Abstract
Species of Sphaerellopsis (sexual morph Eudarluca) are well-known cosmopolitan mycoparasites occurring on a wide range of rusts. Although their potential role as biocontrol agents has received some attention, the molecular phylogeny of the genus has never been resolved. Based on morphology and DNA sequence data of the large subunit nuclear ribosomal RNA gene (LSU, 28S) and the internal transcribed spacers (ITS) and 5.8S rRNA gene of the nrDNA operon, the genus Sphaerellopsis is shown to belong to Leptosphaeriaceae in Dothideomycetes. Sphaerellopsis is circumscribed, and the sexually typified generic name Eudarluca treated as a synonym on the basis that Sphaerellopsis is more commonly used in literature, is the older generic name, and is the morph commonly encountered by plant pathologists in the field. A neotype is designated for Sphaerellopsis filum, and two new species are introduced, S. macroconidialis and S. paraphysata spp. nov. Species previously incorrectly placed in Sphaerellopsis are allocated to Neosphaerellopsis gen. nov. as N. thailandica, and to the genus Acrocalymma, as A. fici. The genus Rhizopycnis is nestled among species of Acrocalymma, and reduced to synonymy based on its morphology and DNA phylogeny, while Acrocalymmaceae is introduced as novel family to accommodate members of this genus in the Dothideomycetes. Furthermore, Sphaerellopsis proved to be phylogenetically closely allied to a lichenicolous complex of phoma-like taxa, for which the new genera Diederichomyces and Xenophoma are established. Several new combinations are introduced, namely D. xanthomendozae, D. ficuzzae, D. caloplacae, D. cladoniicola, D. foliaceiphila, and X. puncteliae combs. nov, while Paraphaeosphaeria parmeliae sp. nov. is newly described.
Keywords: Ascomycota, Dothideomycetes, Eudarluca, Fungiculous fungi, ITS, LSU, Pleosporales, Rust fungi, systematics
INTRODUCTION
Sphaerellopsis filum (Dothideomycetes, Pleosporales, Leptosphaeriaceae) and its purported sexual morph Eudarluca caricis is a well-known cosmopolitan mycoparasite occurring on a wide range of rust species. The species has been commonly recorded in North and South America, Europe, and Asia. Most records are as S. filum, as E. caricis is not so commonly observed (Yuan et al. 1998). Given the wide host range, S. filum is thought to be a common rust mycoparasite, and its potential role as biocontrol agent has received some attention (Kuhlman et al. 1978, Whelan et al. 1997, Pei et al. 2003, Nischwitz et al. 2005). Little is known, however, about the ecology, and genetic diversity within the taxon.
Sphaeria filum was originally described from rust species on Convolvulus sepium and Populus nigra in Sicily (Bivona-Bernadi 1813–16). Fries (1823) transferred S. filum to Phoma, while Castagne (1851) established the genus Darluca based on Darluca vagans, treating Sphaeria filum as a synonym. Eriksson (1966) pointed out, however, that even though synonymous, the species epithet “filum” had priority over “vagans”.
Spegazzini (1908) established the genus Eudarluca for an ascomycete associated with uredinia of a rust on Canna sp. in Brazil, assuming it to be the sexual morph of Darluca. Keener (1951) proved the connection between Eudarluca and Darluca, which was confirmed via culture studies (Yuan et al. 1998). Eriksson (1966) introduced the combination Eudarluca caricis for the sexual morph of D. filum, while Sutton (1977) relocated D. filum to the genus Sphaerellopsis. Since this time the application of the names have proven stable, with Sphaerellopsis filum being reported on close to 369 species and 30 genera of rusts in more than 50 countries (Kranz & Brandenburger 1981).
Eriksson (1967) observed Eudarluca caricis to occur as a mycoparasite on Puccinia spp. occurring on Poaceae in Sweden, and also to appear pathogenic to the grass species themselves, though this has not been tested experimentally. Eriksson (1966) provided an overview of the taxonomy, nomenclature, and distribution of E. caricis. He also located some material in Herb. Fries (UPS), consisting of three leaf fragments of a Carex sp., containing asci and ascospores matching that of E. australis, a synonym of E. caricis.
The abolishment of dual nomenclature in July 2011 (Hawksworth 2011, Hawksworth et al. 2011, McNeill et al. 2012, Wingfield et al. 2012), means that the generic name Sphaerellopsis 1883 has priority over Eudarluca 1908. Sphaerellopsis presently has two acknowledged species (Nag Raj 1993), while Eudarluca has eight, though the genus is in need of revision. Because Sphaerellopsis is more commonly used in literature, is the older generic name, and is the morph commonly encountered by plant pathologists in the field, we propose to retain only Sphaerellopsis on the list of protected generic names (Kirk et al. 2013), and reduce Eudarluca to synonymy. This listing will avoid the necessity of making a formal separate proposal to retain Eudarluca and awaiting its rejection before taking up Sphaerellopsis as required under the current Art. 57.6 of the ICN (McNeill et al. 2012), which is in any case to be proposed for deletion (Hawksworth 2014).
Liesebach & Zaspel (2004) compared 77 isolates of S. filum isolated from Populus spp., Parthenium hysterophorus and Bellis perennis in Europe. Based on ITS sequence data, they revealed two main clades, with each containing further subclades, suggesting as many as five species to be present in their samples, and also showing that different Sphaerellopsis species could occur on the same rust samples. They refrained from naming any new taxa, however, and referred all isolates to S. filum.
By conducting inoculation experiments with S. filum isolates obtained from various rust and host species, Nischwitz et al. (2005) were able to demonstrate a strong level of host specificity. Furthermore, in their phylogenetic analysis, isolates grouped in four separate clades, again suggesting several species to be present, with isolates from grass hosts clustering separately to those obtained from poplar. Based on the morphological continuum observed among isolates, however, Nischwitz et al. (2005) also refrained from naming any new species.
The aim of the present paper was thus to conduct a DNA phylogenetic study of the S. filum isolates available to us from the CBS-KNAW Fungal Biodiversity Centre (CBS) culture collection (Utrecht, The Netherlands), supplemented with fresh collections from Brazil, South Africa, Thailand, and The Netherlands. A further aim was also to delineate Sphaerellopsis from genera that are phylogenetically closely related, or morphologically similar.
MATERIAL AND METHODS
Isolates
Fresh collections were made from rust sori on diverse hosts. Single conidial colonies were established from sporulating conidiomata on Petri dishes containing 2 % malt extract agar (MEA; Crous et al. 2009). Additional strains were obtained from the culture collection of the CBS. Colonies were subcultured onto potato-dextrose agar (PDA), oatmeal agar (OA) (Crous et al. 2009), MEA, and pine needle agar (PNA) (Smith et al. 1996), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Voucher strains were deposited in CBS.
DNA isolation, amplification and analyses
Genomic DNA was extracted from fungal colonies growing on MEA using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Solana Beach, CA) according to the manufacturer’s protocol. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify the nuclear rDNA operon spanning the 3’ end of the 18S rRNA gene, the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region and the 5’ end of the 28S rRNA gene. The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009a) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. Part of the translation elongation factor 1-alpha (TEF-1α) was amplified and sequenced using primers EF1-728F (Carbone & Kohn 1999) and EF-2 (O’Donnell et al. 1998), while T1 (O’Donnell & Cigelnik 1997) and Bt-2b (Glass & Donaldson 1995) were used for the beta-tubulin (TUB) gene region. Amplification conditions for ITS, LSU and TEF-1α followed Crous et al. (2013) and for TUB, Lee et al. (2004). Megablast searches (Altschul et al. 1997) using the ITS and LSU sequences were performed in NCBI’s GenBank nucleotide sequence database to identify the closest matching sequences, which were added to the sequence alignment. The sequence alignment and subsequent phylogenetic analyses for all the above were carried out using the methods in Crous et al. (2006). Sequences derived in this study were lodged at GenBank, the alignments and trees in TreeBASE (www.treebase.org/treebase/index.html), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Morphology
Observations were made with a Zeiss V20 Discovery stereo-microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and an AxioCam MRc5 camera and software. Measurements and photographs were made from structures mounted in clear lactic acid. The 95 % confidence intervals were derived from 30 observations (× 1000), with the extremes given in parentheses. Ranges of the dimensions of other characters are given. Colony characters and pigment production were noted after 2 wk of growth on different media incubated at 25 ºC. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970). Morphological descriptions were based on cultures sporulating on MEA.
RESULTS
Phylogeny
Four phylogenies were generated; the first is based on LSU sequences and was used to determine the familial relationships of the studied species (Fig. 1), the second is based on an ITS alignment of stagonospora- and phoma-like isolates (Fig. 2), the third is based on an ITS alignment of Acrocalymma and related species (Fig. 3), and the final tree on an ITS alignment of Sphaerellopsis isolates (Fig. 4). The ITS alignments were split to facilitate more robust multiple alignments of the included sequences rather than having an ambiguous alignment containing all of the ITS sequences in a single analysis. The TEF-1α and TUB sequences (Table 1) confirmed the ITS results and were therefore not subjected to a separate phylogenetic analysis.
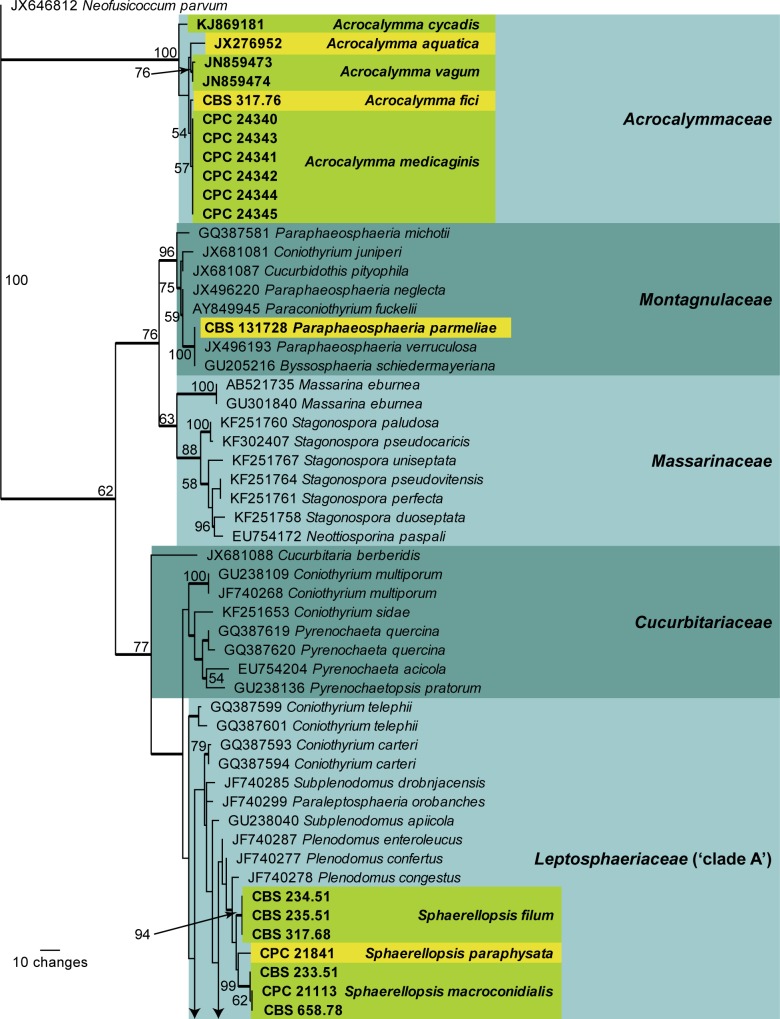
Fig. 1.
