Abstract
Purpose
To assess the clinical efficacy of cultivated oral mucosal epithelial transplantation (COMET) for the treatment of persistent epithelial defect (PED).
Methods
We treated 10 eyes of nine patients with PED (Stevens–Johnson syndrome: three eyes; thermal/chemical injury: five eyes; ocular cicatricial pemphigoid: two eyes) with COMET at Kyoto Prefectural University of Medicine, Kyoto, Japan from 2002 to 2008.
Results
Preoperatively, PED existed on over more than 50% of the corneal surface in seven eyes. Severe ocular surface inflammation with fibrovascular tissue surrounded the PED in all 10 eyes. At 24-weeks postoperative, PED had improved in all cases except 1 in which the patient was unable to return to the hospital (95% CI, 55.5–99.7; Wilcoxon signed-rank test, p = 0.0078). The preoperative median of logarithmic minimum angle of resolution was 1.85 (range 0.15–2.70), and 1.85, 1.85, and 1.52 at the 4th, 12th, and 24th postoperative week, respectively. The mean total preoperative ocular surface grading score was 7.0 (range 4–17). At 4 and 12 weeks postoperative, the total ocular surface grading score had improved significantly (p = 0.0020, p = 0.0078), and at 24 weeks postoperative, it was 3.0 (range 2–12, p = 0.0234). During the follow-up period (median 23.3 months, range 5.6–39.7 months), no recurrence of PED was observed in any eye, and long-term ocular surface stability was obtained.
Conclusion
COMET enabled complete epithelialization of PED and stabilization of the ocular surface in patients with severe ocular surface disease, thus preventing end-stage cicatrization and vision loss at a later stage.
Keywords: acute inflammatory activity, cultivated oral mucosal epithelial transplantation (COMET), limbal stem cell deficiency (LSCD), persistent epithelial defect (PED)
Introduction
Corneal renewal and repair are mediated by corneal epithelial stem cells located mainly in the limbus, the narrow region between the cornea and the bulbar conjunctiva (Kinoshita et al. 2001). Damage or depletion of the corneal epithelial stem cells, known as limbal stem cell deficiency (LSCD), leads to conjunctival invasion that results in vascularization of the cornea with an associated profound loss of vision (Kinoshita et al. 2001). LSCD can be caused by Stevens–Johnson syndrome (SJS), ocular cicatricial pemphigoid (OCP) and thermal or chemical injury, which are all characterized by the loss of corneal epithelial stem cells. Such LSCD may cause severe ocular surface diseases (OSDs) in which cicatrization resulting from conjunctival fibrosis, symblepharon and severe dry eye greatly disrupt visual function.
Limbal stem cell deficiency can be classified into two categories, acute and chronic, based on the onset and progression pattern of the disease. In cases such as SJS and thermal/chemical injury, epithelial defect occurs suddenly and is accompanied by massive inflammation on the ocular surface. During the several weeks that follow, epithelialization progresses with subsequent scar tissue formation, such as that associated with conjunctival shrinkage. In contrast, the onset of OCP is difficult to determine. In OCP cases, cicatrization progresses gradually without an acute episode. However, those patients are sometimes observed with acute manifestations consisting of intense conjunctival hyperaemia and localized epithelial defects. Acute OCP causes rapid shrinkage of the conjunctiva (Mondino et al. 1979), similar to that which occurs in a thermal/chemical injury or in the acute phase of SJS.
Regardless of the causes, such LSCD in severe OSDs may sometimes cause persistent epithelial defects (PED) with prolonged inflammation on the ocular surface. A PED occurring in the subacute phase of LSCD is very difficult to treat. Massive inflammation on the ocular surface is often uncontrollable, even with the use of systemic and/or local steroids. The effects of local treatment with eye ointment or a medical-use soft contact lens are limited. Exposure of the corneal stroma can induce infectious or non-infectious corneal stromal thinning and perforation. Even if epithelialization can be achieved, long-standing inflamed PED eventually results in symblepharon, conjunctivalization and neovascularization of the cornea, leading to blindness in the long term. Although early reports demonstrated the effectiveness of amniotic membrane transplantation for PED (Shimazaki et al. 1997; Gomes et al. 2003), further research by our group and others showed that the effects of this technique on PED are limited (Azuara-Blanco et al. 1999; Meller et al. 2000; Tamhane et al. 2005; Saw et al. 2007; Rahman et al. 2009; Tandon et al. 2011; Hino et al. 2012).
