Abstract
Purpose
To investigate the relationship between rising patterns of prostate-specific antigen (PSA) before chemotherapy and PSA flare during the early phase of chemotherapy in patients with castration-resistant prostate cancer (CRPC).
Materials and Methods
This study included 55 patients with CRPC who received chemotherapy and in whom pre-treatment or post-treatment PSA levels could be serially obtained. The baseline parameters included age, performance, Gleason score, PSA level, and disease extent. PSA doubling time was calculated using the different intervals: the conventional interval from the second hormone manipulation following the nadir until anti-androgen withdrawal (PSADT1), the interval from the initial rise after anti-androgen withdrawal to the start of chemotherapy (PSADT2), and the interval from the nadir until the start of chemotherapy (PSADT3). The PSA growth patterns were analyzed using the ratio of PSADT2 to PSADT1.
Results
There were two growth patterns of PSA doubling time: 22 patients (40.0%) had a steady pattern with a more prolonged PSADT2 than PSADT1, while 33 (60.0%) had an accelerating pattern with a shorter PSADT2 than PSADT1. During three cycles of chemotherapy, PSA flare occurred in 11 patients (20.0%); of these patients, 3 were among 33 (9.1%) patients with an accelerating PSA growth pattern and 8 were among 22 patients (36.4%) with a steady PSA growth pattern (p=0.019). Multivariate analysis showed that only PSA growth pattern was an independent predictor of PSA flare (p=0.034).
Conclusion
An exponential rise in PSA during anti-androgen withdrawal is a significant predictor for PSA flare during chemotherapy in CRPC patients.
Keywords: Prostate cancer, castration-resistant, prostate-specific antigen, prostate-specific antigen doubling time, prostate-specific antigen flare
INTRODUCTION
Prostate cancer is the most common cancer in Western countries, and its incidence has been rapidly rising in Asia.1,2,3,4 Androgen deprivation therapy treats advanced prostate cancer effectively for a considerable amount of time; however, most patients with the disease eventually progress to castration-resistant prostate cancer (CRPC), which is refractory to any hormone manipulation.5 CRPC remains the main cause of prostate cancer-related mortality. Recently, randomized trials with docetaxel-based chemotherapy reported a significant improvement in overall survival in patients with CRPC.6 Since then, systemic chemotherapy has become the standard first-line treatment in the management of CRPC.7,8
As most CRPC patients have non-measurable skeletal metastases, response assessment during chemotherapy generally relies on a traditional serum marker, prostate-specific antigen (PSA). However, PSA is occasionally inaccurate when assessing disease status in advanced prostate cancer, as poorly differentiated advanced prostate cancer may not produce PSA.9,10 Recent studies have reported the existence of PSA flare phenomenon after the onset of chemotherapy in patients with CRPC.11,12,13,14,15,16, PSA flare is not related with progression and does not impact outcomes negatively. However, considering that there has been no predictor for an occurrence of PSA flare in CRPC patients, an inefficient treatment must be maintained for more than 12 weeks.9,11,13,16
Changes in PSA over time (i.e., PSA dynamics) have been advocated for risk stratification of prostate cancer across the spectrum of the disease.17 PSA doubling time represents the relative rate of PSA change over time and is defined as the time needed for the PSA value to double; it also takes into account the exponential nature of neoplastic growth and thus requires logarithmic analysis.18 To date, PSA doubling time has emerged as a potentially useful tool in predicting the prognosis of patients with CRPC. However, there has been no study to investigate this calculation as a pretreatment predictor for an occurrence of PSA flare during chemotherapy.
PSA doubling time is generally calculated using the period from the nadir until the start of the next treatment, usually anti-androgen withdrawal.17,18 However, although PSA increases after the next hormonal manipulation until the start of chemotherapy, the values during this period are not included in the calculation of PSA doubling time. We postulated that PSA changes immediately before chemotherapy may be associated with PSA changes immediately after chemotherapy and subsequently focused on the interval of antiandrogen withdrawal, the final interval immediately before the start of chemotherapy in patients with CRPC. In this study, we report the relationship between pretreatment PSA doubling time during antiandrogen withdrawal and PSA flare after chemotherapy.
