Abstract
High amylase activity in dogs is associated with a drastic increase in copy numbers of the gene coding for pancreatic amylase, AMY2B, that likely allowed dogs to thrive on a relatively starch-rich diet during early dog domestication. Although most dogs thus probably digest starch more efficiently than do wolves, AMY2B copy numbers vary widely within the dog population, and it is not clear how this variation affects the individual ability to handle starch nor how it affects dog health. In humans, copy numbers of the gene coding for salivary amylase, AMY1, correlate with both salivary amylase levels and enzyme activity, and high amylase activity is related to improved glycemic homeostasis and lower frequencies of metabolic syndrome. Here, we investigate the relationship between AMY2B copy numbers and serum amylase activity in dogs and show that amylase activity correlates with AMY2B copy numbers. We then describe how AMY2B copy numbers vary in individuals from 20 dog breeds and find strong breed-dependent patterns, indicating that the ability to digest starch varies both at the breed and individual level. Finally, to test whether AMY2B copy number is strongly associated with the risk of developing diabetes mellitus, we compare copy numbers in cases and controls as well as in breeds with varying diabetes susceptibility. Although we see no such association here, future studies using larger cohorts are needed before excluding a possible link between AMY2B and diabetes mellitus.
Keywords: canine genetics, comparative genetics, starch digestion
Introduction
Adaptation to a new diet during dog domestication
A recent comparison of genome-wide patterns of genetic variation in a large panel of dogs and wolves identified genomic regions that were affected by directional selection during early dog domestication (Axelsson et al. 2013). Through functional characterization of genes residing in these domestication regions, new light was shed on characteristics of adaptive advantage to early dogs. These analyses identified several genes involved in digestion and energy metabolism, suggesting that the transition from wolf to dog was accompanied by a change in diet. Augmented by evidence from expression analyses and enzyme assays, it was concluded that changes in three consecutive steps in the pathway responsible for starch digestion and subsequent glucose absorption allowed dogs to rely on a diet rich in starch relative to the carnivorous wolf diet (Axelsson et al. 2013).
AMY2B and amylase activity
Pancreatic amylase (AMY2B) serves as the first step in the digestion of starch to glucose in the small intestine (Mocharla et al. 1990) by catalyzing the breakdown of starch to oligosaccharides maltose and maltriose. Axelsson et al. (2013) specifically demonstrated that selection had acted on a series of duplication events to favor the accumulation of additional copies of AMY2B, resulting in an average sevenfold copy number increase in dogs relative to in wolves, and that this increase corresponds to higher pancreatic AMY2B expression as well as higher serum amylase activity. Although these observations argue that dogs in general digest starch more efficiently than do wolves, considerable variation in AMY2B copy numbers within the dog population, with diploid copy numbers ranging from 4 to 30 (n = 136) (Axelsson et al. 2013), indicates that the ability to handle starch may vary significantly among dogs. In support of this idea, wide reference values for serum amylase activity in blood biochemistry panels indicate strong variability in amylase activity among dogs. Based on the simultaneous increase in AMY2B copy number and amylase activity in dogs relative to in wolves (Axelsson et al. 2013), it is reasonable to hypothesize that amylase activity is associated with AMY2B copy number within the dog population. Yet, a previous analysis within the dog population did not support this hypothesis (Axelsson et al. 2013).
AMY2B and breed variation
Although it is clear that AMY2B copy number varies considerably among dogs, it is not known how much of this variation is confined to breeds. Strong genetic drift associated with the shifting demographic histories of dog breeds is likely to have resulted in breed-specific patterns of copy abundance, but is also possible that regional dietary constraints may have resulted in differential selective regimes that may have shifted average copy numbers among breeds. Regardless of how, it is thus possible that the average ability to digest starch varies among dog breeds.