Ophthalmologists are continually challenged with the task of developing treatments for severe OSDs in which no medical or surgical treatments exist. To that end, beginning in 2002, our group was the first in the world to perform ocular surface reconstruction using tissue-engineered autologous oral mucosal epithelial sheets (Nakamura et al. 2004), and since that time we have continued to perform autologous cultivated oral mucosal epithelial transplantation (COMET) after careful determination of the surgical indication (Ang et al. 2006; Inatomi et al. 2006a; Inatomi et al. 2006b; Nakamura et al. 2011). In the clinical setting, deciding the indication and postoperative management are extremely important.
To clarify the effectiveness, disease-specific outcomes and safety of COMET, we analysed the clinical data for all 72 patients treated with COMET at our facility since 2002. We reported that for 46 eyes of 40 LSCD patients, COMET resulted in favourable long-term visual acuity (VA) outcomes (Sotozono et al. 2013). The purpose of this present study was to summarize the long-term clinical outcomes of 10 eyes of nine of those 72 patients who underwent COMET with the primary objective of treating PED with acute inflammatory activity.
Methods
Study design
The medical records of all patients who underwent COMET at the Department of Ophthalmology, Kyoto Prefectural University of Medicine, Kyoto, Japan, from June 2002 until December 2008 were retrospectively examined and recorded on case report forms. All of the data relating to the oral mucosal epithelial sheets, surgical outcomes and adverse events were evaluated, and statistical analyses were conducted using the data obtained. This retrospective outcome study protocol was approved by the Ethical Review Board of Kyoto Prefectural University of Medicine in 2009.
The study included 81 eyes of 72 patients, and those eyes were classified into one of the following four categories according to the purpose of treatment with COMET: (i) corneal reconstruction for visual improvement, (ii) corneal reconstruction for the treatment of PED with acute inflammatory activity, (iii) conjunctival fornix reconstruction and (iv) others (Fig. 1). In this present study, we report the results of COMET for the purpose of corneal reconstruction to treat PED with acute inflammatory activity.
Fig 1.
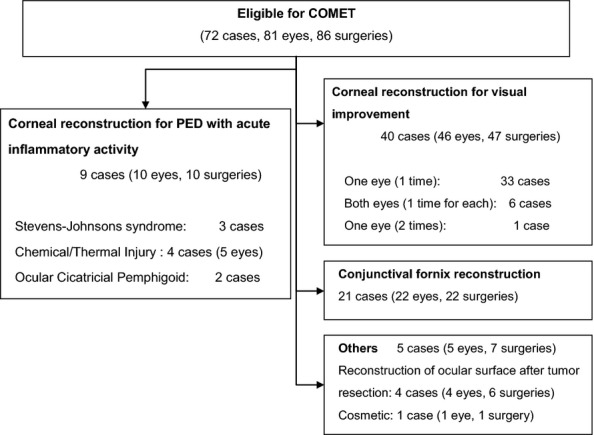
Study flow diagram. Seventy-two patients (81 eyes) underwent cultivated oral mucosal epithelial transplantation (COMET) between June 2002 and December 2008, and nine patients (10 eyes) with persistent epithelial defect (PED) with accompanying acute inflammatory activity were included in this study.
Patients
Cultivated oral mucosal epithelial transplantation was performed on nine consecutive patients (10 eyes) with LSCD who were diagnosed with PED accompanying acute inflammatory activity on the ocular surface. Inclusion criteria were as follows: (i) LSCD, (ii) a history of an acute episode of epithelial defect, (iii) a PED existing for more than 1 month, (iv) persistent ocular surface inflammation even in the use of systemic and/or local steroids, (v) resistance to conventional therapy such as eye ointments or a medical-use soft contact lens and (vi) fibrovascular tissue surrounding the PED.
For each patient, the final decision to perform COMET was made by the team of corneal specialists at our university hospital. As eyes with stem cell deficiency can be asymptomatic carriers of methicillin-resistant Staphylococcus aureus (MRSA) (Sotozono et al. 2002), a conjunctival swab was cultured preoperatively and antibiotics were used when deemed necessary.