MATERIALS AND METHODS
Study sample
From 2002 to 2008, 98 patients with CRPC received systemic chemotherapy at our institution. Of them, the patients who had been followed up from the start of androgen deprivation therapy until 3 months after chemotherapy were included in this study. We excluded patients who did not undergo anti-androgen withdrawal before chemotherapy and those for whom serum PSA levels were not measured. Ultimately, a total of 55 patients were assessed in this study. Chemotherapy continued until disease progression, uncontrolled toxicity, or deterioration of performance occurred. The Institutional Review Board of our institution approved the execution of this retrospective study.
Calculation of PSA doubling time
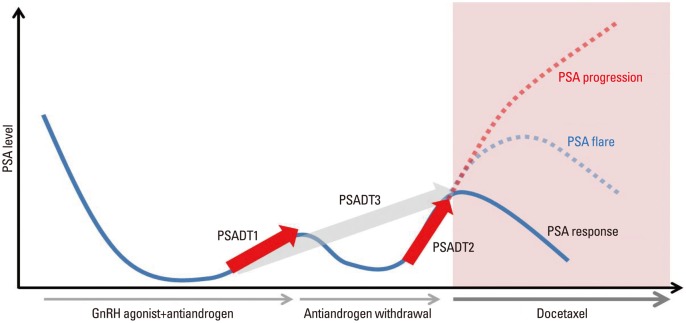
PSA doubling time was calculated using the log slope method.18 Briefly, the PSA rate variable was calculated by taking the natural log of each PSA measurement, plotting the results versus time, and then measuring the slope of the linear regression through the data points ln [PSAt]=ln [PSAinitial]+mt (t=time). PSADT was then calculated by dividing ln 2 by m.18 PSADT1 was defined as PSA doubling time calculated with the traditional interval from the first rise greater than the nadir during androgen deprivation therapy until androgen withdrawal. PSADT2 was defined as the PSA doubling time from the initial rise after anti-androgen withdrawal until the start of chemotherapy (Fig. 1). PSADT3 was defined as the PSA doubling time during the total interval from the nadir until the start of chemotherapy. The ratios of PSA doubling times were analyzed using the ratio of PSADT1 to PSADT2 (Fig. 1).
Fig. 1.
Schematic graph of PSA doubling times according to different intervals during the transient period from the diagnosis of CRPC until the end of anti-androgen withdrawal and PSA response during docetaxel chemotherapy. PSA, prostate-specific antigen; PSADT1, PSA doubling time calculated with the traditional interval from the first rise greater than the nadir during androgen deprivation therapy until androgen withdrawal; PSADT2, PSA doubling time from the initial rise after anti-androgen withdrawal until the start of chemotherapy; PSADT3, PSA doubling time during the total interval from the nadir until the start of chemotherapy.
Definition of PSA flare
PSA flare was defined as a pattern of serum PSA level initially rising after the start of chemotherapy and then falling below the baseline prechemotherapy PSA level within 12 weeks.
Data collection
The baseline parameters included patient age, performance status (Karnofsky score), serum PSA level, Gleason score, extent of disease, and previous treatments. The end point of this study was an occurrence of PSA flare after the start of chemotherapy.
Statistical considerations
For univariate analysis, the Mann-Whitney U test was used to compare the median values of the two groups, and the chi-square test and Fisher's exact test were used to compare binominal variables. Logistic regression analysis was used in the multivariate analysis. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS), version 17.0, for Windows (SPSS Inc., Chicago, IL, USA). All tests were two-sided and performed at the 5% significance level.
