Abstract
Purpose
The aim of this study was to evaluate perioperative complications of robot-assisted laparoscopic surgery in gynecology.
Materials and Methods
Patients who underwent elective robot-assisted laparoscopic surgery between February 2006 and December 2013 were identified. Robotic procedures were performed using the da Vinci robotic system. Patient demographic data and operative outcomes were prospectively collected in a computerized database and extracted for this study.
Results
Two hundred and ninety eight patients were identified during the study period. One case was converted to conventional laparoscopy due to mechanical failure of the robot system before the procedure and excluded from review. The median age and body mass index of patients were 48 years and 23.0 kg/m2, respectively. The majority (n=130, 43.6%) of operative procedures was radical hysterectomy, followed by endometrial cancer staging (n=112, 37.6%), total hysterectomy (n=39, 13.1%), and myomectomy (n=17, 5.7%). The median operative time, estimated blood loss, and postoperative hospital stay were 208.5 min, 184.8 mL, and 8.9 days, respectively. The overall complication rate was 18.8% and that for only oncologic cases was 16.1%. Intraoperative complications (n=5, 1.7%) consisted of three vessel injuries, one bowel content leakage during an appendectomy during endometrial cancer staging and one case of bladder injury during radical hysterectomy. Early and late postoperative complications were 14.4% and 2.7%, respectively. Five patients (1.7%) experienced grade 3 complications according to Clavien-Dindo classification and therefore needed further intervention.
Conclusion
Robot-assisted laparoscopic surgery is a feasible approach in gynecology with acceptable complications.
Keywords: Robotics, postoperative complications, laparoscopy
INTRODUCTION
Laparoscopic surgery offers significant postoperative advantages, including faster recovery time, improved cosmesis, shorter length of hospital stay, lower cost, and reduced pain, compared to laparotomy.1,2 However, conventional laparoscopy still has its drawbacks, such as limited mobility of laparoscopic instruments, two dimensional view, poor ergonomic position for the surgeon, and a steep learning curve. Robot-assisted laparoscopic surgery is considered an alternative approach that addresses the current limitations of conventional laparoscopic surgery. The robotic platform improves visualization and allows for greater precision and a shorter learning curve in performing surgical tasks.3,4 Numerous reports have demonstrated the feasibility of robotic surgery in gynecology.5,6 Therefore, the use of robotic systems in minimally invasive gynecologic surgeries has increased substantially over the past decade.
Nevertheless, there are several shortcomings of robotic platforms, such as the absence of tactile feedback in the robotic arms and the need for ports of larger diameter, compared to conventional laparoscopy. These weaknesses might lead to increases in operative complications. Although a number of previous studies have addressed the complications of conventional laparoscopic approaches in gynecology, only a few studies have reported on complications during robot-assisted laparoscopic procedures using a standardized tool.7,8,9,10 As well, most of the literature describes the complications of robotic surgery using four robotic arms and categorizes complications as either major or minor. Meanwhile, accurate assessment of the complication rates of robot-assisted laparoscopic procedures may be challenging due to underestimation by reporting bias, incomplete data collection, and the lack of standardization in defining complications among institutions: unbiased reports of the risks associated with robotic surgery are important for training purposes, as well as for future development of advanced operative instruments and techniques.
Accordingly, the purpose of this study was to identify the overall and specific complications associated with robot-assisted laparoscopic gynecologic surgery using three robotic arms at a single high-volume institution.
MATERIALS AND METHODS
Between February 2006 and December 2013, patients who underwent robotic surgery at the Department of Obstetrics and Gynecology, Severance Hospital for gynecologic conditions were identified. All robot-assisted surgery was performed using the S or Si da Vinci system (Intuitive Surgical, Inc., Sunnyvale, CA, USA). Data pertaining to patient demographics, diagnosis, perioperative outcomes and complications were prospectively collected in a computerized database. Data were retrieved from the database for a retrospective review. This study was approved by the Institutional Review Board of Yonsei University College of Medicine.
All procedures were performed by surgeons experienced and proficient in advanced laparoscopic gynecologic procedures. The surgical team consisted of a chief resident or fellow as surgical assistants at the bedside or at the caudal part of the patient for uterine manipulation. A RUMI uterine manipulator equipped with a Koh colpotomy ring and a vaginal balloon pnuemo-occluder (Cooper Surgical Inc., Trumball, CT, USA) was routinely placed for adequate pelvic exposure, unless failure occurred due to a bulky cervical mass or cervical stenosis. All robotic surgeries were performed using Maryland bipolar forceps on the left robotic arm and a permanent cautery spatula or needle holder on the right robotic arm. Total operative time was recorded as the time from the first skin incision to the last port site skin closure. Docking time was defined as the time to position the robotic column and install the robotic arms securely to the port sites. Console time was defined as the time the surgeon spent at the robotic console during the main procedure. Ports were placed after creating pneumoperitoneum by Veress needle insertion or by an open Hasson method at the umbilicus. Four trocars with three robotic arms were used as a routine port placment: a 12-mm conventional laparoscopic trocar at the umbilicus for the camera (2 cm upwards from the umbilicus for endometrial cancer staging); two 8-mm lateral robotic trocars at each lower quadrant of the abdomen at 2 to 3 cm below the umbilical level; and a fourth conventional trocar (either 5 or 10 mm) at the mid-distance between the umbilicus and the left robotic arm for the bedside assistant. The bedside assistant assisted in procedures, such as suction, irrigation, retraction of tissues, and lymph node retrieval, through the 5 or 10 mm trocar placed on the left side of the patient.