AMY2B and DM in dogs
In contrast to dogs, humans have acquired expression of amylase in saliva via an ancient gene duplication and a subsequent insertion of a retroviral promoter upstream of the duplicated gene copy (Ting et al. 1992). Parallel to the increase in AMY2B copy number during dog domestication, the copy number of the gene coding for salivary amylase, AMY1, has risen threefold in humans relative to in chimpanzees (Bank et al. 1992; Perry et al. 2007), and copy numbers are higher in human populations, such as American Europeans and Japanese, that traditionally relied on a diet that was relatively rich in starch (Perry et al. 2007), suggesting that dogs and humans have adapted to a similar change in diet. Both amylase activity and protein levels in human saliva correlate with AMY1 copy number, arguing that efficient starch digestion in saliva is directly linked to copy number abundance (Perry et al. 2007; Mandel et al. 2010). In humans, high salivary amylase activity is furthermore associated with a rapid insulin response accompanied by a quick reduction in blood glucose levels following starch ingestion (Mandel & Breslin 2012), whereas low serum amylase activity is associated with an increased risk of cardiometabolic disorders (Lee et al. 2011; Nakajima et al. 2011a,b; Mandel & Breslin 2012; Muneyuki et al. 2012).
Although these associations are based on measures of amylase activity rather than AMY1 copy number directly, it is feasible that genetically determined variation in amylase activity may predispose to conditions such as obesity and diabetes mellitus (DM) in humans (Lee et al. 2011; Nakajima et al. 2011a,b; Mandel & Breslin 2012; Muneyuki et al. 2012). DM is one of the most common metabolic disorders affecting both man and dog. In dogs, the exact etiology of DM is not known. Although dogs do not develop classical type 2 DM as do humans and cats (Catchpole et al. 2013), DM can occur secondary to gestation or diestrous in females due to hormonal changes. Other common secondary causes of diabetes are pancreatitis and Cushing's disease. It is estimated that more than 1 percent of all dogs will be affected by diabetes (Fall et al. 2007); however, incidence varies substantially among dog breeds, arguing that genetic components may contribute to this disease (Fall et al. 2007; Catchpole et al. 2013).
In this study, we estimated AMY2B copy numbers in a large number of dog samples to (i) test whether amylase activity is associated with AMY2B copy number in dogs, (ii) investigate how AMY2B copy number segregates within and among dog breeds and (iii) test for a potential link between AMY2B copy number and susceptibility for developing DM.
Materials and methods
Dog samples
To investigate the relationship between amylase activity and AMY2B copy numbers, EDTA blood and serum were obtained from leftover patient material at the Clinical Pathology service at the Swedish University of Agricultural Sciences in Uppsala, Sweden. Samples were collected randomly without any regard to age, sex or health status, with the exception of excluding all suspected pancreatitis cases from further analyses to avoid any potential confounding effects on amylase activity. In total, 55 samples from 35 different breeds (Table S1) were collected to have both amylase activity and AMY2B copy number measured (Fig. S1).
To study breed-specific patterns of AMY2B variation and to investigate the relationship between copy numbers and diabetes susceptibility, we collected eight dogs each from 19 different dog breeds (Table S2), as well as 19 Greenland Sledge dogs (total number of dogs, n = 171). All samples except the Greenland Sledge dogs, which were sampled on location in Greenland, were collected from the Canine Biobank at Uppsala University and the Swedish University of Agricultural Sciences. Breed selection was based on primarily the availability of breed-specific DM prevalence statistics (data available for 16 of the 20 breeds; Fall et al. 2007), whereas additional breeds were included as previous observations indicated deviant AMY2B copy numbers.
AMY2B copy numbers were compared in eight diabetic dogs and eight healthy controls (dogs ages >7 without diabetes) respectively in five different breeds (Samoyed, Australian Terrier, Border Collie, Swedish Elkhound and Norwegian Elkhound), all of which have shown an increased risk of developing DM (Fall et al. 2007; Catchpole et al. 2013) (total number of dogs, n = 80). The 40 control dogs were also used in the breed-specific amylase copy analysis mentioned above. The Swedish and Norwegian Elkhounds as well as the Border Collies were all females and the cases were classified as hormone-dependent DM (gestational/diestral). The Samoyed and Australian Terrier populations were a mix of both females and males having adult-onset insulin-dependent DM without further classification.