RESULTS
Patient characteristics
Patient characteristics of the study sample are summarized in Table 1. The median age was 69 years old (range, 54 to 84), and the median follow-up period from the start of chemotherapy was 13.5 months (range, 3.0 to 47.0). Most patients (91.0%) had a good performance score at the start of chemotherapy. There was no mortality within the 3 months after the start of chemotherapy. During three cycles of docetaxel chemotherapy, PSA flare occurred in 11 patients (20.0%). After the PSA flare, the PSA level declined to the level of response in 3 patients (27.3%) and to the level of stabilization in 8 patients (72.7%).
Table 1.
Baseline Patient Characteristics
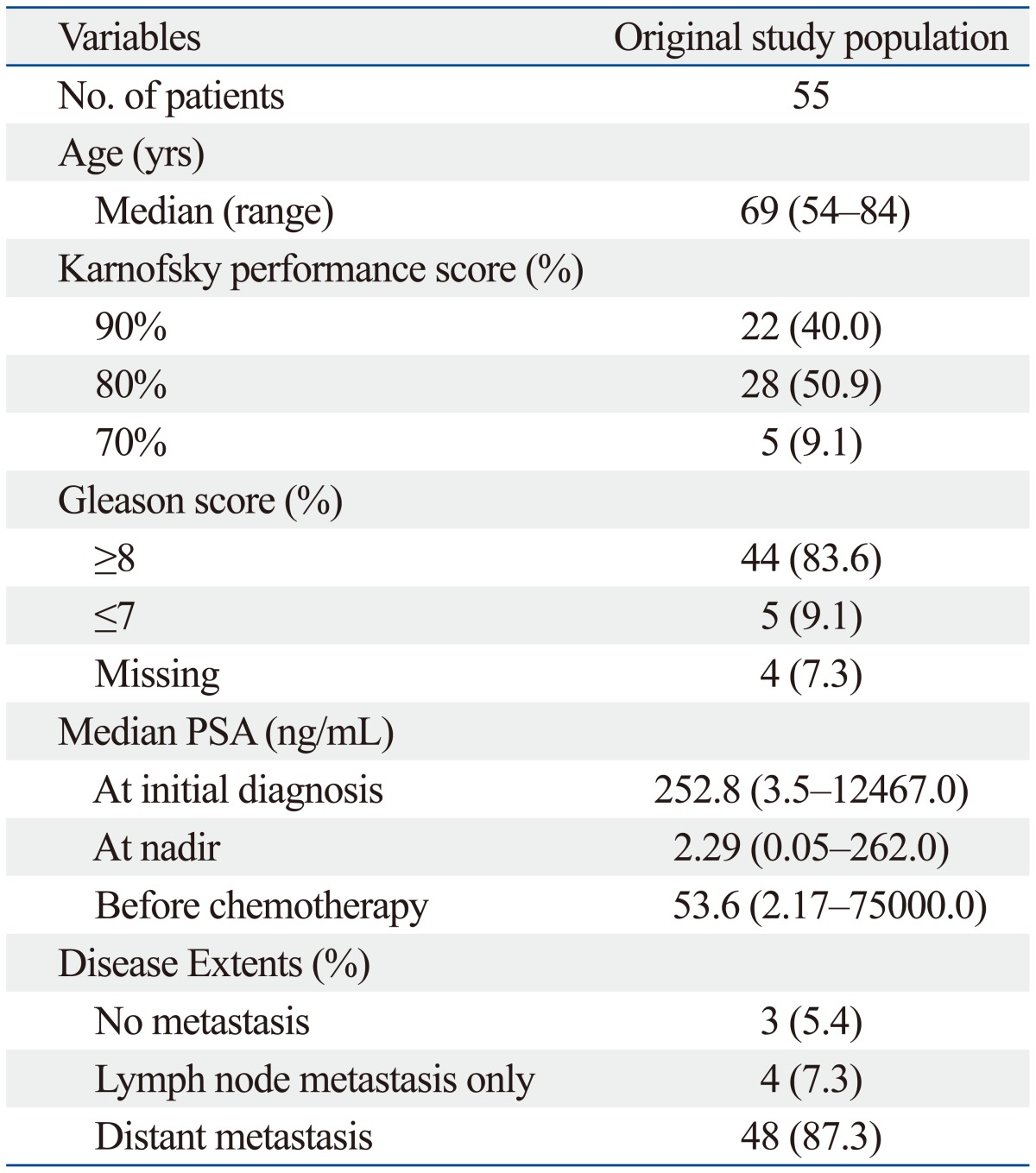
PSA, prostate-specific antigen.