The surgical management of cervical cancer included radical hysterectomy with removal of bilateral pelvic lymph nodes, as described in our previous report.7 All pelvic lymphadenectomies were performed in a standard da Vinci system setup (robotic tower in between the patients' legs in a steep Trendelenburg position). The decision to perform paraaortic lymph node dissection was made at the surgeon's discretion. Modified radical or radical hysterectomy was performed according to stage. For surgical staging in endometrial cancer, extended total hysterectomy with bilateral pelvic lymph node dissection, as well as routine paraaortic lymph node dissection, was performed. The vaginal cuff was closed either intracorporeally using interrupted/continuous sutures of 1-Vicryl (Ethicon, Piscataway, NJ, USA) or extracorporeally using a Clarke-Reich knot pusher. For myomectomy, the uterine wall was repaired with interrupted sutures of 1-Vicryl or continuously with barbed suture, V-loc (Covidien, Dublin, Ireland). Excised myomas were morcellated or cut and extracted from the umbilicus after placing the specimen into a Lap bag (Sejong Medical, Paju, Korea). Upon completion of the procedure, the fascia of the port sites greater than 8 mm in diameter were closed with interrupted suture using 1-0 Vicryl (Ethicon, Piscataway, NJ, USA) or by an Endoclose suture device (Tyco Auto Suture International Inc., Norwalk, CT, USA). The skin at trocar sites was not sutured, but approximated with Steri-Strips (3M, St. Paul, MN, USA).
Patients who underwent a myomectomy, hysterectomy, or adnexectomy received only prophylactic antibiotics 30 minutes before surgery. For those patients who underwent staging surgery and radical hysterectomy, antibiotics were used for postoperative five days. Indwelling bladder catheters were removed on postoperative day one for those who underwent a myomectomy, day two for those who underwent a hysterectomy or endometrial cancer staging surgery, and on days 7 to 12 after radical hysterectomy. A hemovac drain was left in the pelvic cavity in case of a radical hysterectomy and staging surgery, and removed around postoperative day 5, according to the drainage amount. All patients were permitted sips of water beginning six hours after an inadvertent benign operation. A clear liquid diet was offered as the first meal after passing flatus. The next meal consisted of soft foods, followed by a general diet.
Complications were categorized as intraoperative and postoperative events. Postoperative complications were further divided into early (less than postoperative 6 weeks) and late (after postoperative 6 weeks) events. Clavien-Dindo classification was used to stratify complications into five grades according to their therapeutic interventions.11
