Abstract
Purpose
The purpose was to evaluate the incidence and risk factors for rebleeding during cerebral angiography in ruptured intracranial aneurysms.
Materials and Methods
Among 1896 patients with ruptured intracranial aneurysms between September 2006 and December 2013, a total of 11 patients who experienced rebleeding of the ruptured aneurysms during digital subtraction angiography (DSA) were recruited in this study.
Results
There were 184 patients (9.7%) who had suffered rebleeding prior to the securing procedure. Among them, 11 patients experienced rebleeding during DSA and other 173 patients at a time other than DSA. Eight (72.7%) of the 11 patients experienced rebleeding during three-dimensional rotational angiography (3DRA). The incidence of rebleeding during DSA was 0.6% in patients with ruptured intracranial aneurysms. Multivariate logistic regression analysis showed that aneurysm location in anterior circulation [odds ratio=14.286; 95% confidence interval (CI), 1.877 to 250.0; p=0.048] and higher aspect ratio (odds ratio=3.040; 95% CI, 1.896 to 10.309; p=0.041) remained independent risk factors for rebleeding during DSA.
Conclusion
Ruptured aneurysms located in anterior circulation with a high aspect ratio might have the risk of rebleeding during DSA, especially during 3DRA.
Keywords: Cerebral angiography, intracranial aneurysm, rebleeding, subarachnoid hemorrhage
INTRODUCTION
Recent developments in neurointerventional technology and neurosurgical treatment demand a greater understanding of lesions and adjacent anatomic structures in three dimensions. Cerebral angiography, especially three-dimensional rotational angiography (3DRA), allows for acquisition of high-quality images of the cerebral arteries, showing delineation of the aneurysm neck, shape, and relationship to adjacent arteries from multiple directions in order to determine the appropriate working projection for coil embolization.1,2 3DRA has recently been regarded as an important component in the treatment of cerebral aneurysms and for proper selection for therapeutic procedures. Nevertheless, cerebral angiography carries a risk of rebleeding in patients with ruptured intracranial aneurysms. Extravasation of contrast medium due to rebleeding during cerebral angiography occurs in 0.1-8.7% of cases and is an independent factor for mortality.3,4,5 Findings from previous studies show a high rate of mortality after rebleeding during cerebral angiography.6,7,8 After 3DRA is applied to neurointerventional field, however, the incidence of rebleeding and risk factors related to rebleeding during cerebral angiography have to be re-evaluated. Thus, the purpose of this study was to evaluate the incidence and risk factors for rebleeding of ruptured intracranial aneurysms during cerebral angiography.
MATERIALS AND METHODS
This retrospective study was approved by our Institutional Review Board, and the requirement for informed consent was waived. Between September 2006 and December 2013, 1896 patients who had ruptured intracranial aneurysms underwent digital subtraction angiography (DSA) using a biplane neuroangiographic unit (Integris Allura; Philips Medical Systems, Best, the Netherlands). 3DRA was performed in order to evaluate the anatomy of the aneurysm and to determine the type of securing procedure (microsurgical clipping or endovascular coiling) required. Among the 1896 patients, a total of 184 patients experienced rebleeding prior to the securing procedure. Eleven patients who experienced rebleeding DSA were recruited in this study.