PSA doubling times
Table 2 shows the mean and median values of PSA doubling times using different intervals for the 55 patients. Using the conventional PSA doubling time interval, the mean was 3.61 months, and the median was 2.37 months. In contrast, the mean and median values of the PSA doubling time during anti-androgen withdrawal were 2.42 months and 1.76 months, respectively. The mean and median PSADT2 values were shorter than the mean and median values for both PSADT1 and PSADT3. As for the ratio of PSADT2 to PSADT1, PSADT2 was shorter than PSADT1 in 33 patients (60.0%) and longer than PSADT1 in 22 patients (40.0%).
Table 2.
Pretreatment PSA Doubling Time Values Using Different Intervals in the Calculation and Ratio of PSA Doubling Times
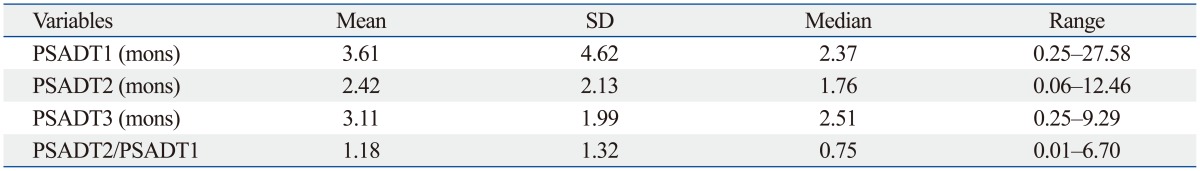
PSA doubling time was calculated using the log slope method.14 Briefly, the PSA rate variable was calculated by taking the natural log of each PSA measurement, plotting them versus time and then measuring the slope of the linear regression through the data points ln [PSAt]=ln [PSAinitial]+mt (t=time). PSADT was then calculated by dividing ln 2 by m.14 PSADT1, PSA doubling time calculated using the conventional interval from the first rise greater than the nadir during androgen deprivation therapy until the start of the next treatment; PSADT2, PSA doubling time calculated using the period from the initial rise after anti-androgen withdrawal until the start of chemotherapy; PSADT3, PSA doubling time calculated using the total period from the first rise greater than the nadir during androgen deprivation therapy until the start of chemotherapy; PSADT2/PSADT1, the ratio of PSADT2 to PSADT1; SD, standard deviation; PSA, prostate-specific antigen.
Univariate and multivariate analyses
In univariate analyses with baseline clinical parameters, no clinical factors were associated with an occurrence of PSA flare after chemotherapy (Table 3). PSA flare occurred in 9 of 29 patients who received docetaxel-based chemotherapy, while it occurred in only 2 of 26 patients who received estramustine-based chemotherapy. In univariate analyses with PSA parameters, pretreatment PSA and PSA doubling times were not associated with an occurrence of PSA flare; however, the ratio of PSA doubling time (PSADT2 to PSADT1) was associated with PSA flare (p=0.019) (Table 3 and 4). PSA flare occurred in 8 of 22 patients (36.4%) in whom PSADT2 was shorter than PSADT1; conversely, only 3 of 33 patients (9.1%) in whom PSADT2 was longer than PSADT1 experienced PSA flare. Multivariate logistic regression analysis also revealed that ratio of PSA doubling time was independently associated with an occurrence of PSA flare during chemotherapy (odds ratio=24.618; p=0.034) (Table 5).