The first of 1 000 equally most parsimonious trees (TL = 610; CI = 0.430; RI = 0.848; RC = 0.364) resulting from a parsimony analysis of the LSU sequence alignment. The bootstrap support values from 1 000 replicates are indicated at the nodes and the scale bar represents the number of changes. Thickened branches reflect those branches present in the strict consensus tree. Family names based on literature are indicated to the right of the tree in darker and lighter blocks. Species of interest are shown in bold text and are highlighted in the yellow and green blocks. The tree was rooted to Neofusicoccum parvum (GenBank JX646812)
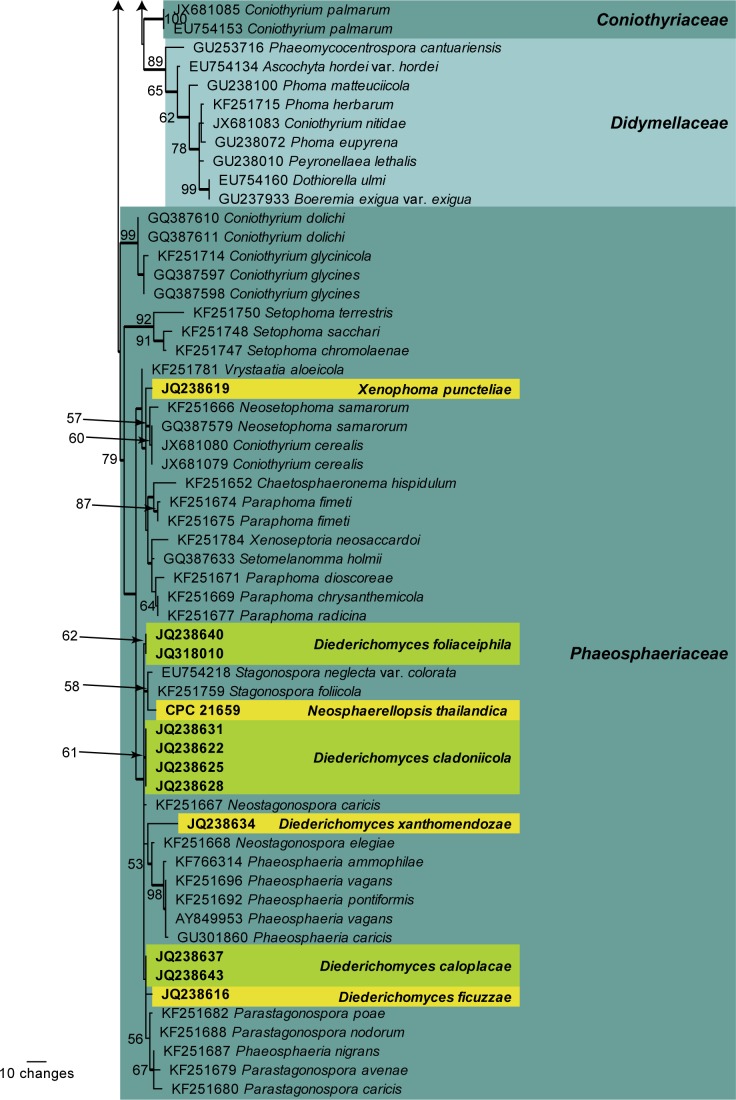
Fig. 2.
The first of 60 equally most parsimonious trees (TL = 1090; CI = 0.517; RI = 0.707; RC = 0.365) resulting from a parsimony analysis of the ITS alignment representing stagonospora-like and phoma-like genera. The bootstrap support values from 1 000 replicates are indicated at the nodes and the scale bar represents the number of changes. Thickened branches reflect those branches present in the strict consensus tree. Different genera are highlighted in the yellow and green blocks, with the genera of interest shown in bold text. The tree was rooted to Alternaria consortialis (GenBank KC584247)
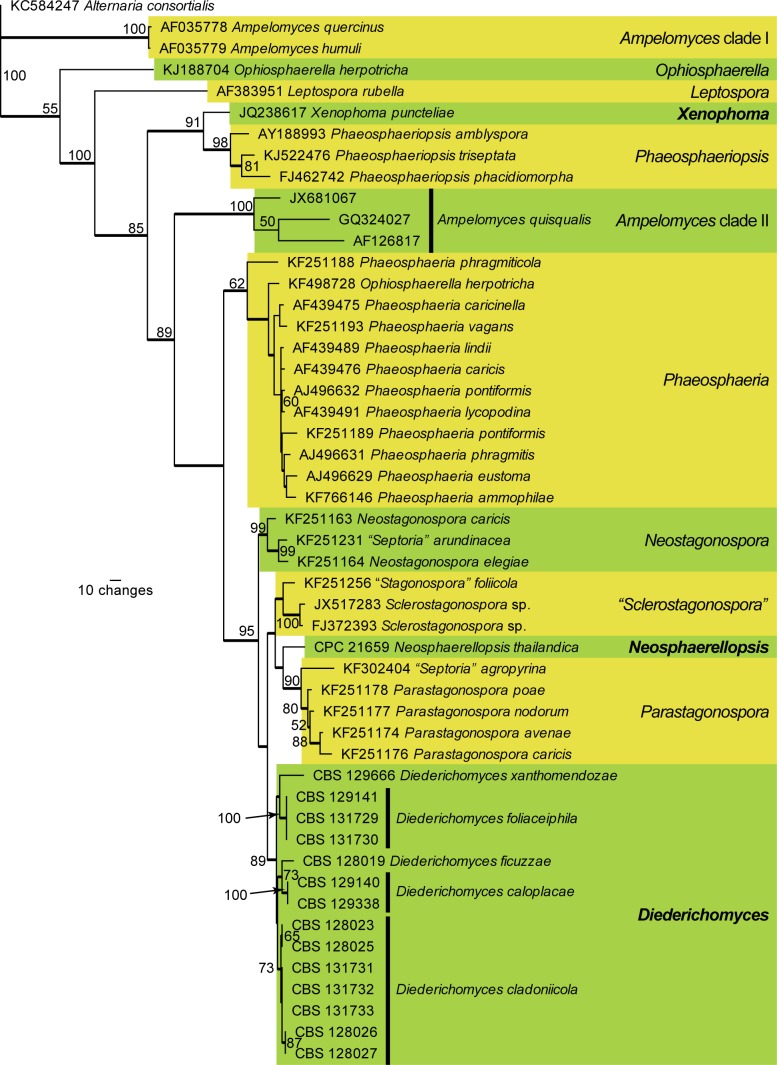
Fig. 3.
The first of two equally most parsimonious trees (TL = 404; CI = 0.735; RI = 0.871; RC = 0.640) resulting from a parsimony analysis of the ITS alignment representing Acrocalymma and related genera. The bootstrap support values from 1 000 replicates are indicated at the nodes and the scale bar represents the number of changes. Thickened branches reflect those branches present in the strict consensus tree. Species of Acrocalymma are highlighted in the yellow and green blocks. Additional species names of interest to this study are shown in bold text. The tree was rooted to Alternaria consortialis (GenBank KC584247)
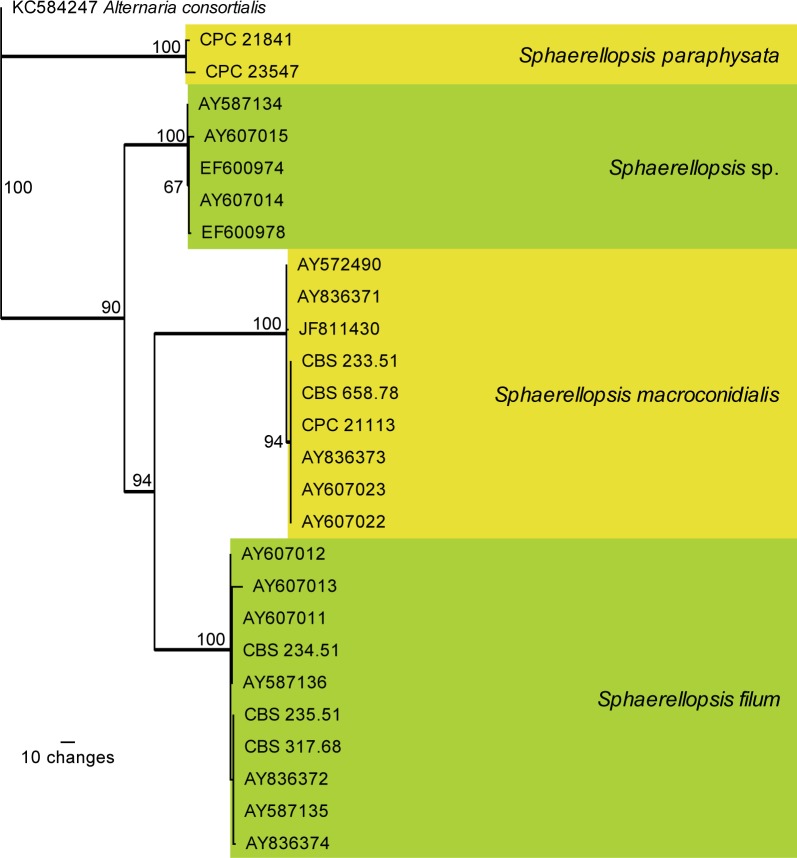
Fig. 4.
The first of 288 equally most parsimonious trees (TL = 319; CI = 0.881; RI = 0.964; RC = 0.850) resulting from a parsimony analysis of the ITS alignment representing Sphaerellopsis isolates. The bootstrap support values from 1 000 replicates are indicated at the nodes and the scale bar represents the number of changes. Thickened branches reflect those branches present in the strict consensus tree. The four species are highlighted in the yellow and green blocks. The tree was rooted to Alternaria consortialis (GenBank KC584247)
Table 1.
Details of fungal strains included in the molecular and morphological analyses.