In total, 266 dogs [55 + 171 + 40 (the 40 DM controls were included among the 171 dogs)] were assayed for AMY2B copy number in this study.
DNA extraction
DNA was extracted from EDTA blood using either manual salt extraction (Miller et al. 1988) or the QIASymphony DNA Midi kit (Qiagen) on the QIASymphony robot (Qiagen).
Amylase activity assay
Serum amylase activity was analyzed at the Clinical Pathology service (Swedish Agricultural University) using an Architect e400 instrument using the amylase reagents 7D58-21 (Abbott Laboratories).
Copy number assay
AMY2B copy number variation in dogs was previously studied using traditional qPCR (Axelsson et al. 2013). In this study, droplet digital PCR (ddPCR) was used, which allows for an absolute measure of DNA molecules partitioned into thousands of droplets. This enables a more precise estimation of DNA copy numbers, which in particular has the potential to overcome the limited capacity of qPCR to resolve high copy number gene duplications accurately (Hindson et al. 2011; Pinheiro et al. 2012). Probe and primers for the AMY2B target gene region and the CCZ1B (previous c7orf28b) reference gene were designed as described in Axelsson et al. (2013). Droplet digital PCR was performed using the QX100 third-generation droplet digital PCR system provided by Bio-Rad (Hindson et al. 2011). DNA was digested with DRAI (New England Biolabs) to separate individual amylase copies to allow for better partitioning. Raw copy number data were rounded to the nearest whole number.
Statistics
A mixed linear regression model was used to assess the association of AMY2B copy number with amylase activity in the 55 dogs. The stata procedure ‘xtmixed’ was used for this purpose, and the variable dog breed (average 1.5 dogs per breed, range 1–6) was entered into the model as a random effect. Preliminary modeling showed non-normality of residuals; therefore, the dependent variable amylase activity was transformed to the natural logarithm scale before modeling.
A one-way ANOVA test was used to test whether mean AMY2B copy numbers differ among dog breeds and to establish how much of individual copy number variability could be ascribed to breed origin. We used Pearson's correlation coefficient to test for a correlation between mean copy number and DM incidence and two-way ANOVA tests to determine whether AMY2B copy numbers differed between DM cases and controls. These statistical analyses were performed using graphpad prism™ software.
Ethics
Dog samples were obtained with the owner's consent. The sampling conformed to the decision of the Swedish Animal Ethical Committee (no. C62/10) and the Swedish Animal Welfare Agency (no.31-1711/10).
Results
Serum amylase activity correlates with AMY2B copy number
AMY2B copy number and amylase activity were estimated in 55 dogs of 35 different breeds (Table S1 and Fig. S1). Amylase activity in serum varied widely throughout the samples. Median activity was 12.1 μkat/μl (IQR 8.6–17.3; range 4.9–34.5). We also observed considerable variation in AMY2B copy numbers among the 55 dogs. Mean diploid copy number was 2n = 10.3 ± 2.5 with individual values ranging from 2n = 4 to 2n = 18 (Table S1). Mixed linear regression modeling adjusting for breed showed a positive association of the number of AMY2B copies with ln amylase [β = 0.05 (95% CI, 0.1–0.9; P-value = 0.011)]. This corresponds to an increase of 5.4 percent in amylase activity for each extra copy. The copy number variation was estimated to explain 14.8% of the variance in amylase activity.