Table 3.
Univariate Analyses of Pretreatment Binomial Variables for Occurrences of PSA Flare during Chemotherapy in Patients with CRPC
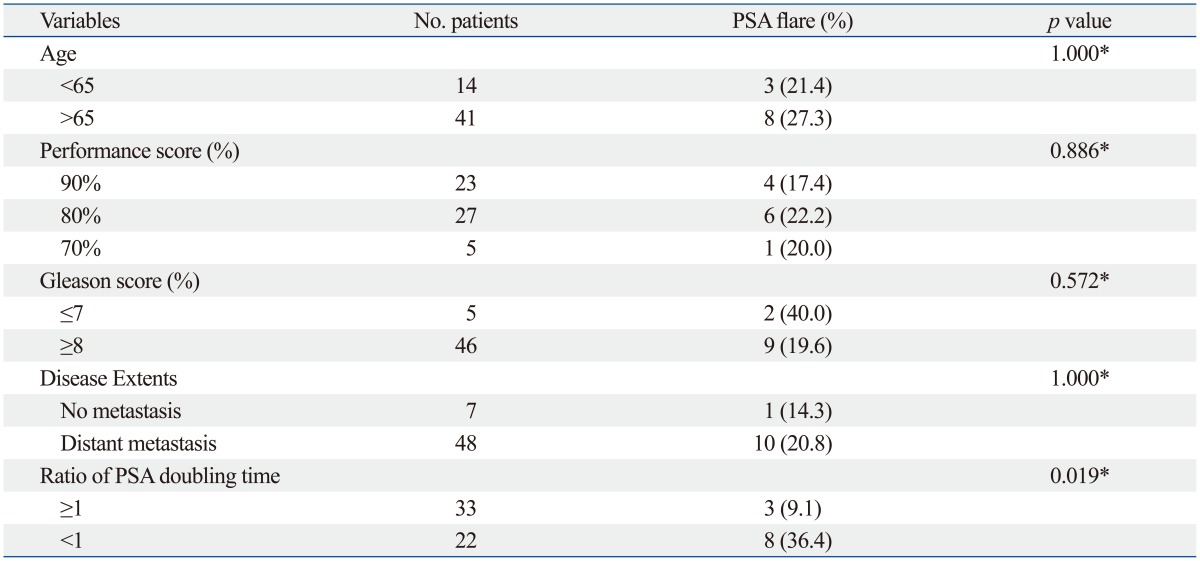
PSA, prostate-specific antigen; CRPC, castration-resistant prostate cancer.
*Fisher's exact test.
Table 4.
Univariate Logistic Regression Analyses of Pretreatment Continuous Variables for Occurrences of PSA Flare during Chemotherapy in Patients with CRPC
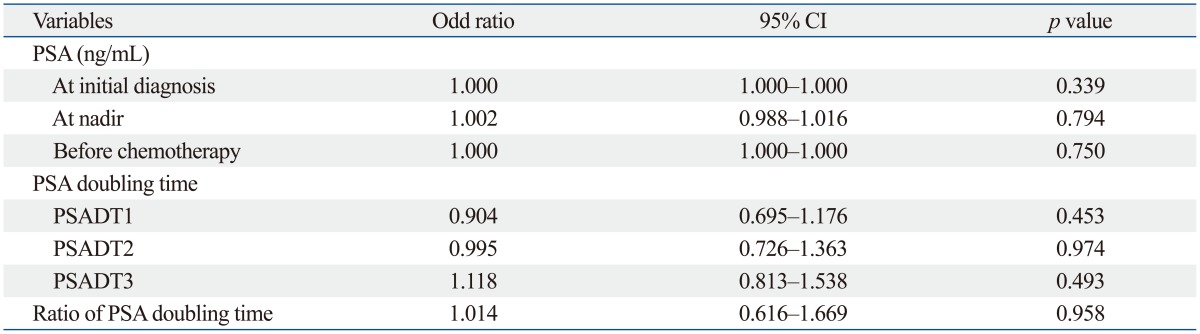
PSA, prostate-specific antigen; CRPC, castration-resistant prostate cancer; CI, confidence interval; PSADT1, PSA doubling time calculated using the conventional interval from the first rise greater than the nadir during androgen deprivation therapy until the start of the next treatment; PSADT2, PSA doubling time calculated using the period from the initial rise after anti-androgen withdrawal until the start of chemotherapy; PSADT3, PSA doubling time calculated using the total period from the first rise greater than the nadir during androgen deprivation therapy until the start of chemotherapy; ratio of PSA doubling time, PSADT2/PSADT1.