| Species name in mmmanuscript | Strain accession number1 | Substrate of isolation | Origin | GenBank accession numbers2 | |||
|---|---|---|---|---|---|---|---|
| ITS | LSU | TEF-1α | TUB | ||||
| Acrocalymma fici | CBS 317.76 ex-type | Bark of Ficus sp. | India | KP170619 | KP170712 | KP170663 | KP170687 |
| Acrocalymma medicaginis | CPC 24340 = BRIP 5876a = IMI 165613 ex-type | Medicago sativa | Australia: QLD | KP170620 | KP170713 | — | — |
| CPC 24341 | Medicago sativa | Australia: QLD | KP170621 | KP170714 | — | — | |
| CPC 24342 = BRIP 14544a | Medicago sativa | Australia: QLD | KP170622 | KP170715 | — | — | |
| CPC 24343 | Medicago sativa | Australia: QLD | KP170623 | KP170716 | — | — | |
| CPC 24344 = BRIP 15915a | Medicago sativa | Australia: QLD | KP170624 | KP170717 | — | — | |
| CPC 24345 | Medicago sativa | Australia: QLD | KP170625 | KP170718 | — | — | |
| CPC 24346 = BRIP 16416a | Medicago sativa | Australia: SA | — | KP170719 | — | — | |
| CPC 24347 | Medicago sativa | Australia: SA | — | KP170720 | — | — | |
| Acrocalymma vagum | CPC 24216 = Rv-17 | Water melon | Spain | KP170626 | — | — | — |
| CPC 24217 = Rv-25 | Cucumis melo | Spain | KP170627 | — | — | — | |
| CPC 24218 = Rv-43 | Cucumis melo | Spain | KP170628 | — | — | — | |
| CPC 24219 = Rv-55 | Cucumis melo | Spain | KP170629 | — | — | — | |
| CPC 24220 = Rv-77 | Vitis vinifera | Spain | KP170630 | — | — | — | |
| CPC 24221 = Rv-86 | Amaranthus sp. | Spain | KP170631 | — | — | — | |
| CPC 24222 = Rv-110 | Cucumis melo | USA: Texas | KP170632 | — | — | — | |
| CPC 24223 = Rv-0103 | Cucurbita rootstock | Spain | KP170633 | — | — | — | |
| CPC 24224 = Rv-0703 | Citrullus lanatus | Spain | KP170634 | — | — | — | |
| CPC 24225 = Rv-1403 | Cucumis sativus | Spain | KP170635 | — | — | — | |
| CPC 24226 = Rv-0504 | Cucurbita rootstock | Spain | KP170636 | — | — | — | |
| CPC 24227 = Rv-0106 | Eriobotrya japonica | Spain | KP170637 | — | — | — | |
| Diederichomyces caloplacae | CBS 129140 | Caloplaca cerina | Canada | KP170638 | JQ238637 | KP170664 | KP170688 |
| CBS 129338 | Caloplaca cerina | Canada | KP170639 | JQ238643 | KP170665 | KP170689 | |
| Diederichomyces cladoniicola | CBS 128023 | Squamarina cartilaginea | Belgium | KP170640 | JQ238622 | KP170666 | KP170690 |
| CBS 128025 | Squamarina cartilaginea | Belgium | KP170641 | JQ238625 | KP170667 | KP170691 | |
| CBS 128026 | Cladonia sp. | Spain | KP170642 | JQ238628 | KP170668 | KP170692 | |
| CBS 128027 | Parmelina tiliacea | Spain | KP170643 | JQ238631 | KP170669 | KP170693 | |
| CBS 131731 | Ramalina pollinaria | France | KP170644 | — | KP170670 | KP170694 | |
| CBS 131732 | Cladonia symphycarpa | France | KP170645 | — | KP170671 | KP170695 | |
| CBS 131733 | Cladonia rangiformis | France | KP170646 | — | KP170672 | KP170696 | |
| Diederichomyces ficuzzae | CBS 128019 | Ramalina fastigiata | France | KP170647 | JQ238616 | KP170673 | KP170697 |
| Diederichomyces foliaceiphila | CBS 129141 | Cladonia squamosa | Belgium | KP170648 | JQ238640 | KP170674 | KP170698 |
| CBS 131729 | Cladonia | Belgium | KP170649 | — | KP170675 | KP170699 | |
| CBS 131730 | Parmelia sulcata | Belgium | KP170650 | — | KP170676 | KP170700 | |
| Diederichomyces xanthomendozae | CBS 129666 ex-type | Xanthomendoza hasseana | Canada | KP170651 | JQ238634 | KP170677 | KP170701 |
| Neosphaerellopsis thailandica | CPC 21659 ex-type | Bothriochloa bladhii | Thailand | KP170652 | KP170721 | KP170678 | KP170702 |
| Neottiosporina paspali | CBS 331.37 | Paspalum notatum | USA: Florida | KP170653 | EU754172 | — | — |
| Paraphaeosphaeria parmeliae | CBS 131728 ex-type | Parmelia sulcata | Belgium | KP170654 | KP170722 | KP170679 | KP170703 |
| Sphaerellopsis filum | CBS 234.51 = ATCC 22603 | Puccinia coronata on Lolium italicum | Switzerland | KP170655 | KP170723 | KP170680 | KP170704 |
| CBS 235.51 = ATCC 22604 | Puccinia hordei on Ornithogalum divergens | Portugal | KP170656 | KP170724 | KP170681 | KP170705 | |
| CBS 317.68 ex-neotype | Puccinia deschampsiae uredinium, on Deschampsia caespitosa | Germany | KP170657 | KP170725 | KP170682 | KP170706 | |
| Sphaerellopsis macroconidialis | CBS 233.51 = ATCC 11100 = VKM F-2880 | Uromyces caryophylli on Dianthus caryophyllus | Italy | KP170658 | KP170726 | KP170683 | KP170707 |
| CBS 658.78 ex-type | Puccinia allii sori, on Allium schoenoprasum | Netherlands | KP170659 | KP170727 | KP170684 | KP170708 | |
| CPC 21113 | Rust on Carex acutiformis | Netherlands | KP170660 | KP170728 | — | KP170709 | |
| Sphaerellopsis paraphysata | CBS 137231 = CPC 23547 | Ravenelia macowania on Vachellia karroo | South Africa | KP170661 | — | — | — |
| CPC 21841 ex-type | Pennistum sp. | Brazil | KP170662 | KP170729 | KP170685 | KP170710 | |
| Xenophoma puncteliae | CBS 128022 ex-type | Punctelia rudecta | USA | JQ238617 | JQ238619 | KP170686 | KP170711 |
1 ATCC: American Type Culture Collection, Virginia, USA; BRIP: Plant Pathology Herbarium, Department of Primary Industries, Queensland, Australia; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS; IMI: International Mycological Institute, CABI-Bioscience, Egham, UK; VKM: All-Russian Collection of Microorganisms, Russian Academy of Sciences, Institute of Biochemistry and Physiology of Microorganisms, 142292 Pushchino, Moscow Region, Russia.
2 ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: large subunit (28S) of the nrRNA gene operon; TEF-1α: partial translation elongation factor 1-alpha gene; TUB: partial beta-tubulin gene.
The first analysis (LSU) is based on 112 isolates (including the outgroup sequence) and the resulting dataset of 751 characters, including alignment gaps which are treated as fifth base, consisted of 546 constant characters, 64 variable parsimony-uninformative characters and 141 parsimony-informative characters. The maximum of 1000 equally most parsimonious trees were retained (TL = 610; CI = 0.430; RI = 0.848; RC = 0.364), the first of which is presented in Fig. 1. It was not possible to determine a more precise phylogenetic position of Acrocalymma, neither in the phylogeny nor by megablast searches of NCBI’s GenBank nucleotide database (closest matches being Pyrenochaeta quercina and Pyrenochaetopsis pratorum with 97 % identity over approximately 1195 nucleotides), therefore a new family name is introduced below to accommodate it. Sphaerellopsis is shown to belong to Leptosphaeriaceae (‘clade A’ sensu de Gruyter et al. 2013), while the three newly recognised genera in this study, Diederichomyces, Neosphaerellopsis and Xenophoma, are allied to Phaeosphaeriaceae. The new species Paraphaeosphaeria parmeliae is placed in Montagnulaceae. The large number of nodes without support in this phylogeny shows that LSU alone does not have the resolution to resolve the complexity of many genera and families in Pleosporales.
The second analysis (ITS alignment focussed on stagonospora-like and phoma-like species) is based on 50 isolates (including the outgroup sequence) and the resulting dataset of 522 characters, including alignment gaps which are treated as fifth base, consisted of 240 constant characters, 62 variable parsimony-uninformative characters and 220 parsimony-informative characters. Sixty equally most parsimonious trees were retained (TL = 1090; CI = 0.517; RI = 0.707; RC = 0.365), the first of which is presented in Fig. 2. The phylogenetic placement of the three newly described genera, Diederichomyces, Neosphaerellopsis and Xenophoma, are shown as being sister to Phaeosphaeriopsis, Parastagonospora and the broader lineage “Sclerostagonospora” / Neosphaerellopsis / Parastagonospora, respectively. Five species of Diederich-omyces are distinguished in the phylogeny.
The third analysis (ITS alignment focussed on Acrocalymma and related species) is based on 48 isolates (including the outgroup sequence) and the resulting dataset of 401 characters, including alignment gaps which are treated as fifth base, consisted of 218 constant characters, 37 variable parsimony-uninformative characters and 146 parsimony-informative characters. Two equally most parsimonious trees were retained (TL = 404; CI = 0.735; RI = 0.871; RC = 0.640), the first of which is presented in Fig. 3. Eight distinct lineages represent Acrocalymma in this phylogeny, including A. fici, which is described as a taxonomic novelty below. Massarina walkeri is nestled inside the broader Acrocallyma lineage, distinct from M. eburnea, the type species of the genus Massarina, and is therefore allocated to Acrocalymma. Likewise, Rhizopycnis vagum is included here as A. vagum.
The fourth analysis (ITS alignment focussed on Sphaerellopsis isolates) is based on 27 isolates (including the outgroup sequence) and the resulting dataset of 518 characters, including alignment gaps which are treated as fifth base, consisted of 326 constant characters, 50 variable parsimony-uninformative characters and 142 parsimony-informative characters. A total of 288 equally most parsimonious trees were retained (TL = 319; CI = 0.881; RI = 0.964; RC = 0.850), the first of which is presented in Fig. 4. Four distinct, well-supported clades are found, of which two are newly named below, as S. paraphysata and S. macroconidialis.
TAXONOMY
Although the present study focuses of Sphaerellopsis, several isolates deposited under this name turned out to be unrelated, and to belong to other genera, phylogenetically allied to a complex of phoma-like species. The type species of the genus Sphaerellopsis is neotypified below, and new generic names are introduced to accommodate other taxa in this complex.
Phoma-like genera
The morphology of the lichenicolous phoma-like species has been well studied in the past (Hawksworth 1981, Diederich et al. 2007, von Brackel 2008, Lawrey et al. 2012). These species are considered to be host-specific to varying extents, being confined to a single species, a single genus, or a few closely related genera (Diederich et al. 2007). However, based on recent cultural and DNA phylogenetic data, Lawrey et al. (2012) questioned the past practise of identifying lichenicolous Phoma species based on host preference, echoing the caution needed in naming lichenicolous fungi generally (Hawksworth 1977, 2003). Furthermore, as shown here (Fig. 1), the lichenicolous Phoma species are not congeneric with the genus Phoma (Aveskamp et al. 2010, de Gruyter et al. 2010, 2013), and thus need to be accommodated elsewhere.
Diederichomyces Crous & Trakunyingcharoen, gen. nov.
MycoBank MB810828
Etymology: Named after Paul Diederich, who contributed significantly to our present knowledge of lichenicolous fungi.
Diagnosis: Conidiomata globose, brown, uni- to multilocular, ostiolate, frequently with brown setae around ostiolar area. Paraphyses mostly absent. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, ampulliform to doliiform, mono- to polyphialidic, at times with percurrent proliferation. Conidia dimorphic, forming fusoid-ellipsoid and globose conidia in same conidioma. Frequently forming orange crystals in the agar.
Type species: Diederichomyces xanthomendozae (Diederich & Freebury) Crous & Trakunyingcharoen 2014 (syn. Phoma xanthomendozae Diederich & Freebury 2013),
Description: Conidiomata globose, brown, superficial to immersed, solitary to aggregated, uni- to multilocular, ostiolate, frequently with brown setae around ostiolar area; wall layers of dark brown textura angularis, becoming thin-walled and hyaline toward the inner region. Paraphyses mostly absent, hyaline, cylindrical, 1–2-septate, with rounded ends. Conidiophores reduced to conidiogenous cells, or with a supporting cell. Conidiogenous cells hyaline, ampulliform to doliiform, mono- to polyphialidic, with prominent periclinal thickening, with outer collarette, and at times with percurrent proliferation. Conidia solitary, hyaline, smooth, thin-walled, 1–3 guttulate, dimorphic, forming fusoid-ellipsoid and globose conidia in same conidioma. Frequently forming orange crystals in the agar.
Notes: Diederichomyces is distinct from Phoma in that it has dimorphic conidia, and forms orange crystals in culture. Features that are expressed in some species in the genus include the presence of ostiolar setae, paraphyses, and polyphialidic conidiogenous cells with prominent collarettes, rendering it morphologically variable. Furthermore, based on the LSU phylogeny (Fig. 1) the genus appears to be paraphyletic, but more collections would be required to suitably delineate these taxa, and identify the synapomorphies associated with potential additional genera.