Descriptive statistical analysis was performed with IBM SPSS software version 20 for Windows (SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was used to verify standard normal distributional assumptions. Analysis of variance was used for multivariable analysis of continuous variables.
RESULTS
Two hundred ninety nine patients underwent robotic surgery during the study period. One patient who was diagnosed with cervix cancer and initially planned to undergo robot-assisted radical surgery was converted to conventional laparoscopy due to mechanical failure of the robotic system. Finally, the surgical outcomes of 298 patients were evaluated in this study.
Table 1 lists the patient demographics. The median age of the patients was 48 years (range 24-78 years). The median body mass index was 23.0 kg/m2 (range 17.0-40.0 kg/m2). Fifty-four patients (18.1%) reported having at least one prior abdominal-pelvic surgery. One or more medical co-morbidities were reported in approximately one fourth of the patients (26.8%). Operative procedures included 17 myomectomies (5.7%), 39 total hysterectomy with or without adnexectomy (13.1%), 112 staging surgeries for endometrial cancer (37.6%), and 130 radical hysterectomies for cervical cancer (43.6%). Ten out of 39 patients (25.6%) underwent hysterectomy±adnexectomy due to uterine and/or adnexal invasion of rectal, stomach, or bladder cancer.
Table 1.
Patient Demographics
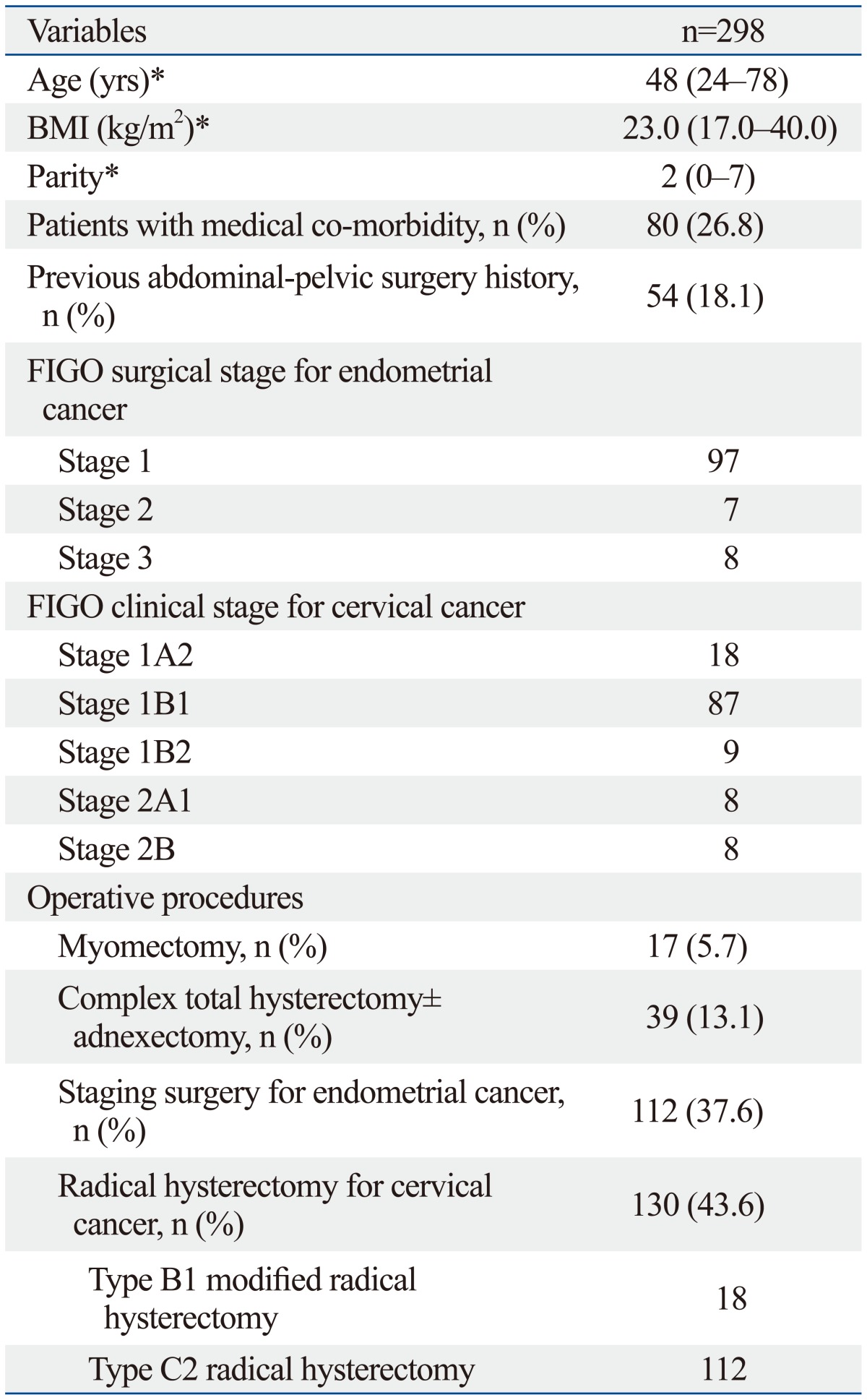
BMI, body mass index; FIGO, Federation of Gynecology and Obstetrics.
*Data are given as median (range).
Surgical outcomes are shown in Table 2. The overall mean operative time was 208.5±73.9 minutes, console time was 159.2±66.4 minutes, and docking time was 5.1±3.8 minutes. The mean estimated blood loss (EBL) was 184 mL. The longest console time and highest EBL were recorded after myomectomy, with mean console time of 276.4 minutes and blood loss of 280 mL, respectively. Myomectomy was the latest procedure type commenced at our institution (year 2010). The overall mean hospital stay was 8.9 days. Longest hospital stay was seen after radical hysterectomy (mean 11.4±5.2 days) due to postoperative bladder catheter training. There was no conversion to laparotomy. Accurate measurement of docking time, blood loss, and hospital stay were not possible for hysterectomy cases, since 25% were performed as a co-operation with other departments.
Table 2.
Surgical Outcomes According to Procedure
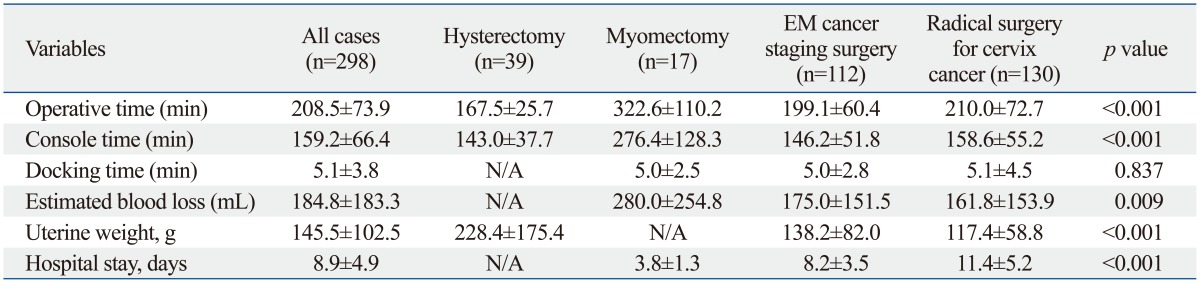
EM, endometrial; N/A, not applicable.
Data are given as mean±standard deviation.