Cell culture
All COMET sheets were prepared at the Good Manufacturing Practices (GMP)-graded Cell Processing Center at Kyoto Prefectural University of Medicine as described previously (Nakamura et al. 2004; Ang et al. 2006; Inatomi et al. 2006a,b). Autologous oral mucosal epithelial cells were obtained from a 6-mm-diameter biopsy specimen taken from the patient's buccal mucosa and were then co-cultured with mitomycin C-inactivated 3T3 fibroblasts (NIH-3T3-4; RIKEN Cell Bank, Tsukuba, Japan) on an amniotic membrane spread on the bottom of a culture insert. The cultured cells were submerged in medium for approximately 1 week and then exposed to air by lowering the level of the medium (airlifting) for 1–2 days. All amniotic membrane was obtained from caesarean sections, and the preparation method was performed as described previously (Nakamura et al. 2004). Although foetal bovine serum was initially used as the culture medium, autologous serum was subsequently selected as the serum of choice to reduce the risk of transmitting non-human pathogens (Ang et al. 2006).
Transplantation and postoperative management
For each patient, the surgical procedure and postoperative management were performed as previously described (Ang et al. 2006; Inatomi et al. 2006a,b; Nakamura et al. 2011). In patients with symblepharon or a large area of bare sclera exposed during surgery, amniotic membrane was transplanted onto the bare sclera in order to reconstruct the conjunctival fornices (Solomon et al. 2003). In patients with a cataract, phaco-emulsification/aspiration plus intraocular lens implantation was carried out simultaneously with COMET. In patients with an eyelid abnormality, eyelid surgery was combined with COMET (Takeda et al. 2011).
Systemic corticosteroid (betamethasone, 1 mg/day) and cyclosporine (2–3 mg/kg/day) were administered to prevent postoperative inflammation and an immunological response and then tapered depending on the clinical findings. Dexamethasone (0.1%) and antibiotic eye drops were instilled four times per day. Dry eye patients received a topical administration of artificial tears. A therapeutic soft contact lens was used for at least 1 month to protect the transplanted epithelium from mechanical ablation.
Clinical outcomes
The primary outcome was defined as epithelialization of the PED. Secondary outcomes included a change in best-corrected VA (BCVA) and changes in the ocular surface grading score.
The ocular surface conditions including corneal appearance (epithelial defects, clinical conjunctivalization, neovascularization, opacification, keratinization and symblepharon) were alternately graded by 2 of 3 ophthalmologists (C.S., T.I. and T.N.) on a scale from 0 to 3 according to their severity, in accordance with our previously reported grading system (Sotozono et al. 2007). Furthermore, findings on upper and lower fornix shortening were included when grading the conjunctival cicatrization. The sum of each grading score was defined as the ocular surface grading score (maximum score 24) (Table 2).
Table 2.
Ocular surface grading system for COMET.
| Variable |
Variable scores |
||||
|---|---|---|---|---|---|
| Category | Variable name | 0 | 1 | 2 | 3 |
| Corneal appearance | Symblepharon | No symblepharon | Involving only the conjunctiva | Less than 1/2 of the corneal surface | More than 1/2 of the corneal surface |
| Epithelial defect | No defect | Less than 1/4 of the corneal surface | 1/4–1/2 of the corneal surface | More than 1/2 of the corneal surface | |
| Conjunctivalization | Absence of conjunctivalization | Less than 1/4 of the corneal surface | 1/4–1/2 of the corneal surface | More than 1/2 of the corneal surface | |
| Neovascularization | No neovascularization | Confined to the corneal periphery | Extending up to the pupil margin | Extending beyond the pupil margin into the central cornea | |
| Opacification | Iris details clearly visualized | Partial obscuration of the iris details | Iris details poorly seen with pupil margin visible | Complete obscuration of iris and pupil details | |
| Keratinization | No corneal keratinization | Less than 1/4 of the corneal surface | 1/4–1/2 of the corneal surface | More than 1/2 of the corneal surface | |
| Conjunctival appearance | Fornix shortening (Upper) | Normal depth | Shortened by less than 1/4 | Shortened by 1/4–1/2 | Shortened by more than 1/2 |
| (Lower) | Normal depth | Shortened by less than 1/4 | Shortened by 1/4–1/2 | Shortened by more than 1/2 | |
This scoring system as previously reported has been refined by the findings on the prognostic significance of the degree or severity of ocular surface disorders obtained in this work. The scoring system could be classified broadly as corneal complications and conjunctival complications, respectively.
