Abstract
Background
Data on relative safety, efficacy, and role of different percutaneous left ventricular assist devices for hemodynamic support during the ventricular tachycardia (VT) ablation procedure are limited.
Methods and Results
We performed a multicenter, observational study from a prospective registry including all consecutive patients (N=66) undergoing VT ablation with a percutaneous left ventricular assist devices in 6 centers in the United States. Patients with intra-aortic balloon pump (IABP group; N=22) were compared with patients with either an Impella or a TandemHeart device (non-IABP group; N=44). There were no significant differences in the baseline characteristics between both the groups. In non-IABP group (1) more patients could undergo entrainment/activation mapping (82% versus 59%; P=0.046), (2) more number of unstable VTs could be mapped and ablated per patient (1.05±0.78 versus 0.32±0.48; P<0.001), (3) more number of VTs could be terminated by ablation (1.59±1.0 versus 0.91±0.81; P=0.007), and (4) fewer VTs were terminated with rescue shocks (1.9±2.2 versus 3.0±1.5; P=0.049) when compared with IABP group. Complications of the procedure trended to be more in the non-IABP group when compared with those in the IABP group (32% versus 14%; P=0.143). Intermediate term outcomes (mortality and VT recurrence) during 12±5-month follow-up were not different between both groups. Left ventricular ejection fraction ≤15% was a strong and independent predictor of in-hospital mortality (53% versus 4%; P<0.001).
Conclusions
Impella and TandemHeart use in VT ablation facilitates extensive activation mapping of several unstable VTs and requires fewer rescue shocks during the procedure when compared with using IABP.
Keywords: catheter ablation, intra-aortic balloon pumping, tachycardia, ventricular
Radiofrequency catheter ablation of ventricular tachycardia (VT) is indicated and often necessary in patients with structurally abnormal hearts and drug refractory, recurrent VT resulting in multiple implantable cardiac defibrillator (ICD) shocks.1 Comprehensive evaluation of VT in the electrophysiology laboratory ideally comprises activation and entrainment mapping in addition to substrate mapping. This enables us to define the VT circuit, identify the isthmus of the circuit, and perform ablation during VT, which provides an immediate end point of efficacy. An ablation approach comprising VT induction also assures targeting the clinical arrhythmia(s) and limits unnecessary ablation potentially decreasing procedure complications while maintaining efficacy. Unfortunately, 50% to 80% of the patients with structural heart disease referred for VT ablation have unstable VT.2,3
Catheter ablation in patients with unstable VT is primarily performed with substrate and pace mapping techniques. As such, extensive ablation for substrate modification is often required because complete delineation of the VT(s) circuits’ cannot be performed. Extensive ablation can affect subsequent cardiac function, requires extensive catheter manipulation, and if the linear ablation is not complete may facilitate novel macroreentrant arrhythmias. Complicating the peri-procedural management of unstable VT(s), patients are often referred for ablation in the setting of frequent VT shocks and VT storm. Patients that present in this manner often have a more acutely decompensated hemodynamic status.
In patients with unstable VT(s), options are available for peri-procedural blood pressure support.4 Few centers use intra-aortic balloon pump (IABP) routinely for hemodynamic support during unstable VT ablation, although some limited data suggest that IABP support may not be adequate when compared with other newer percutaneous left ventricular assist devices (pLVADs).5 Alternatives, with more pressure support, are newer pLVADs; however, large prospective studies are lacking.5–8 We intended to evaluate the relative safety and efficacy of using IABP versus non-IABP (Impella or TandemHeart) for unstable VT ablation in a multicenter study.
Methods
We report findings from a multicenter, prospective registry including all consecutive patients who underwent VT ablation with a pLVAD in 6 participating centers across United States between March 2006 and December 2011. The study was approved by the institutional review board at each of the participating centers. VT ablation was indicated for recurrent ICD therapies, despite being on antiarrhythmic medications in all patients. A pLVAD was implanted prophylactically for VT ablation at the discretion of the operator. Patients who underwent pLVAD for cardiogenic shock but later on underwent VT ablation were excluded. The pLVADs used were (1) IABP (Arrow International Inc, Reading, PA; Datascope, Montvale, NJ; Abiomed Inc, Danvers, MA); (2) Impella microcirculatory axial blood flow pump (Abiomed Inc, Danvers, MA); and (3) TandemHeart (Cardiac Assist, Inc, Pittsburg, PA). The choice of the device was at the physician’s discretion. See Table I in the Data Supplement for distribution of the device use across the centers.
pLVAD Placement
A total of 66 patients underwent VT ablation during the study period with one of the above pLVADs of which, IABP, Impella, and TandemHeart devices were implanted in 22 (33%), 25 (38%), and 19 (29%) patients, respectively. Eight receiving TandemHeart and included in this study were part of a previously published series.6 All the pLVADs were placed in the electrophysiology laboratory by the operating electrophysiology or an interventional cardiologist using standard techniques as described elsewhere, before the placement of diagnostic catheters.4,9,10 See Data Supplement and Figures 1 to 3 for implantation details. Low left ventricular ejection faction (LVEF) was defined as LVEF ≤15%. All procedures were performed under general anesthesia and continuous intra-arterial blood pressure monitoring was performed throughout the procedure.
