Abstract
Purpose
Although conventional neuro-navigation is a useful tool for image-guided glioma surgery, there are some limitations, such as brain shift. We introduced our methods using an identifiable marker, a "tailed bullet", to overcome the limitation of conventional neuro-navigation. A tailed bullet is an identifiable tumor location marker that determines the extent of a resection and we have introduced our technique and reviewed the clinical results.
Materials and Methods
We have developed and used "tailed bullets" for brain tumor surgery. They were inserted into the brain parenchyma or the tumor itself to help identify the margin of tumor. We retrospectively reviewed surgically resected glioma cases using "tailed bullet". Total 110 gliomas included in this study and it contains WHO grade 2, 3, and 4 glioma was 14, 36, and 60 cases, respectively.
Results
Gross total resection (GTR) was achieved in 71 patients (64.5%), subtotal resection in 36 patients (32.7%), and partial resection in 3 patients (2.7%). The overall survival (OS) duration of grade 3 and 4 gliomas were 20.9 (range, 1.2-82.4) and 13.6 months (range, 1.4-173.4), respectively. Extent of resection (GTR), younger age, and higher initial Karnofsky Performance Status (KPS) score were related to longer OS for grade-4 gliomas. There was no significant adverse event directly related to the use of tailed bullets.
Conclusion
Considering the limitations of conventional neuro-navigation methods, the tailed bullets could be helpful during glioma resection. We believe this simple method is an easily accessible technique and overcomes the limitation of the brain shift from the conventional neuro-navigation. Further studies are needed to verify the clinical benefits of using tailed bullets.
Keywords: Glioma, image-guided surgery, neuro-navigation, tailed bullet
INTRODUCTION
The role of surgery is still in debate regarding the management of glioma. Even though there is little doubt for low-grade gliomas, but there is growing evidence that radical excision is associated with improved outcomes in the management of low grade glioma and high grade glioma.1,2,3,4,5,6,7,8,9,10,11 To perform radical resection, many modalities have been used to determine the surgical margin, which is difficult to identify due to the infiltrating features of glioma. Image-guided neurosurgery is a promising modality for maximal resection. Conventional neuro-navigation is the most widely used image-guided modality but has some critical limitation such as brain shift. To overcome this limitation, intraoperative imaging using magnetic resonance image (MRI) or computed tomography (CT) has been drawing attention. However, these techniques also have some drawbacks, of which the two main examples are difficulty of use and high cost.12
We have used a new method of image-guided surgery technique, using reference points marked as 'tailed bullets'. Here, we introduce the technical details and review clinical results and its limitations.
MATERIALS AND METHODS
Tailed bullet
We have developed and used the new image-guided surgery method and reported our preliminary results.13 The "tailed bullets" was invented to identify the surgical margin during brain tumor surgery and consists of 3 parts: bullet, string, and the tag (Fig. 1). All materials were made as single use only. The bullet is made from a metal cylinder with smooth rounded head which was coated with silicon (5×2×2 mm). It is inserted into the brain parenchyma by stereotactic methods, to help identify the boundary of tumor margin. Delineation of tumor and normal brain tissue is difficult, or sometimes impossible, even under a surgical microscope. To identify each bullet when multiple bullets are used, the numbered tags were attached to each bullet, using the string between the bullets and numbered tags. The string, which connects the bullet and numbered tag, hold tight during the bullet insertion and is then buried in the brain parenchyma during operation. The string prevents missing of inserted bullet in the brain parenchyma. We used tailed bullet for two purposes: maximal surgical resection and minimal morbidity. For maximal resection, we placed the bullet on the border of tumor and normal brain, based on preoperative MRI. For minimal morbidity, if a tumor infiltrates the eloquent area, post-operative neurological worsening might be expected, and the resection is limited to the location of bullets, which are placed in relative safe areas within/around the lesion. The number of tailed bullets used in a given operation was dependent on the location, size, and shape of a lesion and the purpose of bullet insertion. We inserted more bullets near the pyramidal tract on diffusion tensor tractography merged image. We had used 5-15 (average 8-10) bullets per single surgery to fence the most part lesion.
Fig. 1.