Amylase copy number variation within and among dog breeds
AMY2B copy numbers also varied considerably in a larger set of 171 dogs from 20 different breeds [eight dogs from each of 19 breeds (Table S2) and 19 Greenland Sledge dogs]. Mean diploid AMY2B copy number was 2n = 11.2 ± 4.0, and individual estimates ranged from 2n = 2 to 2n = 21. To investigate to what extent individual copy number variability depends on breed origin, we carried out a one-way ANOVA test. We found that copy numbers vary significantly among breeds and that nearly 70 percent of the individual variation can be attributed to breed (P > 0.0001, R2 = 0.67) (Fig.1 and Table S2). Among the 20 breeds analyzed here, AMY2B copy numbers were least abundant in Greenland Sledge dogs (mean diploid AMY2B copy number 2n = 4.3 ± 2.7), and we identified several Greenland Sledge dogs with only two AMY2B copies. To exclude a potential bias on the breed-based analysis due to this unusual copy number distribution, we excluded all Greenland Sledge dogs and reanalyzed the data. Mean copy numbers still varied significantly among breeds, and breed origin explained more than 50 percent of copy number variability (P > 0.0001, R2 = 0.51). In addition to Greenland Sledge dogs, we also note that, in general, Samoyeds carry few AMY2B copies (mean diploid AMY2B copy number 2n = 6.9, SD = 2.6), whereas German Shepherds (mean diploid AMY2B copy number 2n = 15.8, SD = 1.9) and English Springer Spaniels (mean diploid AMY2B copy number 2n = 17.3, SD = 3.4) consistently carry diploid copy numbers above 10. AMY2B copy number varied in all breeds studied (Table S2), and Beagles in particular are highly variable at this locus (mean diploid AMY2B copy number 2n = 11.9, SD = 4.7), whereas Polish Lowland Sheepdogs (PON) are relatively homogenous (mean diploid AMY2B copy number 2n = 10.8, SD = 1.0).
Figure 1.

Tukey boxplot showing AMY2B copy number diversity in different dog breeds. Horizontal bars represent the median copy number within each breed. The 25th to 75th percentiles are boxed and the remaining distribution marked by a vertical line. Outside dots mark major outliers. 1 = Greenland Sledge Dog, 2 = Samoyed, 3 = Poodle, 4 = Shar Pei, 5 = Alaskan Malamute, 6 = Bearded Collie, 7 = PON, 8 = Drever, 9 = Boxer, 10 = Labrador, 11 = Beagle, 12 = Norwegian Elkhound, 13 = Border Collie, 14 = Golden Retriever, 15 = Rottweiler, 16 = Australian Shepherd, 17 = Swedish Elkhound, 18 = Duck Tolling Retriever, 19 = German Shepherd, 20 = English Springer Spaniel.
Testing for an association between diabetes and AMY2B
Among the dogs analyzed above, reliable diabetes incidence rates have been estimated for 16 of 20 breeds (Fall et al. 2007; Table1). To investigate a potential link between AMY2B copy number and susceptibility to DM, we first compared mean copy numbers and DM incidence across these breeds. We specifically note that incidence is high (ranked second) in Samoyeds, which generally carry few AMY2B copies; however, we see no general association between low copy numbers and high DM incidence when all breeds are analyzed jointly (Pearson's correlation test, one-tailed P = 0.30).
Table 1.
Mean copy number and diabetes incidence per 10 000 dog years at risk (DYAR) for 16 dog breeds. Incidence values were published in Fall et al. (2007).
| Breed | Mean AMY2B copy number | Diabetes incidence pr. 10 000 DYAR |
|---|---|---|
| Boxer | 11.55 | 0 |
| Bearded Collie | 10.05 | 1 |
| Golden Retriever | 12.91 | 1 |
| Poodle | 9.451 | 2 |
| Duck Tolling Retriever | 14.25 | 3 |
| German Shepherd | 15.71 | 4 |
| Labrador | 11.86 | 13 |
| English Springer Spaniel | 17.28 | 13 |
| Norwegian Elkhound | 11.96 | 17 |
| Rottweiler | 13.66 | 23 |
| Beagle | 11.88 | 24 |
| Drever | 11.65 | 36 |
| Border Collie | 12.74 | 36 |
| Swedish Elkhound | 13.66 | 45 |
| Samoyed | 6.878 | 104 |
| Australian Terrier | 13.68 | 183 |
We then examined AMY2B copy numbers in eight DM cases and eight healthy controls from five different breeds (Samoyed, Australian Terrier, Border Collie, Swedish Elkhound and Norwegian Elkhound), all of which have shown an increased risk of developing DM (Fall et al. 2007; Catchpole et al. 2013). Although we note that AMY2B copy numbers on average tend to be lower in all cases (2n = 11.4, SD = 2.0, n = 40) than in all controls (2n = 11.8, SD = 2.853, n = 40, P = 0.41) and in four of five comparisons within breeds (Fig.2, Table S3), no differences are significant. Furthermore, a two-way ANOVA analysis investigating the joint affects of breed origin and disease status indicates that breed origin accounts for 51.7 percent of the AMY2B copy number variation (P < 0.0001), whereas disease status is not related to copy number (proportion of variation explained = 0.45%, P = 0.41, Table S4).