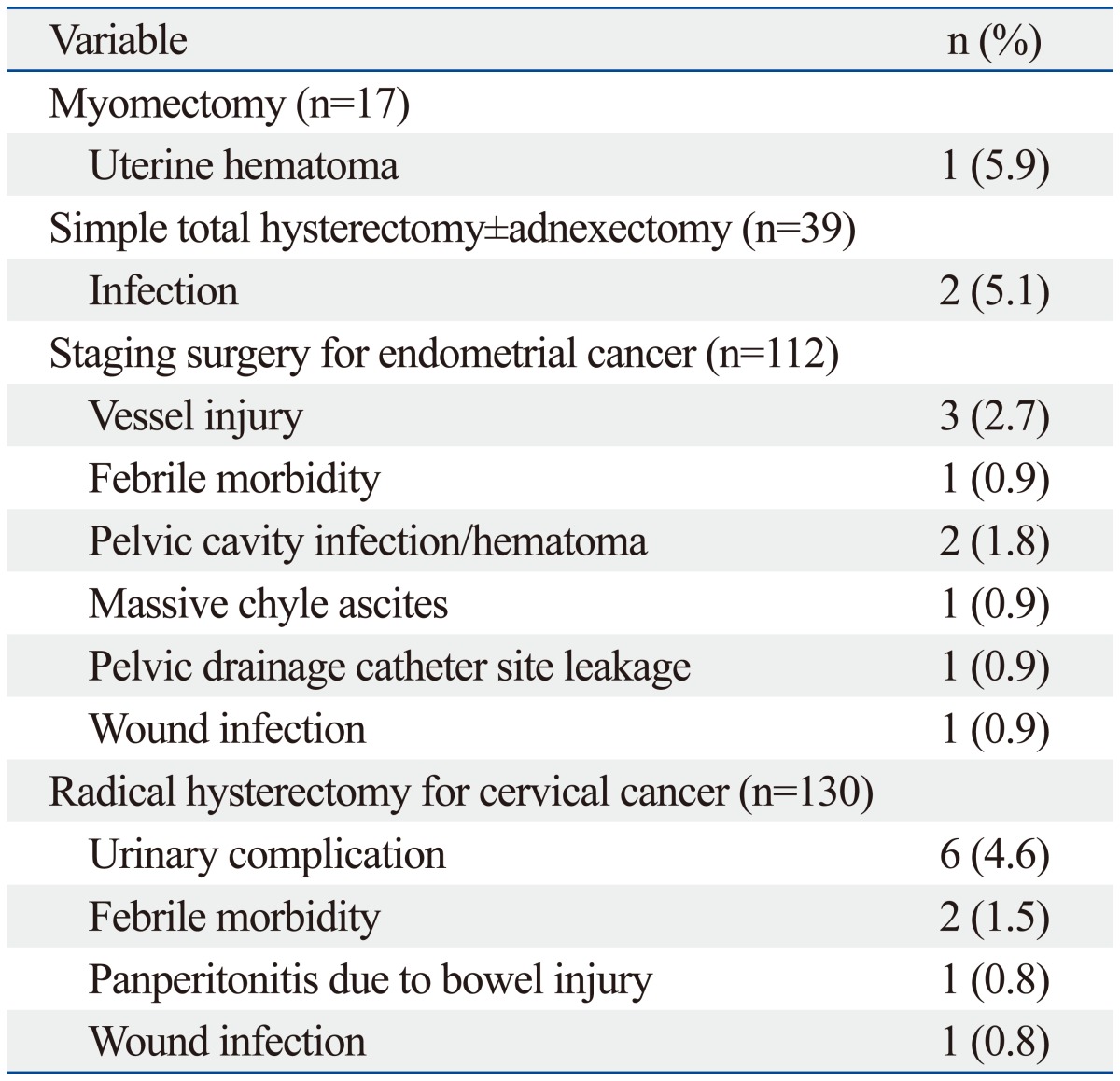
Complication data are shown in Table 3 and 4. One case planned for radical hysterectomy was converted to laparoscopy due to mechanical malfunction of the robotic system. The plan for a robotic procedure was aborted at the initial set-up prior to the patient's entrance into the operating room. There were no intra-operative device failures that resulted into an unexpected conversion. The overall complication rate was 18.8%, including both minor and major complications. Intra-operative complications including a bladder injury and a bowel content leakage during appendectomy and adhesiolysis occurred during radical hysterectomy and pelvic lymph node dissection. Three cases of vessel injury [renal vein (n=1), aortal (n=1), external iliac vein (n=1)] occurred during the staging and lymph node dissection for endometrial cancer. However, these injuries were managed robotically without conversion to another surgical approach. The common early complications occurring before postoperative 6 weeks were febrile events (n=6), lymphedema (n=4), vaginal vault bleeding in case of hysterectomy (n=5), voiding difficulty (n=4), peritonitis (n=4), and hemovac site leakage (n=5). The frequencies of late postoperative events were less than those in the early postoperative period. Three patients with voiding difficulty that underwent radical hysterectomy were managed conservatively. Patients that had an infection or lymphocele were discharged after conservative management with antibiotics. No patient experienced a bowel obstruction, incisional hernia, or vaginal vault dehiscence. A patient who had neuropathy in the early postoperative period, including obturator nerve and femoral cutaneous nerve neurapraxia, had improved after six weeks with rehabilitation. Most of the early and late postoperative complications were spontaneously resolved by conservative management (grade 1 or 2). Complications greater than grade 3 by Clavien-Dindo classification, defined as surgical postoperative complications requiring surgical or radiological intervention, occurred in 5 patients (1.7%). The most severe complication (grade IIIB) occurred in one patient who presented with panperitonitis due to bowel perforation that had developed postoperatively after radical hysterectomy. Diagnostic laparoscopy of this patient revealed extensive stool soilage in the abdominal cavity, leaking from an approximately 1-cm sized laceration site on the anterior wall of the upper rectum. Low anterior resection with diverting ileostomy was proceeded via a laparotomy. Ileostomy was successfully repaired 3 months after low anterior resection. Among 4 patients with grade IIIA complications, two patients received ureteral stent insertion due to ureterovaginal fistula and unilateral hydronephrosis. One hemovac insertion site dehiscence was treated with antibiotics and resuturing under local anesthesia. The last grade IIIA complication was a patient with severe chyle ascites that necessitated paracentesis at the emergency unit. No patient experienced organ failure or death after surgery. When stratified according to procedure types (Table 4), the most frequent complications greater than or equal to grade 2 in the myomectomy group were one case of uterine incision site hematoma, two cases (5.1%) of infection related morbidity in simple hysterectomy group, vessel injury (three cases, 2.7%) among the endometrial cancer staging group, which occurred during lymph node dissection, and six cases (4.6%) of urinary complication in the radical hysterectomy group.
