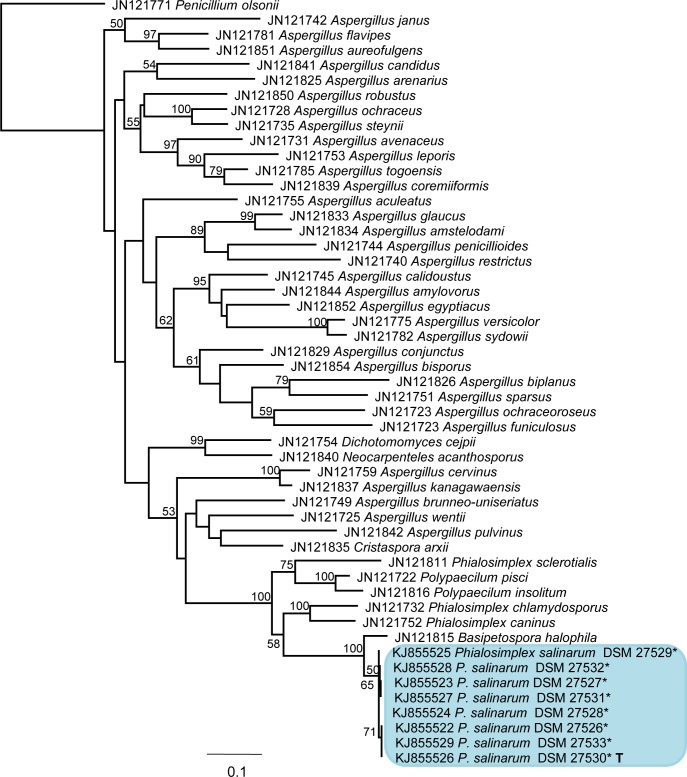
The TSR1 sequences obtained from the sequenced strains (Table 2) cluster together with all known Phialosimplex spp., Polypaecilum insolitum, P. pisci, and Basipetospora halophila in a phylogeny with selected sequences of Eurotiales (Fig. 4). The phylogenetic analysis confirms the affiliation of Phialosimplex to Aspergillus, which was already shown by Houbraken & Samson (2011).
Fig. 4.
Phylogenetic relationships of Eurotiomycetes as inferred from TSR1 sequence data. Most likely tree found by PhyML. Taxon names are preceded by the GenBank accession number. Support values above 70 % are noted above the respective branches. Sequences obtained from this study are marked with an asterisk. T = ex-type culture. Penicillium olsonii was used as outgroup.
The analysis based on a narrower taxon selection, but on basis of ITS sequences, saw the isolates of P. salinarum close to sequences of uncultured fungi obtained from an archaeological excavation in China (JQ410084, JQ410077), an endophytic fungus of Aquilaria sinensis (FN667943), and several isolates from mummies in a catacomb in Italy. Together with further environmental sequences, the representatives of Phialosimplex form a well-delimited and clade which obtained 70 % bootstrap support (BS).
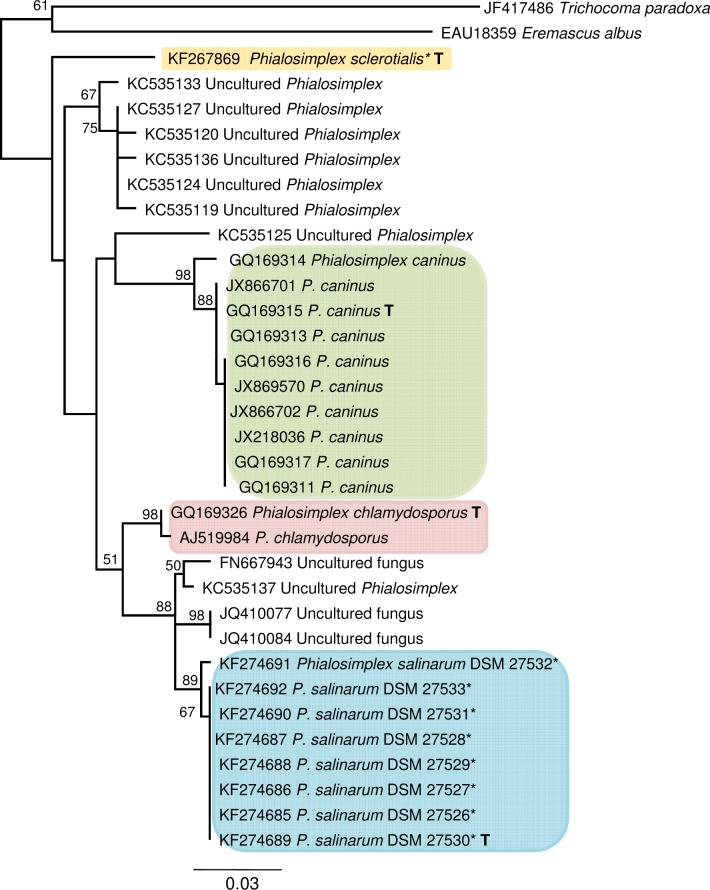
Three species (Phialosimplex chlamydosporus, P. sclerotialis, and P. caninus) were previously assigned to the genus Phialosimplex, established by Sigler et al. (2010). The eight strains of P. salinarum cluster together with these in a well-supported clade within Trichocomaceae (Fig. 5). The phylogenetically closest relatives to P. salinarum are four uncultured fungi, one of which was assigned to Phialosimplex, followed by P. chlamydosporus. However, the eight newly isolated strains are separated from their closest relatives. Furthermore, all ITS sequences from P. caninus cluster in a distinct clade. Sequences of Sagenomella are distinguishable from Phialosimplex, confirming the phylogenetic relationship described by Sigler et al. (2010). The clustering of P. sclerotialis (CBS 366.77) in the Phialosimplex clade of our ITS phylogeny is in accordance with its phylogenetic position in an 18S rRNA gene tree including a broader range of taxa of Eurotiomycetes (Houbraken & Samson 2011).