Figure 2.
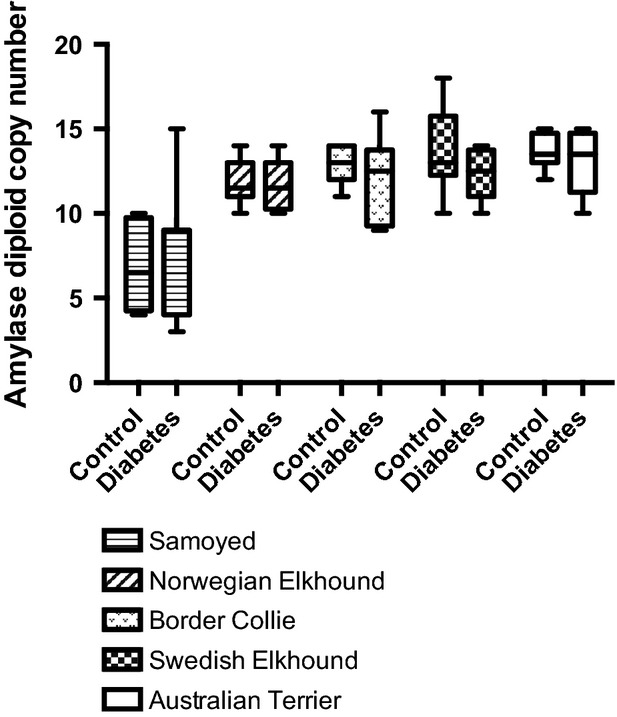
Tukey boxplot showing the distribution of AMY2B copy numbers in diabetic cases and healthy controls from 5 different high-risk dog breeds. The horizontal bar marks the median for each group. The 25th to 75th percentiles are boxed and the remaining distribution marked by a vertical line.
Discussion
AMY2B copy number and serum amylase activity
Evidence for selection at the entire pathway responsible for starch digestion and glucose absorption indicates that efficient use of energy stored in starch was crucial to the survival and fitness of dogs during the domestication process (Axelsson et al. 2013). In the first step of this pathway, alpha-amylase initializes starch digestion by catalyzing the hydrolysis of starch to oligosaccharides maltose and maltriose. Increased amylase activity in dogs relative to wolves is associated with high AMY2B copy numbers in dogs, arguing that efficient starch digestion is linked to high copy numbers at this locus. However, this association has so far not been confirmed within the dog population (Axelsson et al. 2013). A lack of association may potentially question a causal link between copy number and amylase activity, or alternatively, reflect a combination of a limited sample size in the previous study, physiological effects on AMY2B activity (Swanson et al. 2000; Piccione et al. 2008; Nakajima et al. 2011a) and the limited precision of qPCR to accurately resolve high copy number gene duplications (Hindson et al. 2011). Independent of that, serum amylase activity measurements are currently of limited diagnostic value (Strombeck et al. 1981). To gain a better diagnostic use of this blood biochemistry analyte and to provide additional insight into the evolution of this trait during dog domestication, we measured AMY2B copy numbers and serum amylase activity in 55 dogs from a diverse set of breeds. We note that the variation in amylase activity throughout this sample (range 4.9–34.5 μkat/μl) largely recapitulates the wide reference values for serum amylase activity assays (the in-house laboratory normal reference values for the amylase test used were 5–25 μkat/μl). Similarly, in agreement with previous observations (Axelsson et al. 2013), it is also clear that AMY2B copy numbers vary substantially among individuals. By comparing amylase activity and AMY2B copy number, we then show that amylase activity increases linearly with copy number in this sample of dogs. Our result argue that starch digestion indeed is more efficient in dogs with many AMY2B copies compared with in individuals carrying few copies. This in turn strengthens the argument that selection for efficient starch digestion caused the increase in AMY2B copy numbers during dog domestication and that this change likely allowed dogs to thrive on a diet that was relatively rich in starch (Axelsson et al. 2013).