A review of radiographic images and clinical data was conducted retrospectively in order to determine risk factors that might contribute to rebleeding during DSA. Patient factors [age, gender, hypertension, diabetes, smoking, initial Hunt-Hess grade (HHG), combined intracerebral hemorrhage, and combined hydrocephalus], characteristics of aneurysms [aneurysm location (anterior or posterior circulation), size, bottleneck factor, aspect ratio, size ratio, and aneurysm type], and time from the last bleeding episode to DSA were evaluated. Non-saccular type aneurysms indicated blood blister-like aneurysms or fusiform aneurysms. Initial clinical status of the patients was graded according to the HHG. A good clinical grade was defined as HHG 1, 2, or 3 whereas a poor clinical grade was defined as HHG 4 or 5. Aneurysm size was defined as the maximum perpendicular height of the dome from the neck plane on 3DRA.9 Bottleneck factor was defined as the ratio of the maximum width of the dome to the average neck diameter on 3DRA.10 Aspect ratio was defined as the ratio of the maximum perpendicular height to the average neck diameter on 3DRA. Size ratio was defined as the ratio of the maximum aneurysm height to the average parent vessel diameter on 3DRA.9
Cerebral angiography and follow-up computed tomography scan were reviewed by two independent investigators in order to confirm rebleeding. Rebleeding was confirmed by extravasation of contrast medium from a ruptured aneurysm during DSA. Independent determination of clinical outcome, assessed based on the Glasgow Outcome Scale (GOS), was made by the two investigators during the follow-up periods (mean 25.4 months). A favorable outcome was defined as a GOS of 4 or 5 (moderate disability or better), and a poor outcome was defined as a GOS of 2 or 3 (severe disability or vegetative state). A GOS of 1 was indicative of death.
Digital subtraction cerebral angiography
All catheterization procedures were performed by means of a transfemoral approach using the Seldinger technique. A 6-Fr femoral sheath was inserted into the right common femoral artery. A 5-Fr standard diagnostic catheter was used in the angiographic procedure. Selective three- or four-vessel angiography was performed in the Towne anteroposterior and lateral projections with injection of a target vessel, such as the internal carotid artery (ICA) or the vertebral artery (VA) using a power injector. The injector pressure was 300 pounds-per-square-inch. Following performance of 2D-DSA, 3DRA was performed for a vessel with an aneurysm or a suspected aneurysm. 2D-DSA was performed using 5 mL of contrast medium (Visipaque, Nycomed Imaging, Oslo, Norway) injected at a rate of 3 mL per second in the ICA and 4 mL of contrast medium injected at a rate of 2 mL per second in the VA. 3DRA was performed with a 4.1-second 270° rotational run, with acquisition of 120 images and injection of 2 or 3 mL contrast medium per second for 6 seconds, with a delay of several seconds before the start of imaging, in the VA or the ICA, respectively. Using these parameters, the vessel tree was well-filled from the start of acquisition of images.
Statistical analysis
Statistical analysis was performed with SPSS 18.0 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used for numeric variables. The chi-square test or Fisher exact test was used for nominal variables. Univariate analysis was performed to determine the association of rebleeding with other factors. Forward stepwise logistic regression analysis was then performed on variables with an unadjusted effect, with a p value of <0.10 by univariate analysis, to determine the independent association of rebleeding with other factors. A p-value of less than 0.05 for a 95% confidence interval (CI) was considered statistically significant.
RESULTS
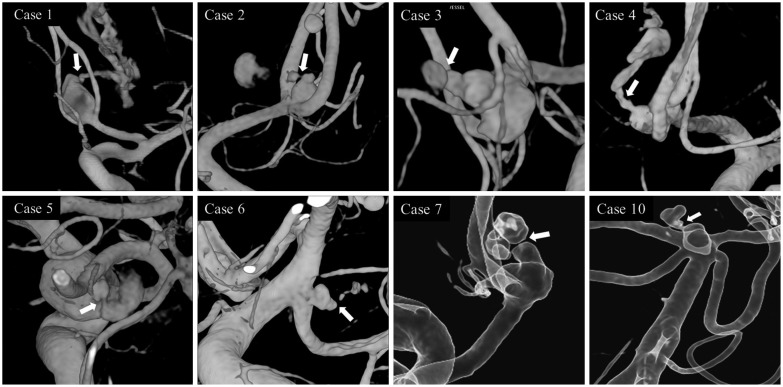
Among the 1896 patients with ruptured aneurysms, 184 patients (9.7%) suffered rebleeding prior to the securing procedure. Eleven patients experienced rebleeding during DSA and the remaining 173 patients at a time other than during DSA. The incidence of rebleeding during DSA was 0.6% in the ruptured aneurysm group (n=1896) and 5.9% in the rebleeding group (n=184). All data about the 11 patients are shown in Table 1. In eight (72.7%) of the 11 patients, rebleeding was confirmed by extravasation of contrast medium from a ruptured aneurysm during 3DRA (Fig. 1) and the other three (27.3%) patients during 2D-DSA. DSA was performed within 6 hours of the last incidence of bleeding in 7 (63.6%) patients and the mean aneurysm size was 5.65±1.75 mm (range, 3.5-9.6 mm). The mean bottleneck factor, aspect ratio, and size ratio were 1.72±0.49 (range, 0.83-2.44), 2.19±0.76 (range, 0.83-3.26), and 2.10±0.87 (range, 0.95-3.41), respectively. All 11 patients underwent endovascular coiling (simple coiling in 9 patients and stent-assisted coiling in 2 patients). Among them, 5 (45.5%) patients had favorable outcomes, but the other 6 (54.5%) had unfavorable outcomes including 5 deaths (45.5%) during the clinical follow-up periods (mean 25.4 months).