Table 5.
Multivariate Logistic Regression Analysis for Occurrences of PSA Flare during Chemotherapy in Patients with CRPC
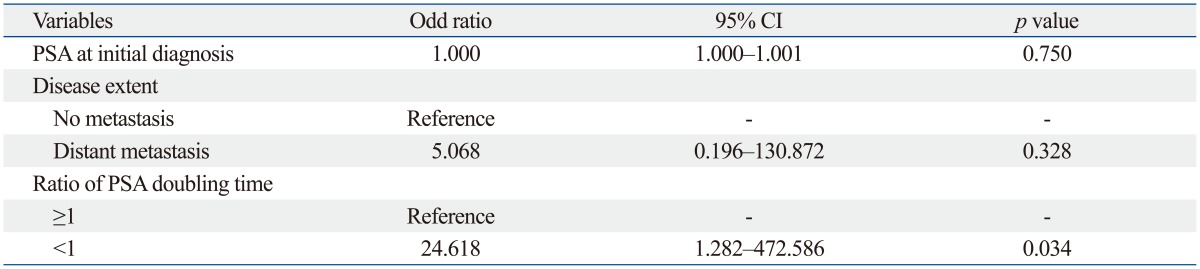
PSA, prostate-specific antigen; CRPC, castration-resistant prostate cancer; CI, confidence interval; PSADT1, PSA doubling time calculated using the conventional interval from the first rise greater than the nadir during androgen deprivation therapy until the start of the next treatment; PSADT2, PSA doubling time calculated using the period from the initial rise after anti-androgen withdrawal until the start of chemotherapy; ratio of PSA doubling time, PSADT2/PSADT1.
DISCUSSION
The PSA flare often observed during the early phase of chemotherapy suggests that PSA monitoring is occasionally inappropriate for evaluating the response of prostate cancer during the early phase of chemotherapy.9 Prediction of this phenomenon has an important implication in selecting the proper management of CRPC. Variations in PSA levels immediately before chemotherapy may be closely associated with PSA level changes during the early phase of chemotherapy. In this study, we focused on the relationship between the variation patterns of PSA levels immediately before chemotherapy and those after chemotherapy in patients with CRPC. To our knowledge, this is the first study to demonstrate a pretreatment predictor for PSA flare during chemotherapy in patients with CRPC.
PSA changes after anti-androgen withdrawal are usually not included in the conventional calculation of PSA doubling time.16,17 However, we assessed an additional PSA doubling time that was calculated using the PSA values during this period and then used the ratio of two different PSA doubling times to evaluate the PSA growth pattern during the period from nadir to chemotherapy. In this study, PSA doubling time after anti-androgen withdrawal was different from the conventional PSA doubling time in a significant proportion of patients. It is possible that the latter PSA doubling time is shorter than the former PSA doubling time, as the progression of prostate cancer is based on exponential growth. However, during anti-androgen withdrawal, 60% of patients experienced a shortening of PSA doubling time, whereas 40% experienced a prolonging of PSA doubling time. This difference was significantly correlated with the occurrence of the PSA flare phenomenon during the initial phase of chemotherapy on the basis of multivariate analysis.