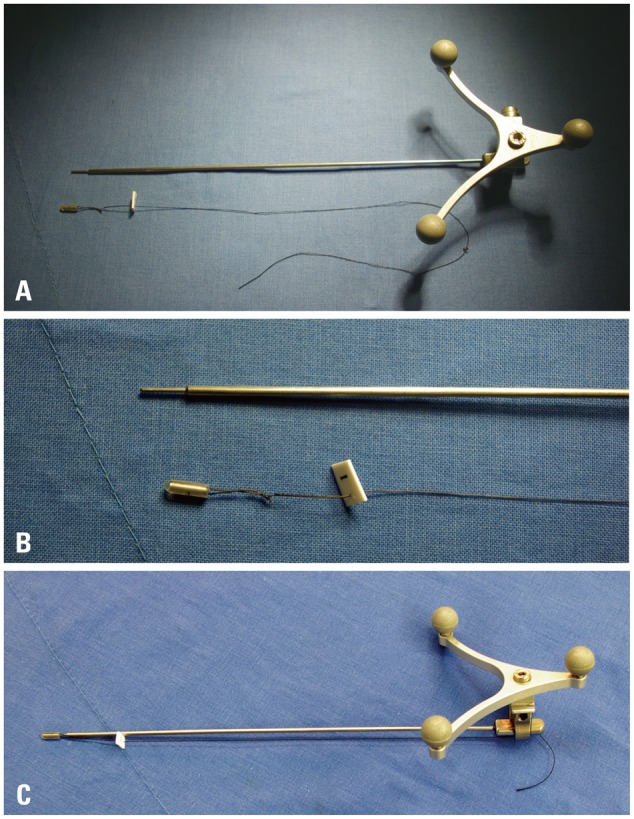
Photos of tailed bullet used in image-guided surgery. (A) A tailed bullet and probe for the insertion. Optical sensor for navigation is attached to the probe. (B) Enlarged view of the bullet. The bullet is in a cylindrical shape and is coated with silicon. The hollow space is fitted to the probe. A number is tagged to each bullet. (C) Navigation probe fitted with a bullet.
Operation procedures
During our initial experience from 1995 to 2000, we used stereotactic frame based methods to insert the bullets. Although stereotactic methods could be more accurate than the navigation method, the stereotactic frame itself caused technical difficulties during the operation (space limitation), in addition to discomfort to patients during frame fixation. After neuro-navigation became available, we have used the neuro-navigation system (Stealth Treon, Medtronic, Minneapolis, MN, USA) for bullet placements. After craniotomy, a minimal multiple dural incisions were made for the bullets' insertion to decrease the cerebrospinal fluid (CSF) drain and prevent parenchymal herniation before dural opening (Fig. 2A). We are trying to choose the bullet route avoiding the ventricle system as if tumor biopsy. After bullet insertion, resections were performed in the usual manner until the bullets were encountered. At this point, the resection was considered to have been carried out to the critical margin (Fig. 2B). Because the tailed bullets were inserted during initial steps of surgery, brain shift was minimal to none.
Fig. 2.
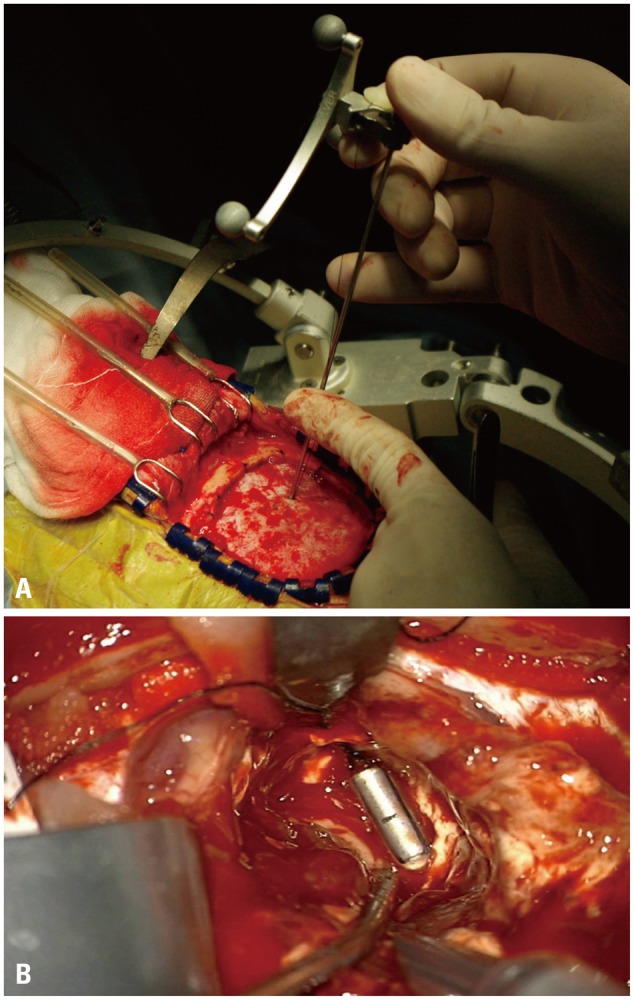
Intraoperative photos of bullet insertion. (A) After craniotomy, a small dura incision was made for tailed bullet insertion under navigation guidance. (B) Buried bullet in brain parenchyma. When the bullet is encountered, the resection has carried out to the tumor margin or eloquent location.
