Abstract
The morphologically diverse genus Ceuthospora has traditionally been linked to Phacidium sexual morphs via association, though molecular or cultural data to confirm this relationship have been lacking. The aim of this study was thus to resolve the relationship of these two genera by generating nucleotide sequence data for three loci, ITS, LSU and RPB2. Based on these results, Ceuthospora is reduced to synonymy under the older generic name Phacidium. Phacidiaceae (currently Helotiales) is suggested to constitute a separate order, Phacidiales (Leotiomycetes), as sister to Helotiales, which is clearly paraphyletic. Phacidiaceae includes Bulgaria, and consequently the family Bulgariaceae becomes a synonym of Phacidiaceae. Several new combinations are introduced in Phacidium, along with two new species, P. pseudophacidioides, which occurs on Ilex and Chamaespartium in Europe, and Phacidium trichophori, which occurs on Trichophorum cespitosum subsp. germanicum in The Netherlands. The generic name Allantophomopsiella is introduced to accommodate A. pseudotsugae, a pathogen of conifers, while Gremmenia is resurrected to accommodate the snow-blight pathogens of conifers, G. abietis, G. infestans, and G. pini-cembrae.
Keywords: Bulgariaceae, coelomycete, discomycete, Gremmenia, Helotiales, LSU, Phacidiales, RPB2, systematics
INTRODUCTION
The generic name Phacidium was introduced by Fries (1815) for P. coronatum and P. integerrimum. Phacidium integerrimum was subsequently transferred to Excipula, and P. coronatum to Coccomyces. Phacidium lacerum, a species described later by Fries (1818), was therefore considered the type species of the genus (von Höhnel 1917), even though it was not one of the two species originally described by Fries. Subsequent authors have accepted this typification (Terrier 1942, von Arx & Müller 1954, Reid & Cain 1962, Korf 1973, Dennis 1978). In order to regularise this situation, Hawksworth & Sherwood (1981) proposed the conservation of Phacidium with P. lacerum as the conserved type species; this proposal was accepted by the Committee for Fungi and Lichens and incorporated into the appendices of the Berlin Code of 1988 (Greuter et al. 1988). While the proposal to conserve the type was under discussion, DiCosmo et al. (1984) followed Höhnel (1917) and accepted P. lacerum as the type. Under the Melbourne Code (McNeill et al. 2013), types for sanctioned names can be selected either from the original protologue (in this case Fries 1815) or the sanctioning work (in this case Fries 1823, where P. lacerum was included), so there would be no objection now to the acceptance of P. lacerum as type species of Phacidum but that option was not available in 1981.
Tulasne & Tulasne (1861–65) recognised that Phacidium ilicis was a single fungus with two morphs (pleomorphic), and the conidiomatal states to be representative of Cytisporae (spermatial) and Ceuthosporae (asexual). Ceuthospora has been linked as an asexual morph to Phacidium (Sutton 1980, Nag Raj 1993). As with Phacidium, however, the typification of the asexual name was also beset by problems.
The generic name Ceuthospora was published by Fries (1825) with two species, Sphaeria phaeocomes and Sclerotium inclusum. Greville (1828) included another three species, C. phacidioides, C. lauri, and C. phaeocomes, all accepted by Fries (1832). Fries (1849) introduced the generic name Pyrenophora, and referred both Sphaeria phaeocomes and Sclerotia inclusum to that genus. The genus Ceuthospora, was thus not clearly typified. Fries (1832) effectively selected Sphaeria phaeocomes as type, when he excluded Sclerotium inclusum from the genus. However, Pyrenophora phaeocomes was selected as type species of Pyrenophora by Shoemaker (1961), which effectively leaves Pyrenophora as a later nomenclatural synonym of Ceuthospora. To resolve this situation, Sutton (1972) proposed the conservation of Ceuthospora Grev. 1826 with C. lauri as type, over Ceuthospora Fr. 1825 with C. phaeocomes as type; this was accepted by the Committee for Fungi and Lichens and the name included in the appendices to the Leningrad Code (Stafleu et al. 1978).
The family name Phacidiaceae was introduced by Fries (1849) and accepted by Karsten (1871) who stressed that members had a particular apothecium type with a reduced exciple. Nannfeldt (1932) recognised 14 genera in the family, but Terrier (1942) felt that the family contained different elements, and probably should only include five genera. Von Arx & Müller (1954) regarded eight genera as belonging to the family. The circumscription remained a bone of contention, as Kreisel (1969) recognised four genera, Korf (1973) nine, Dennis (1978) 13, and Lanier et al. (1978) seven. DiCosmo et al. (1984) listed 24 genera in Phacidiaceae, stating that it was heterogeneous and in need of further study. In a recent phylogenetic study of Leotiomycetes (Wang et al. 2006b), Helotiales (incl. Phacidiaceae) was left untreated as a large, polyphyletic assemblage, due to inadequate molecular data and sampling. However, they did refer to Phacidiaceae, based on analyses including a LSU sequence of Phacidium lacerum (data not shown), which suggested a sister group relationship with Phacidiopycnis pyri (sexual morph: Potebniamyces pyri), previously questionably placed in Rhytismatales). In the SSU-LSU-5.8S phylogeny (Wang et al. 2006b), P. pyri formed a strongly supported monophyletic group with Bulgaria inquinans (Bulgariaceae), and P. pyri together with Holwaya mucida (although without support) was moved to Bulgariaceae. Bulgaria has large, brown-black to black, turbinate, gelatinous apothecia with brown-walled ascospores, and is thus quite different from Phacidium and Potebniamyces in morphology. In the LSU & SSU phylogeny generated by Lantz et al. (2011), however, these three genera clustered together in a well-supported clade.
Given the recent decision to abolish dual nomenclature for fungi (Hawksworth 2011, Hawksworth et al. 2011, Wingfield et al. 2012), we wanted to resolve the issue of whether Phacidium and Ceuthospora were really congeneric (Johnston et al. 2014) and fix the application of these names using genetic data, and further to determine the phylogenetic position of Phacidiaceae within Helotiales.
MATERIAL AND METHODS
Isolates
Isolates used in this study were obtained from the culture collection of the CBS-KNAW Fungal Biodiversity Centre (CBS). Utrecht, The Netherlands (Table 1). Colonies were established on Petri dishes containing 2 % malt extract agar (MEA), potato-dextrose agar (PDA), and oatmeal agar (OA) (Crous et al. 2009b), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation.
Table 1.
Collection details and GenBank accession numbers of isolates included in this study.
| Species | Isolate no.1 | Host | Location | Collector | GenBank accession no.2 | ||
|---|---|---|---|---|---|---|---|
| LSU | ITS | RPB2 | |||||
| Allantophomopsiella pseudotsugae | CBS 288.37 | Picea abies | United Kingdom | – | KJ663863 | KJ663824 | KJ663904 |
| CBS 320.53 | Pseudotsuga menziesii | Norway | H. Robak | KJ663864 | KJ663825 | KJ663905 | |
| CBS 321.53 | Picea abies | Norway | H. Robak | KJ663865 | KJ663826 | KJ663906 | |
| CBS 437.71 | Pinus sylvestris | Netherlands | J. Gremmen | KJ663866 | KJ663827 | KJ663907 | |
| CBS 562.63 | Pinus sylvestris | Norway | F. Roll-Hansen | KJ663867 | KJ663828 | KJ663908 | |
| CBS 841.91 | Pinus sp. | Germany | P. Schumacher | KJ663868 | KJ663829 | KJ663909 | |
| Allantophomopsis cytisporea | CBS 262.85 | Roots of Conifer species | Germany | H. Courtois | KJ663869 | KJ663830 | KJ663910 |
| ‘Allantophomopsis’ sp. | CBS 109.22 | Oxycoccus macrocarpos | USA | C.L. Shear | KJ663861 | KJ663822 | KJ663902 |
| Allantophomopsis sp. | CBS 322.36 | Pinus radiata | New Zealand | P.C. Birch | KJ663880 | KJ663839 | KJ663921 |
| Bulgaria inquinans | CBS 118.31 | Forest floor | Germany | BIHM | KJ663870 | KJ663831 | KJ663911 |
| CBS 129.58 | Forest floor | Germany | – | KJ663871 | KJ663832 | KJ663912 | |
| CBS 145.55 | – | Germany | H. Lyr | KJ663872 | KM216394 | KJ663913 | |
| CBS 315.71 | Quercus robur | Switzerland | E. Müller | KJ663873 | KJ663833 | KJ663914 | |
| Coleophoma sp. | CBS 449.70 | Liriodendron tulipifera | Netherlands | H.A. van der Aa | KJ663874 | KJ663834 | KJ663915 |
| Gremmenia infestans | CBS 396.48 | – | Sweden | E. Björkman | KJ663876 | KM216393 | KJ663917 |
| Hyaloscypha sp. | CBS 109453 | Miconia sp. | Venezuela | I. Hernandez | KJ663875 | KJ663835 | KJ663916 |
| Neofabraea sp. | CBS 135481 = CPC 22154 | Polygonatum sp. | Netherlands | U. Damm | KF251745 | KF251242 | KJ663942 |
| Phacidium calderae | CBS 287.72 | Arbutus unedo | Italy | W. Gams & J.A. Stalpers | KJ663878 | KJ663837 | KJ663919 |
| CBS 372.81 | Pistacia terebinthus | Spain | H.A. van der Aa | KJ663879 | KJ663838 | KJ663920 | |
| Phacidium fennicum | CBS 457.83 | Pinus sylvestris | Finland | H. Butin | KJ663881 | KJ663840 | KJ663922 |
| Phacidium lacerum | CBS 130.30 | Pinus sylvestris | Netherlands | J.Y. van Vliet | KJ663882 | KJ663841 | KJ663923 |
| CBS 338.70 | Ilex aquifolium | Netherlands | H.A. van der Aa | KJ663883 | KJ663842 | KJ663924 | |
| CBS 400.81 | Juniperus communis | France | O. Petrini | KJ663884 | KJ663843 | KJ663925 | |
| CBS 540.70 | Pinus sylvestris | Netherlands | H.A. van der Aa | KJ663885 | KJ663844 | KJ663926 | |
| CBS 557.70 | Sciadopitys verticillata | Netherlands | H.A. van der Aa | KJ663886 | KJ663845 | KJ663927 | |
| CBS 761.73 | Pinus sylvestris | France | M. Morelet | KJ663887 | KJ663846 | KJ663928 | |
| Phacidium lauri | CBS 198.68 | Vinca minor | Netherlands | H.A. van der Aa | KJ663890 | KJ663849 | KJ663930 |
| CBS 308.68 | Prunus laurocerasus | Netherlands | H.A. van der Aa | KJ663891 | KJ663850 | KJ663931 | |
| CBS 443.71 | Ilex aquifolium | Netherlands | L. Marvanová | KM216397 | KM216392 | KM216396 | |
| CBS 589.67 | Ilex aquifolium | Netherlands | H.A. van der Aa | KJ663892 | KJ663851 | KJ663932 | |
| Phacidium mollerianum | CBS 365.72 | Eucalyptus sp. | Italy | W. Gams | KJ663888 | KJ663847 | KJ663929 |
| CBS 574.66 | Polygonatum odoratum | Netherlands | H.A. van der Aa | KJ663889 | KJ663848 | KM216395 | |
| Phacidium pseudophacidioides | CBS 497.72 | Chamaespartium sagittale | Switzerland | E. Müller | KJ663893 | KJ663852 | KJ663933 |
| CBS 590.69 | Ilex aquifolium | Netherlands | H.A. van der Aa | KJ663894 | KJ663853 | KJ663934 | |
| Phacidium trichophori | CBS 138246 = CPC 22952 | Trichophorum cespitosum subsp. germanicum | Netherlands | W. Quaedvlieg | KJ663895 | KJ663854 | KJ663935 |
| Phacidium vaccinii | CBS 444.71 | Vaccinium vitis-idaea | Netherlands | L. Marvanová | KJ663896 | KJ663855 | KJ663936 |
| Phlyctema vincetoxici | CBS 123726 | Vincetoxicum officinale | Czech Republic | G. Verkley | KJ663897 | KJ663856 | KJ663937 |
| CBS 123727 | Vincetoxicum officinale | Czech Republic | G. Verkley | KJ663898 | KJ663857 | KJ663938 | |
| CBS 123743 | Vincetoxicum officinale | Czech Republic | G. Verkley | KJ663899 | KJ663858 | KJ663939 | |
| Potebniamyces pyri | CBS 282.55 | Pyrus communis | Netherlands | Zweede | KJ663862 | KJ663823 | KJ663903 |
| CBS 322.63 | Pyrus communis | Netherlands | G.S. Roosje | KJ663900 | KJ663859 | KJ663940 | |
| Pseudophacidium ledi | CBS 377.59 | Picea abies | Switzerland | J. Gremmen | KJ663901 | KJ663860 | KJ663941 |
| Sarcotrochila longispora | CBS 273.74 | Pinus contorta | British Columbia | W.G. Ziller & A. Funk | KJ663877 | KJ663836 | KJ663918 |
1CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS.