Although serum amylase activity thus depends on AMY2B copy number in dogs, only a relatively small proportion of the variation of serum amylase is explained by this variable (R2 = 14.8%), indicating that additional factors must be invoked to explain the majority of the variation. We can think of several such potential additional factors. First, the samples used in this study were taken from dogs that had blood samples taken for diagnostic purposes. Although no dogs that were suspected of having pancreatitis were included in this study, in principle, these dogs could be affected by other conditions that may affect serum amylase activity. Second, and probably more important, serum amylase activity is influenced by dietary habits, age and circadian rhythm, factors that have not been taken into account in this study (Piccione et al. 2008). Although serum amylase activity clearly is associated with AMY2B copy number in dogs, care should thus be taken at this point not to interpret activity measures as a direct indicator of inherited ability to handle starch. Future studies using larger cohorts under controlled settings may potentially allow us to establish copy-number-specific reference values for serum amylase activity that can be used to detect abnormal activity.
AMY2B copy number variability within and among breeds
In line with our previous observation, AMY2B copy numbers varied considerably among the 266 dogs analyzed here with individual measures ranging from 2n = 2 to 2n = 21. We note that the maximum copy number detected in this study (2n = 21) contrasts with our previous study in which the maximum AMY2B copy number was 2n = 30. This discrepancy is likely due to the improved accuracy of ddPCR over traditional qPCR at resolving high copy number gene duplications, and 2n = 21 likely represents the better estimate of the upper bound of the AMY2B copy number distribution in dogs.
AMY2B copy numbers vary significantly among breeds, and at least 50 percent of the individual copy number variability can be attributed to breed origin. This finding has relevance for the use of serum amylase activity measures for diagnostic purposes, as it provides a first framework for interpreting activity measures based on a breed origin. A strikingly high amylase activity measure may be considered more unusual in, for instance, a Samoyed than an English Springer Spaniel. Differences in AMY2B copy numbers among breeds are expected based on the highly divergent demographic histories for several of the breeds studied here. Bottlenecks associated with the creation of breeds likely resulted in a random inclusion of small subsets of the entire range of AMY2B haplotypes into different breeds. This effect is likely reflected in our observations of restricted variation in AMY2B copy numbers in PON, whereas Beagles display a much wider copy number range. The PON breed was almost extinct around 1950 with only a few individuals serving as founders for the population we see today (Augustowska 2007), whereas the Beagle breed is based on a larger founder population and subdivided into working dogs and laboratory dogs, which adds to a larger expected diversity in this breed.
In addition to these demographic effects, based on the strong evidence for selection for increased AMY2B copy numbers during dog domestication, it is also tempting to speculate that selection has continued to mold copy number variation among dog breeds. Among the breeds analyzed here, we find two breeds, Greenland Sledge dogs and Samoyeds, with markedly lower copy numbers compared with other breeds. The Samoyed presumably represents an old breed that was developed among hunting and pastoralist populations in Siberia. Greenland Sledge dogs represent a breed that through many years has been isolated geographically and, more recently by laws, from other dog breeds. Both of these breeds have probably relied on a largely protein-based diet, including meat and fish. It is thus possible that the relatively low AMY2B copy numbers reflect a relaxation of the directional selections at this locus in these breeds. However, we cannot rule out that the observation of several Greenland Sledge dogs with a wolf-like haplotype in this study is the result of recent cross-breeding between wolves and sledge dogs, a practice that has been documented historically (Walker & Frison 1982). A more detailed analysis of dogs from traditionally protein-based vs. starch-based food cultures is needed to understand whether selection may have contributed to breed differences in average AMY2B copy numbers.