Each patient's epithelial defect, logarithmic minimum angle of resolution (logMAR), ocular surface grading score and data on adverse events related to COMET or postoperative management were recorded before surgery, at the 4th, 12th and 24th postoperative week and at the final follow-up examination.
Statistical analysis
The change in epithelial defect, BCVA and ocular surface grading score from baseline at each visit, except for the final visit, was analysed using the Wilcoxon signed-rank test. All statistical analyses were conducted at the Translational Research Informatics Center (Kobe, Japan) using sas software, version 9.1 (SAS Institute Inc., Cary, NC, USA) or jmp software, version 8.2 (SAS Institute Inc.).
Results
Patient characteristics
A total of 10 surgeries (10 eyes in nine patients) were performed on three eyes with SJS, five eyes with thermal or chemical injury and two eyes with OCP (Fig. 1, Table 1). Epithelial defects persisted, with extensive ocular surface inflammation accompanying the progress of cicatrization of the ocular surface (Fig. 2). The duration of the PED ranged from 1 to 15 months (median 3 months). In all patients, the condition was intractable and unresponsive to medical treatments including systemic and local steroids. Amniotic membrane transplantation carried out at previous hospital was failed in both eyes with severe chemical injury (Case 1). In all three patients with SJS, MRSA was detected in both eyes, necessitating both preoperative and postoperative anti-MRSA therapy.
Table 1.
Summary of the clinical outcomes of COMET.
| Case No. | Disease | Age/sex | Duration of the disorder (months) | Duration of PED (months) | Eye(L/R) | Prior surgery (Y/N) | Combination surgery | Visual acuity |
Total ocular surface score |
Follow-up month | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 24W | Last | Pre | 24W | Last | |||||||||
| 1 | Chemical injury | 33/M | 3 | 3 | R | Y | No | 0.002 | 0.002 | 0.002 | 8 | 5 | 7 | 27.2 |
| L | Y | No | 0.03 | 0.1 | 0.4 | 6 | 3 | 4 | 27.2 | |||||
| 2 | Thermal injury | 27/M | 3 | 3 | L | N | AMT+Eyelid surgery | 0.002 | 0.03 | 0.04 | 6 | 5 | 7 | 31.4 |
| 3 | Thermal injury | 29/M | 1 | 1 | R | N | Eyelid surgery | 0.7 | 0.15 | 0.03 | 4 | 6 | 9 | 16.5 |
| 4 | Thermal injury | 61/M | 3 | 3 | R | N | AMT | 0.01 | – | 0.01 | 6 | 3 | 3 | 33 |
| 5 | SJS | 8/F | 8 | 8 | R | N | No | 0.7 | 0.1 | 0.002 | 8 | 2 | 5 | 39.7 |
| 6 | SJS | 30/M | 14 | 14 | R | N | AMT | 0.02 | 0.004 | 0.01 | 12 | 12 | 17 | 18.4 |
| 7 | SJS | 62/M | 15 | 15 | L | N | AMT+CS+Other | 0.01 | 0.01 | 0.01 | 6 | 3 | 4 | 19.4 |
| 8 | OCP | 73/M | 42 | 3 | R | N | AMT | 0.002 | 0.06 | 0.09 | 17 | 3 | 3 | 9.5 |
| 9 | OCP | 83/M | 18 | 7 | L | Y | No | 0.02 | – | 0.03 | 17 | – | 2 | 5.6 |
COMET, autologous cultivated oral mucosal epithelial transplantation; SJS, Stevens–Johnson syndrome; OCP, ocular cicatricial pemphigoid; AMT, amniotic membrane transplantation; CS, cataract surgery; F, female; M, male; Y, yes; N, no.
Visual acuity below 0.01 is shown in italics. Counting fingers, hand motion and light perception were determined to be 0.004, 0.002 and 0.001, respectively.
Asterisks (*) indicates cases in which foetal bovine serum (FBS) was used as a culture medium for the epithelial sheet. We used autologous serum (AS) in other cases.
Fig 2.