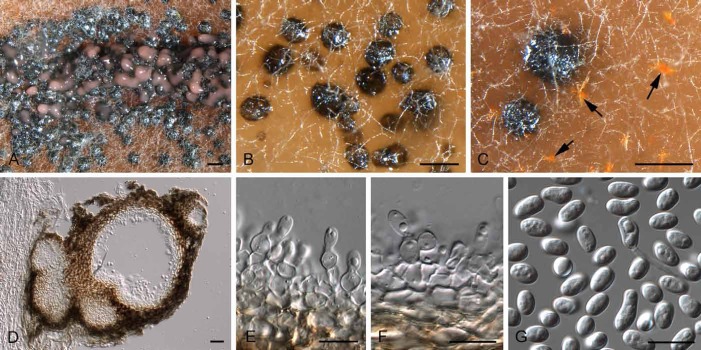
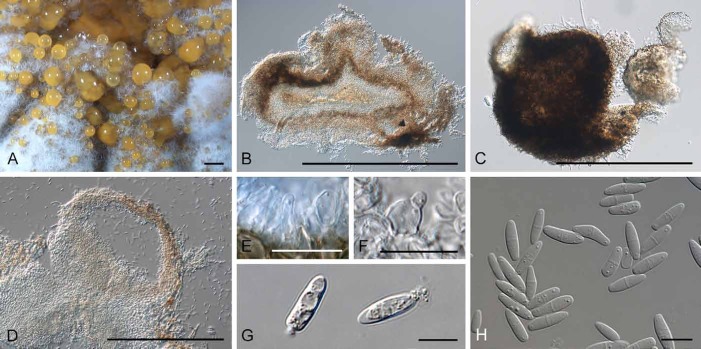
Diederichomyces caloplacae (D. Hawksw.) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810829
(Fig. 5)
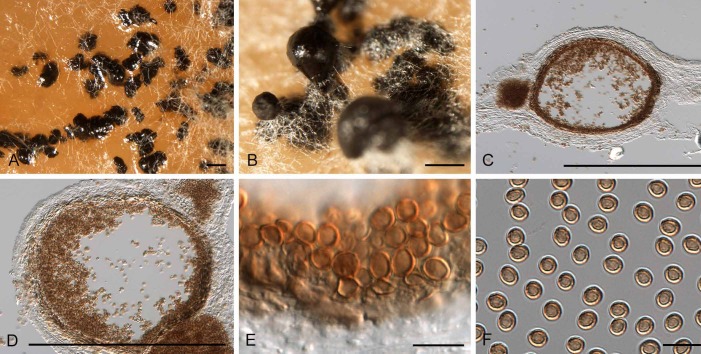
Fig. 5.
Diederichomyces caloplacae (CBS 129140). A–C. Conidiomata on MEA (arrows indicate red crystals formed in agar). D. vertical section through conidioma. E, F. Conidiogenous cells. G. Conidia. Bars: A–D = 200 μm, E–G = 10 μm
Basionym: Phoma caloplacae D. Hawksw., Bull. Brit. Mus. (Nat. Hist.), Bot. 9: 50 (1981).
Materials examined: Canada: Saskatchewan: lichenicolous on Caloplaca cerina, C. Freebury (CBS 129140, CBS 129338).
Note: The species was originally described from apothecia of Caloplaca cerina from the former Soviet Union (Hawksworth 1981).
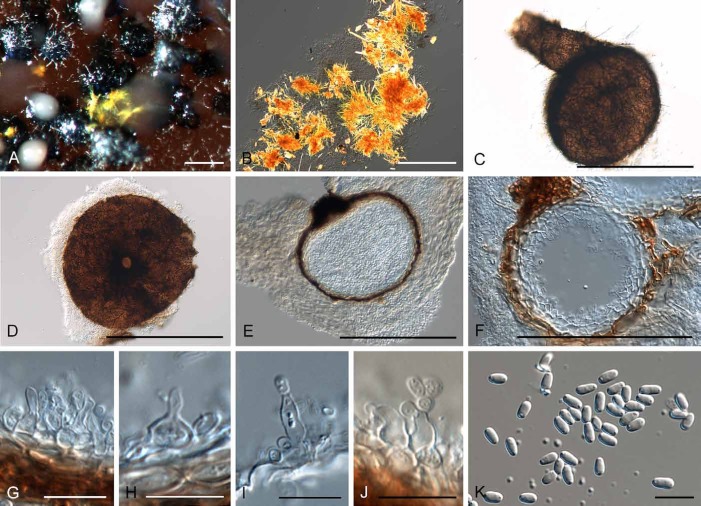
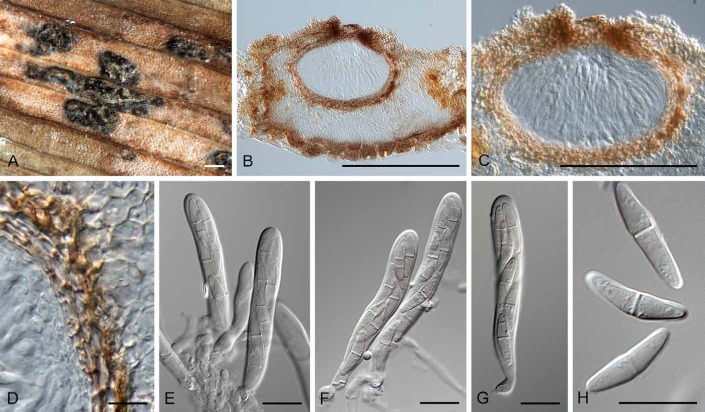
Diederichomyces cladoniicola (Diederich et al.) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810830
(Fig. 6)
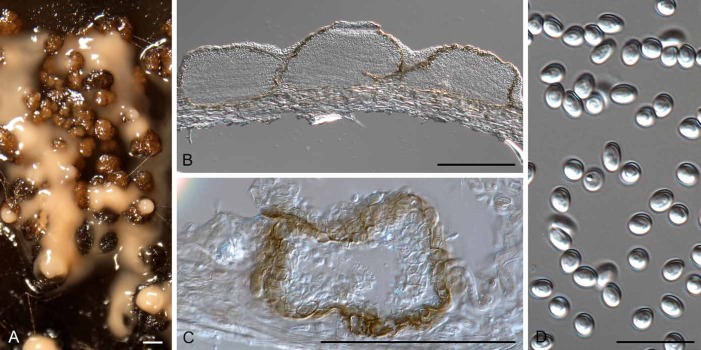
Fig. 6.
Diederichomyces cladoniicola (CBS 128023). A. Conidioma on MEA. B. Red crystals formed in agar. C, D. Conidiomata. E, F. Sections through conidiomata. G–J. Conidiogenous cells. K. Conidia. Bars: A, C–F = 300 μm, B = 100 μm, G–K = 10 μm
Basionym: Phoma cladoniicola Diederich et al., Lichenologist 39: 157 (2007).
Materials examined: Belgium: parasitic on lichen Squamarina cartilaginea, D. Ertz (CBS 128023, CBS 128025). – Spain: Mallorca, parasitic on Cladonia sp., P. Diederich (CBS 128026, CBS 128027). – France: Ardennes, Chooz, on thallus of Ramalina pollinaria, D. Ertz (CBS 131731); Ardennes, Chooz, on thallus of Cladonia symphycarpa, D. Ertz (CBS 131732); Ardennes, Chooz, on thallus of Cladonia rangiformis, D. Ertz (CBS 131733).
Notes: This species was originally described from the thallus of Cladonia pyxidata, collected in Minnesota, USA. Conidia are ellipsoid, biguttulate, (3.5–)4.5–6(–7.5) × (2–)2.5–3 μm, corresponding to those of the isolates studied here (see Materials examined).
Diederichomyces ficuzzae (Brackel) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810831
Basionym: Phoma ficuzzae Brackel, Sauteria 15: 109 (2008).
Material examined: France: Boulonnais, parasitic on lichen Ramalina fastigata, D. van den Broeck (CBS 128019).
Notes: This species was originally described from Ramalina fastigata growing on the bark of Pyrus amygdaliformis in Sicily, Italy. It lacks an ex-type strain (von Brackel 2008), and ideally an isolate should be obtained and sequenced to fix the genetic application of the name.
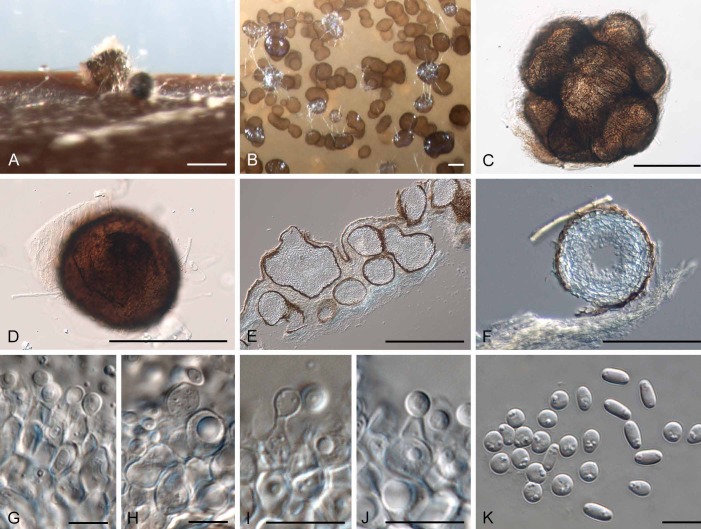
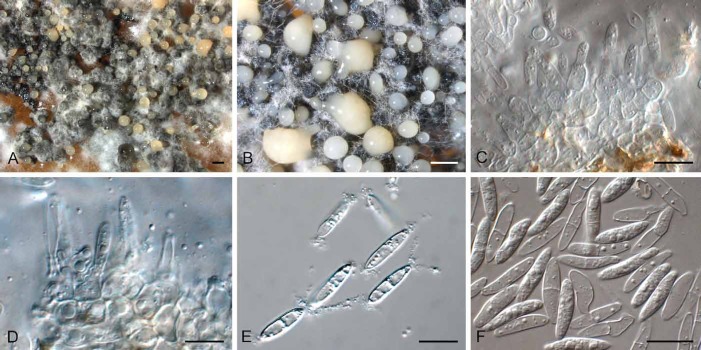
Diederichomyces foliaceiphila (Diederich et al.) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810832
(Fig. 7)
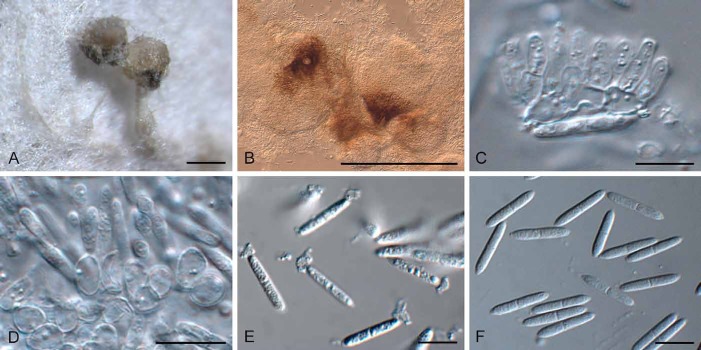
Fig. 7.
Diederichomyces foliaceiphila (CBS 129141). A–D. Conidiomata in culture. E, F. Sections through conidiomata. G–J. Conidiogenous cells. K. Dimorphic conidia. Bars: A–E = 250 μm, F = 200 μm, G–K = 10 μm
Basionym: Phoma foliaceiphila Diederich et al., Lichenologist 39: 159 (2007).
Materials examined: Belgium: lichenicolous on Cladonia squamosa, P. Diederich (CBS 129141); Ardenne district, on thallus of Cladonia sp., D. Ertz, (CBS 131729); Ardenne district, on thallus of Parmelia sulcata, D. Ertz (CBS 131730).
Notes: This species was originally described from the thallus of Cladonia foliacea collected in the Czech Republic. No cultures were made from the type collection.
Diederichomyces xanthomendozae (Diederich & Freebury) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810833
Basionym: Phoma xanthomendozae Diederich & Freebury, Fungal Div. 55: 208 (2013).
Material examined: Canada: Quebec: Les Collines-de-l’Outaouais RCM, Gatineau Park, near Wakefield, grassy ditch beside Route 05, 45°37.8′N, 75°56.4′W, on fallen Salix, on Xanthomendoza hasseana, 3 May 2010, C. Freebury (CANL – holotype; JL451-10, CBS 129666 – ex-type cultures).
Paraphaeosphaeria parmeliae Crous & Trakunyingcharoen, sp. nov.
MycoBank MB810834
(Fig. 8)
Fig. 8.
Paraphaeosphaeria parmeliae (CBS 131728). A, B. Conidiomata in culture. C, D. Sections through conidiomata. E. Conidiogenous cells. F. Conidia. Bars: A–D = 200 μm, E, F = 10 μm
Etymology: Named after the lichen genus on which it occurs, Parmelia.