Patient population
A retrospective review was performed for 110 cases of glioma resected using the tailed bullet method. Medical records were reviewed for detailed clinical, radiologic, and pathologic findings. Pre- and postoperative MRI studies were reviewed through the final report by radiologists. The length of survival was calculated from the date of pathologic diagnosis to the date of death, as acquired though chart review and follow-up telephone interviews. Volumetric measurements from immediate postoperative MRIs (within 48 hours for enhancing tumors, 4 months after surgery for non-enhancing tumors) were used to evaluate the extent of resection. Enhancing tumors were defined as gross total resection (GTR) if the postoperative MRI revealed no evidence of an enhancing lesion. For non-enhancing tumors, fluid-attenuated inversion recovery and T2-weighted image were used to determine the extent of resection. A resection was designated as a subtotal resection (STR), if more than 80% of the tumor was resected. Otherwise, a resection was deemed to be a partial resection (PR) if less than 80% of the tumor was resected.
Statistical analysis
We analyzed various factors affecting survival. Statistical analyses were performed with SPSS version 19.0.0 (SPSS Inc., Chicago, IL, USA). Dichotomous data were assessed using the chi-square test. Survival was evaluated using the Kaplan-Meier estimates. Potential factors influencing survival were assessed in a multivariate analysis using the Cox proportional hazards model. Variables included in this analysis were patient age, KPS score, number of bullets used, and extent of resection (GTR vs. non-GTR). p-values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
Demographic data of the patients are summarized in Table 1. The mean age was 46.64 years (range 11-75 years), with 57 males and 53 females. The location of lesions was frontal in 57 patients (51.8%), temporal in 28 patients (25.5%), parietal in 22 patients (20.0%), occipital in 13 patients (11.8%), and cerebellar in 2 patients (1.8%). Twelve patients (10.9%) had lesions involving 2 or more lobes. By the WHO classification of glial tumors, 60 patients (54.5%) had grade 4 disease, 36 patients (32.7%) with grade 3, and 14 patients (12.7%) with grade 2 disease. GTR, STR, and PR had been achieved in 71 (64.5%), 36 (32.7%), and 3 (2.7%) patients, respectively.
Table 1.
Demographic Data and Characteristics in 110 Patients in This Study
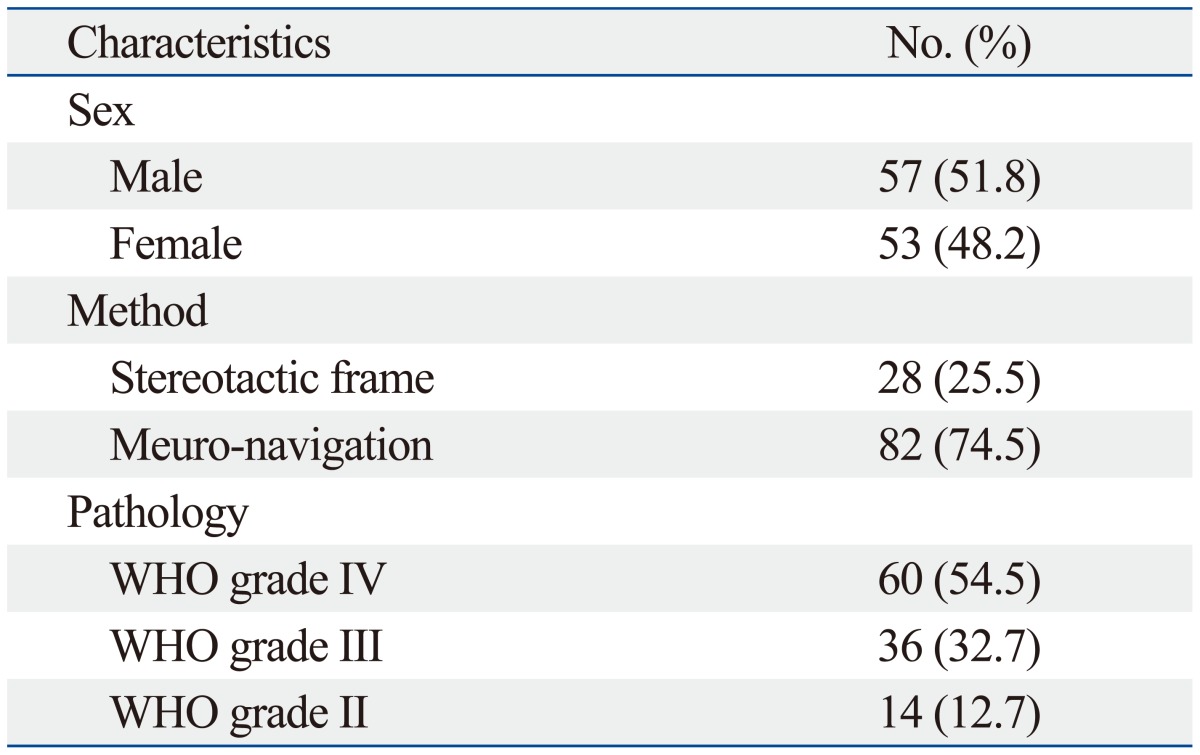
WHO, World Health Organization.