2LSU: large subunit (28S) of the nrRNA gene; ITS: internal transcribed spacers and intervening 5.8S nrDNA; RPB2: partial RNA polymerase II second largest subunit gene.
DNA isolation, amplification and analyses
Genomic DNA was extracted from fungal colonies growing on MEA using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Solana Beach, CA) according to the manufacturer’s protocol. The primers LSU1Fd (Crous et al. 2009a) and LR5 (Vilgalys & Hester 1990) were used to amplify the partial 28S rRNA gene (LSU), ITS5 and ITS4 (White et al. 1990) were used to amplify the internal transcribed spacer region (ITS), fRPB2-5F (Liu et al. 1999), and fRPB2-414R (Quaedvlieg et al. 2011) were used to amplify the partial RNA polymerase II second largest subunit locus (RPB2). A basic alignment of the obtained sequence data was first done using MAFFT v. 7 (http://mafft.cbrc.jp/alignment /server/index. html; Katoh et al. 2002) and if necessary, manually improved in BioEdit v. 7.0.5.2 (Hall 1999). To check the congruency of the individual datasets, a 70 % neighbour-joining (NJ) reciprocal bootstrap was performed (Mason-Gamer & Kellogg 1996, Lombard et al. 2010). A Bayesian analysis (critical value for the topological convergence diagnostic set to 0.01) was performed on both the Multi order LSU (Fig. 1) and the concatenated ITS, LSU and RPB2 (Fig. 2) datasets using MrBayes v. 3.2.1 (Huelsenbeck & Ronquist 2001) as described by Crous et al. (2006) using nucleotide substitution models that were selected using the Akaike Information Criterion implemented in MrModeltest v. 2.3 (Nylander 2004). Novel sequences derived from this study were lodged at GenBank, and the alignments and phylogenetic trees in TreeBASE (www.treebase.org/treebase/index.html).
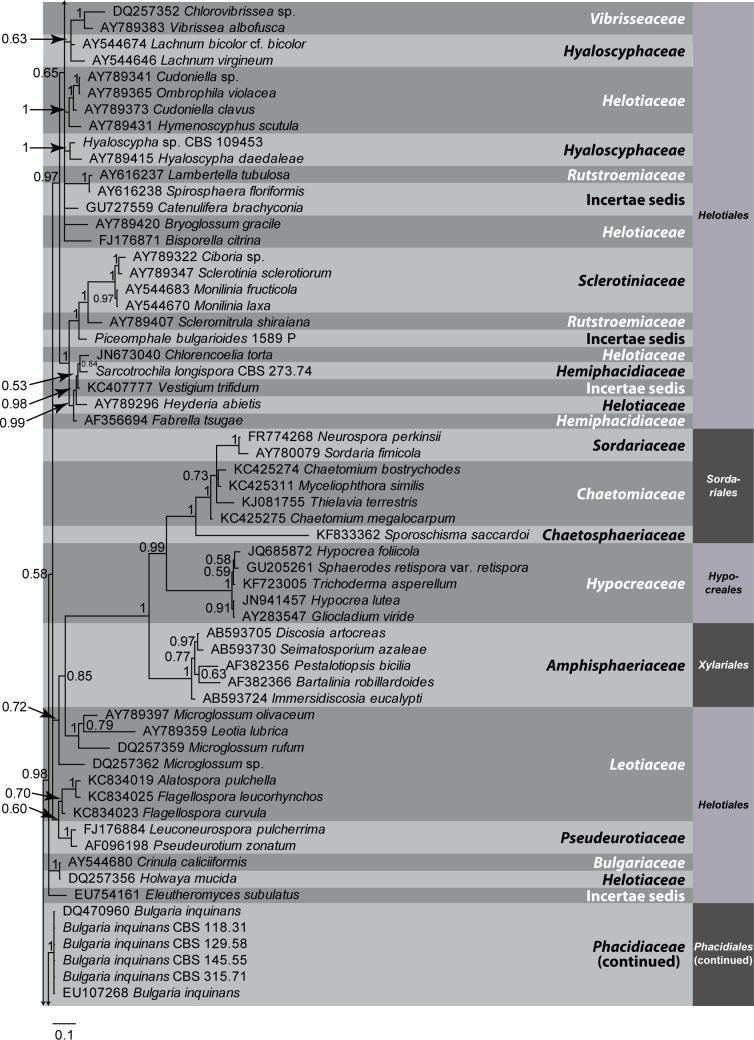
Fig. 1.
A Bayesian 50 % majority rule LSU consensus tree containing representative isolates belonging to Leotiomycetes and related classes. Bayesian posterior probabilities support values for the respective nodes are displayed in the tree. A stop rule (set to 0.01) for the critical value for the topological convergence diagnostic was used for the Bayesian analysis. The tree was rooted to Saccharomyces cerevisiae (GenBank J01355). The scale bar indicates 0.1 expected changes per site
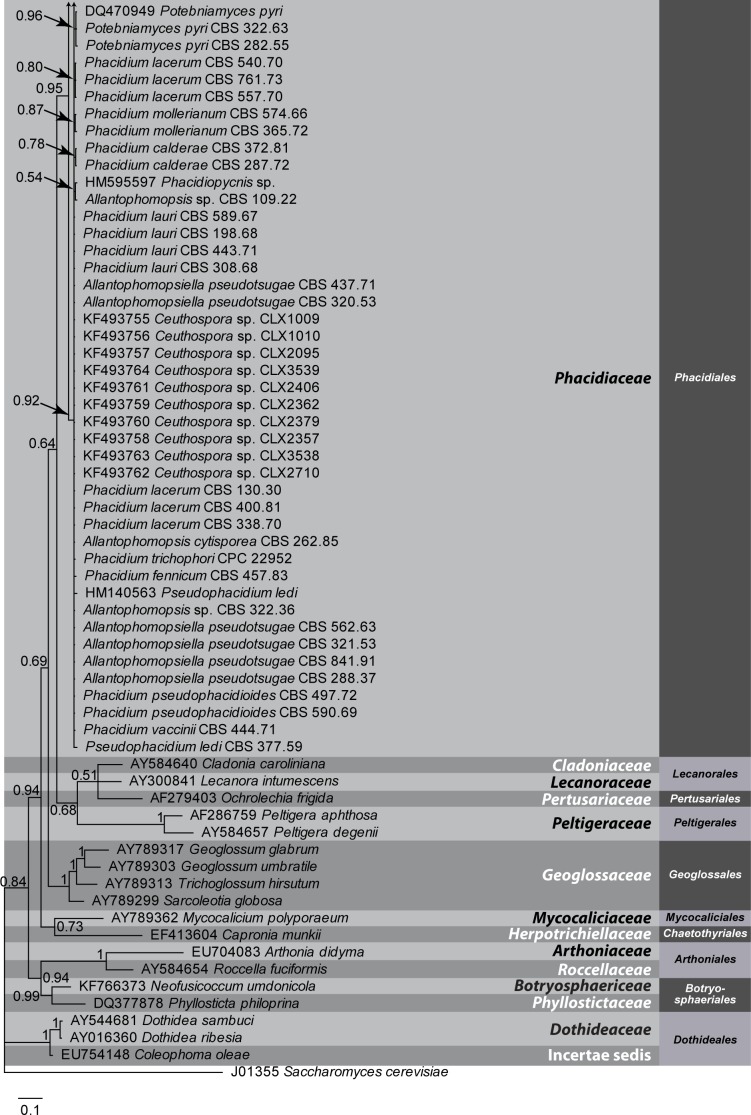
Fig. 2.
A Bayesian 50 % majority rule combined ITS, LSU and RPB2 consensus tree containing representative isolates belonging to the Phacidiales and related orders. Bayesian posterior probabilities support values for the respective nodes are displayed in the tree. A stop rule (set to 0.01) for the critical value for the topological convergence diagnostic was used for the Bayesian analysis. The tree was rooted to Sarcotrochila longispora (CBS 273.74). The scale bar indicates 0.01 expected changes per site
Morphology
Observations were made with a Zeiss V20 Discovery stereo-microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and an AxioCam MRc5 camera and Zen software. Colony characters and pigment production were noted after 2 wk of growth on MEA, PDA and OA incubated at 25 °C. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970). Morphological descriptions were based on cultures sporulating on PDA, and taxonomic novelties and metadata were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
RESULTS
Phylogeny
The ITS, RPB2 and LSU sequence datasets of 43 sequences (including the outgroup) did not show any conflicts in their tree topology for the 70 % reciprocal bootstrap trees, allowing us to combine them in the multigene analyses. For Fig. 1, the LSU overview dataset of 160 sequences (including the outgroup) contained 830 characters, of which 461 contained unique site patterns. For Fig. 2, the LSU dataset contained 819 characters, of which 101 contained unique site patterns, the RPB2 dataset contained 355 characters, of which 155 contained unique site patterns, and the ITS dataset contained 537 characters, of which 203 contained unique site patterns.