Table 1.
Data of the Patients Who Had Experienced Rebleeding of Their Ruptured Aneurysms during DSA
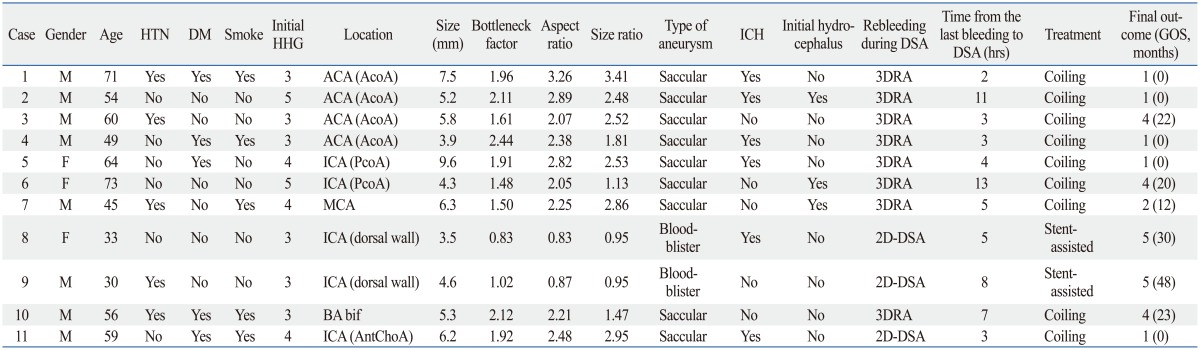
AcoA, anterior communicating artery; AntChoA, anterior choroidal artery; BA bif, basilar artery bifurcation; DM, diabetes mellitus; DSA, digital subtraction angiography; GOS, Glasgow Outcome Scale; HHG, Hunt-Hess grade; HTN, hypertension; ICA, internal carotid artery; ICH, intracerebral hemorrhage; MCA, middle cerebral artery; PcoA, posterior communicating artery; 3DRA, three-dimensional rotational angiography; ACA, anterior cerebral artery.
Fig. 1.
All pictures of patients with rebleeding during 3-dimensional rotational angiography.
Analysis of risk factors for rebleeding during DSA is shown in Table 2. Gender, combined intracerebral hemorrhage, aneurysm location in anterior circulation, bottleneck factor and aspect ratio were significant risk factors for rebleeding during DSA (p=0.042, 0.036, 0.044, 0.033, and 0.012, respectively) on univariate analysis, suggesting that male patients, patients with combined intracerebral hemorrhage, aneurysms located in anterior circulation, aneurysms with a higher bottleneck factor, or aneurysms with higher aspect ratio had a tendency toward rebleeding during DSA. Multivariate logistic regression analysis showed that aneurysm location in anterior circulation (odds ratio=14.286; 95% CI, 1.877 to 250.0; p=0.048) and aspect ratio (odds ratio=3.040; 95% CI, 1.896 to 10.309; p=0.041) remained independent risk factors for rebleeding during DSA.
Table 2.