Surgical results
Among the 60 cases of grade-4 malignant glioma patients, GTR was achieved in 47 (78.3%) patients. The median length of survival was 19.3 months (range 1.4-173.4 months) for the GTR group and 10.4 months (range 1.8-48.8) for the non-GTR group (p=0.012). Survival, according to various factors, including age, sex, extent of resection, performance status (KPS score), and number of bullets used, was also analyzed. Age (p=0.012), KPS (p=0.041), and GTR (p=0.002) were associated with significant improvements in median overall survival for grade-4 malignant glioma. The number of bullets had no relation with GTR rate (p=0.90). The tailed bullets were used with the frame-based stereotactic method in 28 patients and with the neuro-navigation guided method in 82 patients. Although the accuracy of placement might be better in frame based on the stereotactic method than the neuro-navigation guided method, there was no statistical difference in the GTR rate between these two methods (p=0.861). Among the 14 cases of grade-2 gliomas, GTR was achieved in 13 patients with STR in 1 patient. There was no death or disease progressing during the follow-up period for these 14 cases.
Complications
Among the 110 cases, postoperative bleeding had occurred in 4 (3.6%) patients, with postoperative infection in 3 patients (2.7%). There were one case each of neurological worsening (motor functions), post-operative pneumonia, and pulmonary-thromboembolism.
Illustrative case 1, tailed bullet surgery for high grade glioma
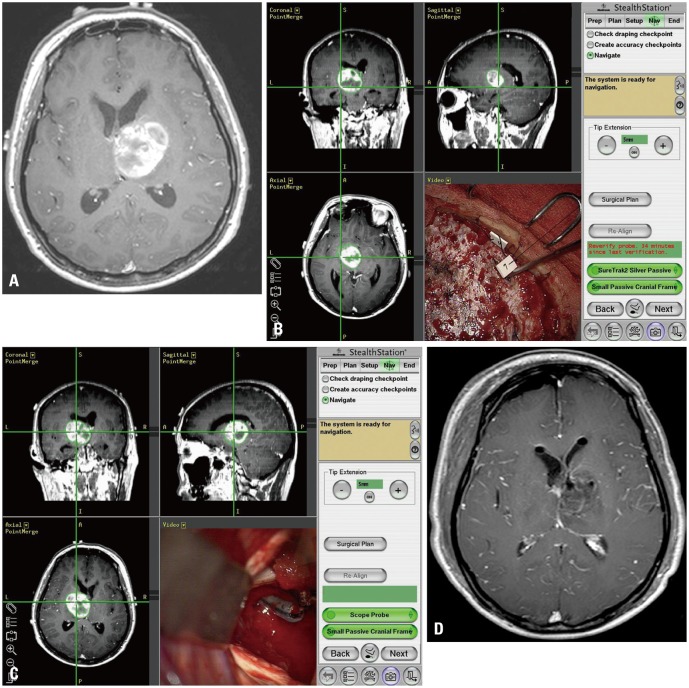
A 45-year-old female patient presented with aphasia and right-sided weakness. Neurological examination revealed a grade-3 hemiparesis on the right side. A work-up MRI study identified a mass lesion in the left basal ganglia (Fig. 3A). Initial impression was a malignant brain tumor, and a resection with tailed bullet guidance was planned. Under general anesthesia and in supine position, a three-pin rigid head fixator was applied, along with a neuro-navigation system. A C-shape craniotomy was performed around the left frontal area, above the superior sagittal sinus. After a minimal dura incision, 6 bullets were inserted to mark the tumor margins under navigation guidance (Fig. 3B and C). After bullet insertion, the dura was incised widely in a circular fashion. Resection was performed through the inter-hemispheric approach (Fig. 3D). Post operation course was uneventful. There was no further neurological deterioration, immediately after the operation. Postoperative MRI revealed the resection to be a GTR. The pathologic diagnosis was confirmed as a glioblastoma.