Diagnosis: Conidiomata globose, dark brown, unilocular. Conidiophores reduced to conidiogenous cells that are brown, ampulliform, enteroblastic, 4.5–6.5 × 3.5–7 μm. Conidia globose, brown, aseptate, thick-walled, smooth to rough, (3–)3.5–4(–4.5) × 3–4(–4.5) μm.
Type: Belgium: Ardenne district, on thallus of Parmelia sulcata, 2010, D. Ertz (CBS H-21850 – holotype; CBS 131728 – ex-type culture).
Description: Conidiomata globose, dark brown, semi-immersed to immersed, solitary to aggregated, unilocular, ostiolate, thin-walled, 120–175 × 150–220 μm. Conidiophores reduced to conidiogenous cells. Conidiogenous cells brown, ampulliform, enteroblastic, proliferation at the same level with visible periclinal thickening, 4.5–6.5 × 3.5–7 μm. Conidia globose, brown, aseptate, thick-walled, smooth to rough, (3–) 3.5–4(–4.5) × 3–4(–4.5) μm.
Culture characteristics: Colonies on OA with white fluffy and moderately dense mycelium, abundant black pycnidia forming semi-immersed into the culture media, exuding a dark brown-black conidial mass. Colony surface on OA pale olivaceous grey, reverse olivaceous grey. Colony surface on MEA dirty white to pale olivaceous grey, reverse sienna with patches of umber.
Notes: The isolate on which Paraphaeosphaeria parmeliae is based, was originally identified as Phoma foliaceiphila. It differs from this taxon by forming brown, thick-walled conidia. The genus Paraphaeosphaeria was shown to not be congeneric with Paraconiothyrium (Verkley et al. 2013).
Xenophoma Crous & Trakunyingcharoen, gen. nov.
MycoBank MB810835
Etymology: Named after its morphological similarity to the genus Phoma.
Diagnosis: Conidiomata uni- to multilocular, irregular to cauliflower-shaped. Paraphyses absent. Conidiophores reduced to conidiogenous cells, hyaline, ampulliform, monophialidic. Conidia solitary, hyaline, smooth, thin-walled, guttulate, subspherical to ellipsoid.
Type species: Xenophoma puncteliae (Diederich & Lawrey) Crous & Trakunyingcharoen 2014 (syn. Phoma puncteliae Diederich & Lawrey 2013)
Description: Conidiomata globose to subglobose, uni- to multilocular, irregular to cauliflower-shaped, solitary to aggregated, mostly solitary, ostiolate; wall layers of 2–3 layers of dark brown textura angularis, becoming thin-walled and hyaline toward the inner region. Paraphyses absent. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, hyaline, ampulliform, monophialidic, with prominent periclinal thickening. Conidia in creamy white masses, solitary, hyaline, smooth, thin-walled, guttulate, subspherical to ellipsoid.
Notes: Xenophoma is morphologically similar to the genus Phoma, the only differences being the cauliflower-shaped, uni- to multilocular conidiomata, and subspherical to ellipsoid conidia.
Xenophoma puncteliae (Diederich & Lawrey) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810836
(Fig. 9)
Fig. 9.
Xenophoma puncteliae (CBS 128022). A. Conidiomata forming on MEA. B, C. Sections through conidiomata. D. Conidia. Bars: A–C = 50 μm, D = 10 μm
Basionym: Phoma puncteliae Diederich & Lawrey, Fungal Div. 55: 207 (2013).
Type: USA: Maryland: Frederick Co., Catoctin Mt. National Park, Hog Rock Trail, open oak-woodland, 39°38′55.1″N, 77°27′08.6″W, parasitic on Punctelia rudecat on Quercus rubra, 13 Oct. 2009, J.D. Lawrey (BR – holotype; CBS 128022 – ex-type culture).
Note: Based on the ITS phylogeny (Fig. 2), Xenophoma puncteliae clusters basal to species of Phaeosphaeriopsis.
Sphaerellopsis-like genera
As shown in Fig. 1, the genus Acrocalymma represents an undefined lineage of Dothideomycetes that have massarina-like sexual morphs. A new family name is herewith introduced to accommodate these species.
Acrocalymmaceae Crous & Trakunyingcharoen, fam. nov.
MycoBank MB810837
Etymology: Named after the genus Acrocalymma.
Diagnosis: Ascomata globose, opening by central beak with ostiole lined with periphyses; inner layer giving rise to hyaline pseudoparaphyses, septate, anastomosing. Asci cylindrical, sessile in rosette, 8-spored, bitunicate. Ascospores narrowly fusoid, straight to slightly curved, initially hyaline, 1-septate, with a mucoid sheath, becoming transversely 3-septate after discharge, constricted or not, pale brown. Conidiomata pycnidial, papillate or rostrate, globose, with central ostiole. Conidiophores reduced to conidiogenous cells or a supporting cell. Conidiogenous cells ampulliform to doliiform or cylindrical, hyaline, smooth, proliferating inconspicuously percurrently at apex. Conidia hyaline, but becoming pigmented with age, smooth, 0–3-septate, not constricted at septa, with flaring mucoid apical and basal appendages.
Type genus: Acrocalymma Alcorn & J.A.G. Irwin 1987.
Description: Ascomata globose, immersed, becoming erumpent, covered with pale grey hyphae, opening by central beak with ostiole lined with periphyses; wall of textura angularis; inner layer giving rise to hyaline pseudoparaphyses, septate, anastomosing. Asci cylindrical, sessile in rosette, 8-spored, bitunicate, with biseriate ascospores. Ascospores narrowly fusoid, straight to slightly curved, initially hyaline, 1-septate, with a mucoid sheath, becoming transversely 3-septate after discharge, constricted or not, pale brown. Conidiomata pycnidial, papillate or rostrate, globose, erumpent, separate but aggregated in clusters, subhyaline to brown with central ostiole; wall of 3–6 layers of hyaline to brown textura angularis. Conidiophores reduced to conidiogenous cells or a supporting cell. Conidiogenous cells ampulliform to doliiform or cylindrical, hyaline, smooth, proliferating inconspicuously percurrently at apex. Conidia hyaline, but becoming pigmented with age, smooth, guttulate, cylindrical to fusoid with subobtuse apex, acutely tapered at base to a small flattened central scar, 0–3-septate, not constricted at septa, with flaring mucoid apical and basal appendages.
Notes: Shoemaker et al. (1991) linked Acrocalymma to massarina-like sexual morphs in culture, establishing the asexual/sexual connection. Phylogenetically the genus Acrocalymma represents an undefined lineage in the Dothideomycetes, for which Acrocalymmaceae is herewith introduced.
Acrocalymma Alcorn & J.A.G. Irwin, Trans. Brit. mycol. Soc. 88: 163 (1987).
Synonym: Rhizopycnis D.F. Farr, Mycologia 90: 291 (1998).
Description: Conidiomata pycnidial, papillate or rostrate, globose, erumpent, separate but aggregated in clusters, subhyaline to brown with central ostiole; wall of 3–6 layers of hyaline to brown textura angularis. Conidiophores reduced to conidiogenous cells or a supporting cell. Conidiogenous cells ampulliform to doliiform or cylindrical, hyaline, smooth, proliferating inconspicuously percurrently at apex. Conidia hyaline, but becoming pigmented with age, smooth, guttulate, cylindrical to fusoid with subobtuse apex, acutely tapered at base to a small flattened central scar, 0–3-septate, not constricted at septa, with flaring mucoid apical and basal appendages, originating from a sheath surrounding developing conidia.
Type species: Acrocalymma medicaginis Alcorn & J.A.G. Irwin 1987.
Acrocalymma fici Crous & Trakunyingcharoen, sp. nov.
MycoBank MB810838
(Fig. 10)
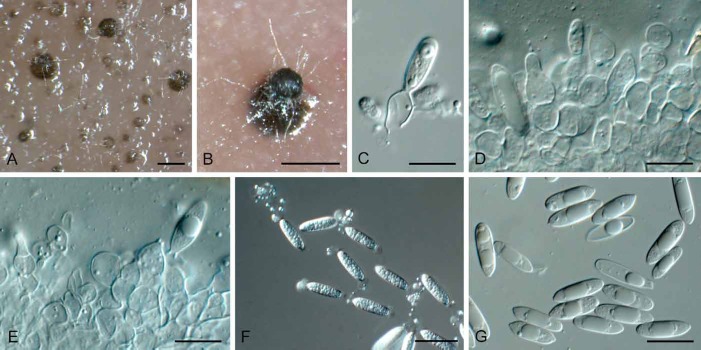
Fig. 10.
Acrocalymma fici (CBS 317.76). A, B. Conidiomata forming in agar. C, D. Conidiogenous cells. E, F. Conidia. Bars: A, B = 200 μm, C–F = 10 μm
Etymology: Named after the host genus on which it occurs, Ficus.
Diagnosis: Conidiomata pycnidial, globose, up to 200 μm diam. Conidiophores reduced to conidiogenous cells, ampulliform to doliiform, hyaline, smooth, 5–12 × 3–5 μm; inconspicuous percurrent proliferation visible at apex. Conidia hyaline, smooth, guttulate, cylindrical with subobtuse apex, medianly 1-septate, not constricted at septum, (12–)13–15(–16) × 2.5(–3) μm, with flaring mucoid apical appendage.
Type: India: New Delhi: on Ficus sp., 23 Dec. 1975, G. Malhotra (CBS H-11698 – holotype; CBS 317.76 – ex-type culture).
Description: Conidiomata pycnidial, globose, up to 200 μm diam, erumpent, separate but aggregated in clusters, subhyaline with dark brown region around ostiole, 20–30 μm diam; wall of 3–6 layers of hyaline to subhyaline textura angularis. Conidiophores reduced to conidiogenous cells or a supporting cell. Conidiogenous cells ampulliform to doliiform, hyaline, smooth, 5–12 × 3–5 μm; inconspicuous percurrent proliferation visible at apex. Conidia hyaline, smooth, guttulate, cylindrical with subobtuse apex, acutely tapered at base to a small flattened central scar, medianly 1-septate, not constricted at septum, (12–)13–15(–16) × 2.5(–3) μm, with flaring mucoid apical appendage (3–5 μm diam), visible in water mounts.
Culture characteristics: Colonies on MEA flat, spreading, with moderate aerial mycelium, and smooth, even margins; surface smoke-grey in centre, pale olivaceous grey in outer region, smoke-grey in reverse.
Acrocalymma medicaginis Alcorn & J.A.G. Irwin, Trans. Brit. mycol. Soc. 88: 163 (1987).
(Fig. 11)
Fig. 11.
Acrocalymma medicaginis (BRIP 5876a). A, B. Conidiomata forming in agar. C–E. Conidiogenous cells. F, G. Conidia. Bars: A, B = 250 μm, C–G = 10 μm
Description: Conidiomata separate, immersed, globose, brown with central ostiole, up to 250 μm diam; wall of 3–8 layers of brown textura angularis, becoming hyaline towards the inner region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity of conidioma, hyaline, smooth, ampulliform to doliiform, 5–10 × 6–7 μm, proliferating with visible periclinal thickening at apex. Conidia solitary, hyaline, smooth, guttulate, thin-walled, straight, subcylindrical, apex obtuse, tapering at base to truncate hilum, 1.5 μm diam, (11–)13–15(–16) × (3.5–)4 μm; ends with mucoid caps, conidia becoming pale olivaceous and 1-septate with age.