Table 3.
Intraoperative and Postoperative Complications
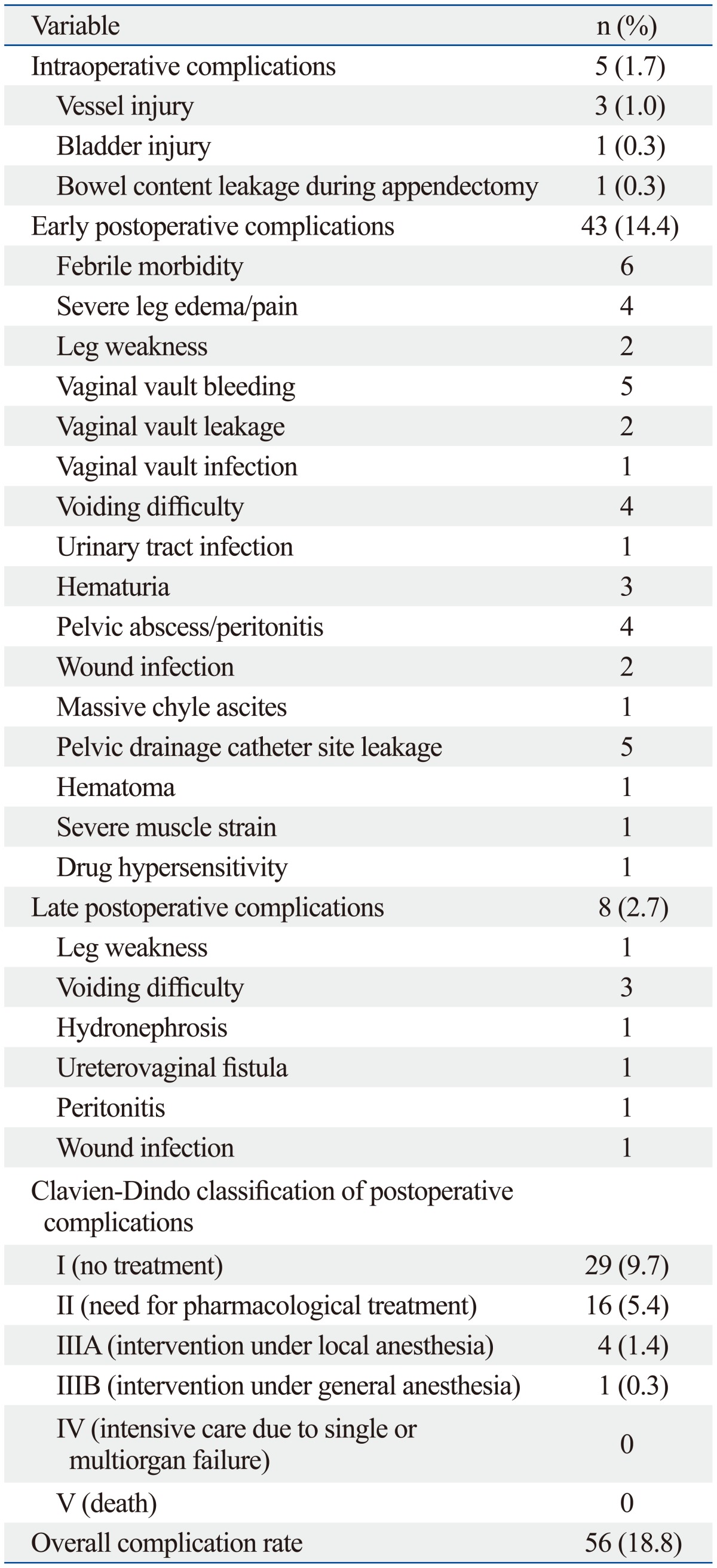
Table 4.
Type of Perioperative Complications (Clavien-Dindo Classification ≥2) According to Treatment Method
DISCUSSION
The results of this study demonstrate that robot-assisted laparoscopic surgery is a feasible approach in gynecology with acceptable complications. The da Vinci robotic system was approved by the United States Food and Drug Administration in the gynecologic field in 2005, with unique characteristics of wristed instruments, tremor elimination, steady three-dimensional visualization, and an ergonomic working position.6 These features may help surgeons overcome some of the limitations associated with conventional laparoscopic surgery. Although there are several studies regarding the feasibility of robot-assisted laparoscopic surgery in gynecology, studies have mainly focused on perioperative morbidity with standardized classification.4,8 Since robotic surgery is in its infancy compared to other conventional laparoscopic or open abdominal approaches, continuous investigation is important.
There are several studies demonstrating the feasibility and complications of robotic surgery using four robotic arms. Bedient, et al.9 reported a peri-operative complication rate of 16% in 40 robot-assisted laparoscopic myomectomy cases. In their cohort, two patients (5%) were readmitted because of ileal perforation, likely attributable to an electrical injury, during surgery and for febrile urinary tract infection. Their postoperative complication rate was 11%, including one pneumonia, two blood transfusions, two wound infections, one bowel injury, and one pelvic abscess. In this study, the number of myomectomies performed was about half than that in the study by Bedient, et al.9 Still, there was only one grade 2 complication of uterine hematoma, which needed conservative treatment with intravenous iron supplementation. Another unusual complication after myomectomy was a patient who complained of immobility of her left arm due to severe trapezoid muscle pain immediately after surgery. Plain radiologic X-rays showed no signs of bone fracture. All patients in this study had both arms tucked to each side, instead of placing them on arm boards, to avoid brachial plexus injury. Also, gel or cushion pads were fastened to the surgical table to support the upper body and shoulders. Nonetheless, severe muscle strain developed in one patient, presumably due to pressure from the steep Trendelenburg position. This was the only case of severe muscle pain after gynecologic robotic surgery at our institution. The authors believe that proper patient positioning is crucial to reducing complications in robotic surgery.