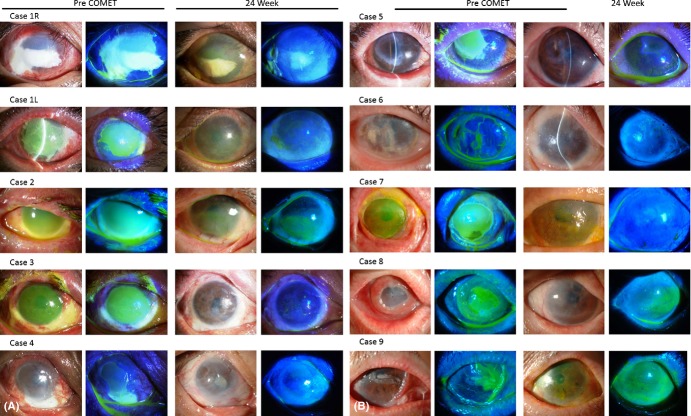
Slit-lamp appearances of all eyes with PED treated by cultivated oral mucosal epithelial transplantation (COMET). (Left two columns) Preoperatively, epithelial defect persisted in all eyes, with massive inflammation on the ocular surface. Note the subconjunctival fibrosis around the PED. (Right two columns) Ocular surface appearance at the 24th postoperative week. Ocular surface stabilization and complete epithelialization were achieved in all eyes.
Contralateral eyes of unilateral treatment with COMET
All three cases with Stevens–Johnson syndrome (Cases 5, 6, and 7) and both cases with ocular cicatricial pemphigoid (Cases 8 and 9) were damaged bilaterally and had PED in both eyes except 1 case with OCP (Case 9). Case 5 with SJS presented at our hospital at 1 month after disease onset. In that case, the PED in the contralateral eye healed 6 months later without undergoing COMET; however, severe conjunctivalization, neovascularization and opacification of the cornea had progressed. Two patients with SJS presented at our hospital following PED and subsequent corneal infection or perforation in the contralateral eye (Fig. 3, Cases 6 and 7). In the contralateral eye of 1 patient with OCP, rapid progression of the cicatrization had previously occurred following PED (Fig. 3, Case 8). In another case with OCP (Case 9), the contralateral eye was already severely cicatrized. In all five cases, the contralateral eye had lost vision prior to undergoing treatment with COMET.
Fig 3.
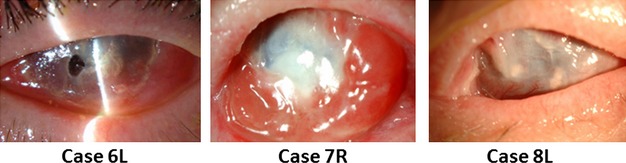
Clinical courses of the contralateral eyes not treated with COMET. Note the non-infectious corneal stromal melting and perforation in Case 6 (left), the MRSA infection in Case 7 (middle) and the cicatrization in Case 8 after PED with acute inflammatory activity (right).
Epithelial sheet cultivation and transplantation
Cultivated autologous oral mucosal epithelial sheets were successfully generated from all patients. In each case, COMET was successfully performed and no epithelial damage was observed during surgery. Amniotic membrane transplantation (AMT) was combined with COMET in five of the 10 surgeries, and cataract surgery was combined with COMET in 1 eye. In two cases with thermal injury, eyelid surgery to correct entropion was combined with COMET (Table 1).
Epithelialization outcomes
Preoperatively, epithelial defects were present on over 50% of the corneal surface (score 3) in seven eyes, on 25–50% of the corneal surface (score 2) in one eye and on <25% of the corneal surface (score 1) in two eyes. Severe ocular surface inflammation with fibrovascular tissue surrounding the PED was observed in all 10 eyes. In all eyes, the transplanted epithelium completely covered the corneal surface during COMET surgery, and ocular surface inflammation decreased during the first few weeks following surgery. At the 4th postoperative week, seven eyes (70%) had achieved complete epithelialization and three eyes showed a small epithelial defect (two cases with severe thermal burn and one case of SJS with severe dry eye). At the 24th postoperative week, PED had improved in all patients except one patient who did not undergo the 24th postoperative week follow-up (95% CI, 55.5–99.7%; Wilcoxon signed-rank test, p = 0.0078) (Figs. 2, 4). The mean follow-up period was 21.5 months (range 5.6–39.7 months; median 23.3 months), and complete epithelialization was maintained in all eyes (Table 1, Figs. 2, 4A).