For the combined dataset, all data partitions used the GTR model but the LSU partition was analysed with MrBayes using dirichlet (1,1,1,1) state frequency distribution and inverse gamma-shaped rate variation across sites, the ITS partition was analysed using a fixed (equal) state frequency distribution and gamma-shaped rate variation across sites, and the RPB2 partition was analysed using fixed (equal) state frequency distribution and inverse gamma-shaped rate variation across sites. For the LSU overview tree, MrModeltest suggested the same Bayesian parameters as for the LSU partition in the combined dataset. During the generation of Fig. 1, 72 262 trees were generated of which 54 198 (75 %) were sampled for the final tree; for Fig. 2, 2 672 trees were generated of which 2 004 (75 %) were sampled for the final tree.
TAXONOMY
The genus Phacidium represents inoperculate discomycetes characterised by erumpent, cleistohymenial apothecia with a covering layer splitting into teeth or lobes, an externally black stroma with vertically arranged pseudoparenchymatous cells, asci that are clavate, (4–)8-spored, with amyloid dehiscence rings, and aseptate, ellipsoid, hyaline ascospores lacking sheaths or appendages. Paraphyses extend above the asci, frequently anastomose, are embedded in mucilage, and arise from the subhymenium. The Ceuthospora asexual morphs have phialidic conidiogenesis, and subcylindrical, hyaline conidia with funnel-shaped, mucoid apical appendages.
Leotiomycetes, Phacidiales, Phacidiaceae
Phacidiales Höhn., Ber. Deutsch. Bot. Ges. 34: 416 (1917).
Saprobic or plant pathogenic. Ascomata circular, superficial, discoid, or immersed, becoming erumpent, opening by irregular tears in upper layer. Asci clavate, unitunicate with or without apical dehiscence ring. Ascospores aseptate, ellipsoid to subcylindrical or irregularly so, straight to curved, hyaline or brown, without sheath. Paraphyses branched or simple, septate, hyaline, anastomosing, invested in mucilage. Conidiomata uni- to multilocular, single to aggregated. Conidiophores hyaline, smooth, branched, or reduced to conidiogenous cells. Conidiogenous cells phialidic, at times proliferating percurrently, invested in mucilage. Conidia subcylindrical, ellipsoid-oblong or subreniform, aseptate, with or without appendages.
Type family: Phacidiaceae Fr. 1849.
Phacidiaceae Fr., Summa veg. Scand. 2: 367 (1849); as “Phacidicei”.
Synonym: Bulgariaceae Fr., Summa veg. Scand. 2: 357 (1849); as “Bulgariacei”.
Foliicolous, caulicolous, corticicolous, parasitic or saprobic (endophytic). Ascomata apothecial, discoid, or circular, initially immersed, becoming erumpent, opening by irregular tears in upper layer, teeth opening to expose hymenium; wall of textura globulosa to textura angularis; inner layer of smooth-walled, hyaline periphysoids invested in mucilage; basal stroma of textura angularis to textura globulosa. Asci arising from croziers, clavate, (4–)8-spored, unitunicate, with or without amyloid dehiscence ring staining blue in Melzer’s reagent. Ascospores aseptate, ellipsoid, fusoid, subcylindrical or irregularly so, hyaline or brown, with or without germ slit, straight to curved, lacking gelatinous appendages. Paraphyses branched or simple, septate hyaline, anastomosing, invested in mucilage or not. Conidiomata uni- to multilocular, single to aggregated, with one to several ostioles. Walls of textura angularis to textura globulosa. Conidiophores hyaline, smooth, branched, or reduced to conidiogenous cells, arising from inner layer of conidioma. Conidiogenous cells phialidic, at times proliferating percurrently, invested in mucilage. Conidia hyaline, smooth, subcylindrical, ellipsoid-oblong or subreniform, with or without apical, mucoid funnel-shaped appendage.
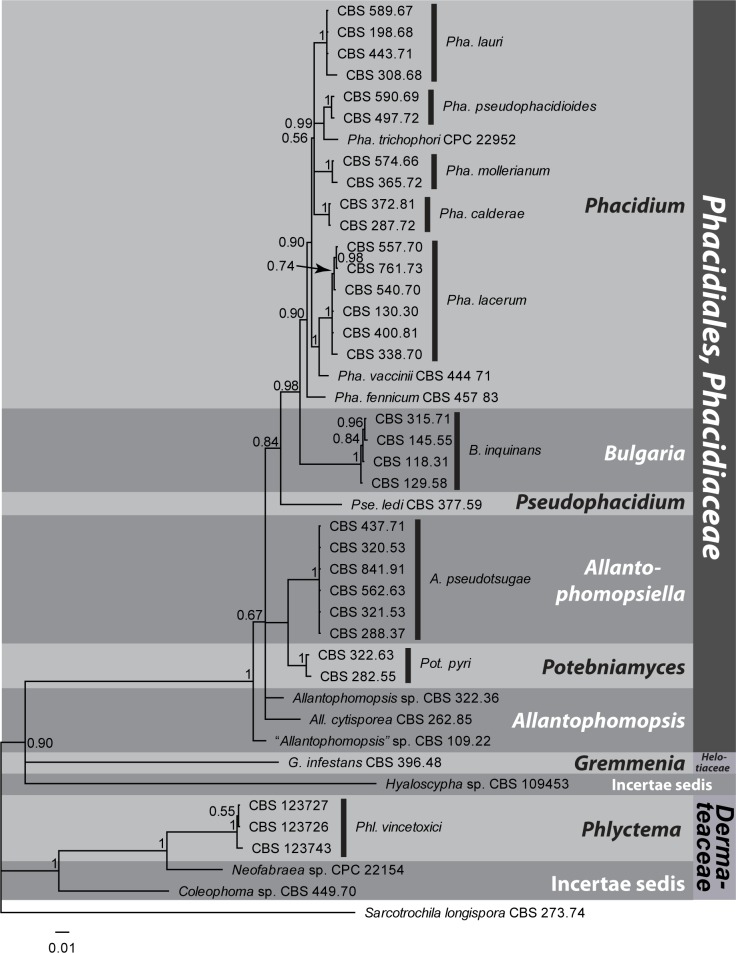
Notes: Bulgaria inquinans (formerly Bulgariaceae) forms a strongly supported monophyletic group with members of the Phacidiaceae, and although its exact placement within this group is unresolved, Bulgariaceae is reduced to synonymy because it is genetically very close to these taxa (Figs 1–2). Holwaya mucida, placed in Bulgariaceae by Lumbsch & Huhndorf (2010) based on the results of Wang (2006b), groups outside Phacidiaceae and does not appear to be closely related (Fig. 1). Morphologically Bulgariaceae resembles the Phacidiaceae in its cleistohymenial development (Bellemère 1968), but is distinguished by having ascomata with a brownish outer exciple, a thick gelatinous medullary exciple, and four of the eight ascospores being dark brown, with a longitudinal germ-slit. Potebniamyces pyri (syn. Phacidiopycnis pyri), which was also placed in Bulgariaceae, clusters in the same clade. The family name Phacidiaceae was published in the same publication as Bulgariaceae, but is chosen here over the latter, as the family name Phacidiaceae is better established in the literature, and has more members.
Type genus: Phacidium Fr. 1815. Other genera accepted in Phacidiaceae based on DNA data include Allantophomopsiella, Allantophomopsis, Bulgaria, Potebniamyces, and Pseudophacidium.
Allantophomopsiella Crous, gen. nov.
MycoBank MB809673
Etymology: Named after its morphological similarity to the genus Allantophomopsis.
Diagnosis: Distinct from Apostrasseria and Allantophomopsis in lacking percurrent proliferation and in having inequilaterally fusiform or naviculate conidia.
Description: Conidiomata pycnidial, immersed, becoming erumpent, irregularly multilocular, dark brown, ostiolate; wall of 3–4 layers of dark brown textura angularis. Conidiophores arising from inner layer of conidioma, at times reduced to conidiogenous cells, branched, septate. Conidiogenous cells integrated or discrete, ampulliform to subcylindrical or lageniform, hyaline, smooth with minute periclinal thickening at apex. Conidia ellipsoid to fusiform, hyaline, smooth, aseptate, guttulate, bearing mucoid apical appendages (Type C sensu Nag Raj 1993), flabelliform to irregular in shape.
Type species: Allantophomopsiella pseudotsugae (M. Wilson) Crous 2014.
Allantophomopsiella pseudotsugae (M. Wilson) Crous, comb. nov.
MycoBank MB809674
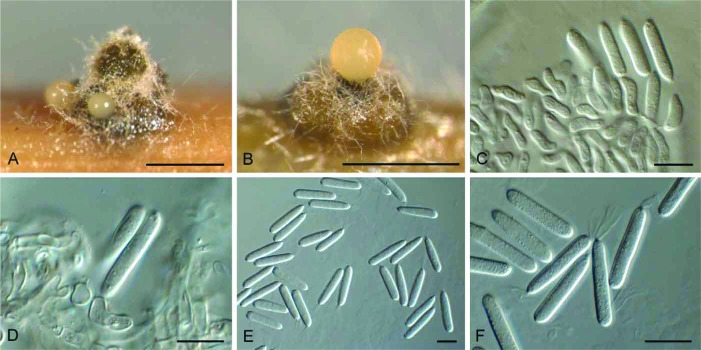
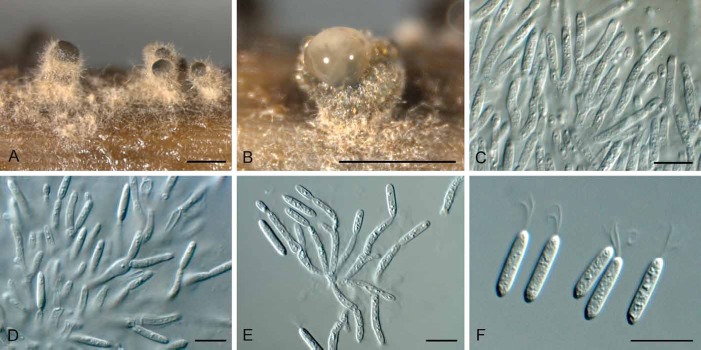
(Fig. 3)
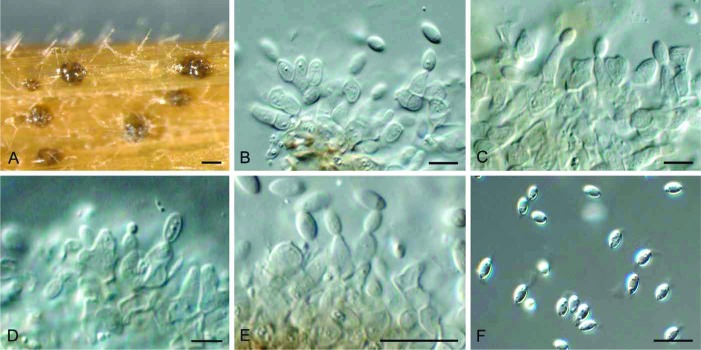
Fig. 3.
Allantophomopsiella pseudotsugae (CBS 841.91). A. Conidiomata forming on autoclaved barley leaves. B–E. Conidiogenous cells giving rise to conidia. F. Conidia. Bars: A = 300 μm, all others = 10 μm
Basionym: Phomopsis pseudotsugae M. Wilson, Trans. R. Scottish Arboricult. Soc. 34(2): 147 (1920).
Synonyms: Allantophomopsis pseudotsugae (M. Wilson) Nag Raj, Coelom. Anam. App. Conidia: 116 (1993).