Complication rates during and after gynecologic oncologic surgeries are expected to be higher than that of benign gynecologic cases due to extensive dissection and broader surgical field. Veljovich, et al.10 reported operative outcomes of 118 robotic surgeries in gynecologic oncology. The major complication rate therein was 6.8%, including two vessel injuries, two venous thromboembolisms, two vaginal cuff dehiscences, one readmission for small bowel obstruction, and one intensive care unit admission. Two patients were converted to laparotomy. The first had a body mass index (BMI) of 49 kg/m2 and was eventually converted for laparotomic endometrial cancer staging; the second was undergoing a radical hysterectomy with extensive distortion and obliteration of normal avascular anatomic planes. Boggess, et al.12 reported an overall complication rate of 6.8% for endometrial cancer staging, which included one bowel injury, one port site hernia, one pulmonary embolism, and one lymphedema in 103 cases. Three cases (2.8%) were converted to laparotomy in their report. Persson, et al.6 demonstrated a peri/postoperative complication rate of 41% of in robotic assisted laparoscopic radical hysterectomies. Five patients (6%) developed vaginal cuff dehiscence, three patients had port site herniation (4%), one patient (1%) had a ureter stricture, and one patient (1%) experienced reversible partial obturator nerve palsy. In two cases, the small bowel was incarcerated through the opening at the site of a 15-mm trocar. In the current analysis, the rate of complications that needed pharmacological intervention (Clavien-Dindo classification ≥2) was as expected. During endometrial cancer staging operations, our policy is to do a full lymphadenectomy up to the renal vein level unless the patient is medically inapt for prolonged surgery. Therefore, vessel injury was the highest type of complications that needed intraoperative intervention. During radical hysterectomy for cervical cancer, urinary complications were expected to be the most frequent events during the extensive dissection of the ureter and bladder. These speculations were confirmed in our data (Table 4).
In the present study, the authors used only three robotic arms and an assistant conventional port for all procedures. The overall complication rate was 18.8% and that for only the oncologic cases was 16.1%; complication rates in our cohort did not significantly deviate from other rates (6.8-41%) reported in previous studies that used four robotic arms.3,6,13,14 In addition, the intra-operative complication rate was low (1.7%), and all cases were continued robotically without conversion to laparoscopy or an open method. Table 5 summarizes data from a review of published robot-assisted laparoscopic studies, and shows that the complication rates of our cohort are comparable to previous reports. In this study, one patient was converted to a laparoscopic radical hysterectomy due to mechanical failure. The patient had her procedures aborted secondary to system failure at the initial set-up prior to the patient's entrance into the operating room. There were no intra-operative device failures that resulted in a case conversion. Technical errors that could potentially handicap a surgeon have not occured in our institution so far. In previous studies, the mean recoverable and non-recoverable fault rates per procedure were reported to be 0.21 and 0.05, respectively, since the last computer system upgrade.15 However, one external iliac vein injury during pelvic lymph node dissection occurred by a small tear in the elastic sheath of the robotic instrument, which seemed to have lacerated the vein when the instrument head brushed near the vessel. Accordingly, regular surveillance on the mechanical and technical status of the robotic system is crucial to prevent unexpected complications during surgical procedures.
Table 5.
Complications of Robotic Oncologic Surgeries (Literature Review and Present Study)
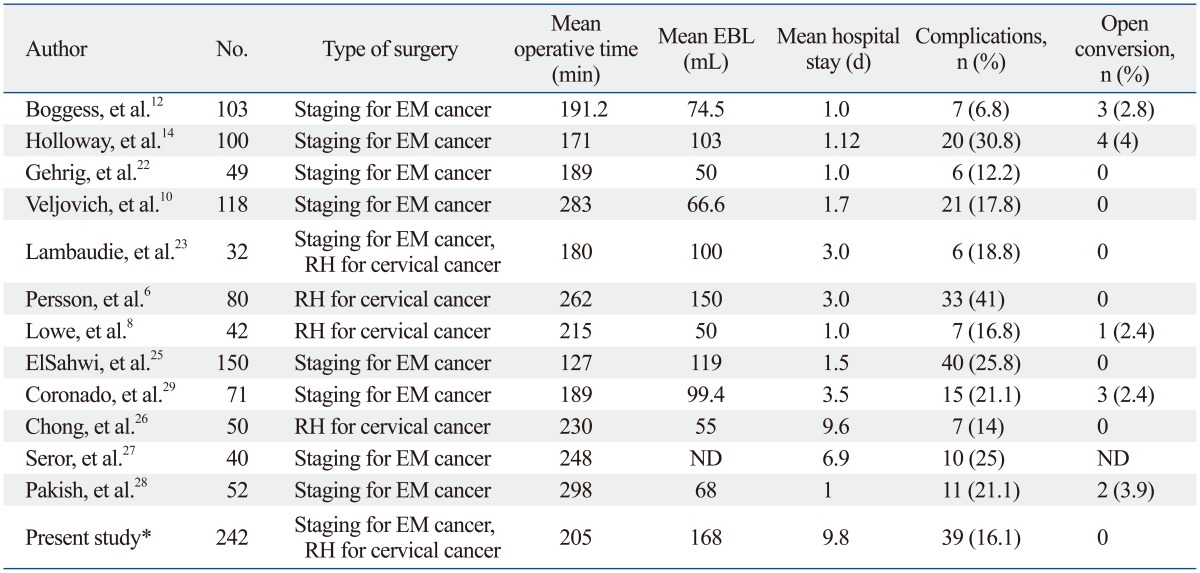
EBL, estimated blood loss; EM, endometrial; RH, radical hysterectomy; ND, not defined.