Fig 4.
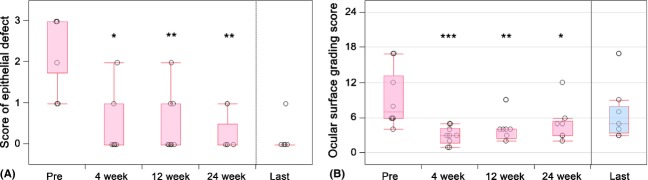
Preoperative and postoperative scores of ocular surface. (A) Preoperative and postoperative scores of the corneal epithelial defects. The epithelial defect scores for each patient were calculated using the previously reported grading system (Sotozono et al. 2007). The scores before surgery and at the 4th, 12th and 24th postoperative weeks and at the final follow-up examination were calculated. The change from baseline at each visit, except for the final visit, was analysed using the Wilcoxon signed-rank test. The bottom and top lines of the box represent the 25th and 75th percentiles, respectively. The circles represent the individual scores of the patients. The horizontal lines below and above the box represent the lowest and highest values, respectively (or are located 1.5 times the interquartile range away from the box). *p = 0.0156; **p = 0.0078 (Wilcoxon signed-rank test). (B) Preoperative and postoperative ocular surface grading score. Ocular surface grading scores for each patient were calculated using the previously reported grading system (Sotozono et al. 2007). Scores for 8 components of the ocular surface were calculated by the grading system. The total scores before surgery and at the 4th, 12th and 24th postoperative weeks and last follow-up examination were calculated. The change in ocular surface grading score from baseline at each visit, except for the last visit, was analysed using the Wilcoxon signed-rank test. The circles represent the individual scores of the patients. The horizontal line within each box represents the median value, the bottom and top lines of the box represent the 25th and 75th percentiles, respectively, and the horizontal lines below and above the box represent the lowest and highest values, respectively (or are located 1.5 times the interquartile range away from the box). *p = 0.0234; **p = 0.0078; ***p = 0.0020 (Wilcoxon signed-rank test).
VA outcomes
In all patients, no significant difference was found between preoperative and postoperative VA. The median logMAR was 1.85 before COMET (range 0.15–2.70) and 1.85, 1.85 and 1.52 at 4, 12 and 24 weeks (Wilcoxon signed-rank test, p = 0.8, 0.6, 0.8), respectively, postoperative; that is, VA was maintained in all cases.
Ocular surface grading score outcomes
Preoperatively, the median ocular surface grading score was 7.0 (range 4–17). While the epithelial defect score was high, other grading scores of cicatricial changes were low. At 4, 12 and 24 weeks postoperative, the ocular surface grading score improved significantly (p = 0.0020, p = 0.0078, and p = 0.0234, respectively). The median ocular surface grading score at 4, 12, and 24 weeks postoperative and at the final follow-up was 3.0, 4.0, 3.0 and 5.0, respectively.
Adverse events
Adverse events included slight elevation of intraocular pressure (IOP) due to steroid use in two patients, although in both cases the IOP returned to the normal range after steroid use was reduced. Moderate MRSA-related corneal infection occurred in one patient; however, the infection healed without perforation. No systemic adverse events occurred in any of the patients.
Discussion
Persistent epithelial defect with LSCD at an acute inflammatory stage has been a major problem for ophthalmologists. The clinical course is progressive with subsequent conjunctivalization and neovascularization of the cornea, as well as fornix shortening and subconjunctival fibrosis. Limbal transplantation and AMT do not guarantee epithelialization in such severely inflamed eyes (Azuara-Blanco et al. 1999; Rao et al. 1999; Meller et al. 2000; Tamhane et al. 2005; Saw et al. 2007; Rahman et al. 2009; Tandon et al. 2011; Hino et al. 2012). In fact, in two eyes in this study, AMT had previously been performed unsuccessfully at another hospital. To date, there is no reliable therapy to achieve epithelialization in such cases, and inhibition of the progression of cicatrization is very difficult to achieve.