Phacidiella coniferarum G.G. Hahn, Mycologia 49: 227 (1957).
Phacidium coniferarum (G.G. Hahn) DiCosmo, et al., Canad. J. Bot. 61: 37 (1983).
Additional synonyms are provided in Nag Raj (1993).
Specimens examined: Germany: on Pinus wood, Dec. 1991, P. Schumacher (CBS 841.91). – The Netherlands: Groesbeek, on needles of Pinus sylvestris, Nov. 1970, J. Gremmen (CBS 437.71). – Norway: on needles of Pinus sylvestris, July 1963, F. Roll-Hansen (CBS H-15946, culture CBS 562.63); Førde in Sunnfjord, shoot of Pseudotsuga menziesii, Apr. 1948, H. Robak (CBS 320.53); Gyl in Nordmøre, dead bark of Picea abies, June 1948, H. Robak (CBS 321.53). – UK: near Dumfries, dieback of 30-yr-old Picea abies, Oct. 1937, Peace (CBS 288.37).
Sporulating on PNA: Conidiomata pycnidial, immersed, becoming erumpent, irregularly multilocular, to 600 μm diam, dark brown, ostiolate; wall of 3–4 layers of dark brown textura angularis. Conidiophores arising from inner layer of conidioma, at times reduced to conidiogenous cells, branched, septate, 5–15 × 2.5–3.5 μm. Conidiogenous cells integrated or discrete, ampulliform to subcylindrical or lageniform, hyaline, smooth with minute periclinal thickening at apex, 5–8 × 2.5–3 μm. Conidia (4–)5–6(–7) × (2–)3 μm, ellipsoid to fusiform, hyaline, smooth, aseptate, guttulate, bearing mucoid apical appendages (Type C sensu Nag Raj 1993, only visible in water), flabelliform to irregular in shape.
Culture characteristics: Colonies spreading, flat with sparse aerial mycelium and feathery margins. On PDA surface olivaceous grey, reverse iron-grey. On OA surface olivaceous grey with patches of iron-grey.
Notes: Distinct from Apostrasseria and Allantophomopsis in that it lacks percurrent proliferation on its conidiogenous cells, and has inequilaterally fusiform or naviculate conidia.
Gremmenia: snow-blight pathogens of conifers
Gremmen (1953) described Phragmonaevia gigaspora as a new pathogen associated with needle blight (snow mould disease) of Pinus cembra in Europe. In his revision of Helotiales occurring on conifers, Korf (1962) established the genus Gremmenia to accommodate this pathogen, as it was clearly distinct from Phacidium s. str., lacking a distinct upper stroma in its apothecia. He also disagreed with Petrak (1957), who concluded that P. gigaspora represented old and abnormal material of Phacidium infestans, which occurs on Pinus sylvestris, and its synonym, Phacidium pini-cembrae, which occurs on Pinus cembra. Korf (1962) distinguished these two species based on the fact that Phacidium pini-cembrae frequently has less than eight ascospores in its asci, a feature not observed in P. infestans. In a detailed comparison of the two species made by DiCosmo et al. (1984), G. gigaspora was reduced to synonymy with Phacidium infestans. Phacidium infestans and P. pini-cembrae are morphologically similar, but can be distinguished based on different hosts, as well as ascus and ascospore morphologies (DiCosmo et al. 1984). Ascospores of P. infestans are elongate-ellipsoid, straight, curved, or curved-fusiform, eight per ascus, with apothecia formed on needles of two-needled pines. In contrast, ascospores of P. pini-cembrae are slightly narrower, elongate-ellipsoid, frequently less than eight per ascus, and apothecia occur on needles of five-needled pines. Although DiCosmo et al. (1984) listed Phragmonaevia gigaspora as synonym of Phacidium infestans, this was incorrect, as a Phragmonaevia gigaspora occurs on Pinus cembra, and frequently has less than 8-ascospores (clearly illustrated by Gremmen 1953), and thus is more correctly placed in synonymy with Phacidium pini-cembrae. A third species of this complex is P. abietis, which causes disease on Abies and Pseudotsuga. Although further research is required to define the host range of these species, they are clearly not members of Phacidium s. str. (Fig. 2), and thus the genus Gremmenia is herewith resurrected to accommodate them.
Gremmenia Korf, Mycologia 54: 27 (1962).
Plant pathogenic, foliicolous. Ascomata scattered, gregarious or separate, circular to elliptical in outline, subepidermal, raising host tissue, bursting open via 4–8 teach that curve backwards, exposing the creamy hymenium at maturity; apothecial roof of textura globulosa, brown, appearing like a clypeus when viewed in section when ascomata are immature, becoming hyaline towards interior, with inner layer giving rise to periphysoids, invested in mucilage; subhymenium of hyaline smooth-walled hyphae, forming a textura angularis. Paraphyses simple or branched, septate, hyaline, smooth-walled, invested in mucilage, sometimes slightly swollen at the tip. Asci club-shaped, (1–)8-spored, tapering towards base, stipitate, flattened at apex, giving blue reaction in Melzer’s reagent. Ascospores biseriate, ellipsoid-elongate, straight to curved or reniform, aseptate, finely guttulate, hyaline, smooth.
Type species: Gremmenia gigaspora (Gremmen) Korf 1962 (i.e. G. pini-cembrae (Rehm) Crous 2014).
Gremmenia abietis (Dearn.) Crous, comb. nov.
MycoBank MB809676
Basionym: Phacidium infestans var. abietis Dearn., Mycologia 18: 237 (1926).
Synonym: Phacidium abietis (Dearn.) J. Reid & Cain, Mycologia 54: 482 (1963) [“1962”] (nom. illegit., Art. 53.1; non P. abietis Rabenh. 1844).
Notes: Gremmenia abietis causes snow-blight of Abies spp. and Pseudotsuga menziesii (Dearness 1926, Faull 1930). Bega (1978) reported that the disease could reach epidemic proportions in Idaho and Oregon. No cultures are presently available of this pathogen, and it will have to be recollected to clarify its phylogenetic position.
Gremmenia infestans (P. Karst.) Crous, comb. nov.
MycoBank MB809677
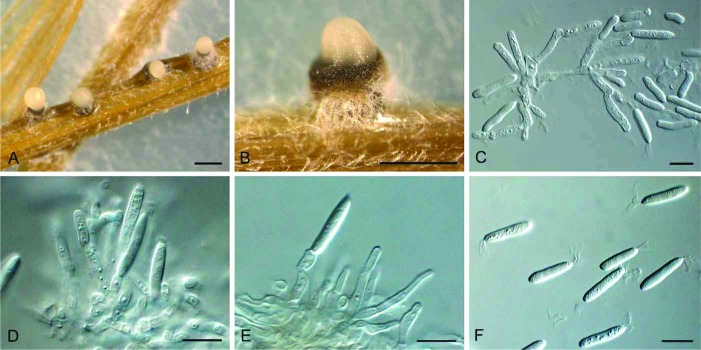
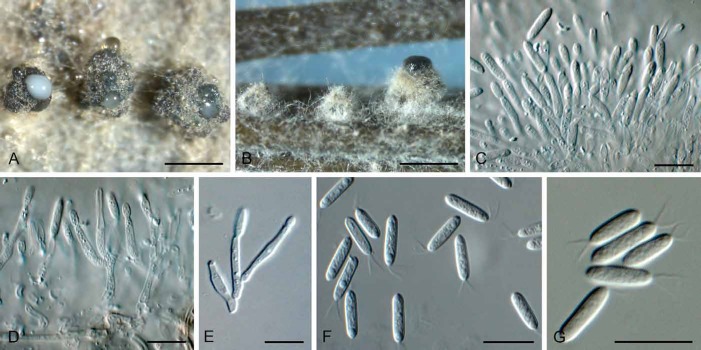
(Fig. 4)
Fig. 4.
Gremmenia infestans (S-F207441). A, B. Ascomata on needles. C, D. Vertical section through ascomata. E–G. Asci and ascospores (F in Meltzer’s solution). H. Ascospores. Bars: A = 1 mm, B = 0.5 mm, all others = 10 μm
Basionym: Phacidium infestans P. Karst., Hedwigia 25: 232 (1886).
Description and illustrations: DiCosmo et al. (1984).
Specimens examined: Sweden, Vindeln, host unknown, 1946, E. Björkman, CBS 396.48; location unknown, on needles of Abies balsamea, Oct 1931, J.H. Faull, CBS 265.31; on needles of A. balsamea, 30 Nov. 1928, J.H. Faull, CBS 264.31; on needles of Picea, 22 Nov. 1928, J.H. Faull, CBS 263.31. Lappland, Jokkmokk, Kassavare, on dead needles of Pinus sylvestris, still attached to a young tree, 31 Aug. 2011, K. Hansen & I. Olariaga (S).
Notes: The isolates listed here show variation in the DNA sequence data, suggesting that they could represent more than one taxon. As the cultures proved to be sterile, fresh collections and epitypification is required to settle the application of the name.
Gremmenia pini-cembrae (Rehm) Crous, comb. nov.
MycoBank MB809678
Basionym: Phacidium lacerum f. pini-cembrae Rehm, Ber. Bayer. Bot. Ges. 13: 124 (1912).
Synonyms: Phacidium pini-cembrae (Rehm) Terrier, Beitr. Kryptog-fl. Schweiz 9: 73 (1942).
Phragmonaevia gigaspora Gremmen, Sydowia 7: 141 (1953).
Gremmenia gigaspora (Gremmen) Korf, Mycologia 54: 27 (1962).
Description and illustrations: DiCosmo et al. (1984).
Phacidium Fr., Observ. mycol. 1: 167 (1815); nom. cons.
Synonyms: Phacidiostroma Höhn., Ber. dt. bot. Ges. 35: 420 (1917).
Ceuthospora Grev., Scot. Crypt. Flora 5: 253 (1827); nom. cons.
Foliicolous or caulicolous. Ascomata amphigenous, scattered or gregarious, circular, immersed, becoming erumpent, rupturing host tissue by irregular stellate splits, of dark brown pseudoparenchymatal cells of textura globulosa, inner layer with periphysoids, invested in mucilage. Hymenium of asci and paraphyses; basal stroma present or absent. Asci clavate, (4–)8-spored, with amyloid (staining blue in Melzer’s reagent) apical discharge mechanism. Ascospores aseptate, ellipsoid to ellipsoid-fusoid, uni- to biseriate, hyaline, smooth, lacking mucoid appendages. Paraphyses septate, hyaline, smooth, branched, anastomosing, invested in mucilage. Conidiomata pycnidial, immersed, becoming erumpent, uni- to multilocular, brown, with ostiole; wall of textura angularis to textura globulosa. Conidiophores branched or simple, septate, hyaline, smooth, invested in mucilage. Conidiogenous cells phialidic, at times proliferating percurrently, subcylindrical to ampulliform, smooth, hyaline, invested in mucilage. Conidia subcylindrical, aseptate, hyaline, smooth, with irregular funnel-shaped apical mucilaginous appendage.
Type species: Phacidium lacerum Fr. 1818, typ. cons.
Phacidium calderae (Urries) Crous, comb. nov.