AMY2B copy number and DM
In humans, high salivary amylase activities have been associated with a rapid insulin response that in turn results in a rapid reduction in blood glucose levels (Mandel & Breslin 2012). This association may hint at a possible association between AMY2B copy numbers and risk of developing DM. In this study, however, we do not find any association between DM and AMY2B copy numbers in dogs, neither when comparing mean copy numbers in breeds with varying DM incidence nor when comparing cases and controls. Although these observations may rule out AMY2B copy number as a strong monogenic risk factor for DM in dogs, it is premature to rule out a link due to several factors. First, onset of DM is likely triggered by a multitude of factors including several environmental factors, indicating that the effect of individual factors, such as potentially AMY2B copy number, may be small. Furthermore, in regard to this multifactorial nature of DM susceptibility, it is very likely that the limited sample size of our case–control comparisons is insufficient to detect a risk allele with a weaker effect within a multigenic disease. Finally, incidence values used in this study represent all diagnosed cases of diabetes without distinguishing particular subtypes. Specific effects of AMY2B copy number on a particular diabetes subtype within individual breeds may hence go undetected in this comparison. A lack of association between DM incidence rates and mean AMY2B copy numbers among breeds could thus reflect the diversity of DM types involved in this comparison. Future studies including a larger collection of carefully characterized cases and controls should help disentangle these possibilities in more detail.
Acknowledgments
We thank Merete Fredholm at the University of Copenhagen, the Swedish Canine Biobank at Uppsala University and the Swedish University for Agricultural Sciences and the Clinical Pathology service at the Swedish University of Agricultural Sciences in Uppsala for providing samples. The project was funded by a grant from the Swedish Research Council to E.A. and a EURYI to K.L.-T. funded by the ESF.
Supporting Information
Additional supporting information may be found in the online version of this article.
AMY2B copy number variation relative to serum amylase activity. Scatter plot showing the relationship between AMY2B copy number and serum amylase activity.
Breed, serum amylase activity and diploid AMY2B copy number for the 55 dogs used to test for an association between AMY2B copy number and serum amylase activity.
Distribution of diploid AMY2B copy numbers in 20 different dog breeds
Mean diploid AMY2B copy number in diabetic mellitus cases and controls respectively for five dog breeds with high risk of developing diabetes.
Summary of the two-way ANOVA statistics analyzing how much of the variation in diploid AMY2B copy numbers is explained by diabetes and breed respectively.
References
- Augustowska E. Polish Lowland Sheepdog. Allenhurst, NJ: Kennel Club Books; 2007. [Google Scholar]
- Axelsson E, Ratnakumar A, Arendt ML, Maqbool K, Webster MT, Perloski M, Liberg O, Arnemo JM, Hedhammar A. Lindblad-Toh K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495:360–4. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- Bank RA, Hettema EH, Muijs MA, Pals G, Arwert F, Boomsma DI. Pronk JC. Variation in gene copy number and polymorphism of the human salivary amylase isoenzyme system in Caucasians. Human Genetics. 1992;89:213–22. doi: 10.1007/BF00217126. [DOI] [PubMed] [Google Scholar]
- Catchpole B, Adams JP, Holder AL, Short AD, Ollier WE. Kennedy LJ. Genetics of canine diabetes mellitus: are the diabetes susceptibility genes identified in humans involved in breed susceptibility to diabetes mellitus in dogs? The Veterinary Journal. 2013;195:139–47. doi: 10.1016/j.tvjl.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Fall T, Hemlin HH, Hedhammer Å, Kämpe O. Egenvall A. Diabetes mellitus in a population of 180,000 insured dogs: incidence, survival, and breed distribution. Journal of Veterinary Internal Medicine. 2007;21:1209–16. doi: 10.1892/07-021.1. [DOI] [PubMed] [Google Scholar]