Fig. 5.
Phylogenetic relationships of Phialosimplex spp. as inferred from ITS rRNA sequence data. Most likely tree found by PhyML. Taxon names are preceded by the GenBank accession number. Support values above 50 % are noted above the respective branches. Sequences obtained from this study are marked with an asterisk. T = ex-type culture. Eremascus albus and Trichocoma paradoxa are used as outgroup.
The TSR1 and ITS phylogenies both support the current delimitation of the genus Phialosimplex, which so far has primarily been based on morphological traits.
Taxonomy
Phialosimplex salinarum Greiner, Peršoh, Weig & Rambold, sp. nov.
MycoBank MB809044
(Fig. 3)
Etymology: From the Latin salinae meaning saltern or saline, referring to the origin of the fungus from saline lakes inside a salt mine.
Diagnosis: Coloniae velociter crescentes 20 ºC ad 30 ºC, pulveraceae vel albae ad 15 % et 25 % salem, sine sale vel cum 4 % sale coloniae crescentes fasciculatae vel aurantiaco-griseae et flavoalbae vel luteae. Cellulae conidiogenae phialides simplices, laterales, monophialidicae. Phialides hyalinae, cylindricae, interdum deorsum vel prope medium modice inflatae, cum collari indistincto ad apicem, 1.5–8.5 μm longae, 1–1.5 μm latae ad basim, et 1.0–1.5 μm latae ad apicem. Conidia in catenis longis vel raro in capitulis aggregata, hyalina, perlucida, plerumque globosa ad subglobosa, 3.0–4.0 μm longa et 3.0–4.0 μm lata. Ascomata, sclerotia, et chlamydosporae absunt.
Type: Germany: Bavaria: Upper Bavaria, Berchtesgaden salt mine, 47 38′17.99″N, 13 1′3.21″E, isolated from water samples, flowing water and water surface of a large brine of ca. 10 m diam, being unaffected by salt mining activities for about 20 yr, 6 June 2011, K. Greiner, A. Weig & G. Rambold (DSM 27530 – holotype [maintained in a metabolically inactive state]; CBS 138583 –– isotype); ITS rRNA gene sequence deposited at GenBank, accession number KF274689; TSR1 sequence deposited under accession numbers KJ855522–KJ855529.