MycoBank MB809679
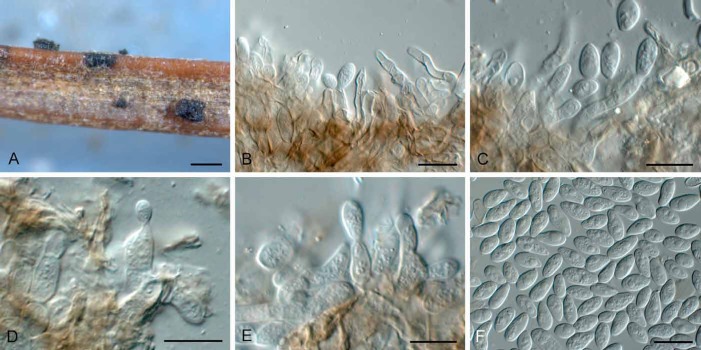
(Fig. 5)
Fig. 5.
Phacidium calderae (CBS 372.81). A, B. Conidiomata. C, D. Conidiogenous cells. E, F. Conidia. Bars: A, B = 200 μm, all others = 10 μm
Basionym: Ceuthospora calderae Urries, An. Inst. bot. A.J. Cavanilles 14: 165 (1956) [“1955”].
Description: Conidiomata multilocular, with large central ostiole, papillate. Conidiophores frequently reduced to conidiogenous cells, branched, 1–3-septate, up to 30 μm long, 3–4 μm diam. Conidiogenous cells hyaline, smooth, terminal and subterminal, 5–15 × 2.5–3.5 μm, proliferating with periclinal thickening (characteristic of this species). Conidia subcylindrical, smooth, granular, hyaline, (17–)18–20(–22) × 3.5(–4) μm, with a flared, funnel-shaped apical mucoid appendage.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and feathery margins. On PDA and OA surface and reverse olivaceous grey.
Specimens examined: Italy: Sardinia: Tacco di Sta Barbara, fallen leaf of Arbutus unedo, 10 May 1971, W. Gams & J.A. Stalpers (CBS 287.72). – Spain: Ibiza: Cala Llonga, fallen leaves of Pistacia terebinthus, 17 Apr. 1981, H.A. van der Aa (CBS H-10269, culture CBS 372.81).
Notes: Ceuthospora calderae was originally described from leaves of Pistacia lentiscus, collected from the Canary Islands. Culture CBS 372.81 closely matches the morphology of this species; CBS 287.72 was now sterile.
Phacidium fennicum Butin, Sydowia 37: 21 (1984).
Description and illustration: Butin (1984).
Specimen examined: Finland: Lempäälä Kulju, on needles of Pinus sylvestris, 18 Apr. 1982, U. Söderholm (CBS 457.83 – ex-type culture).
Phacidium lacerum Fr., Observ. mycol. 2: 312 (1818) : Fr., Syst. mycol. 2: 575 (1823).
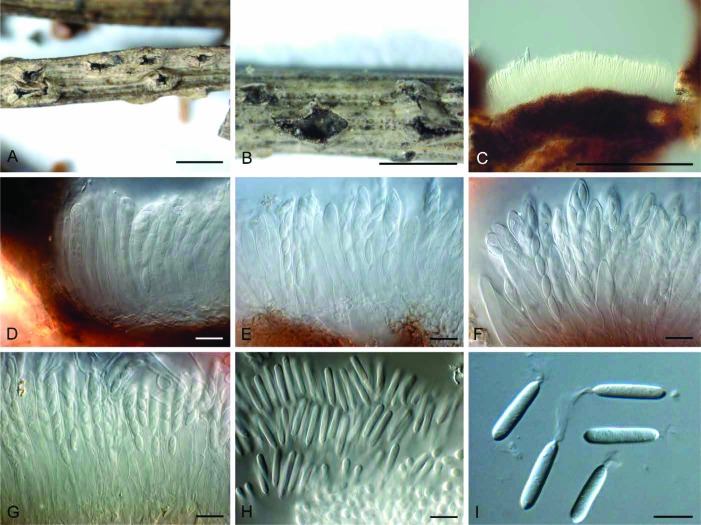
Fig. 6.
Phacidium lacerum (CBS 761.73). A, B. Conidiomata on autoclaved barley leaves. C–E. Conidiogenous cells. F. Conidia. Bars: A, B = 300 μm, all others = 10 μm
Fig. 7.
Phacidium lacerum (K(M) 189269). A, B. Ascomata on needles. C–E. Vertical section through ascomata. F, G. Asci and ascospores. H, I. Conidia. Bars: A, B = 1000 μm, all others = 10 μm
Synonyms: Dothidea pinastri Fr., Elench. fung. 2: 123 (1828).
Ceuthospora pinastri (Fr.) Höhn., Mitt. bot. Inst. tech. Hochsch. Wien 2(4): 104 (1925).
Additional synonyms are included in Nag Raj (1993).
Description: Foliicolous or on cone scales. Ascomata amphigenous, circular, 300–2000 μm diam, aggregated to solitary, initially immersed, subepidermal, becoming erumpent, opening by 3–5 teeth of brown textura globulosa, to expose hymenium; inner layer of periphysoids, covered in mucilage. Asci clavate, 8-spored, with amyloid (straining blue in Melzer’s reagent) discharge mechanism, 70–110 × 7–10 μm. Ascospores aseptate, hyaline, smooth, guttulate, biseriate, 9.5–12.5 × 3–3.5 μm. Paraphyses simple, septate, hyaline, smooth, anastomosing, invested in mucilage. Conidiomata pycnidial, flask-shaped to irregularly subglobose, amphigenous, scattered, gregarious, to aggregated, subepidermal, splitting the epidermis, multilocular, 500–1500 μm diam, brown from surface view, with individual locules up to 170 μm diam, with individual ostioles up to 25 μm diam. Condiophores simple or branched, septate, often reduced to phialidic conidiogenous cells, lining the inner layer of conidioma, hyaline, smooth-walled, (5–)7–15 × 2–3(–4) μm, invested in mucilage; proliferating percurrently, or with visible periclinal thickening. Conidia aseptate, subcylindrical, base bluntly rounded with central, flattened scar, apex with funnel-shaped mucilaginous appendage, (10–)13–15(–18) × (2.5–) 3(–4) μm.
Culture characteristics: Colonies spreading with sparse to moderate aerial mycelium. On OA surface pale olivaceous grey. On PDA surface olivaceous grey, reverse iron-grey.
Specimens examined: France: Mt. Ventoux, living needle of Juniperus communis, May 1981, O. Petrini (CBS 400.81); Hagenau, Bas Rhin, on needles of Pinus sylvestris, 26 Aug. 1968, M. Morelet (CBS H-10302 – neotype for Phacidium lacerum designated here, MBT178726; CBS 761.73 – ex-neotype culture). – Germany: Baden, Bastadt, on pine needles, Apr. 1877, Schroeter [ex herbs Thuemen & Grove] (K(M) 189268); Königstein, on needles of P. sylvestris, 30 Apr. 1887, W. Krieger [Fungi Saxonici exs. 290] (K(M) 189269). – The Netherlands: on needle of Pinus sylvestris, Feb. 1930, J.Y. van Vliet (CBS 130.30); Baarn, Cantonspark, on leaves of Sciadopitys verticillata, 27 Apr. 1970, H.A. van der Aa (CBS 557.70); Baarn, Maarschalksbos, on leaves of Ilex aquifolium, July 1969, H.A. van der Aa (CBS H-10292, culture CBS 338.70); Hulshorsterzand, on needles of Pinus sylvestris, 12 Apr. 1970, H.A. van der Aa (CBS 540.70). – Sweden: on needles of Pinus sylvestris, ex-herb Fries (UPS).
Notes: Phacidium lacerum was originally described from European collections on needles from Pinus sylvestris, and is widely distributed throughout Europe, where it occurs commonly on this host. The material in UPS referred to as the “original collection” by (DiCosmo et al. 1984) in UPS was stated to be depauperate. In the sanctioning work, Fries (1823) refers to “(Exs. ined.)” and later “S.S.” (Fries 1849) implying that he had intended to distribute material in his exsiccate Scleromycetes Suecici. This was not, however, done and the name is listed as “material unknown” by Holm & Nannfeldt (1962: 38). The UPS specimen is unlocalised and has no date, and appears to have been collected after Fries came to Uppsala in 1835 according to Stefan Ekman (pers. comm.) (Fig. 8). That specimen is not, therefore, the “original collection” Fries had before him in 1818 or 1823 and so a neotype is designated for Fries’ name here.
Fig. 8.

Phacidium lacerum (UPS – Fries’ specimen). Photo: Stefan Ekman
Phacidium lauri (Sow.) Crous & D. Hawksw., comb. nov.
MycoBank MB810293
Basionym: Sphaeria lauri Sow., Col. Fig. Br. Fungi 3: sine pagin. [53] (18031).
Synonyms: Cryptosphaeria lauri (Sow.) Grev., Fl. Edin.: 361 (1824).
Ceuthospora lauri (Sow.) Grev., Scott. Crypt. Fl. 5: 254 (1826) : Fr., Syst. mycol., Index: 167 (1832).
Xyloma multivalve DC., Fl. franç., 3rd edn 2: 303 (1805).
Phacidium multivalve (DC.) J.C. Schmidt, Mykol. Heft. 1: 42 (1817) : Fr., Syst. mycol. 2: 576 (1823).
Ceuthospora phacidioides Grev., Scott. Crypt. Fl. 5: 253 (1826); nom. illegit. (Art. 52,1).
Additional synonyms are listed in Nag Raj (1993).
Descriptions and illustrations: Sutton (1972, a sexual morph), DiCosmo et al. (1984; sexual and asexual morph) and Nag Raj (1993; asexual morph only).
Specimens examined: The Netherlands: Baarn, Drakenburgerweg, on Ilex aquifolium, 8 Oct. 1967, H.A. van der Aa (CBS 589.67, as C. phacidioides); Baarn, Cantonspark, on leaves of Prunus laurocerasus, 2 Mar. 1968, H.A. van der Aa (CBS H-10276 – epitype of Sphaeria lauri designated here, MBT178727; CBS 308.68 – culture ex-epitype); Wageningen, on leaves of Ilex aquifolium, May 1969, L. Marvanová (CBS 443.71, as C. phacidioides); Baarn, Eemnesserweg 90, on leaves of Vinca minor, 3 Mar. 1968, H.A. van der Aa (CBS H-10271, culture CBS 198.68, as C. feurichii). – UK: sine loc., on dead leaves of Prunus laurocerasus, G.B.W. Kirby (K – holotype of Sphaeria lauri [not traced]; K (M) IMI 153020 – slides ex holotype).