- Hindson BJ, Ness KD, Masquelier DA, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Analytical Chemistry. 2011;83:8604–10. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Park SW, Cho BM, Lee S, Kim YJ, Jeong DW, Yi YH. Cho YH. Serum amylase and risk of the metabolic syndrome in Korean adults. Clinica Chimica Acta. 2011;412:1848–53. doi: 10.1016/j.cca.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Mandel AL. Breslin PA. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. Journal of Nutrition. 2012;142:853–8. doi: 10.3945/jn.111.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel AL, Peyrot des Gachons C, Plank KL, Alarcon S. Breslin PA. Individual differences in AMY1 gene copy number, salivary alpha-amylase levels, and the perception of oral starch. PLoS ONE. 2010;5:e13352. doi: 10.1371/journal.pone.0013352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD. Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocharla H, Mocharla R. Hodes ME. Alpha-amylase gene transcription in tissues of normal dog. Nucleic Acids Research. 1990;18:1031–6. doi: 10.1093/nar/18.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneyuki T, Nakajima K, Aoki A, et al. Latent associations of low serum amylase with decreased plasma insulin levels and insulin resistance in asymptomatic middle-aged adults. Cardiovascular Diabetology. 2012;11:80. doi: 10.1186/1475-2840-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Muneyuki T, Munakata H. Kakei M. Revisiting the cardiometabolic relevance of serum amylase. BMC Research Notes. 2011a;4:419. doi: 10.1186/1756-0500-4-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Nemoto T, Muneyuki T, Kakei M, Fuchigami H. Munakata H. Low serum amylase in association with metabolic syndrome and diabetes: a community-based study. Cardiovascular Diabetology. 2011b;10:34. doi: 10.1186/1475-2840-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry GH, Dominy NJ, Claw KG, et al. Diet and the evolution of human amylase gene copy number variation. Nature Genetics. 2007;39:1256–60. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccione G, Giannetto C, Fazio F. Giudice E. Daily rhythm of serum lipase and alpha-amylase activity in fed and fasted dogs. Journal of Veterinary Diagnostic Investigation. 2008;20:795–9. doi: 10.1177/104063870802000614. [DOI] [PubMed] [Google Scholar]
- Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, Bhat S. Emslie KR. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Analytical Chemistry. 2012;84:1003–11. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strombeck DR, Farver T. Kaneko JJ. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. American Journal of Veterinary Research. 1981;42:1966–70. [PubMed] [Google Scholar]
- Swanson KC, Matthews JC, Matthews AD, Howell JA, Richards CJ. Harmon DL. Dietary carbohydrate source and energy intake influence the expression of pancreatic alpha-amylase in lambs. Journal of Nutrition. 2000;130:2157–65. doi: 10.1093/jn/130.9.2157. [DOI] [PubMed] [Google Scholar]
- Ting CN, Rosenberg MP, Snow CM, Samuelson LC. Meisler MH. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes & Development. 1992;6:1457–65. doi: 10.1101/gad.6.8.1457. [DOI] [PubMed] [Google Scholar]
- Walker DN. Frison GC. Studies on Amerindian dogs, 3: prehistoric wolf/dog hybrids from the northwestern plains. Journal of Archaeological Science. 1982;9:125–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AMY2B copy number variation relative to serum amylase activity. Scatter plot showing the relationship between AMY2B copy number and serum amylase activity.
Breed, serum amylase activity and diploid AMY2B copy number for the 55 dogs used to test for an association between AMY2B copy number and serum amylase activity.
Distribution of diploid AMY2B copy numbers in 20 different dog breeds
Mean diploid AMY2B copy number in diabetic mellitus cases and controls respectively for five dog breeds with high risk of developing diabetes.
Summary of the two-way ANOVA statistics analyzing how much of the variation in diploid AMY2B copy numbers is explained by diabetes and breed respectively.