All patients were anticoagulated with unfractionated heparin to maintain an activated clotting time >300 seconds just before or immediately after the transseptal puncture (TandemHeart) or placement of the arterial sheath (in case of IABP and Impella). The arteriotomies in patients with Impella and TandemHeart devices were closed using a double Perclose technique, and prolonged manual compression was used for IABP patients.
Mapping and Ablation Procedure
After placement of diagnostic catheters at standard locations (right ventricular apex, coronary sinus, His bundle, and high right atrium in some cases), transseptal puncture with Brockenbrough needle and Mullin’s sheath was performed under intracardiac echocardiography guidance in patients with IABP and Impella, whereas a retrograde LV access was obtained in patients with TandemHeart. A few patients underwent additional pericardial access using standard techniques as described elsewhere.11 Mean arterial pressures were obtained from the continuous blood pressure monitoring at the start of the procedure. Electroanatomic mapping was performed with CARTO or EnSite mapping systems. Remote magnetic navigation system (Stereotaxis Inc, St. Louis, MO) was also used in some cases at the discretion of the operator. Mapping and ablation were performed with ThermoCool (or ThermoCool RMT in case of Stereotaxis) 3.5-mm externally irrigated catheter (Biosense Webster, Inc, Diamond Bar, CA). All patients underwent dense substrate/scar mapping using standard definitions of scar: <0.5 mV of bipolar local electrogram designated as scar and the same between 0.5 and 1.5 mV designated as border zone.
In all patients VT was induced and attempts were made to perform activation and entrainment mapping if possible. VT was terminated prematurely if the mean arterial blood pressure was dropping <45 mm Hg. In patients in whom activation map could be obtained, entrainment mapping was performed when possible and the optimal site of ablation was identified and targeted with the intention to terminate VT with ablation. Patients in whom VT could not be tolerated in spite of maximal circulatory support, pace mapping with local electrogram characteristics during sinus/paced rhythm was used to identify optimal sites of ablation. Initial goal of the ablation was to make the clinical VT noninducible. Further attempts to target easily inducible, nonclinical VTs with substrate or activation mapping were made by the operators at their discretion.
Data Collection and Follow-Up
Data were collected prospectively, as a part of a multicenter registry, at each of the participating centers. Baseline characteristics, medication use, ICD therapies, procedural variables, and short-term and long-term outcomes were collected. Patients were followed up as per the standard of practice. Repeat VT ablation procedures were performed in a few patients when deemed necessary. In cases of death, the reason for death was also recorded when available.
Statistical Analysis
Based on previous studies and our experience with the degree of circulatory support obtained with each of the devices, we divided the population into 2 groups: IABP and non-IABP groups. The non-IABP group included patients with either an Impella or a TandemHeart device. Univariate analyses were performed using χ2 test with Fisher exact test wherever required for categorical variables and ANOVA or Wilcoxon Mann–Whitney rank test for continuous variables. We also assessed the predictors of in-hospital and long-term mortality using univariate and multivariate analyses. Cox-regression analysis was used after selecting 3 predictors, which were significant in the univariate analyses with the least P value. A 2-tailed P value of <0.05 was considered statistically significant. All analyses were performed with SPSS version 19.0 for Windows (SPSS, Inc, Chicago, IL).
Results
Baseline Characteristics
All 66 patients who underwent VT ablation during the study period with a pLVAD in one of our participating centers during the study period are included in current study. Of these IABP, Impella and TandemHeart devices were implanted in 22 (33%), 25 (38%), and 19 (29%) patients, respectively (Table 1; Table II in the Data Supplement). Mean age of the study population was 67±12 years with 94% men, and 70% having ischemic cardiomyopathy with no significant differences between both the groups. Mean LVEF was 28±12% with no significant difference between both the groups. The primary reason for implantation of pLVAD was also not significantly different across both the groups. In 17 (26%) patients pLVAD was implanted for borderline hemodynamic status at baseline. In the remaining patients, it was implanted either for unstable VT (31; 48%) or for low LVEF (17; 26%). There were no significant differences between the comorbidities, medication use, proportion with prior VT ablation, mean number of ICD shocks, ATP therapies, and antiarrhythmics failed between both the groups.
Table 1.