Fig. 3.
Illustrative case for high grade glioma. (A) A preoperative MRI reveals a 2 cm left thalamic enhancing mass. (B) After craniotomy, several bullets were inserted at tumor margins. (C) During resection, the bullets delineate tumor margin. (D) Postoperative MRI reveals no residual enhancing lesion.
Illustrative case 2, tailed bullet surgery for low grade glioma
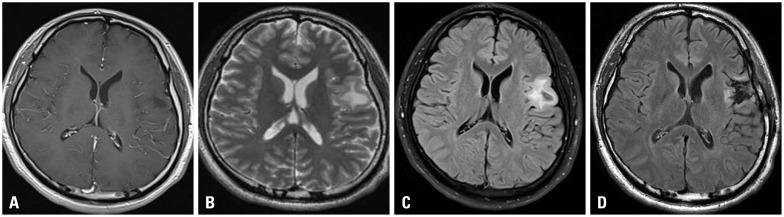
A 45-year-old female patient presented with seizure. Neurological examination revealed no abnormal finding on admission. A work-up MRI study identified a mass lesion in the left parietal area without enhancement (Fig. 4A and B). As the same manner, we performed surgical resection under tailed bullet guidance. After a minimal dura incision, 7 bullets were inserted to mark the tumor margins under navigation guidance. Post-operation course was uneventful. There was no further neurological deterioration after the operation. Postoperative MRI revealed no residual tumor (Fig. 4C and D). The pathologic diagnosis was confirmed as a diffuse astrocytoma.
Fig. 4.
Illustrative case for low grade glioma. A preoperative MRI reveals non-enhancing mass on left inferior frontal gyrus on T1 weighted image (A). The mass was shown in high signal intensity on T2 weighted image (B) and FLAIR image (C). Postoperative MRI reveals no residual high signal intensity on FLAIR image (D). FLAIR, fluid-attenuated inversion recovery.
DISCUSSION
Surgical role in glioma
In the absence of prospective randomized clinical trials, the association of aggressive tumor removal and survival in gliomas have been debated for a long time.2,3,6 For low grade glioma, though some debate, there is growing evidence that radical excision is associated with improved outcomes.5,8,9,10,11 Current standard treatment of glioblastoma is maximal safe resection, followed by concurrent radiation and chemotherapy (temozolomide) and adjuvant chemotherapy.14 Despite following this guideline, outcomes have been dismal, and thus, many clinical trials are being attempted. Stummer, et al.7 analyzed 3-randomized phase 3 trials on the role of surgery in glioblastoma and concluded that, if radical GTR can be achieved safely, complete resection appears to improve the surgical outcomes and increases the efficacy of adjuvant therapies. Thus, complete resection is the only single prognostic factor that can be modified by surgical means in the management of this disease.
Limitation of conventional image guided surgery
In order to maximize the surgical resection, especially for gliomas, several techniques have been developed in recent years. On the past time awake surgery is considered as the method of choice to precisely identify the eloquent location with intraoperative monitoring such as motor evoked potential and somatosensory evoked potential. Fluorescence dye (5-aminolevulinic acid, ALA), is also considered as the very effective tool for maximal resection.5,15,16,17 Although the classical image-guided surgery using neuro-navigation is widely used in brain tumor surgery, this technique requires the assumption that intraoperative anatomy of the brain will not change from the preoperative image, which has been found to be untrue due to parenchymal changes (brain shift and/or edema) and CSF drainage.18,19,20,21 To overcome this limitation, intraoperative MRI have been used during the resection.22 However, this method also presents considerable technical and logistical obstacles.