*The present study indicates only oncologic cases for overall comparison.
The data from this cohort is different from other previous studies in regards to certain types of complications. One of them is that there was no trocar site hernia among our cases. Trocar site hernia is a rare but serious complication when bowel strangulation and infection occurs. One of the known risk is when large ports (10 mm in diameter or larger) are used; trocar site hernia is uncommon for ports less than 10 mm in diameter, ranging from 0 to 1.2%. Nevertheless, Seamon, et al.16 reported a case of bowel herniation through an 8-mm robotic port site, and Persson, et al.6 also observed port site hernia in three of 80 cases of robotic radical hysterectomy. In contrast, a recent article that used bladeless 12-mm and 8-mm trocars reported no hernias and only one port site dehiscence, recommending that fascial closure is unnecessary when using bladeless trocars.17 In our cohort, about 70% of patients underwent fascial closure using Endoclose suture device to seal incisions that were larger than 8 mm. This device allows for the approximation of tissues and percutaneous suturing of port sites laparoscopically. The differences in port site herniation might also be explained by the low BMI of our patients, compared to other reports of Caucasian patients (median BMI of 23.0 kg/m2). Obese patients have a greater risk of developing hernias at an incision site due to the larger preperitoneal space and elevated intra-abdominal pressure.18 Furthermore, we only used four ports, including three robotic arms and one assistant 10-mm port, for all operations. In general, five to six ports were placed in the patients' abdomen in order to perform robot assisted laparoscopic surgery in most studies. The low number of trocars in our approach may have decreased the chance of trocar site hernia. Additionally, due to the relatively lower average BMI of Korean women than that of other Western countries,19 it was feasible to access the upper para-aortic area with only standard docking (instead of side docking).
The other difference in postoperative complication is that there was no vaginal cuff dehiscence in our study. In a study by Kho, et al.,20 the rate of vaginal cuff dehiscence was 4.1% (21/510) after robotic hysterectomies. The cause of higher incidences of vaginal cuff dehiscence in laparoscopic and robotic hysterectomies is unknown, and multiple factors may be involved.21 Thermal effect, racial difference, the size of genital organs, smoking, and high BMI are suspected, although results inconclusive. Meanwhile, suture method and type of sutures do not seem to promote any increase in vault dehiscence, as shown in a review by Drudi, et al.24 In their study, postoperative chemotherapy and/or brachytherapy were also risk factors for dehiscence, such that those who received adjuvant treatment had a higher rate thereof, compared to patients who did not (3% vs. 0.4%, respectively). In our cohort of oncologic cases, 56 out of 281 patients (19.9%) received adjuvant therapy of chemotherapy, radiotherapy, or both; however, no case of vaginal dehiscence requiring repair occurred. It is our routine practice to educate patients to refrain from coital activity and tub baths at least 2 months after the surgery. Also, only 1.9% of patients were smokers. Finally, there were no embolic events in this cohort.
The limitation of this study is the lack of comparisons with conventional laparoscopy and laparotomy. Thus, it is difficult to make definitive conclusions based on our findings, and further randomized prospective studies in large population settings are crucial to uncovering significant clinical implications. Despite this weakness, the strength of our study is that we evaluated data from a high volume institution and thereby provides a greater perspective on the kinds of perioperative complications that could potentially arise during robot-assisted laparoscopic procedures. Also, modification of the surgical procedure, such as using only three robotic arms, may provide a reference to other surgeons who perform gynecologic surgeries in a similar setting. Although the reason for using only three arms was originally due to cost issues, this method has become our current practice without significant limitations.
In conclusion, three-armed robot-assisted laparoscopic surgery in gynecology is feasible with few major complications. The use of objective grading tools may be helpful to classifying perioperative complications with better standardization. In addition, surgeons should be vigilant on the potential risks of mechanical failure of the robotic system to prevent further unexpected complications.
Footnotes
The authors have no financial conflicts of interest.