This study demonstrated the successful use of COMET to treat PED. The disease status was characterized by a history of an acute episode of PED and prolonged inflammation on the ocular surface. We defined this condition as PED with acute inflammatory activity. In all eyes, the cornea was epithelialized immediately after undergoing COMET, and the inflamed ocular surface stabilized during the first few weeks following surgery. In all patients, sustained clinical remission was achieved within 1 or 2 months postoperative. Long-term follow-up revealed that epithelialization and stabilization of the ocular surface were maintained over a long period.
In eyes with early-stage PED without stromal damage, VA is usually undisturbed. Therefore, the severity and prognosis of the disease are often underestimated. However, prolonged PED induces conjunctivalization of the cornea and/or stromal melting. In fact, in some of the cases, conjunctivalization progressed during the period from the oral mucosal biopsy to transplantation of the prepared epithelial sheet. It should be emphasized that in the bilateral cases, the similarly afflicted contralateral eyes lost vision because of corneal perforation, infection or severe cicatrization. We consider that epithelialization resulting from COMET prevented complications such as corneal ulceration and perforation. The fact that no significant difference was found between preoperative and postoperative VA means that COMET effectively worked to maintain the patient's preoperative VA, thus preventing loss of VA, or in cases of prolonged PED, any further loss of VA. The ocular surface grading score indicates the degree of cicatrization of the ocular surface and is known to correlate well with VA (Sotozono et al. 2007). In this study, it is remarkable that the ocular surface grading score was improved both at the 24th postoperative week and at the final follow-up. Only in one patient with moderate MRSA infection (Case 6) had fornix shortening and conjunctivalization progressed after the remission of infection. We theorize that epithelialization resulting from COMET may have prevented the progression of cicatrization of the ocular surface at a later stage.
We hypothesize that one of the mechanisms by which COMET has a positive treatment effect on subacute PED is the decrease in massive inflammation on the ocular surface. A similar effect can be obtained by cultivated corneal limbal epithelial transplantation (CLET) (Koizumi et al. 2001). Conventional stem cell transplantation such as limbal transplantation (LT) requires several weeks for the corneal epithelium from the donor cornea to migrate and cover the corneal surface. In contrast, transplantation of cultivated epithelium covers the entire cornea during surgery and works to resolve the ocular surface inflammation. Previously, we compared the resolution of inflammation on the ocular surface in the eyes of an SJS patient who underwent CLET for one eye and conventional LT for the fellow eye. Our findings showed that ocular inflammation and IL-8 levels in tears decreased more rapidly in the eye that underwent CLET and that ocular cicatrization was higher in the eye that underwent conventional LT (Ang et al. 2007). Whereas long-term postoperative immunosuppressive therapy to prevent epithelial rejection is needed after allo CLET, it is not necessary after COMET. Thus, we select either CLET or COMET depending on the disease and how severely the eye is affected.
In this present study, we found differences among the three disease categories. In the patients with thermal or chemical injury, the reconstructed ocular surface has been maintained for a long period of time, up until the present date. All three SJS patients required both preoperative and postoperative anti-MRSA therapies. Due to the fact that OCP is a progressive autoimmune disease, conjunctival cicatrization gradually progressed after COMET in the two OCP cases in this study.
Persistent epithelial defect also occurs in the chronic stage of LSCD. Eyes with chronic PED are accompanied by severe dryness of the ocular surface and sometimes blink-related mechanical trauma. Chronic PED is not usually inflamed, and anti-inflammatory medication is ineffective. Surgical treatments such as LT or AMT are also ineffective. Transplanted epithelium easily detaches in eyes with chronic PED due to severe dryness of the eye. Thus, we consider chronic PED to be a contraindication for COMET, so it is important to distinguish chronic PED from subacute PED with inflammatory activity. It should be noted that the management of chronic PED is extremely difficult. Eye ointments, artificial tears and bandaging of the eye are all necessary. Moreover, attention should be paid to infectious or non-infectious stromal melting. However, such intensive care does not guarantee re-epithelialization, and PED with acute inflammatory activity can shift to chronic PED when the condition persists.
Limitations of this study included the small number of the patients, varying aetiology of PED, and that this was a non-randomized study with no control group. Prospective studies are necessary to evaluate and confirm the efficacy of COMET.