The main problem with intraoperative imaging update is the high cost. Despite using a less expensive type of intraoperative MRI (low teslor type), the overall initial capital cost was over US $3 million, with the annual running costs reaching 4% of this investment.12 Making matters worse, the economic lifespan of such an intraoperative MRI system is only 5 years, with zero end-of-life salvage value, according to the 2008 American Hospital Association Health Data Management Group guidelines.23 Additionally, the depreciation life of anesthesia and monitoring equipments are also 5 years, and the depreciation life of infrastructural modifications of an operating room is 10 years. Based on these figures and yearly utilization frequency, depreciation expense per procedure increases threefold over the lifetime of an intraoperative MRI.12 Moreover, high-tesla type intraoperative MRI systems require an even greater amount of investment. All equipment (surgical and anesthetic) used within the operation room has to be compatible with the magnetic field generated by the imaging machine, as well as the construction and outfit of the operation room. The MRI equipment will require an upgrade or be changed for a new one every 3-5 years due to technical developments. In countries with limited financial resources available for healthcare expenditure and in smaller hospitals, the equipment utilization would be unforgivingly high due to the small number of cases, and the cost of adopting intraoperative MRI will be prohibitive in the majority of neurosurgical departments around the world.24
Fluorescent-guided surgery (5-ALA) is another available option for maximal resection of glial tumor. This technique does not require large magnitude of investments; however, this method is limited to enhancing lesions.25,26 And also 5-ALA induced fluorescence is observed in areas that are devoid of tumor cells due to protoporphyrin IX leaks into surrounding edematous area form neoplastic cells. In such reason, only fluorescent-guided surgery may make new neurologic deficits.27,28 Also, although a trial is currently undergoing for 5-ALA in patients with low-grade gliomas, currently its indication is limited to high-grade gliomas (enhancing lesions).29 Compared to the results reported for these methods, we were able to obtain a higher rate of GTR (13/14, 92.9%) for patients with low-grade gliomas. Thus, we believe that the tailed bullet method could be helpful for resection of non-enhancing lesions.30,31
Another limitation of intraoperative MRI is technical challenges and longer operation time.32 Intraoperative image acquisition requires additional time during an operation. If the surgeon needs more accurate image update, the number of imaging will increase, which would increase the operation time-an important prognostic factor in neurosurgery. Imaging not only interrupts the flow of operation but adds substantial extra-nonsurgical time to the overall duration of a procedure.33
Advantages of "tailed bullet" using surgery
There has been several attempts to avoid locational error due to brain shift. Kelly, et al.34 reported their surgical technique using stereotaxic CT scanning data in stereotaxic space. They placed a series of 1-mm stainless steel reference balls at 5-mm intervals through the tumor along the surgical viewline. Yoshikawa, et al.35 reported their experience using navigation-guided fence-post. They try to avoid locational error due to brain shift by developing fence-post procedure. They used silicon tubes as a fence post under navigation guidances.
Similar to the previous attempts, we also tried to avoid locational error. The main advantage of our method is that it could be easily adopted and applied. The system takes one or two minutes to insert the bullets under conventional neuro-navigation guidance. We used the more bullets (up to 20) to increase accuracy of surgical margin for the larger tumor. The bullet is inserted at important landmark locations, which is used as references to adjust for errors from conventional neuro-navigation. Operative methods are not significantly different from conventional neuro-navigation guided biopsy procedure using a biopsy module. Because we insert the tailed bullets during the initial stage of an operation and because the bullets move together with brain parenchyma, errors in obtaining proper tumor margin could be minimized. Additionally, the "tail" itself could be used to guide the orientation of marker's location, and the risk of mis-orientation could also be minimized without risk of missing of bullets. As such, our method more accurately indicates the tumor margin or eloquent area when compared to a conventional neuro-navigation resection. When we remove tumor adjacent to motor tract where the bullets are already inserted, we always confirm the location using stimulation of motor evoked potential. We have been used 5-ALA during image guided surgery with "tailed bullets" for more correct localization for the malignant glioma over recent 5 years, there is sometime not enough to localize the tumor by fluorescent dye even in the malignat glimoas (unpublished data).
Another advantage of the tailed bullet method is its low cost. To reiterate, intraoperative imaging update to overcome the limitation of the conventional navigation is very expensive and need additional facilities. As of 2013, the National Health Insurance does not cover intraoperative imaging surgery in South Korea, and patients routinely pay extra $3000-5000 USD per operation in the country. This cost burden is an obstacle for low-income patients. However, our methods needs only the tailed bullets, and there is no need for additional equipment. The tailed bullets are made from simple materials, and the cost of each tailed bullet is approximately $20 USD. Even if several tailed bullets are used, the total cost of a glioma resection would be much lower than that of an intraoperative imaging method.