Notes: Conidia of the present strains (12–)13–15(–16) × 3(–3.5) μm fall into the variation of C. lauri (7–17 × 2–3 μm, Nag Raj 1993), which has several synonyms, including C. phacidioides (Nag Raj 1993). Furthermore, DiCosmo et al. (1984) link C. phacidioides (conidia 10–17 × 2.5–3 μm) to Phacidium multivalve as sexual morph. Ceuthospora lauri, which is the type species of the genus Ceuthospora (on Prunus laurocerasus, Europe), and C. phacidioides (Ilex aquifolium, Europe), have been commonly confused in the past, but appear to be synonymous based on the cultures we investigated in this study. Di Cosmo et al. (1984) designated a lectotype for Xyloma multivalve in G but did not provide more details. However, we have been unable to locate the original material of all these synonyms (also see Sutton 1980, Nag Raj 1993), and suspect that they have been lost. The names Ceuthospora lauri and Phacidium multivalve were both sanctioned by Fries, and as the earliest species epithet which is legitimate under the ICN is Sphaeria lauri, that name has priority. Ceuthospora lauri also appears more commonly used in literature than Phacidium multivalve. Note that the name Ceuthospora phacidioides is illegitimate as Xyloma multivalve was listed as a synonym when it was introduced; it is therefore a superfluous name.
The choice of the epithet “lauri” by Sowerby is explained by Grove (1935: 291–292): “Owing to the custom, in Britain, of speaking of Prunus Laurocerasus as ‘the Laurel’, great confusion has arisen. It has frequently been erroneously regarded as the true Bay Laurel (Laurus nobilis), and its leaves are still sometimes used by our cooks as such.” He also confirmed that Greville’s specimens were on the Prunus and not Laurus, contrary to his publications, which led to that error being perpetuated although the misidentification was soon recognized, for example, by Berkeley (1836: 283) who gave it the English name “Cherry-laurel Ceuthospora” and stated “On dead leaves of Prunus Lauro-cerasus (not Laurus nobilis as is stated by Dr. Greville)”.
Sowerby (1803) referred to a single unlocalized, but presumably English (as his work was devoted to fungi from that country) collection made by the Rev. William Kirby (1759–1850) when introducing the name Sphaeria lauri (Fig. 9); Kirby was born in and died in Suffolk, England. The original material was located in K by Sutton (1972) who prepared sections (now preserved as K(M) IMI 153020; Fig 10) and published drawings made from Kirby’s specimen (Sutton 1972: 323 fig. 1). That specimen could not now be re-located in K, despite extensive searches by Begoña Aguirre-Hudson, Heidi Döring, and D.L.H. Sutton did, however, include a sketch of the material he saw which evidently comprised two specimens, the right-hand one of which had Kirby’s name below it and is therefore the holotype of Sowerby’s name.
Fig. 9.

Sowerby’s original diagnosis (A) and illustrations (B) of Sphaeria lauri. From Sowerby (1803)
Fig. 10.
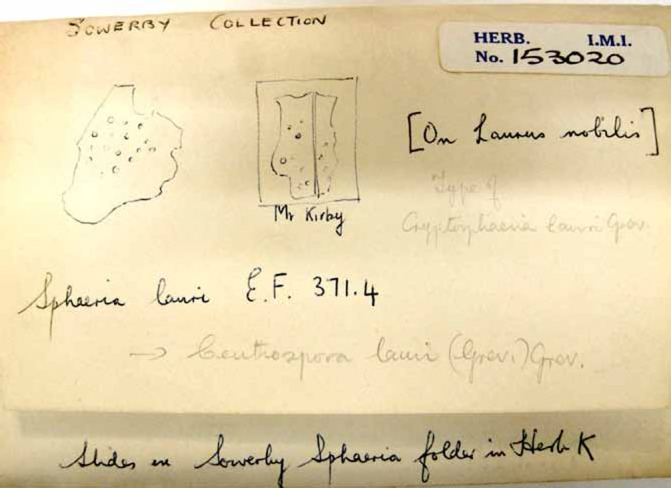
Sphaeria lauri packet with slides made Brian Sutton from Sowerby’s holotype (K(M) IMI 153020)
Sutton (1972, 1980) attributed the epithet “lauri” to Greville and not Sowerby, in accordance with the Code then in operation which ruled that names of “Fungi caeteri” published before 1 January 1821 were not validly published; that situation changed with the deletion of the later starting point dates for fungi at the Sydney IBC in 1981. In using the term “lectotype”, Sutton meant as the type of Ceuthospora lauri “Grev.”, and that is clear from his annotation on the packet with the slides (Fig. 10) and not Sowerby’s original binomial. The use of that term was correct for Greville’s name, under the pre-1981 Code, as Greville had studied collections other than Sowerby’s.
Phacidium mollerianum (Thüm.) Crous, comb. nov.
MycoBank MB809680
(Fig. 11)
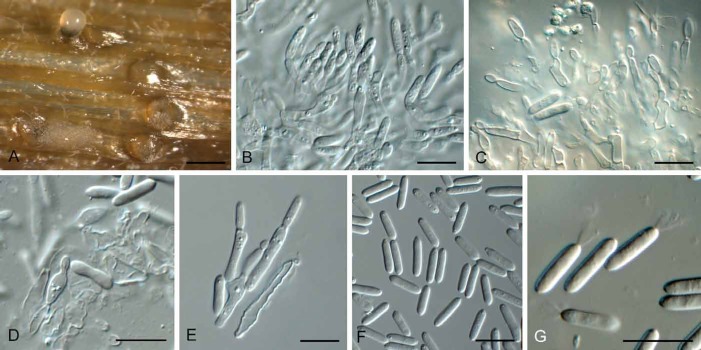
Fig. 11.
Phacidium molleriana (CBS 574.66). A. Conidiomata on autoclaved barley leaves. B–E. Conidiogenous cells. F, G. Conidia. Bars: A = 300 μm, all others = 10 μm
Basionym: Sphaeropsis molleriana Thüm., Inst. Coimbra 27: 40 (1879).
Synonyms: Phoma molleriana (Thüm.) Sacc., Syll. fung. 3: 110 (1884).
Macrophoma molleriana (Thüm.) Berl. & Voglino, Syll. fung., Addit. I: 314 (1886).
Ceuthospora molleriana (Thüm.) Petr., Annls mycol. 23: 29 (1925).
Description: Conidiomata uni- to multilocular, up to 400 μm diam. Conidiophores branched, up to 45 μm long, 2–3 μm diam. Conidiogenous cells terminal and lateral, 5–10 × 2–3 μm, with prominent periclinal thickening, rarely proliferating percurrently. Conidia hyaline, smooth, granular, subcylindrical, (9–)10–12(–13) × (2–)2.5 μm; apical mucoid appendage only visible when mounted in water.
Culture characteristics: Colonies spreading with sparse aerial mycelium and feathery margins. On PDA and OA surface and reverse olivaceous grey.
Cultures examined: Italy: Sardinia: Su Cologone, on leaves of Eucalyptus, 6 May 1971, W. Gams (CBS H-10285, 10286; CBS 365.72 – culture). – The Netherlands: Baarn, leaf spot on Polygonatum odoratum, 8 Aug. 1966, H.A. van der Aa (CBS 574.66).
Notes: Ceuthospora molleriana was originally described from Eucalyptus leaves collected in Portugal (conidia 10–13 × 2–2.5 μm, Petrak 1925), and closely fits the morphology observed in the present collections. Although originally described from Eucalyptus, this taxon appears to have a wider host range.
Phacidium pseudophacidioides Crous, sp. nov.
MycoBank MB809681
(Fig. 12)
Fig. 12.
Phacidium pseudophacidioides (CBS 590.69). A, B. Conidiomata on autoclaved barley leaves. C–E. Conidiophores, conidiogenous cells and conidia. F. Conidia. Bars: A = 300 μm, B = 400 μm, all others = 10 μm
Etymology: Named after its morphological similarity to the asexual morph, Ceuthospora phacidioides.
Diagnosis: Conidiogenous cells discrete or integrated, subcylindrical with perominent periclinal thickening or percurrent proliferation, 7–19 × 2–2.5 μm. Conidia hyaline, smooth, granular, subcylindrical, tapering at ends, bearing a funnel-shaped mucoid apical appendage, (11–)11.5–12.5(–13) × (2–)2.5 μm.
Type: The Netherlands: Baarn, Eenmnesserweg 92, on Ilex aquifolium, 8 Aug. 1968, H.A. van der Aa (CBS H-10289 – holotype; CBS 590.69 – ex-type culture).
Description: Conidiomata stromatic, pycnidioid, scattered, black, subepidermal, multiloculate, up to 600 μm diam, with papillate ostioles. Conidiophores arising from inner layers of cavity, subcylindrical, hyaline, smooth, extensively branched, up to 120 μm long, 2.5–3 μm diam, invested in mucus. Conidiogenous cells discrete or integrated, subcylindrical with perominent periclinal thickening or percurrent proliferation, hyaline, smooth, 7–19 × 2–2.5 μm. Conidia hyaline, smooth, granular, subcylindrical, tapering at ends, apex subobtuse, base with truncate hilum, 1 μm diam, bearing a funnel-shaped mucoid apical appendage, (11–)11.5–12.5(–13) × (2–)2.5 μm.
Other specimen examined: Switzerland: Zürich: Weiacherberg, on dead leaf of Chamaespartium sagittale, 3 May 1952, E. Müller (CBS 497.72).
Notes: The two isolates of C. pseudophacidioides were originally identified as C. phacidioides (i.e. P. lauri). However, P. lauri has larger conidia, and the conidiophores are much more extensively branched than in P. pseudophacidioides.
Phacidium trichophori Crous & Quaedvlieg, sp. nov.
MycoBank MB809682
(Fig. 13)
Fig. 13.
Phacidium trichophori (CPC 22952). A, B. Conidiomata in culture. C–E. Conidiophores, conidiogenous cells and conidia. F, G. Conidia. Bars: A, B = 300 μm, all others = 10 μm
Etymology: Named after the host genus on which it was collected, Trichophorum.
Diagnosis: Conidiogenous cells discrete or integrated, subcylindrical with prominent periclinal thickening, 5–12 × 2–3 μm. Conidia hyaline, smooth, granular, subcylindrical, tapering towards a truncate basal scar, 0.5–1 μm diam, bearing a funnel-shaped mucoid apical appendage, 3–5 μm long, 2–4 μm diam at apex, (9–)10–11(–13) × (2–)2.5(–3) μm.
Type: The Netherlands: Korenburgerveen, Winterswijk, on Trichophorum cespitosum subsp. germanicum, 29 Apr. 2013, W. Quaedvlieg (CBS H-21816 – holotype; CBS 138246 = CPC 22952 – ex-type culture).
Description: Foliicolous. Conidiomata pseudostromatic, pycnidioid, scattered to gregarious, black, subepidermal, uniloculate, up to 300 μm diam; walls of the pseudostroma 40–90 μm thick. Conidiophores arising from inner layers of cavity, subcylindrical, hyaline, smooth, thin-walled, branched, to 30 μm long, invested in mucus. Conidiogenous cells discrete or integrated, subcylindrical with prominent periclinal thickening, hyaline, smooth, 5–12 × 2–3 μm. Conidia hyaline, smooth, granular, subcylindrical, tapering towards a truncate basal scar, 0.5–1 μm diam, apex subobtuse, bearing a funnel-shaped mucoid apical appendage, 3–5 μm long, 2–4 μm diam at apex, (9–)10–11(–13) × (2–)2.5(–3) μm.