Description: Colonies with a velvety to powdery appearance and a white surface on yeast malt agar containing 15 % or 25 % salt. On agar ≤4 % salt content colonies have a yellowish and granular surface structure (Fig. 3). All strains except DSM 27526 and DSM 27527, are not capable of growth on media without additional salt. Growth on YMA is slow, reaching 20–27 mm diam on 15 % salt YMA and of 15–23 mm on 25 % salt YMA in 10 wk at 15 °C, showing the same growth characteristics on PDA with the same salt concentrations. Hyphae 1.5–2.5 μm wide and 9.5–16 μm long between the septa. Conidiogenous cells simple phialides arising laterally on vegetative hyphae. Phialides (monophialidic) discrete and mostly cylindrical to occasionally slightly broadened at the base or swollen below the centre, tapering at the neck; 1.5–8.5 μm in length, consistently 1.0–1.5 μm in width from the base to the tip. Conidia formed in long chains or occasionally in heads, hyaline, smooth, mostly globose to subglobose, 3.0–4.0 μm diam (Fig. 3). Chlamydospores or sclerotia not observed.
Ecology and distribution: Known only from a salt mine in Upper Bavaria (Germany), where it was found together with Penicillium brevicompactum, P. biourgeianum, P. chrysogenum, P. expansum, P. crustosum, and a Cladosporium sp. Under laboratory conditions cultures exhibited growth at a temperature range between 4–35 °C and on YMA with salt concentrations between 4–25%.
Discussion: Phialosimplex salinarum differs from its known most related species, P. chlamydosporus, P. sclerotialis, and P. caninus, in the globose conidia (Table 3). In addition, it differs from P. chlamydosporus in the lack of chlamydospores, from P. sclerotialis in the lack of sclerotia, and from P. caninus in the lack of a diffusible yellow pigment. The strain can also be distinguished from Basipetospora halophila, a phylogenetically closely related species, in producing smaller globose, and not ovoid conidia. The common characteristics shared by P. salinarum with all other Phialosimplex spp. currently are colour and shape of the colonies on agar, simple phialides being formed laterally on hyphae producing conidia in long chains or in heads. The diagnostic value of these traits at generic level needs to be evaluated in context of a monographic treatment.
Additional isolates examined: Germany: Bavaria: Upper Bavaria, Berchtesgaden salt mine, 47 38′17.99″N, 13 1′3.21″E, isolated from water samples, water bodies and water surface of a large brine of ca. 10 m diam, being unaffected by salt mining activities for about 20 yr, 6 June 2011, K. Greiner, A. Weig & G. Rambold (DSM 27526, DSM 27527, DSM 27528, DSM 27529, DSM 27531, DSM 27532, and DSM 27533). ITS rRNA GenBank Accession Numbers KF274685, KF274686, KF274687, KF274688, KF274690, KF274691, and KF274692.
DISCUSSION
Combining molecular and phenotypic data, this study describes the new species Phialosimplex salinarum, a representative of the genus Phialosimplex (Eurotiales), only known as asexual morphs. The strains of P. salinarum are undoubtedly native inhabitants of the salt mine, because they were isolated from four independent samples from two different sampling sites inside the mine and handled under sterile conditions. Furthermore, all but one strain were found to be clearly halophilic, a trait rarely found among potential contaminants.
Phylogeny and morphology
In the TSR1 phylogeny, the new species clusters with members of Phialosimplex (generic type species: P. caninus) and “Basipetospora” halophila in a clade which is sister to two species of Polypaecilum (generic type species: P. insolitum) and Phialosimplex sclerotialis. Together with Dichotomomyces, Neocarpenteles, and Cristaspora this group forms the “Polypaecilum-Phialosimplex clade”, which makes Aspergillus paraphyletic and so could support a further splitting of Aspergillus into several units at generic level (Peterson 2008, Houbraken & Samson 2011).
Despite the subgrouping within the Polypaecilum-Phialosimplex clade being only weakly supported in the TSR1 phylogeny, an assignment of the new taxon to Phialosimplex seems appropriate, as the ITS sequence fits with those of all other fungi considered as Phialosimplex species (including the generic type). Furthermore, the sequences were almost unalignable to those of Polypaecilum (P. insolitum and P. pisci) (TreeBASE Submission ID 14493). However, a final decision on the generic assignment will only be possible in the context of a profound revision of this group. This will be achieved in the frame of a monographic treatment (Houbraken, pers. comm.) and is beyond the scope of our study.