We do not use this technique for superficial location adjacent motor or language area to avoid to the functional damage. Although we experienced several complications in our series, no significant relationship existed between our method and these complications. Because of the small size of a bullet (less than 2 mm of diameter, smaller than a usual biopsy needle), direct visualization, and bleeding control during operation, we do not think that the 4 cases of post-operative bleeding was related to bullet placements. Other complications, such as postoperative infection or neurological deterioration, also occur after resections of any kind. Our overall complication rate is not significantly higher than those published for conventional resections.36 In this context, the tailed bullet method is relatively safe with no additional harms to patients above and beyond what is generally accepted for benefit/harm ratios of glioma resections.
Limitations of this study
As with study findings of conventional resection methods, our series also reveals that the GTR group demonstrates significant longer survival duration than that of STR group (p=0.012) for grade 4 gliomas.1,3,6,7 Additionally, we were also able to achieve a relatively high rate (92.9%) of GTR in non-enhancing glioma, such that the method appears to be a valid option to increase the accuracy of conventional neuro-navigation. However, our study has some limitations. First, there is no control group (conventional neuro-navigation group). Because this study represents the initial experience of this surgical technique, further study such as randomized trial or case control study is required. Second, the proportion of high-grade glioma cases was little high relative to the number of low-grade glioma cases. In our experience, the tailed bullet method appears more helpful for tumors with more obscure margins and for non-enhancing tumor (difficult to use 5-ALA), such as low-grade gliomas. However, our study contains more patients with grade-4 glioma (60 patients, 54.5%) than with low-grade glioma (14 patients, 12.7%). Because of this, it is difficult to say our method significantly increase the possibility of GTR in low-grade glioma, though actual GTR rate was high for this group of patients (92.9%, 13 GTR out of 14 low-grade glioma). Another limitation of our method is incomplete fencing of the lesion. Because we used a limited numbers of bullets, we inserted more bullets at functional eloquent area among the whole lesion. The last limitation is with the actual locations of inserted tailed bullets. In the early experience of tailed bullet resections, we had obtained CT scans after the insertion of "tailed bullets" prior to the resection to confirm the location (unpublished data). However, this step may increase infection potential with time consuming, and subsequently, we have stopped verifying the bullet insertion locations. Thus, it is possible that there is minute possibility of target deviation, but this has not been a problem in our experience.
In conclusion, we describe a resection technique using tailed bullets as an alternative technique to overcome the limitations of conventional neuro-navigation guided surgery. We believe that this method could easily be applied, save time, and be relatively safe in glioma surgery. Considering the disadvantages of intraoperative imaging surgery or conventional neuro-navigation guided surgery, our method could be considered as a cost effective image-guided surgery method that overcomes the limitation of conventional neuro-navigation. Further studies should be performed to compensate for the limitations of this study.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Chaichana KL, Martinez-Gutierrez JC, De la Garza-Ramos R, Weingart JD, Olivi A, Gallia GL, et al. Factors associated with survival for patients with glioblastoma with poor pre-operative functional status. J Clin Neurosci. 2013;20:818–823. doi: 10.1016/j.jocn.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moliterno JA, Patel TR, Piepmeier JM. Neurosurgical approach. Cancer J. 2012;18:20–25. doi: 10.1097/PPO.0b013e3183243f6e3. [DOI] [PubMed] [Google Scholar]
- 3.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 4.Stummer W, Meinel T, Ewelt C, Martus P, Jakobs O, Felsberg J, et al. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol. 2012;108:89–97. doi: 10.1007/s11060-012-0798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffau H. Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir (Wien) 2012;154:575–584. doi: 10.1007/s00701-011-1216-x. [DOI] [PubMed] [Google Scholar]
- 6.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 7.Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien) 2011;153:1211–1218. doi: 10.1007/s00701-011-1001-x. [DOI] [PubMed] [Google Scholar]