Only limited information is available regarding PSA flare during the early phase of chemotherapy.19 PSA flare associated with luteinizing hormone-releasing hormone (LHRH) agonist is a well-known phenomenon; however, PSA flare during chemotherapy differs in several aspects.19 In contrast with PSA flare during LHRH agonist therapy, which is related to the transient increase in testosterone levels, PSA flare during chemotherapy is generally not related with testosterone levels or clinical symptoms and is not associated with survival outcomes in CRPC patients.12,13,16,19 PSA flare after chemotherapy appears not to be related with overall disease progression. From our results, pretreatment risk assessment of PSA flare in CRPC patients using PSA growth pattern analysis can help in decision-making with patients who have a PSA increase during the early phase of chemotherapy.
CRPC contains more heterogeneous tumor cell populations than androgen-sensitive prostate cancer in terms of androgen independence and PSA production.20 Highly metastatic prostate cancer cells are poorly differentiated and are usually less capable of producing PSA.10,20 After the acquisition of androgen independence, prostate cancer cells may be changed to tumor cells with different characteristics. Time to response with chemotherapy can also differ according to tumor cell characteristics. Differences in the proportion of tumor cell populations related to PSA production can result in different levels of initial PSA after the initiation of chemotherapy, regardless of overall response. A transient increase in PSA after the initiation of chemotherapy can reflect a relatively dominant acquisition of the tumor cell population, which delays the response to chemotherapy after the acquisition of androgen independence. Hence, the characteristics of tumor cells are not stable over time. However, current calculation methods for PSA doubling time do not reflect different tumor characteristics, as dynamic changes in several PSA values are averaged into one slope for the PSA doubling time. Therefore, comparison of PSA doubling time using an alternative calculation for PSA doubling time after anti-androgen withdrawal can be a suitable method for showing the changing characteristics of CRPC within a patient before chemotherapy during the period from nadir to the start of chemotherapy.
In summary, PSA doubling time after anti-androgen withdrawal is a useful tool for evaluating the characteristics of tumor progression of CRPC before chemotherapy, and comparison of this alternative PSA doubling time with conventional PSA doubling time is a significant pretreatment parameter for predicting an occurrence of PSA flare after the early phase of chemotherapy. However, due to the limitations of the retrospective nature of this study and the small cohort, a prospective study with a larger cohort is required to confirm our results.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Research Foundation of Korea (2013R1A1A2010724 and 2013R1A1A1005025).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Koo KC, Lee DH, Kim KH, Lee SH, Hong CH, Hong SJ, et al. Unrecognized kinetics of serum testosterone: impact on short-term androgen deprivation therapy for prostate cancer. Yonsei Med J. 2014;55:570–575. doi: 10.3349/ymj.2014.55.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YM, Park S, Kim J, Park S, Lee JH, Ryu DS, et al. Role of prostate volume in the early detection of prostate cancer in a cohort with slowly increasing prostate specific antigen. Yonsei Med J. 2013;54:1202–1206. doi: 10.3349/ymj.2013.54.5.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 6.Vaishampayan U, Hussain M. Update in systemic therapy of prostate cancer: improvement in quality and duration of life. Expert Rev Anticancer Ther. 2008;8:269–281. doi: 10.1586/14737140.8.2.269. [DOI] [PubMed] [Google Scholar]