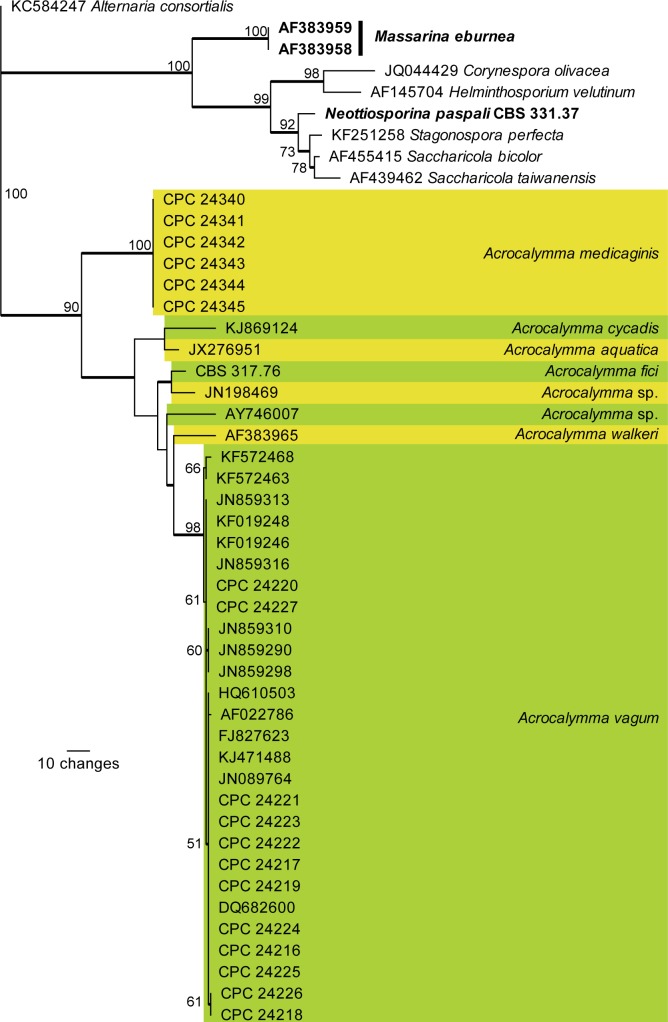
Materials examined: Australia: Queensland: Hermitage, on Medicago sativa, 10 Mar. 1972, J.A.G. Irvin (CPC 24340, CPC 24340, BRIP 5876a, IMI 165613 – ex-type cultures); Gatton, 14 Nov. 1984, J.A.G. Irvin (CPC 24342, CPC 24343, BRIP 14544a); Gatton, DPI Research Station, 22 Jul. 1987, J.A.G. Irvin (CPC 24344, BRIP 15915a). Southern Australia: Langhorne Creek, 8 Oct. 1975, A. Nikandrow (CPC 24346, CPC 24347, BRIP 16416a).
Notes: The isolate we describe here as A. fici clusters with two genera, namely Acrocalymma (based on A. medicaginis) and Rhizopycnis (based on R. vagum). These two genera were established a few years apart to accommodate two root pathogens, namely A. medicaginis on Medicago in Australia, and R. vagum on Cucumis in Texas (Alcorn & Irwin 1987, Farr et al. 1998). Furthermore, Acrocalymma medicaginis has been linked to “Massarina” walkeri as sexual morph (Shoemaker et al. 1991). Morphologically Acrocalymma resembles Rhizopycnis (pycnidial conidiomata, phialidic conidiogenous cells, cylindrical to fusoid, 1–3-septate conidia, that turn brown with age). Reported differences between the two genera are that R. vagum lacks mucoid conidial caps, has phialidic conidiogenesis with periclinal thickening, and conidia turn brown with age (Farr et al. 1998). However, when ex-type strains of A. medicaginis were studied in culture, they exhibited phialidic conidiogenesis, and conidia also become septate and pigmented with age, explaining why isolates of R. vagum clustered among those of Acrocalymma. Rhizopycnis is therefore reduced to synonymy under Acrocalymma, and a new combination introduced for R. vagum.
Recently, a second species of Acrocalymma, A. aquatica, was described from freshwater in Thailand (Zhang et al. 2012), while Crous et al. (2013) introduced A. cycadis from leaves of Cycas calcicola in Australia. Acrocalymma aquatica is similar to A. fici, except that it has slightly wider conidia (12–17 × 3–4 μm), while those of A. cycadis are larger (25–35 × 4–5 μm).
Acrocalymma vagum (D.F. Farr) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810839
Basionym: Rhizopycnis vagum D.F. Farr, Mycologia 90: 291 (1998).
Specimens examined: Spain: Castellón, Almenara, on Amaranthus sp., CPC 24221 = Rv-86); on Cucumis sativus, CPC 24225 = Rv-1403; on Cucurbita rootstock, CPC 24223 = Rv-0103, CPC 24226 = Rv-0504; Ciudad Real, Daimiel, on Vitis vinifera, CPC 24220 = Rv-77; on Loquat, CPC 24227 = Rv-0106; Ciudad Real, Argamasilla de Alba, on Cucumis melo, CPC 24217 = Rv-25; Valencia, El Romaní, CPC 24218 = Rv-43; Murcia, La Palma, CPC 24219 = Rv-55; Valencia, Silla, on Citrullus lanatus, CPC 24216 = Rv-17, CPC 24224 = Rv-0703. – USA: Texas: on Cucumis melo, CPC 24222 = Rv-110.
Acrocalymma walkeri (Shoemaker et al.) Crous & Trakunyingcharoen, comb. nov.
MycoBank MB810840
Basionym: Massarina walkeri Shoemaker et al., Canad. J. Bot. 69: 569 (1991).
Type: Australia: Queensland: on Medicago sativa cv. Hunter River, 22 Jul. 1987, J.A.G. Irwin (DAOM 198791a – holotype).
Notes: Acrocalymma medicaginis, which is also known from Medicago sativa in Queensland, was originally assumed to represent the asexual morph of Massarina walkeri. The two species are, however, phylogenetically distinct, and A. medicaginis has somewhat smaller conidia than those of A. walkeri, which are 11–21 × 3.5–5 μm (Shoemaker et al. 1991).
Sphaerellopsis-like isolates associated with rust sori on Bothriochloa bladhii in Thailand proved to represent yet another genus, which is phylogenetically distinct from Sphaerellopsis s. str.
Neosphaerellopsis Crous & Trakunyingcharoen, gen. nov.
MycoBank MB810841
Etymology: Named after the genus Sphaerellopsis, which it superficially resembles.
Diagnosis: Morphologically similar to Sphaerellopsis, but phylogenetically distinct based on sequence data of the ITS and LSU regions. Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Phaeosphaeria avenaria (GenBank FJ623271; Identities = 535/571 (94 %), Gaps = 11/571 (1 %)), Parastagonospora poagena (GenBank KJ869116; Identities = 528/564 (94 %), Gaps = 9/564 (1 %)), and Phaeosphaeria avenaria f. sp. triticae (GenBank AY196988; Identities = 534/571 (94 %), Gaps = 9/571 (1 %)). The LSU sequence of Neosphaerellopsis thailandica differs from Stagonospora neglecta var. colorata (GenBank EU754218) at positions 57 (C), 94 (A), 96 (T), 114 (C), 167 (T), and 168 (A); and from Stagonospora foliicola KF251759 at positions 94 (A), 114 (C), 167 (T), and 168 (A).
Type species: Neosphaerellopsis thailandica Crous & Trakunyingcharoen 2014.
Description: Conidiomata globose, superficial, solitary or aggregated, mostly unilocular, sometimes multilocular within the same stromata, with central ostiole; outer layers composed of pale to medium brown textura angularis, becoming thin-walled and hyaline toward the inner region, ostiolate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, phialidic with visible periclinal thickening, at times with 1–2 minute apical percurrent proliferations, dolliform to ampulliform. Conidia hyaline, narrowly ellipsoidal, the base truncate, with central median septum, with flaring mucoid appendage at one end.
Notes: There is no clear morphological difference between Sphaerellopsis and Neosphaerellopsis, and these genera are chiefly distinguished based on DNA phylogeny. Neo-sphaerellopsis clusters (Fig. 1) between Parastagono-spora and Sclerostagonospora in Phaeosphaeriaceae (Quaedvlieg et al. 2013), but can be distinguished from them in that neither have mucoid conidial appendages. Neosphaerellopsis is also reminiscent of Tiarospora, except that the latter has deeply immersed, stromatic pycnidia, percurrent proliferating conidiogenous cells, and broadly ellipsoidal conidia that turn brown with age (Nag Raj 1993).
Neosphaerellopsis thailandica Crous & Trakunyingcharoen, sp. nov.
MycoBank MB810842
(Fig. 12)
Fig. 12.
Neosphaerellopsis thailandica (CBS 138578). A, C. Conidiomata forming in agar. B, D. Sections through conidiomata. E, F. Conidiogenous cells. G, H. Conidia. Bars: A–D = 300 μm, E–H = 10 μm
Etymology: Named after the country where the fungus was first collected, Thailand.
Diagnosis: Conidiomata globose, sometimes multilocular. Conidiophores reduced to conidiogenous cells, hyaline, phialidic with visible periclinal thickening, dolliform–ampulliform, 3.5–7 × 2.5–4 μm. Conidia hyaline, narrowly ellipsoidal, the base truncate, with central median septum, (10.5–)11–13(–15) × (3–)3.5–4(–4.5) μm; with flaring mucoid appendage at one end.
Type: Thailand: Royal Project, N18º09’24.8” E98º23’19.6”, on uredinio rust sori on leaves of Bothriochloa bladhii (Poaceae), 29 Oct. 2012, P.W. Crous (CBS H-21847 – holotype; CPC 21659, CBS 138578 – ex-type cultures).
Description: Conidiomata globose, superficial, solitary or aggregated, mostly unilocular, sometimes multilocular within the same stromata, with central ostiole; outer layers composed of pale to medium brown textura angularis, becoming thin-walled and hyaline toward the inner region, ostiolate, to 400 μm diam; conidiomata exude a yellow-orange conidial mass. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, phialidic with visible periclinal thickening, at times with 1–2 minute apical percurrent proliferations, dolliform–ampulliform, 3.5–7 × 2.5–4 μm. Conidia hyaline, narrowly ellipsoidal, the base truncate, with central median septum, (10.5–)11–13(–15) × (3–)3.5–4(–4.5) μm; with flaring mucoid appendage at each end, visible in water mounts.
Culture characteristics: Colonies on MEA with white moderate aerial mycelium, flat to low convex, surface folded towards the centre, orange-brown on surface and reverse. Colonies on PDA with grey-brown, moderate aerial mycelium, immersed mycelium greenish, margin fimbriate, forming dark brown pycnidia in media, exuding a creamy conidial mass; greenish grey in reverse. Colonies on OA with white-grey flat mycelium, surface folded towards the centre, smoky grey in reverse.
Sphaerellopsis Cooke, Grevillea 12 (6): 23 (1883).
Synonyms: Darluca Castagne, Suppl. Cat. Pl. Marseille: 53 (1851).
Eudarluca Speg., Revta Mus. La Plata 15: 22 (1908).
Additional generic synonyms are listed in Sutton (1980) and Nag Raj (1993).
Description: Mycelium immersed, branched, septate, pale brown. Conidiomata eustromatic, pycnidioid, immersed, but becoming erumpent, locules often appearing as separate pycnidia, dark brown to black in vivo, pale brown to brown in vitro, uni- or multilocular, each locule with a separate simple ostiole; basal wall composed of pale brown textura angularis, locular wall of dark brown, thick-walled textura angularis. Paraphyses when present hyaline, filiform, septate, with end-round tip, sometimes branching. Conidiophores reduced to conidiogenous cells, occasionally with a supporting cell. Conidiogenous cells phialidic, indeterminate, cylindrical to doliiform, hyaline to pale brown, smooth, often with 1–3 percurrent proliferations, or determinate with visible periclinal thickening. Conidia hyaline, becoming pale brown and irregularly verruculose, 0–1(–3)-euseptate, constricted at septa, apex obtuse, base truncate, straight, fusoid-ellipsoidal, occasionally Y-shaped or digitate; ends with mucoid polar appendages (type H sensu Nag Raj 1993). Microconidia subcylindrical to ellipsoid or globose, aseptate, smooth, hyaline. Stromata developing in rust sori, brown in outer zone, hyaline in inner part; loci immersed, subglobose to ampulliform, with protruding papillate neck and ostiole; wall of a few layers of textura prismatica. Pseudoparaphyses cellular, septate, branched, hyaline. Asci numerous, 8-spored, bitunicate, fissitunicate, cylindrical-clavate, short stipitate. Ascospores irregularly biseriate, fusoid, hyaline to pale yellow, (1–)2(–3)-septate, constricted at the primary septum; with a mucoid cupula at each polar end.