- 8.Pouratian N, Asthagiri A, Jagannathan J, Shaffrey ME, Schiff D. Surgery Insight: the role of surgery in the management of low-grade gliomas. Nat Clin Pract Neurol. 2007;3:628–639. doi: 10.1038/ncpneuro0634. [DOI] [PubMed] [Google Scholar]
- 9.Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308:1881–1888. doi: 10.1001/jama.2012.12807. [DOI] [PubMed] [Google Scholar]
- 10.Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012;117:1039–1052. doi: 10.3171/2012.8.JNS12393. [DOI] [PubMed] [Google Scholar]
- 11.Bianco Ade M, Miura FK, Clara C, Almeida JR, Silva CC, Teixeira MJ, et al. Low-grade astrocytoma: surgical outcomes in eloquent versus non-eloquent brain areas. Arq Neuropsiquiatr. 2013;71:31–34. doi: 10.1590/s0004-282x2012005000017. [DOI] [PubMed] [Google Scholar]
- 12.Makary M, Chiocca EA, Erminy N, Antor M, Bergese SD, Abdel-Rasoul M, et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J Magn Reson Imaging. 2011;34:1022–1030. doi: 10.1002/jmri.22739. [DOI] [PubMed] [Google Scholar]
- 13.Cho KG, Ahn YH. Stereotactic resection of the brain tumor using 'tailed bullets': technical note. J Korean Neurosurg Soc. 1998;27:619–624. [Google Scholar]
- 14.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 15.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 16.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 17.Uhl E, Zausinger S, Morhard D, Heigl T, Scheder B, Rachinger W, et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009;64(5 Suppl 2):231–239. doi: 10.1227/01.NEU.0000340785.51492.B5. [DOI] [PubMed] [Google Scholar]
- 18.Nimsky C, Ganslandt O, Hastreiter P, Fahlbusch R. Intraoperative compensation for brain shift. Surg Neurol. 2001;56:357–364. doi: 10.1016/s0090-3019(01)00628-0. [DOI] [PubMed] [Google Scholar]
- 19.Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47:1070–1079. doi: 10.1097/00006123-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Nabavi A, Black PM, Gering DT, Westin CF, Mehta V, Pergolizzi RS, Jr, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48:787–797. doi: 10.1097/00006123-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Reinges MH, Nguyen HH, Krings T, Hütter BO, Rohde V, Gilsbach JM. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien) 2004;146:369–377. doi: 10.1007/s00701-003-0204-1. [DOI] [PubMed] [Google Scholar]
- 22.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 23.Rutigliano MJ. Cost effectiveness analysis: a review. Neurosurgery. 1995;37:436–443. doi: 10.1227/00006123-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Ramina R, Coelho Neto M, Giacomelli A, Barros E, Jr, Vosgerau R, Nascimento A, et al. Optimizing costs of intraoperative magnetic resonance imaging. A series of 29 glioma cases. Acta Neurochir (Wien) 2010;152:27–33. doi: 10.1007/s00701-009-0430-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsugu A, Ishizaka H, Mizokami Y, Osada T, Baba T, Yoshiyama M, et al. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg. 2011;76:120–127. doi: 10.1016/j.wneu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Sherman JH, Hoes K, Marcus J, Komotar RJ, Brennan CW, Gutin PH. Neurosurgery for brain tumors: update on recent technical advances. Curr Neurol Neurosci Rep. 2011;11:313–319. doi: 10.1007/s11910-011-0188-9. [DOI] [PubMed] [Google Scholar]
- 27.Utsuki S, Miyoshi N, Oka H, Miyajima Y, Shimizu S, Suzuki S, et al. Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol. 2007;24:53–55. doi: 10.1007/s10014-007-0223-3. [DOI] [PubMed] [Google Scholar]
- 28.Utsuki S, Oka H, Sato S, Shimizu S, Suzuki S, Tanizaki Y, et al. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo) 2007;47:210–213. doi: 10.2176/nmc.47.210. [DOI] [PubMed] [Google Scholar]
- 29.Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115:740–748. doi: 10.3171/2011.6.JNS11252. [DOI] [PubMed] [Google Scholar]
- 30.Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 32.Hirschberg H, Samset E, Hol PK, Tillung T, Lote K. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim Invasive Neurosurg. 2005;48:77–84. doi: 10.1055/s-2004-830225. [DOI] [PubMed] [Google Scholar]
- 33.Jolesz FA. Future perspectives for intraoperative MRI. Neurosurg Clin N Am. 2005;16:201–213. doi: 10.1016/j.nec.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Kelly PJ, Kall BA, Goerss S, Earnest F., 4th Computer-assisted stereotaxic laser resection of intra-axial brain neoplasms. J Neurosurg. 1986;64:427–439. doi: 10.3171/jns.1986.64.3.0427. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa K, Kajiwara K, Morioka J, Fujii M, Tanaka N, Fujisawa H, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006;78:91–97. doi: 10.1007/s11060-005-9064-2. [DOI] [PubMed] [Google Scholar]
- 36.Wong JM, Panchmatia JR, Ziewacz JE, Bader AM, Dunn IF, Laws ER, et al. Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurg Focus. 2012;33:E16. doi: 10.3171/2012.7.FOCUS12183. [DOI] [PubMed] [Google Scholar]