Risk Factors for Rebleeding during Cerebral Angiography for Ruptured Intracranial Aneurysms
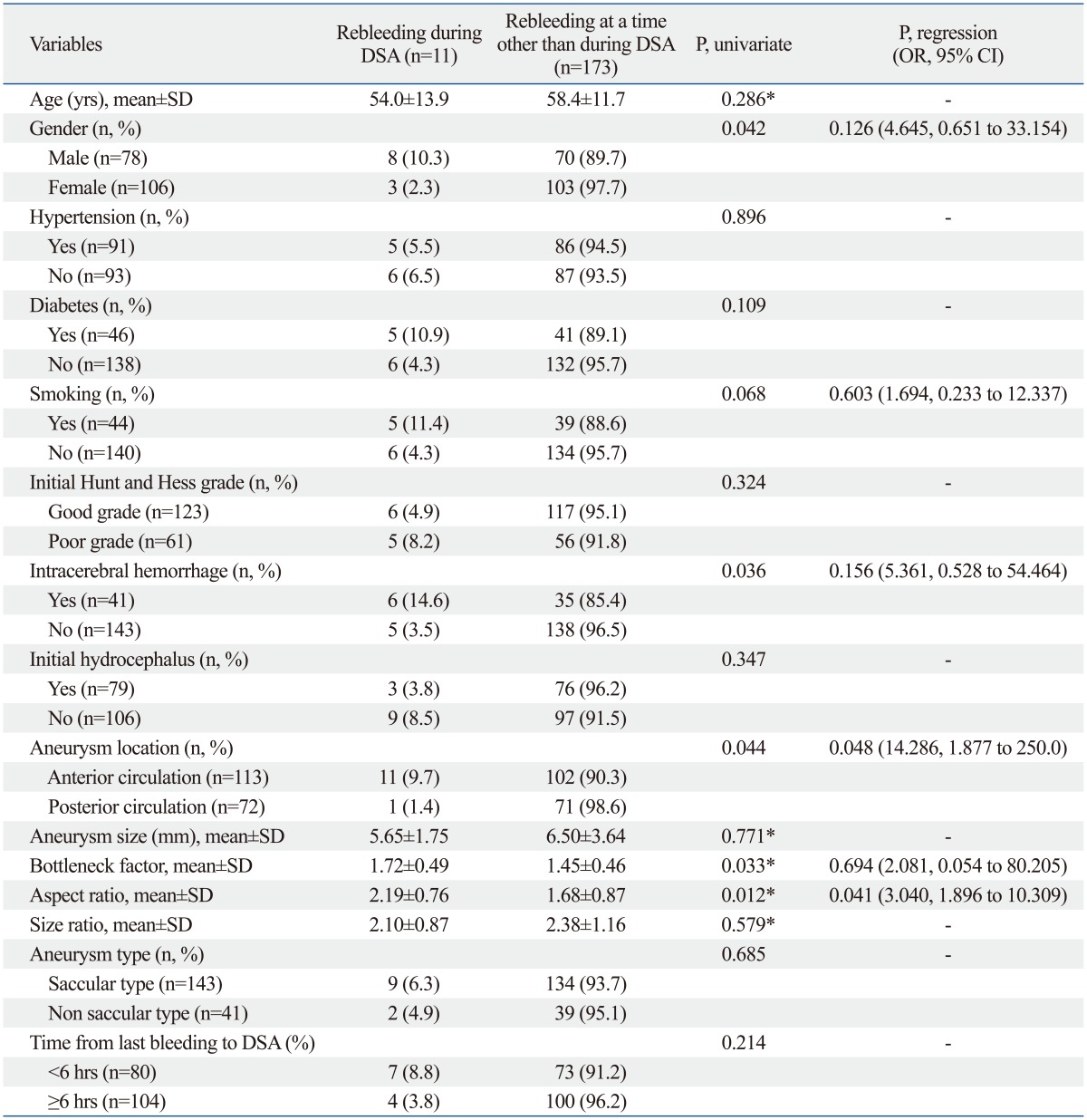
DSA, digital subtraction angiography; OR, odds ratio; CI, confidence interval; SD, standard deviation.