Morphological and phylogenetic analyses concertedly revealed the eight halophilic strains as members of the genus Phialosimplex. Furthermore, the TSR1 sequence data confirmed that the genus is closely related to Aspergillus, Penicillium, Paecilomyces, and Sagenomella (Fig. 2), as already known by RPB1, RPB2, TSR1, and Cct8 sequences (Samson et al. 2011, Houbraken & Samson 2011). While ITS sequences alone are insufficient for species delimitation in Aspergillus and Penicillium (Schoch et al. 2012), in general, the ITS region shows a sufficient gap between intra- and interspecific variability in Phialosimplex and also appears to be suitable for a discrimination of the species P. salinarum.
All strains clustering in the Phialosimplex-clade produce conidia in fragile chains, originating from solitary, cylindrical to flask-shaped phialides. These traits are characteristic of Phialosimplex (incl. Sagenomella p. p.) according to Sigler et al. (2010). Species of Phialosimplex further lack complexly branched conidiophores and frequently have flask-shaped phialides (Sigler et al. 2010). They have been separated from Sagenomella by the production of a diffusible yellow pigment and by exhibiting conidia condensed in heads. However, these two traits are no longer diagnostically valuable since several Sagenomella spp. were included in Phialosimplex (Sigler et al. 2010). By producing hyaline conidia just occasionally in heads and lacking yellow pigments, P. salinarum conforms to the current generic concept, which is therefore not in need of modification or extension. By morphology and ITS and TSR1 sequence data, the isolated strains can therefore be assigned to the genus Phialosimplex.
The main diagnostic characters to distinguish P. salinarum morphologically from other Phialosimplex spp. are the simple, mainly globose, but not ovoid, conidia. Furthermore, P. salinarum has slightly shorter phialides and lacks chlamydospores, sclerotia, and diffusible yellow pigments (Table 3).
Polypaecilum insolitum and P. pisci phylogenetically being closest to Phialosimplex in our trees, can be distinguished from P. salinarum by producing chlamydospores (Pitt & Hocking 1985). Morphological characteristics of the two closely related species ‘Basipetospora’ halophila and Phialosimplex salinarum (Fig. 4), largely agree, but B. halophila is distinguished by much larger conidia (Tubaki 1973).
As B. halophila falls into the same clade as the accepted species of Phialosimplex, and the type species of Basipetospora is a synonym of Monascus and belongs in Monascaceae not Trichocomaceae, the name requires transfer to Phialosimplex1.
Halotolerance
The only taxon known so far, which is unable to grow on salt-deficient media, is Wallemia ichthyophaga, which still grows on NaCl-enriched media at concentrations up to 30 % (Zalar et al. 2005). Phialosimplex salinarum is the first truly halophilic fungus that has been isolated from a subterranean, non-marine habitat. A morphologically similar, but chlamydospore-producing un-named member of Phialosimplex is already known from a hypersaline environment; it was part of a marine fungal community from the Central Indian Basin at ca. 5000 m deep (Singh et al. 2010). Unfortunately no strains or sequences could be obtained for our phylogenetic analysis and it seems to represent another hitherto undescribed taxon (Ravindran et al. 2012).
The genus Phialosimplex includes species that are medically relevant as zoopathogens causing opportunistically disseminated mycoses in dogs (Sigler et al. 2010). Indeed, such a co-occurrence of halophily and zoopathogenicity to warm-blooded animals within one and the same genus is proposed to be likely by de Hoog et al. (2005). After all, both syndromes appear to be only variants of the trait of osmophily, which is considered to be an universal type of stress response within the filamentous fungi (Gunde-Cimerman et al. 2006). To better understand the mechanisms and signalling pathways connected with halophily and zoophily in particular and osmophily in general, gene expression analyses of a broader range of taxa are required (de Hoog et al. 2005).
Habitat and provenience
Strains of Phialosimplex salinarum were isolated from two contrasting sites in the salt mine, but not from all three. This suggests that the species is not omnipresent in the mine, but its absence from site 1 is not yet verified by culture-independent methods. As isolates of P. salinarum only originate from the brine water, the fungus seems not to be able to survive under the probably oxygen-deficient conditions in the sediment, which would be in accordance with the general oxygen-dependency of most filamentous fungi.