Type species: S. filum (Biv.) B. Sutton 1980 (syn. S. quercuum Cooke 1883).
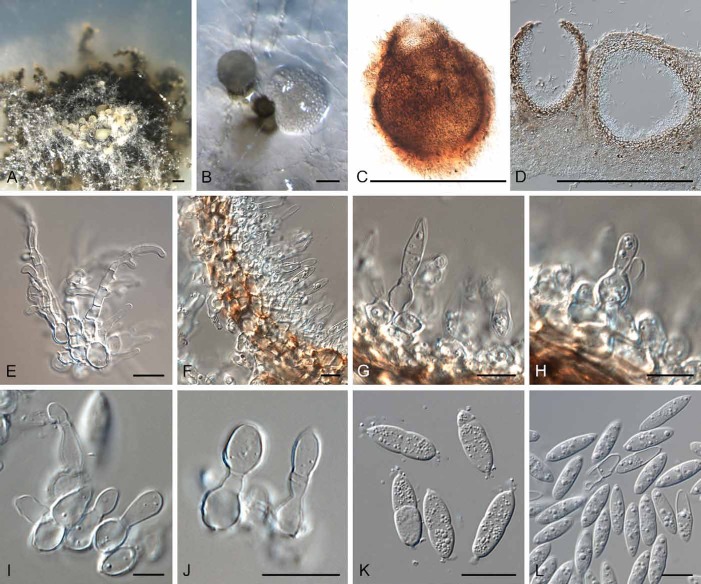
Notes: Although this study confirmed earlier observations (Keener 1951, Yuan et al. 1998) that Sphaerellopsis and Eudarluca (Fig. 13) are congeneric, we could not confirm which Sphaerellopsis species is conspecific with Eudarluca caricis (? syn. E. australis). Eriksson (1966) located what he considered to be “obviously an original collection” of Sphaeria caricis Fr., 1823 in UPS, consisting of three leaf fragments of a Carex sp. from Sweden infected with Puccinia caricina. The specimen has no indication of date, and whether it was collected before he came to Uppsala in 1835, is unknown, and in 1823 Fries evidently was aware of other material apart from his own, such as some of Kunze. The Fries material can confidently be termed “authentic” (i.e. named by the author of the taxon), but it cannot be considered to be a holotype; further, Eriksson did not make an explicit later typification. Although we have one isolate from Carex sp. collected in The Netherlands in the present study (Sphaerellopsis macroconidialis sp. nov. below), we cannot confirm or refute if this could be E. caricis as understood by Fries, as fresh collections of the sexual morph need to be made in Sweden. While there is no doubt about the generic placement of Fries’s name, the application of the specific epithet (a sanctioned name) remains uncertain.
Fig. 13.
Eudarluca caricis (K(M)124143). A. Aggregated ascomata forming on an immersed stroma in leaf tissue, associated with rust pustules. B, C. Vertical section through ascomata. D. Ascomatal wall of textura angularis. E–G. Asci. H. Ascospores. Bars: A–C = 300 μm, D–H = 10 μm
Sphaerellopsis filum (Biv.) B. Sutton, Mycol. Pap. 141: 196 (1977).
(Fig. 14)
Fig. 14.
Sphaerellopsis filum (CBS 317.68). A, B. Conidiomata sporulating on MEA. C, D. Conidiogenous cells. E, F. Conidia. Bars: A, B = 300 μm, C–F = 10 μm
Basionym: Sphaeria filum Biv., Stirp. Rar. Sic. 3: 12 (1815).
Synonyms: See Sutton (1980) and Nag Raj (1993)
Description: Conidiomata pycnidial, erumpent, aggregated, globose, up to 300 μm diam, dark brown with central ostiole, exuding copious amounts of creamy orange conidia; wall of 3–6 layers of dark brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells brown, smooth, thick-walled, ampulliform, to doliiform, 5–10 × 3–5 μm; with prominent periclinal thickening or several prominent, flaring, percurrent proliferations at apex. Conidia hyaline, smooth, guttulate, fusoid to fusoid-ellipsoid, 1(–2)-septate, usually constricted at septa, apex subobtuse, tapering at base to flattened scar, with funnel-shaped mucoid appendages at both ends, (11–)14–16(–18) × (3–)4(–5) μm.
Culture characteristics: Colonies on MEA erumpent, spreading, with sparse aerial mycelium and even, smooth margins; centre olivaceous grey (due to numerous aggregated conidiomata); outer region dirty white (due to mycelial growth in absence of conidiomata). Reverse olivaceous grey in centre, saffron in outer region.
Type: Sicily: on rust of Populus nigra, A. Bivona-Bernadi (holotype not traced and presumably lost). – Germany: Hollingstedt near Husum, on Puccinia deschampsia on Deschampsia caespitosa, Nov. 1966, U.G. Schlösser (CBS H-21851 – neotype designated here MBT200131; CBS 317.68 – ex-neotype culture).
Additional materials examined: Portugal: on Puccinia hordei on Ornithogalum divergens, May 1951, B. d’Oliveira (ATCC 22604, CBS 235.51). – Switzerland: Zürich-Oerlikon, on Puccinia coronata on Lolium italicum, 23 May 1951, E. Müller (ATCC 22603, CBS 234.51).
Notes: Saccardo (1884) stated that conidia of S. filum were 15–18 × 3–4 μm, 1-septate, constricted at the septum. Sutton (1980) examined numerous collections, and gave the conidia as 1-septate, 17–20 × 5.5–6.5 μm, while Nag Raj (1993) regarded conidia as being 1–3-septate, 10–20(–23) × 3–5 μm. The neotype chosen here, closely matches the original description and dimensions provided by Saccardo (1884).
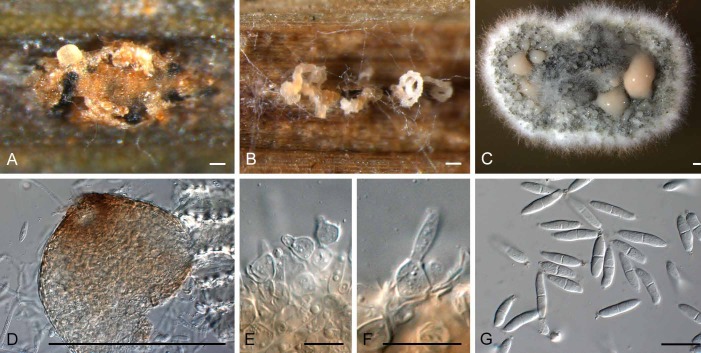
Sphaerellopsis macroconidialis Crous & Trakunyingcharoen, sp. nov.
MycoBank MB810843
(Fig. 15)
Fig. 15.
Sphaerellopsis macroconidialis (CPC 21113). A, B. Conidiomata sporulating inside rust pustules. C. Colony sporulating on MEA. D. Conidioma formed on MEA. E, F. Conidiogenous cells. G. Conidia. Bars: A–D = 250 μm, E–G = 10 μm
Etymology: Named after the large conidial dimensions.
Diagnosis: Conidiomata globose, erumpent to superficial, up to 250 μm diam. Conidiophores reduced to conidiogenous cells, globose to ampulliform, pale brown, smooth, thick-walled with prominent periclinal thickening, or percurrent, 5–8 × 4–10 μm. Conidia fusoid to fusoid-ellipsoid, 1(–3)-septate, with funnel-shaped mucoid appendage at each end, (13–) 17–20(–27) × (3.5–)4.5(–5) μm.
Type: The Netherlands: Nieuwendam, garden, on Puccinia alii sori on Allium schoenoparsum, 8 Oct. 1978, G. van Zanen (CBS H-11700 – holotype; CBS 658.78 – ex-type culture).
Description: Conidiomata globose, erumpent to superficial, also occurring in aerial mycelium, up to 250 μm diam, brown, exuding a creamy white conidial mass. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, globose to ampulliform, pale brown, smooth, thick-walled with prominent periclinal thickening, or also with percurrent proliferation at apex (when ampulliform), 5–8 × 4–10 μm. Conidia fusoid to fusoid-ellipsoid, 1(–3)-septate, hyaline, smooth, guttulate, constricted at septa, base truncate, apex subobtuse to obtusely rounded, with funnel-shaped mucoid appendage at each end, (13–)17–20(–27) × (3.5–)4.5(–5) μm.
Culture characteristics: Colonies on MEA spreading, erumpent, with sparse aerial mycelium, and feathery margin; surface dirty white with patches of olivaceous grey due to profuse sporulation; reverse sienna to umber.
Additional materials examined: Italy: Bologna, on Uromyces caryophylli on Dianthus caryophyllus, June 1951, G. Goidánich (ATCC 11100, CBS 233.51); Veenendal, on rust on Carex acutiformis, 2013, W. Quaedvlieg (CPC 21114, CBS 138761).
Note: Conidia of S. macroconidialis are longer than those of S. filum, which measure (11–)14–16(–18) × (3–)4(–5) μm, and also have up to three septa, though the latter feature is not expressed in all isolates.
Sphaerellopsis paraphysata Crous & Alfenas, sp. nov.
MycoBank MB810844
(Fig. 16)
Fig. 16.
Sphaerellopsis paraphysata (CBS 138579). A. Colony sporulating on SNA. B, C. Conidiomata formed in agar. D. Section through conidiomata. E. Paraphyses. F–H. Section through conidiomatal wall, showing conidiogenous cells. I, J. Conidiogenous cells. K, L. Conidia. Bars: A–D = 450 μm, E–L = 10 μm
Etymology: Named after the presence of conidiomatal paraphysis-like structures.
Diagnosis: Paraphyses hyaline, filiform, 2–5 septate, with end-round tip, sometimes branching, 11.5–49 × 2–6 μm. Conidiophores reduced to conidiogenous cells, ampulliform to doliiform, 4.5–12.5 × 3–8 μm. Conidia fusoid-ellipsoid, 1(–2)-septate, with mucilaginous appendage at both ends, (14.5–)15–18(–20) × (4–)4.5–5.5(–6) μm.
Type: Brazil: Minas Gerais, Viçosa, Universidade Federal de Viçosa campus, on rust on Pennisetum sp., 18 Nov. 2012, A.C. Alfenas (CBS H-21848 – holotype; CPC 21841 = CBS 138579 – ex-type cultures).
Description: Conidiomata brown, superficial to semi-immersed, globose to subglobose, solitary to aggregated, ostiolate, papilate, unilocular, outer layers composed of dark brown textura angularis, becoming thin-walled and hyaline toward the inner region, up to 450 μm diam. Paraphyses hyaline, filiform, 2–5 septate, with end-round tip, sometimes branching, 11.5–49 × 2–6 μm. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, ampulliform to doliiform, hyaline, smooth, monophialidic with inconspicuous percurrent proliferation, 4.5–12.5 × 3–8 μm. Conidia solitary, hyaline, fusoid-ellipsoid, widest in the middle, mostly with 1-median septum, rarely 2-septate, with mucilaginous appendage at both ends, (14.5–)15–18(–20) × (4–)4.5–5.5(–6) μm.
Culture characteristics: Colony on MEA with white cottony aerial mycelium, producing abundant conidiomata, which are covered with white aerial mycelium, sporulating in a yellow-cream conidial mass, crenated, dark brown-grey in reverse. Colony on PDA with grey fluffy aerial mycelium, olivaceous grey, with fimbriate margin, dark olivaceous grey in reverse. Colony on OA with white fluffy aerial mycelium, olivaceous grey on surface with undulate margin; olivaceous grey in reverse.
Additional material examined: South Africa: KwaZulu-Natal, Howick, Amber Valley, on Ravenelia macowania on Vachellia karroo, 4 Aug. 2013, F. Rijkenberg (CBS H-21849, CPC 23548, CPC 23547 = CBS 137231).