In conclusion, the findings of this present study show that in all treated eyes, COMET resulted in complete re-epithelialization as well as ocular surface stabilization, thus preventing end-stage cicatrization and vision loss. COMET provides a new therapeutic modality for the treatment of PED and greatly contributes to the improvement of the visual prognosis in cases of severe OSDs.
References
- Ang LP, Nakamura T, Inatomi T. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006;124:1543–1551. doi: 10.1001/archopht.124.11.1543. [DOI] [PubMed] [Google Scholar]
- Ang LP, Sotozono C, Koizumi N, Suzuki T, Inatomi T, Kinoshita S. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens-Johnson syndrome. Am J Ophthalmol. 2007;143:178–180. doi: 10.1016/j.ajo.2006.07.050. [DOI] [PubMed] [Google Scholar]
- Azuara-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83:399–402. doi: 10.1136/bjo.83.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes JA, dos SM, Cunha MC, Mascaro VL, Barros JN, de SL. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–473. doi: 10.1016/s0161-6420(02)01888-2. [DOI] [PubMed] [Google Scholar]
- Hino T, Sotozono C, Inatomi T. Indications and surgical outcomes of amniotic membrane transplantation. Nihon Ganka Gakkai Zasshi. 2012;116:374–378. [PubMed] [Google Scholar]
- Inatomi T, Nakamura T, Koizumi N, Sotozono C, Yokoi N, Kinoshita S. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006a;141:267–275. doi: 10.1016/j.ajo.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Inatomi T, Nakamura T, Kojyo M, Koizumi N, Sotozono C, Kinoshita S. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am J Ophthalmol. 2006b;142:757–764. doi: 10.1016/j.ajo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Adachi W, Sotozono C. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20:639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001;119:298–300. [PubMed] [Google Scholar]
- Meller D, Pires RT, Mack RJ. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Mondino BJ, Brown SI, Lempert S, Jenkins MS. The acute manifestations of ocular cicatricial pemphigoid: diagnosis and treatment. Ophthalmology. 1979;86:543–555. doi: 10.1016/s0161-6420(79)35486-0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Takeda K, Inatomi T, Sotozono C, Kinoshita S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95:942–946. doi: 10.1136/bjo.2010.188714. [DOI] [PubMed] [Google Scholar]
- Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond) 2009;23:1954–1961. doi: 10.1038/eye.2008.410. [DOI] [PubMed] [Google Scholar]
- Rao SK, Rajagopal R, Sitalakshmi G, Padmanabhan P. Limbal autografting: comparison of results in the acute and chronic phases of ocular surface burns. Cornea. 1999;18:164–171. doi: 10.1097/00003226-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Saw VP, Minassian D, Dart JK. Amniotic membrane transplantation for ocular disease: a review of the first 233 cases from the UK user group. Br J Ophthalmol. 2007;91:1042–1047. doi: 10.1136/bjo.2006.098525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- Solomon A, Espana EM, Tseng SC. Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology. 2003;110:93–100. doi: 10.1016/s0161-6420(02)01441-0. [DOI] [PubMed] [Google Scholar]
- Sotozono C, Inagaki K, Fujita A. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002;21(Suppl):S94–S101. doi: 10.1097/01.ico.0000263127.84015.3f. [DOI] [PubMed] [Google Scholar]
- Sotozono C, Ang LP, Koizumi N. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007;114:1294–1302. doi: 10.1016/j.ophtha.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Sotozono C, Inatomi T, Nakamura T. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology. 2013;120:193–200. doi: 10.1016/j.ophtha.2012.07.053. [DOI] [PubMed] [Google Scholar]
- Takeda K, Nakamura T, Inatomi T, Sotozono C, Watanabe A, Kinoshita S. Ocular surface reconstruction using the combination of autologous cultivated oral mucosal epithelial transplantation and eyelid surgery for severe ocular surface disease. Am J Ophthalmol. 2011;152:195–201. doi: 10.1016/j.ajo.2011.01.046. [DOI] [PubMed] [Google Scholar]
- Tamhane A, Vajpayee RB, Biswas NR. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005;112:1963–1969. doi: 10.1016/j.ophtha.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS, Vajpayee RB. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Br J Ophthalmol. 2011;95:199–204. doi: 10.1136/bjo.2009.173716. [DOI] [PubMed] [Google Scholar]