- 7.Joung JY, Jeong IG, Han KS, Kim TS, Yang SO, Seo HK, et al. Docetaxel chemotherapy of Korean patients with hormone-refractory prostate cancer: comparative analysis between 1st-line and 2nd-line docetaxel. Yonsei Med J. 2008;49:775–782. doi: 10.3349/ymj.2008.49.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong SJ, Cho KS, Cho HY, Ahn H, Kim CS, Chung BH. A prospective, multicenter, open-label trial of zoledronic acid in patients with hormone refractory prostate cancer. Yonsei Med J. 2007;48:1001–1008. doi: 10.3349/ymj.2007.48.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong AJ, Febbo PG. Using surrogate biomarkers to predict clinical benefit in men with castration-resistant prostate cancer: an update and review of the literature. Oncologist. 2009;14:816–827. doi: 10.1634/theoncologist.2009-0043. [DOI] [PubMed] [Google Scholar]
- 10.Tsui KH, Feng TH, Chung LC, Chao CH, Chang PL, Juang HH. Prostate specific antigen gene expression in androgen insensitive prostate carcinoma subculture cell line. Anticancer Res. 2008;28:1969–1976. [PubMed] [Google Scholar]
- 11.Thuret R, Massard C, Gross-Goupil M, Escudier B, Di Palma M, Bossi A, et al. The postchemotherapy PSA surge syndrome. Ann Oncol. 2008;19:1308–1311. doi: 10.1093/annonc/mdn062. [DOI] [PubMed] [Google Scholar]
- 12.Sella A, Sternberg CN, Skoneczna I, Kovel S. Prostate-specific antigen flare phenomenon with docetaxel-based chemotherapy in patients with androgen-independent prostate cancer. BJU Int. 2008;102:1607–1609. doi: 10.1111/j.1464-410X.2008.07873.x. [DOI] [PubMed] [Google Scholar]
- 13.Nelius T, Klatte T, de Riese W, Filleur S. Impact of PSA flare-up in patients with hormone-refractory prostate cancer undergoing chemotherapy. Int Urol Nephrol. 2008;40:97–104. doi: 10.1007/s11255-007-9221-y. [DOI] [PubMed] [Google Scholar]
- 14.Fosså SD, Vaage S, Letocha H, Iversen J, Risberg T, Johannessen DC, et al. Liposomal doxorubicin (Caelyx) in symptomatic androgen-independent prostate cancer (AIPC)--delayed response and flare phenomenon should be considered. Scand J Urol Nephrol. 2002;36:34–39. doi: 10.1080/003655902317259346. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich A, Sommer F, Ohlmann CH, Schrader AJ, Olbert P, Goecke J, et al. Prospective randomized Phase II trial of pegylated doxorubicin in the management of symptomatic hormone-refractory prostate carcinoma. Cancer. 2004;101:948–956. doi: 10.1002/cncr.20455. [DOI] [PubMed] [Google Scholar]
- 16.Olbert PJ, Hegele A, Kraeuter P, Heidenreich A, Hofmann R, Schrader AJ. Clinical significance of a prostate-specific antigen flare phenomenon in patients with hormone-refractory prostate cancer receiving docetaxel. Anticancer Drugs. 2006;17:993–996. doi: 10.1097/01.cad.0000231468.69535.97. [DOI] [PubMed] [Google Scholar]
- 17.Arlen PM, Bianco F, Dahut WL, D'Amico A, Figg WD, Freedland SJ, et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179:2181–2185. doi: 10.1016/j.juro.2008.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daskivich TJ, Regan MM, Oh WK. Prostate specific antigen doubling time calculation: not as easy as 1, 2, 4. J Urol. 2006;176:1927–1937. doi: 10.1016/j.juro.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Nelius T, Filleur S. PSA surge/flare-up in patients with castration-refractory prostate cancer during the initial phase of chemotherapy. Prostate. 2009;69:1802–1807. doi: 10.1002/pros.21024. [DOI] [PubMed] [Google Scholar]
- 20.Tsui KH, Wu L, Chang PL, Hsieh ML, Juang HH. Identifying the combination of the transcriptional regulatory sequences on prostate specific antigen and human glandular kallikrein genes. J Urol. 2004;172(5 Pt 1):2029–2034. doi: 10.1097/01.ju.0000141147.96640.76. [DOI] [PubMed] [Google Scholar]