References
- Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005:CD003677. doi: 10.1002/14651858.CD003677.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Johnson N, Barlow D, Lethaby A, Tavender E, Curr L, Garry R. Methods of hysterectomy: systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;330:1478. doi: 10.1136/bmj.330.7506.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109:86–91. doi: 10.1016/j.ygyno.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Fanning J, Fenton B, Purohit M. Robotic radical hysterectomy. Am J Obstet Gynecol. 2008;198:649. doi: 10.1016/j.ajog.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds RK, Advincula AP. Robot-assisted laparoscopic hysterectomy: technique and initial experience. Am J Surg. 2006;191:555–560. doi: 10.1016/j.amjsurg.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Persson J, Reynisson P, Borgfeldt C, Kannisto P, Lindahl B, Bossmar T. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185–190. doi: 10.1016/j.ygyno.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Kim YT, Kim SW, Hyung WJ, Lee SJ, Nam EJ, Lee WJ. Robotic radical hysterectomy with pelvic lymphadenectomy for cervical carcinoma: a pilot study. Gynecol Oncol. 2008;108:312–316. doi: 10.1016/j.ygyno.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Lowe MP, Chamberlain DH, Kamelle SA, Johnson PR, Tillmanns TD. A multi-institutional experience with robotic-assisted radical hysterectomy for early stage cervical cancer. Gynecol Oncol. 2009;113:191–194. doi: 10.1016/j.ygyno.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201:566. doi: 10.1016/j.ajog.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Veljovich DS, Paley PJ, Drescher CW, Everett EN, Shah C, Peters WA., 3rd Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol. 2008;198:679. doi: 10.1016/j.ajog.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360. doi: 10.1016/j.ajog.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 13.DeNardis SA, Holloway RW, Bigsby GE, 4th, Pikaart DP, Ahmad S, Finkler NJ. Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol Oncol. 2008;111:412–417. doi: 10.1016/j.ygyno.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Holloway RW, Ahmad S, DeNardis SA, Peterson LB, Sultana N, Bigsby GE, 4th, et al. Robotic-assisted laparoscopic hysterectomy and lymphadenectomy for endometrial cancer: analysis of surgical performance. Gynecol Oncol. 2009;115:447–452. doi: 10.1016/j.ygyno.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Zorn KC, Gofrit ON, Orvieto MA, Mikhail AA, Galocy RM, Shalhav AL, et al. Da Vinci robot error and failure rates: single institution experience on a single three-arm robot unit of more than 700 consecutive robot-assisted laparoscopic radical prostatectomies. J Endourol. 2007;21:1341–1344. doi: 10.1089/end.2006.0455. [DOI] [PubMed] [Google Scholar]
- 16.Seamon LG, Backes F, Resnick K, Cohn DE. Robotic trocar site small bowel evisceration after gynecologic cancer surgery. Obstet Gynecol. 2008;112(2 Pt 2):462–464. doi: 10.1097/AOG.0b013e3181719ba8. [DOI] [PubMed] [Google Scholar]
- 17.Boone JD, Fauci JM, Barr ES, Estes JM, Bevis KS. Incidence of port site hernias and/or dehiscence in robotic-assisted procedures in gynecologic oncology patients. Gynecol Oncol. 2013;131:123–126. doi: 10.1016/j.ygyno.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 18.Tonouchi H, Ohmori Y, Kobayashi M, Kusunoki M. Trocar site hernia. Arch Surg. 2004;139:1248–1256. doi: 10.1001/archsurg.139.11.1248. [DOI] [PubMed] [Google Scholar]
- 19.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 20.Kho RM, Akl MN, Cornella JL, Magtibay PM, Wechter ME, Magrina JF. Incidence and characteristics of patients with vaginal cuff dehiscence after robotic procedures. Obstet Gynecol. 2009;114(2 Pt 1):231–235. doi: 10.1097/AOG.0b013e3181af36e3. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Kim S, Bae HS, Lee JK, Lee NW, Song JY. Evaluation of risk factors of vaginal cuff dehiscence after hysterectomy. Obstet Gynecol Sci. 2014;57:136–143. doi: 10.5468/ogs.2014.57.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gehrig PA, Cantrell LA, Shafer A, Abaid LN, Mendivil A, Boggess JF. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol. 2008;111:41–45. doi: 10.1016/j.ygyno.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Lambaudie E, Houvenaeghel G, Walz J, Bannier M, Buttarelli M, Gurriet B, et al. Robot-assisted laparoscopy in gynecologic oncology. Surg Endosc. 2008;22:2743–2747. doi: 10.1007/s00464-008-0116-5. [DOI] [PubMed] [Google Scholar]
- 24.Drudi L, Press JZ, Lau S, Gotlieb R, How J, Eniu I, et al. Vaginal vault dehiscence after robotic hysterectomy for gynecologic cancers: search for risk factors and literature review. Int J Gynecol Cancer. 2013;23:943–950. doi: 10.1097/IGC.0b013e31828f38e1. [DOI] [PubMed] [Google Scholar]
- 25.ElSahwi KS, Hooper C, De Leon MC, Gallo TN, Ratner E, Silasi DA, et al. Comparison between 155 cases of robotic vs. 150 cases of open surgical staging for endometrial cancer. Gynecol Oncol. 2012;124:260–264. doi: 10.1016/j.ygyno.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Chong GO, Lee YH, Hong DG, Cho YL, Park IS, Lee YS. Robot versus laparoscopic nerve-sparing radical hysterectomy for cervical cancer: a comparison of the intraoperative and perioperative results of a single surgeon's initial experience. Int J Gynecol Cancer. 2013;23:1145–1149. doi: 10.1097/IGC.0b013e31829a5db0. [DOI] [PubMed] [Google Scholar]
- 27.Seror J, Bats AS, Huchon C, Bensaïd C, Douay-Hauser N, Lécuru F. Laparoscopy vs robotics in surgical management of endometrial cancer: comparison of intraoperative and postoperative complications. J Minim Invasive Gynecol. 2014;21:120–125. doi: 10.1016/j.jmig.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Pakish J, Soliman PT, Frumovitz M, Westin SN, Schmeler KM, Reis RD, et al. A comparison of extraperitoneal versus transperitoneal laparoscopic or robotic para-aortic lymphadenectomy for staging of endometrial carcinoma. Gynecol Oncol. 2014;132:366–371. doi: 10.1016/j.ygyno.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronado PJ, Herraiz MA, Magrina JF, Fasero M, Vidart JA. Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2012;165:289–294. doi: 10.1016/j.ejogrb.2012.07.006. [DOI] [PubMed] [Google Scholar]