This study did not provide any indications on how P. salinarum reached the salt mine originally. It may originate from “contaminants”, i.e. epigeic populations introduced by biotic carriers such as mine staff, birds, insects, or wind. Alternatively, the subterraneous salt caves may represent its natural environment, and it could have adapted to high salt concentrations during evolution. Nutrients may be introduced into the subterraneous habitat by various biotic or abiotic vectors like humans, animals, or wind, or be provided as debris of lithoautotrophic prokaryotes. The latter scenario seems plausible due to the presence of the archaebacterial species Halococcus salifodinae in the explored salt mines (Denner et al. 1994).
Salt mine-inhabiting Archaea have excited the imagination of various authors, who indicate that these organisms may have derived from populations having been enclosed within the rock salt, i.e. halite, since its formation millions of years ago (Grant et al. 1998, Stan-Lotter et al. 2004, 2011). This would have required long-term survival combined with tolerance of extreme salt concentrations (McGenity et al. 2000, Stan-Lotter et al. 2011). Such a scenario, however, is unlikely for the chemo-heterotrophic fungal inhabitants. It is more probable that these fungi were derived from epigeic populations and spread by animal vectors, as several species of Phialosimplex are associated with infections in animals (Sigler et al. 2010). However, the strains of P. salinarum are obviously well adapted to very high salt concentrations.
1Phialosimplex halophila (F.J.H. Beyma) Greiner, Persoh, Weig & Rambold, comb. nov.
Basionym: Oospora halophila J.F.H. Beyma, Zentbl. Bakt. ParasitKde, Abt. II 88: 134 (1933).
MycoBank MB810542
Synonyms: Basipetospora halophila (J.F.H. Beyma) Pitt & A.D. Hocking, Mycotaxon 22: 198 (1985).
Scopulariopsis halophilica Tubaki, Trans. Mycol. Soc. Japan 14: 367 (1973).
Acknowledgments
We thank the Südsalz GmbH for permission to visit and collect environmental samples in the Berchtesgaden salt mine. Stephan Kellerbauer (Berchtesgaden) is thanked for guidance during an excursion into the salt mine. Christina Leistner (Bayreuth) assisted with the laboratory work. We are also grateful to Joey B. Tanney and Jos Houbraken for critical comments on an earlier version of the manuscript.
REFERENCES
- Atlas RM, Bartha R. (1998) Microbial Ecology: fundamentals and applications. Menlo Park: Benjamin/Cummings Publishing. [Google Scholar]
- Blomberg A, Adler L. (1992) Physiology of osmotolerance in fungi. Advances in Microbial Physiology 33: 145–212. [DOI] [PubMed] [Google Scholar]
- Bibo F-J, Söngen R, Fresenius RE. (1983) Vermehrungsfähige Mikroorganismen in Steinsalz aus primären Lagerstätten. Kali und Steinsalz 8: 367–373. [Google Scholar]
- Bradner JR, Gilings M, Nevalainen KMH. (1999) Qualitative assessment of hydrolytic activities in microfungi grown at different temperatures on solid media. World Journal of Microbiology & Biotechnology 15: 131–132. [Google Scholar]
- Buchalo AS, Nevo E, Wasser SP, Oren A, Molitoris H-P. (1998) Fungal life in the extremely hypersaline water of the Dead Sea: first records. Proceedings of the Royal Society of London B 265: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavicchioli R, Thomas T. (2000) Extremophiles. In: Encyclopedia of Microbiology (Lederberg J, Alexander M, Bloom BR, Hopwood D, Hull R, Iglewski BH, Laskin AI, Oliver SG, Schaechter M, Summers WC, eds): 313–337 San Diego: Academic Press. [Google Scholar]
- de Hoog S, Zalar P, van den Ende BG, Gunde-Cimerman N. (2005) Relation of halotolerance to human-pathogenicity in the fungal tree of live: an overview of ecology and evolution under stress. In: Adaptations to Life at High Salt Concentrations in Archaea, Bacteria and Eukarya (Gunde-Cimerman N, Oren A, Plemenitas A, eds): 185–200 New York: Springer Verlag. [Google Scholar]