P, univariate, p value on univariate analysis; P, regression, p value on logistic regression analysis.
*Mann-Whitney U test.
DISCUSSION
Overall rebleeding rate of ruptured aneurysms has been reported to be about 36%.11 Previous studies revealed that the incidence of rebleeding during cerebral angiography occurred in 0.1-8.7% and was an independent factor for mortality.3,4,5,12 In our study, overall rebleeding rate was 9.7% (184/1896), showing a lower rebleeding rate compared to previous studies, and the incidence of rebleeding during DSA was 0.6% in the ruptured aneurysm group (n=1896) and 5.9% in the rebleeding group (n=184). Multivariate analysis showed that aneurysm location (p=0.048) and AR (p=0.041) was an independent risk factors for rebleeding during DSA.
One reason for rebleeding during DSA is thought to be due to an increase in intra-arterial pressure caused by power injection of contrast medium.4 The abrupt rise in intra-arterial pressure could be transmitted to the vessel wall, which could cause rebleeding of the ruptured aneurysm. In a previous study,13 internal aneurysmal pressure during cerebral angiography was measured, and the injection of contrast medium was found to result in an abrupt increase in intra-aneurysmal pressure of 5-23 mm Hg in the absence of changes in systemic blood pressure. As for the angiographic techniques, the injection volume and pressure of contrast medium and its chemical irritant properties are considered important.14 Using a power injector, the linear rate rise of the injector allows for a linear acceleration of the contrast injection over the first timing interval of the injector. A rate rise in cerebral angiography refers to a progressive acceleration of the rate of contrast over the first second of the injection.15 For 3DRA, a greater volume of contrast medium should be injected over a longer duration so that the pressure wave of the injected contrast medium would overcome the weak wall of the ruptured site.16 van Rooij, et al.17 presented a case of re-ruptured giant carotid-ophthalmic aneurysm during 3DRA. Rebleeding occurred from the previous rupture point at the top with immediate contrast extravasation. Kono, et al.18 incidentally acquired 3-dimensional images before and at the moment of rebleeding of a ruptured aneurysm. On the basis of computational fluid dynamics simulation, they suggested a possible mechanism of rebleeding as follows; low wall shear stress at end diastole caused degeneration and thinning of the aneurysm wall, and high pressure at peak systole resulted in rupture of the thinning wall. The suggested injection rates and volumes in 2D-DSA,15 depending on the vessels, were 3-4 mL/second for a total of 6-8 mL on the VA and 4-5 mL/second for a total of 8-10 mL on the ICA as well as the common carotid artery. In our present study, 3DRA protocol included an injection of 3 or 4 mL contrast medium per second for 6 seconds, with a delay of seconds before the start of imaging, in the VA or the ICA, respectively. The rate of contrast injection and the volume of contrast in our protocol were slower and less amount compared to the suggestion. However, the optimal injection volume should be required to obtain good images while minimizing hemodynamic stress during 3DRA. This might have reduced the rebleeding rate in our practice.
Aspect ratio, defined as aneurysm height divided by the neck diameter, is the most commonly-studied shape parameter and has been found to consistently correlate with rupture of cerebral aneurysms.9,19,20 In the present study, however, a ruptured aneurysm with a high aspect ratio (mean, 2.19±0.76) achieved statistical significance as a risk factor in multivariate analysis (odds ratio=3.040; 95% CI, 1.896 to 10.309; p=0.041) for rebleeding during DSA. In the proximal walls of high aspect ratio sacs, three mural layers were found while these layers are typically absent in the thinner distal wall, which is composed of collagenized tissue. In pulsatile computational fluid dynamics in animal models, a single, stable recirculation zone was present in all low aspect ratio sacs (aspect ratio <1.8), whereas a second, transient recirculation zone was found in the superior aspect of the aneurysm dome for all the aneurysms with high aspect ratio sacs (aspect ratio >2.2).21 Thus, an aneurysm with a high aspect ratio seems to rupture more easily than an aneurysm with a low aspect ratio.