Notes: Phacidium trichophori resembles P. lauri (conidia (12–)13–15(–16) × 3(–3.5) μm), but is morphologically distinct in having smaller conidia. Ceuthospora gaeumannii (conidia 8–11 × 2–2.5 μm, av. 9 × 2.2 μm; Nag Raj 1993) has slightly smaller conidia, much larger conidiomata (500–1500 μm diam).
Phacidium vaccinii Fr., Syst. Mycol. 2(2): 575 (1823).
Synonyms are listed in DiCosmo et al. (1984).
Culture examined: The Netherlands, Loenen, on leaves of Vaccinium vitis-idaea, May 1969, L. Marvanová (CBS 444.71).
Note: Unfortunately this culture proved to be sterile, and thus its morphology could not be confirmed.
Pseudophacidium P. Karst., Acta Soc. Fauna Fl.fenn. 2: 157 (1885) [“1881–1885”].
Synonym: Myxofusicoccum Died., Annls mycol. 19: 68 (1912).
Caulicolous. Ascomata scattered to gregarious, immersed, raising the host epidermis, causing it to rupture; ascomata oblong, hemispherical to irregularly pulvinate, black, carbonaceous; wall of brown textura globulosa, becoming pale brown towards interior; inner layers of subglobose, hyaline cells, invested in mucilage; subhymenium of hyaline textura intricata; basal stroma of pale brown pseudoparenchymatal cells. Asci club-shaped, 8-spored flattened at apex which does not strain in Melzer’s reagent. Ascospores irregularly biseriate, oblong-ellipsoidal, straight to curved, hyaline, smooth-walled. Conidiomata brown, pycnidioid, stromatic, gregarious to crowded, immersed, discoid to orbicular, opening by means of irregular ruptures, multilocular; wall of brown textura globulosa; basal stroma of pale brown textura epidermoidea. Conidiogenous cells phialidic, lining inner cavity, lageniform to ampulliform, hyaline, smooth-walled; proliferating percurrently at apex. Conidia ellipsoid to oblong or subreniform, apex rounded, base with truncate scar, guttulate, hyaline, smooth-walled.
Type species: Pseudophacidium ledi (Alb. & Schwein.) P. Karst. 1885.
Pseudophacidium ledi (Alb. & Schwein.) P. Karst., Acta Soc. Fauna Flora fenn. 2: 157 (1885) [“1881–1885”].
(Fig. 14)
Fig. 14.
Pseudophacidium ledi (CBS 377.59). A. Conidiomata forming on PNA. B–E. Conidiogenous cells giving rise to conidia. F. Conidia. Bars: A = 500 μm, all others = 10 μm
Basionym: Xyloma ledi Alb. & Schwein., Consp. fung.: 60 (1805)l
Description: Sporulating on PNA. Conidiomata stromatic, scattered, erumpent, irregular to pulvinate, brown, opening by irregular rupture, multilocular, up to 500 μm diam. Conidiophores arising from inner cavity, hyaline, subcylindrical, branched or not, 1–3-septate, to 30 μm long, 3–4μm diam, or reduced to condiogenous cells. Conidiogenous cells subcylindrical or ampulliform, phialidic, hyaline, smooth, 5–10 × 3–4 μm, proliferating 1–2 times percurrently near apex. Conidia solitary, ellipsoid to oblong, hyaline, guttulate, thin-walled, mostly widest in upper third, apex subobuse, base truncate, 1 μm diam, (8–)9–11(–12) × (4–)5(–6) μm.
Specimen examined: Switzerland, Graubünden, Bergün, on Picea abies, Apr. 1959, J. Gremmen (CBS 377.59).
Notes: Species of Pseudophacidium are immersed discomycetes that occur on bark of hard- or softwood. Ascomata open by means of an irregular rupture of the covering layer, exposing cream to greyish or brownish discs. The genus contains saprobic, and plant pathogenic species (Smerlis 1969).
Potebniamyces Smerlis, Canad. J. Bot. 40: 352 (1962).
Caulicolous and corticolous. Ascomata scattered to gregarious, initially immersed, becoming erumpent, irregular in outline, opening by irregular rupture to expose hymenium; apical roof of brown textura globulosa, becoming paler towards inner region, which consists of subcylindrical, branched, septate, hyaline periphysoids, invested in mucilage; hymenium red-brown in water, turning blue-green in 2 % KOH. Paraphyses numerous, subcylindrical, branched or septate, hyaline, smooth-walled. Asci club-shaped, 8-spored, with apex not staining in Meltzer’s reagent. Ascospores uni- to biseriate, subglobose to ellipsoid, aseptate, guttulate, hyaline to pale yellow, smooth-walled. Conidiomata scattered to aggregated, immersed, subepidermal, depressed globose, papillate, non-ostiolate, multilocular, brown, opening by irregular rupture; wall of brown textura globulosa to textura epidermoidea above, pale brown below, with wall of yellow-brown textura prismatica. Conidiogenous cells phialidic, lining the inner cavity, subcylindrical, lageniform to ampulliform, straight to curved, hyaline, smooth-walled. Conidia subglobose to obovoid, guttulate, aseptate, base truncate, hyaline to pale yellow, smooth-walled (adapted from Sutton 1980, and DiCosmo et al. 1984).
Type species: Potebniamyces pyri (Berk. & Broome) Dennis 1978.
Potebniamyces pyri (Berk. & Broome) Dennis, Brit. Ascom., 2nd edn: 231 (1978).
Basionym: Stictis lecanora var. pyri Berk. & Broome, Ann. Mag. nat. Hist., ser. 4 17: 144 (1876).
Specimen examined: The Netherlands: Wilhelminadorp, bark of Pyrus communis, Mar. 1963, G.S. Roosje,(CBS 322.63); Wageningen, on peduncle of P. communis, Mar. 1955, Zweede (CBS 282.55).
Notes: Although we assume that Phacidiopycnis is the asexual morph of Potebniamyces, the type species of Phacidiopycnis, P. malorum, still needs to be recollected to confirm this assumption. For this reason the synonymy between these two generic names remains unconfirmed. If shown to be synonymous, the older name, Phacidiopycnis, will have preference for the holomorph (Johnston et al. 2014).
1The text to illustrate the plates in this work are not numbered, and the diagnosis for this fungus appears on the 53rd; the illustration is on pl. 371 fig. 4 (Fig. 9), which has no diagnosis or name, and is dated “1802”, but it seems unlikely that the text was issued before the whole volume was completed in 1803.
DISCUSSION
The primary aim of this study was to obtain molecular support for the assumption that Ceuthospora and Phacidium are congeneric, and at the same time elucidate the position of Phacidiaceae within Leotiomycetes, as the latter family and order were not represented in the phylogenetic study of Wang et al. (2006b).
The order Helotiales contains members that represent a broad range of ecologies, including species that are plant pathogenic, saprobic, endophytic, mycorrhizal or ectomycorrhizal parasites, aquatic saprobes, or wood rotting fungi (Wang et al. 2006a). Although lacking many genera of Helotiales in their phylogenetic analysis, Wang et al. (2006b) concluded that the concept of Helotiales adopted by Eriksson (2005) included many non-monophyletic taxa. They placed 13 families in the order. In spite of earlier work on the class (Gernandt et al. 2001, Lutzoni et al. 2004, Wang et al. 2005), no members of Phacidium were present in the phylogeny of Wang et al. (2006b). By adding Phacidiaceae to Helotiales data present in GenBank, Phacidiales clearly separated from Helotiales, which still appear heterogeneous (Fig. 1). The separation of Phacidiales from Helotiales is also supported by the phylogeny of Wang et al. (2006b), as Bulgaria inquinans and Phacidiophicnes pyri cluster outside of the Helotiales clade in their study.
A surprising result of our study concerns the phylogenetic position of Bulgariaceae. The genus Bulgaria, based on B. inquinans, effectively clusters in Phacidiaceae (based on P. lacerum). A second species has been reported, B. nana (Döring & Triebel 1998, Wang 2006b), but that is considered a species of Austrocenangium (see Gamundi 1997). Bulgaria inquinans is characterised by large (up to 4 cm), dark brown to black turbinate, gelatinous apothecia with brown ascospores. It occurs on bark of fallen trunks and branches, but has also been observed on living trees. The species is considered saprobic, and possibly facultatively plant pathogenic. The relationship of Bulgaria has been debatable. It has been placed close to other species producing gelatinous apothecia in Leotiaceae/Helotiaceae, e.g. Leotia, Neobulgaria, Ombrophila (in Leotieae, Korf 1973; in Ombrophiloideae, Dennis 1978), or considered to be rather distinct, and placed as the sole genus in Bulgariaceae (Helotiales) (e.g. Eriksson 2006). Although some molecular phylogenetic studies have not been able to place Bulgaria in a larger group with support, and instead suggest species such as Leotia, Neobulgaria and Ombrophila (Helotiaceae) are not closely related (Wang 2006a, b), Lantz et al. (2011) placed Bulgaria in a strongly supported clade with Phacidium and Potebniamyces. The relationship between Bulgaria and Phacidium was also confirmed by Hustad & Miller (2011). The ultrastructure of the ascus apical apparatus of Bulgaria, however, is unique compared to other members of Helotiales (Verkley 1992, 1994). For the present, we treat Bulgariaceae as a synonym of Phacidiaceae, but it could be that the family is still heterogeneous, and as more members of Phacidiaceae and Helotiaceae get added in future molecular studies, the Phacidiaceae may yet prove to be paraphyletic.
In our results, the genus Allantophomopsis (based on A. cytisporea), clusters sister to the canker pathogen Potebniamyces pyri (asexual morph: Phacidiopycnis pyri). Potebniamyces produces black, gelatinous discs, erumpent from submerged stromata in tree bark. Although DiCosmo et al. (1984) considered this genus a possible member of Phacidiaceae, Rhytismataceae or Dermateaceae, it appears to be a member of an independent family. As the majority of the families in Helotiales still lack molecular data, we consider it premature to introduce any new family name here to accommodate Potebniamyces. DiCosmo et al. (1984) discussed the morphological differences between Potebniamyces and Phacidium, and Wang et al. (2006b) suggested a possible sister relationship between the two genera.
In the present study we introduce the generic name Allantophomopsiella to accommodate A. pseudotsugae, a pathogen of conifers (Roll Hansen 1992). The genus Gremmenia is resurrected to accommodate the snow-blight pathogens of conifers, namely G. abietis (on Abies spp. and Pseudotsuga menziesii), G. infestans (on Abies balsamea), and G. pini-cembrae (on Pinus cembra).
Finally, the position of the Phacidiaceae is clarified within the resurrected Phacidiales, with respectively a neotype and an epitype designated to fix the phylogenetic application of the names Phacidium lacerum (type of Phacidium) and Sphaeria lauri (type of Ceuthospora) (i.e. Phacidium lauri). Phacidium (1815) is accepted as the correct name for this genus over Ceuthospora (1827), based on nomenclatural priority, as both names are conserved; it also has a greater number of species.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), Mieke Starink-Willemse (DNA isolation, amplification and sequencing), and Emma Hultén (macroscopic photographs of Gremmenia infestans) for their invaluable assistance. In addition, Stefan Ekman searched for material of Phacidium lacerum in UPS and provided information on the single Friesian specimen there, and Begoña Aguirre-Hudson and Heidi Döring kindly gave of their time in quests to re-locate the Sowerby material of Sphaeria lauri in K. We are also thankful for the review and subsequent intense discussions with H.O. Baral, who brought many aspects to our attention, and provided a better insight into this exciting group of fungi. This work was carried out while D.L.H. was in receipt of a grant from the Ministerio de Economía y Competitividad of Spain (project CGL2011-25003).