- Denner EBM, McGenity TJ, Busse H-J, Wanner G, Grant WD, et al. (1994) Halococcus salifodinae sp. nov., an archaeal isolate from an Austrian salt mine. International journal of systematic bacteriology 44: 774–780. [Google Scholar]
- Dombrowski H. (1963) Bacteria from paleozoic salt deposits. Annals of the New York Academy of Sciences 108: 453–460. [DOI] [PubMed] [Google Scholar]
- Grant WD, Gemell RT, McGenity TJ. (1998) Halobacteria: the evidence for longevity. Extremophiles 2: 279–287. [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. (1993) ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2:113–118. [DOI] [PubMed] [Google Scholar]
- Grishkan I, Nevo E, Wasser SP. (2003) Soil micromycete diversity in the hypersaline Dead Sea coastal area (Israel). Mycological Progress 2: 19–28. [Google Scholar]
- Grishkan I, Nevo E, Wasser SP. (2004) Micromycetes from the saline Arubotaim Cave: Mount Sedom, the Dead Sea southwestern shore, Israel. Journal of Arid Environments 57: 431–443. [Google Scholar]
- Gross M. (1998) Life on the Edge: amazing creatures thriving in extreme environments. New York: Plenum Press. [Google Scholar]
- Gunde-Cimerman N, Zalar P, de Hoog GS, Plemenitas A. (2000) Hypersaline waters in salterns – natural ecological niches for halophilic black yeasts. FEMS Microbiology Ecology 32: 235–240. [DOI] [PubMed] [Google Scholar]
- Gunde-Cimerman N, Butinar L, Sonjak S, Turk M, Ursic V, et al. (2006) Halotolerant and halophilic fungi from costal environments in the Arctics. In: Adaptations to Life at High Salt Concentrations in Archaea, Bacteria and Eukarya (Gunde-Cimerman N, Oren A, Plemenitas A, eds): 397–424 New York: Springer Verlag. [Google Scholar]
- Hocking AD. (1993) Responses of xerophilic fungi to changes in water activity. In: Stress Tolerance in Fungi (Jennings DH, ed): 233–256 New York: Marcel Dekker. [Google Scholar]
- Horikoshi K, Grant WD. (1998) Extremophiles: microbial life in extreme environments. New York: Wiley-Liss. [Google Scholar]
- Houbraken J, Samson RA. (2011) Phylogeny of Penicillium and the segregation Trichocomaceae into three families. Studies in Mycology 70: 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerbauer S. (1996) Geologie und Geomechanik der Salzlagerstätte Berchtesgaden. Münchner Geologische Hefte, B: Angewandte Geologie 2: 1–101. [Google Scholar]
- Kis-Papo T, Grishkan I, Oren A, Wasser SP, Nevo E. (2001) Spatiotemporal diversity of filamentous fungi in the hypersaline Dead Sea. Mycological Research 105: 749–756. [Google Scholar]
- Kis-Papo T, Kirzhner V, Wasser SP, Nevo E. (2003) Evolution of genomic diversity and sex at extreme environments: Fungal life under hypersaline Dead Sea stress. Proceedings of the National Academy of Sciences, USA 100: 14970–14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis-Papo T, Weig AR, Riley R, Peršoh D, Salamov A , et al. (2014) Genomic adaptations of the halophilic Dead Sea filamentous fungus Eurotium rubrum. Nature Communications 5: 3745. doi:10.1038. [DOI] [PubMed] [Google Scholar]
- Madigan MT, Martinko JM, Parker J. (1997) Brock’s Biology of Microorganisms. Saddle River, NJ: Prentice Hall. [Google Scholar]
- McGenity TJ, Gemmell RT, Grant WD, Stan-Lotter H. (2000) Origins of halophilic microorganisms in ancient salt deposits. Environmental Microbiology 2: 243–250. [DOI] [PubMed] [Google Scholar]
- Namyslowski MB. (1913) Über unbekannte halophile Mikroorganismen aus dem Inneren des Salzbergwerks Wieliczka. Bulletin of the Academy of Sciences, Krakow, series B 3/4: 88–104. [Google Scholar]
- Nehrkorn A, Schwartz W. (1961) Untersuchungen über Lebensgemeinschaften halophiler Mikroorganismen. I. Mikroorganismen aus Salzseen der Californischen Wüstengebiete und aus einer Natriumchlorid-Sole. Zeitschrift für Allgemeine Mikrobiologie 1: 121–141. [Google Scholar]
- Norton CF, McGenity TJ, Grant WD. (1993) Archaeal halophiles (halobacteria) from two British salt mines. Journal of General Microbiology 139: 1077–1081. [Google Scholar]