Regarding the aneurysm locations, a ruptured aneurysm in the anterior circulation in our study had a tendency to show rebleeding during DSA (odds ratio=14.286; 95% CI, 1.877 to 250.0; p=0.048). Aoyagi and Hayakawa6 reported that aneurysms of the ICA appeared to have an elevated rebleeding rate during cerebral angiography. On the other hand, Sampei, et al.14 indicated that more frequent occurrence of re-rupture was associated with vertebra-basilar aneurysms and anterior communicating artery aneurysms. However, lower rebleeding incidence of aneurysms in the posterior circulation in our study did not agree with the results of Sampei, et al.14
The interval between subarachnoid hemorrhage and DSA has been considered to be a risk factor for rebleeding.6,7,8,22,23 In ruptured intracranial aneurysms, the rebleeding rate was about 30% with peak times of occurrence within 24 hours, especially within 6 hours.4,8 Rebleeding within 6 hours after the initial subarachnoid hemorrhage has been estimated to occur as high as in 48.6% of patients.11 In our study, however, there were 80 (4.2%) of the 1896 patients who experienced rebleeding within 6 hours of the ictus. Regarding the proper timing of cerebral angiography, it has been reported that the most important factor for rebleeding during cerebral angiography appears to be the short time interval from the initial insult to the angiography.8,17,24 Six hours following an episode has been reported as the most dangerous time for rebleeding. The incidence of rebleeding during angiography performed within a period of 6 hours after an episode has been reported as 3.1-3.3%,3,17 which is higher than the rate of rebleeding during angiography beyond 6 hours. Kusumi, et al.7 suggested that cerebral angiography performed within 3 hours of the initial insult carries a high risk of rebleeding, even if the procedure is performed under deep anesthesia with maintenance of normal blood pressure. Compared to the previous data, however, there was no significance in the time from the last bleeding episode to cerebral angiography in our study.
In conclusion, neurointerventionists should not neglect and do consider the incidence of rebleeding during DSA even though the incidence was not very high (0.58%). Ruptured aneurysms located in anterior circulation with a high aspect ratio might have risk of rebleeding during DSA, especially during 3DRA.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Abe T, Hirohata M, Tanaka N, Uchiyama Y, Kojima K, Fujimoto K, et al. Clinical benefits of rotational 3D angiography in endovascular treatment of ruptured cerebral aneurysm. AJNR Am J Neuroradiol. 2002;23:686–688. [PMC free article] [PubMed] [Google Scholar]
- 2.Hirai T, Korogi Y, Suginohara K, Ono K, Nishi T, Uemura S, et al. Clinical usefulness of unsubtracted 3D digital angiography compared with rotational digital angiography in the pretreatment evaluation of intracranial aneurysms. AJNR Am J Neuroradiol. 2003;24:1067–1074. [PMC free article] [PubMed] [Google Scholar]
- 3.Komiyama M, Tamura K, Nagata Y, Fu Y, Yagura H, Yasui T. Aneurysmal rupture during angiography. Neurosurgery. 1993;33:798–803. doi: 10.1227/00006123-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh H, Hayakawa K, Nishimura K, Okuno Y, Teraura T, Yumitori K, et al. Rerupture of cerebral aneurysms during angiography. AJNR Am J Neuroradiol. 1995;16:539–542. [PMC free article] [PubMed] [Google Scholar]
- 5.Zaehringer M, Wedekind C, Gossmann A, Krueger K, Trenschel G, Landwehr P. Aneurysmal re-rupture during selective cerebral angiography. Eur Radiol. 2002;12(Suppl 3):S18–S24. doi: 10.1007/s00330-002-1460-9. [DOI] [PubMed] [Google Scholar]
- 6.Aoyagi N, Hayakawa I. Rerupture of intracranial aneurysms during angiography. Acta Neurochir (Wien) 1989;98:141–147. doi: 10.1007/BF01407340. [DOI] [PubMed] [Google Scholar]