Note: Sphaerellopsis paraphysata has conidia that are slightly longer and wider than those of S. filum, and also has conidiomatal paraphyses, which are absent in S. filum.
DISCUSSION
The present study aimed to elucidate the taxonomy of Sphaerellopsis filum and its purported sexual morph Eudarluca caricis by generating a multigene DNA phylogeny of several fungal isolates tentatively identified under this name. In the process of determining the generic boundaries of Sphaerellopsis, several morphologically similar genera also had to be elucidated, namely Acrocalymma, and a closely related phoma-like complex of lichenicolous fungi.
Species of Sphaerellopsis were shown to be congeneric with Eudarluca, the latter name being treated as a synonym based on the grounds that it is the younger name, and less commonly used in literature, even though it represents the sexual morph. Furthermore, the application of the generic name is fixed in the sense that a neotype is designated for S. filum. As suspected in previous studies (Liesebach & Zaspel 2004, Nischwitz et al. 2005), S. filum was revealed to be a species complex, leading to the introduction of two new species names here, S. paraphysata (on a rust on Pennisetum sp. in Brazil, and on Ravenelia macowania on Vachellia karroo in South Africa), and S. macroconidialis (on Uromyces caryophylli on Dianthus caryophyllus in Italy, and on Puccinia alii on Allium schoenoparsum, and an unidentified rust on Carex acutiformis in The Netherlands). Furthermore, the genus Neosphaerellopsis was introduced to accommodate N. thailandica (occurring on a rust on Bothriochloa bladhii in Thailand), a species morphologically similar to Sphaerellopsis s.str., but phylogenetically distinct.
The genus Acrocalymma (type species A. medicaginis on Medicago in Australia; Alcorn & Irwin 1987) proved to be morphologically similar to an isolate previously incorrectly identified as S. filum (CBS 317.76), which could subsequently be described as A. fici (on Ficus sp. from India). Acrocalymma was also shown to be phylogenetically closely related to the genus Rhizopycnis (type species R. vagum, described from Cucumis sp. in Texas; Farr et al. 1998), the only difference being that R. vagum lacks mucoid conidial caps, and that the conidia turn brown with age. Based on our molecular results and their morphology, these two generic names are congeneric, with R. vagum nestled between A. medicaginis and the recently described A. aquatica (Zhang et al. 2012), and therefore a new combination is proposed for R. vagum in Acrocalymma. Furthermore, species of Acrocalymma represented an undefined lineage in Dothideomycetes, for which the family name Acrocalymmaceae is introduced.
During the course of this study it became obvious that several DNA sequences deposited in GenBank as “Phoma sp.” were unrelated to Phoma s. str. (Aveskamp et al. 2009, 2010, de Gruyter et al. 2009, 2010, 2013), but closely related to Sphaerellopsis. As no genera were available to accommodate these taxa, two new genera were introduced. Diederichomyces is distinguished from Phoma in having dimorphic conidia, and forming orange crystals in culture, while Xenophoma is distinguished from Phoma s. str. in having cauliflower-shaped, uni- to multilocular conidiomata, and subspherical to ellipsoid conidia.
The genus Neottiosporina (type species N. apoda, on Achyrocline saturejoides from Argentina), is still poorly understood, with unknown phylogeny. Neottiosporina apoda, appears to be distinct from several other taxa presently accommodated in the genus, having pigmented, multi-septate conidia. The single species included in the present study and for which DNA data are available, N. paspali (CBS 331.37; from Paspalum notatum, Florida, USA), appears to be closely allied to Stagonospora in Massarinaceae (Fig. 1, also see Quaedvlieg et al. 2013). However, S. paspali (K(M) IMI 175641 ex herb. CUP) was allocated to Neottiosporina on the basis of the conidia being hyaline, 2-septate, and having apical, infundibuliform mucoid appendages (Sutton & Alcorn 1974). Unfortunately CBS 331.37 proved to be sterile, so this matter could not be resolved, but it appears likely that this strain was incorrectly identified.
What started out as a straightforward study to resolve the generic synonymy of Sphaerellopsis and Eudarluca, quickly snowballed into a wider study even including several new phoma-like genera. Although we have tried to designate clear morphological characters to separate these genera, it will be difficult if not impossible to separate Sphaerellopsis from Neosphaerellopsis without the aid of DNA data, and even more so to distinguish all the known, and as yet undescribed species of Sphaerellopsis. Further research on genera of coelomycetes and their sexual morphs is urgently required, merging morphology with DNA phylogenetic data, and fixing the application of these generic names via neo- and epitypification where appropriate.
Acknowledgments
We thank the Royal Golden Jubilee PhD Program (Grant No. PHD/0353/2552) for funding, and the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Alcorn JL, Irwin JAG. (1987) Acrocalymma medicaginis gen. et sp. nov. causing root and crown rot of Medicago sativa in Australia. Transactions of the British Mycological Society 88: 163–167. [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp MM, Verkley GJM, Gruyter J de, Murace MA, Perelló A, et al. (2009) DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101: 363–382. [DOI] [PubMed] [Google Scholar]
- Aveskamp M, Gruyter J de, Woudenberg JHC, Verkley GJM, Crous PW. (2010) Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65: 1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivona-Bernardi A de. (1813–1816) Stirpium rariorum, minusque cognitarum in Sicilia sponte provenientium descriptione, nonnullis iconibus auctae Manipulus I–IV, Palermo, Typis Regiis. [Google Scholar]
- Brackel W von . (2006) Phoma ficuzzae sp. nov. and some other lichenicolous fungi from Sicily, Italy. Sauteria 15: 103–120. [Google Scholar]
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castagne L. (1851) Supplément au catalogue des plantes qui croissent naturellement aux environs de Marseille. Aix: Nicot. [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, et al. (2013) Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al (2006) Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009) Fungal Biodiversity. [CBS Laboratory Manual Series no. 1.] Utrecht: CBS-KNAW Fungal Biodiversity Centre. [Google Scholar]
- Diederich P, Kocourková J, Etayo J, Zhurbenko M. (2007) The lichenicolous Phoma species (coelomycetes) on Cladonia. Lichenologist 39: 153–163. [Google Scholar]
- Eriksson O. (1966) On Eudarluca caricis (Fr.) O. Eriks. comb. nov., a cosmopolitan uredinicolous pyrenomycete. Botaniska Notiser 119: 33–69. [Google Scholar]
- Eriksson O. (1967) On graminicolous pyrenomycetes from Fennoscandia. 2. Phragmosporous and scolecosporous species. Arkiv för Botanik, serie 2, 6: 381–440. [Google Scholar]
- Farr DF, Miller ME, Bruton BD. (1998) Rhizopycnis vagum gen. et sp. nov., a new coelomycetous fungus from roots of melons and sugarcane. Mycologia 90: 290–296. [Google Scholar]
- Fries E. (1823) Systema Mycologicum. Vol. 2 Greifswald: E. Mauritius. [Google Scholar]
- Glass NL, Donaldson G. (1995) Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruyter J de, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, et al. (2009). Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research 113: 508–519. [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, et al. (2010). Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081. [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, et al. (2013). Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL. (1977) Taxonomic and biological observations on the genus Lichenoconium (Sphaeropsidales). Persoonia 9: 159–198. [Google Scholar]
- Hawksworth DL. (1981) The lichenicolous coelomycetes. Bulletin of the British Museum (Natural History), Botany 9: 1–98. [Google Scholar]
- Hawksworth DL. (2003) The lichenicolous fung of Great Britain and Ireland: an annotated checklist. Lichenologist 35: 191–232. [Google Scholar]
- Hawksworth DL. (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus 2: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL. (2014) Possible house-keeping and other draft proposals to clarify or enhance the naming of fungi within the International Code of Nomenclature for algae, fungi, and plants (ICN). IMA Fungus 5: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011) The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende, AHG (1998) Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Keener PD. (1951) An ascigerous stage of Darluca filum (Biv.) Castagne. Plant Disease Reporter 35: 86–87. [Google Scholar]
- Kirk PM, Stalpers JA, Braun U, Crous PW, Hansen K, et al (2013). A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi and plants. IMA Fungus 4: 381–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz J, Brandenburger W. (1981) An amended host list of the rust parasite Eudarluca caricis. Journal of Plant Diseases and Protection 88: 682–702. [Google Scholar]
- Kuhlman E, Matthews F, Tillerson H. (1978) Efficacy of Darluca filum for biological control of Cronartium fusiforme and C. strobilinum. Phytopathology 68: 507–511. [Google Scholar]
- Lawrey JD, Diederich P, Nelsen MP, Freebury C, Van den Broeck D , et al. (2012) Phylogenetic placement of lichenicolous Phoma species in the Phaeosphaeriaceae (Pleosporales, Dothideomycetes). Fungal Diversity 55: 195–213. [Google Scholar]
- Liesebach M, Zaspel I. (2004) Genetic diversity of the hyperparasite Sphaerellopsis filum on Melampsora willow rusts. Forest Pathology 34: 293–305. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, et al. (2012) International Code of Nomenclature for algae, fungi, and plants (Melbourne Code), adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. [Regnum Vegetabile No. 154.] Königstein: Koeltz Scientific Books. [Google Scholar]
- Nag Raj TR. (1993) Coelomycetous Anamorphs with Appendage-bearing Conidia. Waterloo, ON: Mycologue Publications. [Google Scholar]
- Nischwitz C, Newcombe G, Anderson CL. (2005) Host specialization of the mycoparasite Eudarluca caricis and its evolutionary relationship to Ampelomyces. Mycological Research 109: 421–428. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Cigelnik E. (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences, USA 95: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei MH, Hunter T, Ruiz C, Bayon C, Harris J. (2003) Quantitative inoculation of willow rust Melampsora larici-epitea with the mycoparasite Sphaerellopsis filum (teleomorph Eudarluca caricis). Mycological Research 107: 57–63. [DOI] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, Barreto RW, Alfenas AC , et al.(2013) Sizing up Septoria. Studies in Mycology 75: 307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Ronquist F, Teslenko M, Mark P van der, Ayres DL, Darling A, et al. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. (1884) Sylloge Fungorum omnium hucusque cognitorum. Vol. 3 Padova: Abellini. [Google Scholar]
- Shoemaker RA, Babcock CE, Irwin JAG. (1991) Massarina walkeri n. sp., the teleomorph of Acrocalymma medicaginis from Medicago sativa contrasted with Leptosphaeria pratensis, L. weimeri n. sp., and L. viridella. Canadian Journal of Botany 69: 569–573. [Google Scholar]
- Spegazzini C. (1908) Fungi aliquot Paulistani. Revista del Museo de La Plata 15: 7–48. [Google Scholar]
- Smith H, Wingfield MJ, Crous PW, Coutinho TA. (1996) Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88. [Google Scholar]
- Sutton BC. (1977) Coelomycetes VI. Nomenclature of generic names proposed for coelomycetes. Mycological Papers 141: 1–253. [Google Scholar]
- Sutton BC. (1980) The Coelomycetes: Fungi Imperfecti with pycnidia, acervuli and stromata. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Sutton BC, Alcorn JL. (1974). Neottiosporina. Australasian Journal of Botany 22: 517– 530. [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan MJ, Hunter T, Parker SR, Royle DJ. (1997) How effective is Sphaerellopsis filum as a biological control agent of Melampsora willow rust? Aspects of Applied Biology 49: 143–148. [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor SB. (1990)Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ. eds): 315–322 San Diego: Academic Press. [Google Scholar]
- Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, et al (2012) One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology 13: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZW, Pei MH, Hunter T, Royle DJ. (1998) Eudarluca caricis, the teleomorph of the mycoparasite Sphaerellopsis filum, on blackberry rustPhragmidium violaceum. Mycological Research 102: 866–868. [Google Scholar]
- Zhang H, Hyde KD, McKenzie EHC, Bahkali AH, Zhou D. (2012) Sequence data reveals phylogenetic affinities of Acrocalymma aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomyces aquaticus (freshwater coelomycetes). Cryptogamie, Mycologie 33: 333–346. [Google Scholar]