- Peršoh D, Segert J, Zigan A, Rambold G. (2013) Fungal community composition shifts along a leaf degradation gradient in a European beech forest. Plant Soil 362: 175–186. [Google Scholar]
- Peterson RA, Bradner JR, Roberts TH, Nevalainen KMH. (2009) Fungi from koala (Phascolartos cinereus) faeces exhibit a broad range of enzyme activities against recalcitrant substrates. Letters in Applied Microbiology 48: 218–225. [DOI] [PubMed] [Google Scholar]
- Peterson SW. (2008) Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100: 205–226. [DOI] [PubMed] [Google Scholar]
- Pitt JI, Hocking AD. (1985) New species of fungi from Indonesian dried fish. Mycotaxon 22: 197–208. [Google Scholar]
- Ravindran C, Varatharajan GR, Rajasabapathy R, Vijayakanth S, Kumar AH, et al. (2012) A role for antioxidants in acclimation of marine derived pathogenic fungus (NIOCC 1) to salt stress. Microbial Pathogenesis 53: 168–179. [DOI] [PubMed] [Google Scholar]
- Reiser R, Tasch P. (1960) Investigation of the viability of osmophile bacteria of great geological age. Transactions of the Kansas Academy of Science 63: 31–34. [PubMed] [Google Scholar]
- Rothschild LJ, Mancinelli RC. (2001) Life in extreme environments. Nature 409: 1092–1101. [DOI] [PubMed] [Google Scholar]
- Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, et al. (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Studies in Mycology 70: 159–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences, USA 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler L, Sutton DA, Gibas CFC, Summerbell RC, Noel RK, et al. (2010) Phialosimplex, a new anamorphic genus associated with infections in dogs and having phylogenetic affinity to the Trichocomaceae. Medical Mycology 48: 335–345. [DOI] [PubMed] [Google Scholar]
- Sing P, Raghukumar C, Verma P, Shouche Y. (2010) Phylogenetic diversity of culturable fungi from the deep-sea sediments of the Central Indian Basin and their growth characteristics. Fungal Diversity 40: 89–102. [Google Scholar]
- Spötl C. (1988) Sedimentologisch-fazielle Analyse tektonisierter Evaporitserien – Eine Fallstudie am Beispiel des Alpinen Haselgebirges. Geologisch-Paläontologische Mitteilungen Innsbruck 15: 59–69. [Google Scholar]
- Stan-Lotter H, McGenity TJ, Legat A, Denner EBM, Glaser K, et al. (1999) Very similar strains of Halococcus salifodinae are found in geographically separated Permo-Triassic salt deposits. Microbiology 145: 3565–3574. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H, Radax C, McGenity TJ, Legat A, Pfaffenhuemer M, et al. (2004) From intraterrestrials to extraterrestrials – viable haloarchaea in ancient salt deposits. In: Halophilic Microorganisms (Ventosa A, ed.): 89–102 Berlin: Springer. [Google Scholar]
- Stan-Lotter H, Fendrihan S, Legat A, Pfaffenhümer M, Gruber C, et al. (2007) Lebensfähige Halobakterien aus permischem Steinsalz – und im Weltraum? Denisia 66: 313–322. [Google Scholar]
- Stan-Lotter H, Fendrihan S. (2011) Deep biosphere of salt deposits. In: Encyclopedia of Geobiology (Reitner J, Thiel V, eds): 313–317 Heidelberg: Springer Science+Business Media. [Google Scholar]
- Tasch P. (1963) Dead and viable fossil salt bacteria. University of Wichita Bulletin 39: 2–7. [Google Scholar]
- Tubaki K. (1973) An undescribed halophilic species of Scopulariopsis. Transactions of the Mycological Society of Japan 14: 367–369. [Google Scholar]
- Vreeland RH, Huval JH. (1991) Phenotypic characterization of halophilic bacteria from ground water sources in the United States. In: General and Applied Aspects of Microorganisms (Rodríguez-Valera F , ed.): 53–60 New York: Plenum Press. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds): 315–322 San Diego: Academic Press. [Google Scholar]
- Zalar P, de Hoog GS, Schroers H-J, Frank JM, Gunde-Cimerman N. (2005) Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie van Leeuwenhoek 87: 311–328. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, Miller W. (2000) A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7: 203–214. [DOI] [PubMed] [Google Scholar]