- 7.Kusumi M, Yamada M, Kitahara T, Endo M, Kan S, Iida H, et al. Rerupture of cerebral aneurysms during angiography--a retrospective study of 13 patients with subarachnoid hemorrhage. Acta Neurochir (Wien) 2005;147:831–837. doi: 10.1007/s00701-005-0541-3. [DOI] [PubMed] [Google Scholar]
- 8.Yasui T, Kishi H, Komiyama M, Iwai Y, Yamanaka K, Nishikawa M. Very poor prognosis in cases with extravasation of the contrast medium during angiography. Surg Neurol. 1996;45:560–564. doi: 10.1016/0090-3019(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 9.Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008;63:185–196. doi: 10.1227/01.NEU.0000316847.64140.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH, et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007;61:716–722. doi: 10.1227/01.NEU.0000298899.77097.BF. [DOI] [PubMed] [Google Scholar]
- 11.Tanno Y, Homma M, Oinuma M, Kodama N, Ymamoto T. Rebleeding from ruptured intracranial aneurysms in North Eastern Province of Japan. A cooperative study. J Neurol Sci. 2007;258:11–16. doi: 10.1016/j.jns.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 12.Klisch J, Weyerbrock A, Spetzger U, Schumacher M. Active bleeding from ruptured cerebral aneurysms during diagnostic angiography: emergency treatment. AJNR Am J Neuroradiol. 2003;24:2062–2065. [PMC free article] [PubMed] [Google Scholar]
- 13.Sorimachi T, Takeuchi S, Koike T, Minakawa T, Tanaka R. Intra-aneurysmal pressure changes during angiography in coil embolization. Surg Neurol. 1997;48:451–457. doi: 10.1016/s0090-3019(97)00278-4. [DOI] [PubMed] [Google Scholar]
- 14.Sampei T, Yasui N, Mizuno M, Nakajima S, Ishikawa T, Hadeishi H, et al. Contrast medium extravasation during cerebral angiography for ruptured intracranial aneurysm--clinical analysis of 26 cases. Neurol Med Chir (Tokyo) 1990;30:1011–1015. doi: 10.2176/nmc.30.1011. [DOI] [PubMed] [Google Scholar]
- 15.Osborn AG. Diagnostic Cerebral Angiography. 2nd ed. Philadelphia: Lippicott Williams & Wilkins; 1995. pp. 437–439. [Google Scholar]
- 16.Manabe H, Takemura A, Hasegawa S, Nagahata M, Iko Y. Extravasation from rupturing aneurysm demonstrated by 3D digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1370–1371. [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij WJ, Sluzewski M, Peluso JP. Rupture of a giant carotid-ophthalmic aneurysm. Lancet. 2011;378:56. doi: 10.1016/S0140-6736(10)61261-5. [DOI] [PubMed] [Google Scholar]
- 18.Kono K, Fujimoto T, Shintani A, Terada T. Hemodynamic characteristics at the rupture site of cerebral aneurysms: a case study. Neurosurgery. 2012;71:E1202–E1208. doi: 10.1227/NEU.0b013e31826f7ede. [DOI] [PubMed] [Google Scholar]
- 19.Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. 2001;48:495–502. doi: 10.1097/00006123-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Weir B, Amidei C, Kongable G, Findlay JM, Kassell NF, Kelly J, et al. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg. 2003;99:447–451. doi: 10.3171/jns.2003.99.3.0447. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Z, Durka MJ, Kallmes DF, Ding Y, Robertson AM. Can aspect ratio be used to categorize intra-aneurysmal hemodynamics?--A study of elastase induced aneurysms in rabbit. J Biomech. 2011;44:2809–2816. doi: 10.1016/j.jbiomech.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005;62:410–416. doi: 10.1001/archneur.62.3.410. [DOI] [PubMed] [Google Scholar]
- 23.Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001;32:1176–1180. doi: 10.1161/01.str.32.5.1176. [DOI] [PubMed] [Google Scholar]
- 24.Koga H, Kaneko M, Hosaka Y. Extravasation from aneurysms during angiography. Surg Neurol. 1979;12:453–456. [PubMed] [Google Scholar]