REFERENCES
- Arx JA von, Müller E. (1954) Die Gattungen der amerosporen Pyrenomyceten. Beiträge zur Kryptogamen flora der Schweiz 11(1): 1–434. [Google Scholar]
- Bega RV. (1978) Disease of Pacific Coast Conifers. [US Department of Agriculture Handbook No. 521.] US Government Printing Office, Washington, DC: USDA. [Google Scholar]
- Bellemère A. (1968) Contribution a l’étude du dévelopment de l’apothécie chez les discomycètes inoperculés I. Bulletin trimestriel de la Société Mycologique de France 83: 393–640. [Google Scholar]
- Berkeley MJ. (1836) Fungi. [The English Flora by Sir James Edward Smith (Hooker WJ), Vol. 5 (2).] London: Longman; et al. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al. (2009a) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009b) Fungal Biodiversity. [CBS Laboratory Manual Series 1.] Utrecht: CBS-KNAW Fungal Biodiversity Centre. [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ. (2006) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearness J. (1926) New and noteworthy fungi-IV. Mycologia 18: 236–255. [Google Scholar]
- Dennis RWG. (1978) British Ascomycetes. 2nd edn Vaduz: J. Cramer. [Google Scholar]
- DiCosmo F, Nag Raj TR, Kendrick WB. (1984) A revision of the Phacidiaceae and related anamorphs. Mycotaxon 21:1–234. [Google Scholar]
- Döring H, Triebel D. (1998) Phylogenetic relationships of Bulgaria inferred by 18S rDNA sequence analysis. Cryptogamie, Bryologie, Lichénologie 19: 123–136. [Google Scholar]
- Eriksson OE. (ed.) (2005) Notes on ascomycete systematics. Nos. 3912–4284. Myconet 11: 1–51. [Google Scholar]
- Eriksson OE. (ed.) (2006) Outline of Ascomycota – 2006. Myconet 12: 1–82. [Google Scholar]
- Faull JH. (1930) The spread and control of Phacidium blight in spruce plantations. Journal of the Arnold Arboretum 11: 136–147. [Google Scholar]
- Fries EM. (1815) Observationes Mycologicae. Vol 2 Copenhagen: G.Bonnier. [Google Scholar]
- Fries EM. (1823) Systema Mycologicum. Vol. 2 Greifswald: E.Mauritius. [Google Scholar]
- Fries EM. (1825) Systema Orbis Vegetabilis. Vol. 1 Lund: Typographis Academica. [Google Scholar]
- Fries EM. (1832) Systema Mycologicum. Vol. 3 Greifswald: E.Mauritius. [Google Scholar]
- Fries EM. (1849) Summa Vegetabilum Scandinaviae. Vol. 2 Uppsala: Typographia Academica. [Google Scholar]
- Gamundi IJ. (1997) Austrocenangium gen. nov. from southern South America. Mycotaxon 63: 261–268. [Google Scholar]
- Gernandt DS, Platt JL, Stone JK, Spatafora JW, Holst-Jensen A, Hamelin RC, Kohn LM. (2001). Phylogenetics of Helotiales and Rhytismatales based on partial small subunit nuclear ribosomal DNA sequences. Mycologia 93: 915–933. [Google Scholar]
- Gremmen J. (1953) Some noteworthy discomycetous fungi on coniferous hosts. Sydowia 7: 141–145. [Google Scholar]
- Greville RK. (1828) Scottish Cryptogamic Flora. Vol. 6 Edinburgh: MacLachlan & Stewart. [Google Scholar]
- Greuter W, Burdet HM, Chaloner WG, Demoulin V, Grolle R , et al. (eds) (1988) International Code of Botanical Nomenclature adopted by the Fourteenth International Botanical Congress, Berlin, July-August, 1987. [Regnum Vegetabile No. 118.] Königstein: Koeltz Scientific Books. [Google Scholar]
- Grove WB. (1935) British Stem- and Leaf-Fungi (Coleomycetes). Vol. 1 Cambridge: Cambridge University Press. [Google Scholar]
- Hall TA. (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hawksworth DL. (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus 2: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011) The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Sherwood MA.(1981) Proposals for nomina conservanda and rejicienda for ascomycete names (lichenized and non-lichenized). Taxon 30: 338–348. [Google Scholar]
- Holm L, Nannfeldt JA. (1962) Fries’s “Scleromyceti Sueciae”: a study on its editorial history with an annotated check-list. Friesia 7: 10–59. [Google Scholar]
- Höhnel F von. (1917) System der Phacidiales v. H. Berichte der Deutschen Botanischen Gesellschaft 34: 416–422. [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hustad VP, Miller AN. (2011) Phylogenetic placement of four genera within the Leotiomycetes (Ascomycota). North American Fungi 6: 1–13. [Google Scholar]
- Johnston P, Seifert KA, Stone J, Rossman AY, Marvanová L. (2014) Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus 5: 91–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten PA. (1871) Mycologia Fennica 1: Discomycetes. Bidrag till Kännedom om Finlands Natur och Folk 19: 1–263. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf RP. (1962) A synopsis of the Hemiphacidiaceae, a family of the Helotiales (Discomycetes) causing needle-blights of conifers. Mycologia 54: 12–23. [Google Scholar]
- Korf RP. (1973) Discomycetes and Tuberales. In: The Fungi: an advanced treatise. Vol. 4A. A Taxonomic Review with Keys (Ainsworth GC, Sparrow FK, Sussman AS, eds): 249–319 New York: Academic Press. [Google Scholar]
- Kreisel H. (1969) Grundzuege eines natuerlichen Systems der Pilze. Jena: Gustav Fischer Verlag. [Google Scholar]
- Lanier L, Joly P, Bondoux P, Bellemere A. (1978) Mycologie et Pathologie Forestière. Vol. 1 Mycologie Forestière. Paris: Masson. [Google Scholar]
- Lantz H, Johnston PR, Park D, Minter DW. (2011) Molecular phylogeny reveals a core clade of Rhytismatales. Mycologia 103: 57–74. [DOI] [PubMed] [Google Scholar]
- Liu Y, Whelen S, Hall B. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ. (2010) Phylogeny and systematics of the genus Calonectria. Studies in Mycology 66: 31–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, et al. (2004) Where are we in assembling the fungal tree of life, classifying the fungi, and understanding the evolution of their subcellular traits. American Journal of Botany 91: 1446–1480. [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, et al. (eds) (2012) International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. [Regnum Vegetabile No. 154.] Königstein: Koeltz Scientific Books. [Google Scholar]
- Mason-Gamer RJ, Kellogg EA. (1996) Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545. [Google Scholar]
- Nag Raj TR. (1993) Coelomycetous Anamorphs with Appendage-bearing Conidia. Waterloo, ON: Mycologue Publications. [Google Scholar]
- Nannfeldt JA. (1932) Studien über die Morphologie und Systematik der nicht- lichenisierten inoperculaten Discomyceten. Nova Acta Regiae Societatis Scientiarum Upsaliensis, ser. 4 8(2): 1–368. [Google Scholar]
- Nylander JAA. (2004) MrModeltest v2. [Program distributed by the author.] Uppsala: Evolutionary Biology Centre. [Google Scholar]
- Petrak F. (1925) Mykologische Notizen. VIII. Annales Mycologici 23: 1–143 [Google Scholar]
- Petrak F. (1957) Mykologische Bemerkungen. Sydowia 11: 337–353. [Google Scholar]
- Quaedvlieg W, Kema GHJ, Groenewald JZ, Verkley GJM, Seifbarghi S , et al(2011) Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Reid J, Cain RF. (1962) Studies on the organisms associated with “snow-blight” of conifers in North America. II. Some species of the genera Phacidium, Lophophacidium, Sarcotrochila, and Hemiphacidium. Mycologia 54: 481–497. [Google Scholar]
- Roll-Hansen F. (1992) Important pathogenic fungi on conifers in Iceland. Acta Botanica Islandica 11: 9–12. [Google Scholar]
- Shoemaker RA. (1961) Pyrenophora phaeocomes (Reb. ex Fr.) Fr. Canadian Journal of Botany 39: 901–908. [Google Scholar]
- Smerlis E. (1969) Pathogenicity of Pseudophacidium piceae and Pseudophacidium ledi. Plant Disease Reporter 53: 982–983. [Google Scholar]
- Sowerby J. (1801–03) Coloured Figures of English Fungi or Mushrooms. Vol. 3 London: R. Wilks. [Google Scholar]
- Stafleu FA, Demoulin V, Greuter W, Hiepko P, Linczevski IA, et al. (eds) (1978) International Code of Botanical Nomenclature adopted by the Twelfth International Botanical Congress, Leningrad, July 1975. [Regnum Vegetabile No. 97.] Utrecht: Bohn, Scheltema & Holkema. [Google Scholar]
- Sutton BC. (1972) Nomenclature of Ceuthospora, Pyrenophora and Blennoria (Fungi). Taxon 21: 319–326. [Google Scholar]
- Sutton BC. (1980) The Coelomycetes: Fungi imperfecti with pycnidia, acervuli, and stromata. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Terrier CA. (1942) Essai sur la systématíque des Phacidiaceae (Fr.) sensu Nannfeldt (1932). Matériaux Flore Cryptogamique Suise 9(2): 1–99. [Google Scholar]
- Tulasne LR, Tulasne C. (1861–1865) Selecta Fungorum Carpologia. 3 vols Paris: Imperila. [Google Scholar]
- Verkley GJM. (1992) Ultrastructure of the ascus apical apparatus in Ombrophila violacea, Neobulgaria pura and Bulgaria inquinans (Leotiales). Persoonia 15: 3–22. [Google Scholar]
- Verkley GJM. (1994) Ultrastructure of the ascus apical apparatus in Leotia lubrica and some Geoglossaceae (Leotiales, Ascomycotina). Persoonia 15: 405–430. [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Binder M, Schoch CL, Johnston PR, Spatafora JW, Hibbett DS. (2006a) Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Molecular Phylogenetics and Evolution 41: 295–312. [DOI] [PubMed] [Google Scholar]
- Wang Z, Johnston PR, Takamatsu S, Spatafora JW, Hibbett DS. (2006b) Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98: 1065–1075. [DOI] [PubMed] [Google Scholar]
- Wang Z, Binder M, Hibbett DS. (2005) Life history and systematics of the aquatic discomycetes Mitrula (Helotiales, Ascocmycota) based on cultural, morphological, and molecular studies. American Journal of Botany 92: 1565–1574. [DOI] [PubMed] [Google Scholar]
- Wingfield MJ, Beer ZW de, Slippers B, Wingfield BD, Groenewald JZ, et al (2012) One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology 13: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor SB. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds): 315–322 San Diego: Academic Press. [Google Scholar]