Comparison of Baseline Characteristics of Patients Undergoing Ventricular Tachycardia Ablation With Different Percutaneous Left Ventricular Assist Devices
| IABP (N=22) | Non-IABP Combined (N=44) |
Non-IABP Subgroups | Total (N=66) |
P Value (IABP vs Non-IABP) |
||
|---|---|---|---|---|---|---|
| Impella (N=25) | TandemHeart (N=19) | |||||
| Age, y, mean±SD | 69±10 | 66±12 | 68±12 | 62±13 | 67±12 | 0.234 |
| Male sex, % | 21 (96) | 41 (93) | 23 (92) | 18 (95) | 62 (94) | 0.715 |
| Ischemic cardiomyopathy, % | 16 (73) | 29 (66) | 16 (64) | 13 (68) | 45 (68) | 0.575 |
| Previous VT ablation, % | 7 (32) | 15 (34) | 7 (28) | 8 (42) | 22 (33) | 0.854 |
| LVEF, % | 25±10 | 29±15 | 33±14 | 23±13 | 28±13 | 0.213 |
| ICU status, % | 13 (59) | 19 (43) | 9 (36) | 10 (53) | 32 (49) | 0.223 |
| No. of ICD shocks, mean±SD | 11±10 | 11±9.7 | 7±8 | 17±9 | 11±10 | 0.911 |
| No. of ATP, mean±SD | 28±23 | 26±29 | 24±35 | 29±18 | 27±27 | 0.756 |
| No. of AADs failed, mean±SD | 1.8±1.0 | 2.0±1.0 | 1.4±1.0 | 2.6±0.6 | 1.9±1.0 | 0.260 |
AAD indicates antiarrhythmic drug; ATP, antitachycardia pacing; CRT-D, cardiac resynchronization therapy-defibrillator; IABP, intra-aortic balloon pump; ICD, implantable cardiac defibrillator; ICU, intensive care unit; LVEF, left ventricular ejection fraction; and VT, ventricular tachycardia.
Procedural Characteristics
Ten (16%) patients underwent epicardial ablation in addition to the endocardial ablation with no significant differences across both the groups (Table 2). One patient with TandemHeart with significant aortic valvular disease underwent only epicardial ablation. CARTO mapping system was used in 46 (70%) of the patients with no significant difference between both the groups. Stereotaxis was much less likely used in patients with Impella or TandemHeart when compared with patients with IABP (5% versus 36%; P=0.002).
Table 2.
Comparison of Procedural Variables Between Patients With Different Percutaneous Left Ventricular Assist Devices During Ventricular Tachycardia Ablation
| IABP (N=22) | Non-IABP Combined (N=44) |
Non-IABP Subgroups | Total (N=66) |
P Value (IABP vs Non-IABP) |
||
|---|---|---|---|---|---|---|
| Impella (N=25) | TandemHeart (N=19) | |||||
| No. of VTs induced | ||||||
| Mean±SD | 3.3±1.5 | 3.1±1.9 | 2.5±1.7 | 3.9±1.8 | 3.2±1.8 | 0.733 |
| Median (min–max) | 3 (1–7) | 3 (0–7) | 2 (0–7) | 4 (2–7) | 3 (0–7) | 0.556 |
| No. of nonclinical VTs induced | ||||||
| Mean±SD | 1.2±1.1 | 1.0±1.5 | 0.64±1.1 | 1.7±1.9 | 1.1±1.4 | 0.721 |
| Median (min–max) | 1 (0–4) | 0.5 (0–6) | 0 (0–5) | 1 (0–6) | 1 (0–6) | 0.209 |
| No. of VTs ablated | ||||||
| Mean±SD | 1.8±1.0 | 2.4±1.3 | 1.9±1.1 | 3.0±1.2 | 2.2±1.2 | 0.074 |
| Median (min–max) | 1 (1–4) | 2 (1–6) | 1 (1–5) | 3 (1–6) | 2 (1–6) | 0.084 |
| No. of unstable VTs mapped and ablated | ||||||
| Mean±SD | 0.32±0.48* | 1.05±0.78* | 1.12±0.83 | 0.95±0.70 | 0.80±0.77* | <0.001* |
| Median (min–max) | 0 (0–1)* | 1 (0–3)* | 1 (0–3) | 1 (0–2) | 1 (0–3)* | <0.001* |
| No. of VTs RF terminated | ||||||
| Mean±SD | 0.91±0.81* | 1.59±1.00* | 1.16±0.85 | 2.16±0.90 | 1.36±0.99* | 0.007* |
| Median (min–max) | 1 (0–4)* | 1 (0–4)* | 1(0–3) | 2 (1–4) | 1 (0–4)* | 0.003* |
| Entrainment/activation mapping, % | 13 (59)* | 36 (82)* | 20 (80) | 16 (84) | 49 (74)* | 0.046* |
| External rescue shocks | ||||||
| Mean±SD | 3.0±1.5* | 1.9±2.2* | 1.6±2.8 | 2.3±1.2 | 2.3±2.0* | 0.049* |
| Median (min–max) | 3 (0–6)* | 2 (0–13)* | 1 (0–13) | 2 (1–5) | 2 (0–13)* | 0.003* |
| Fluoro time, min, median (Q1–Q3) | 63 (52–72) | 63 (53–73) | 68 (54–74) | 58 (48–70) | 63 (53–73) | 0.935 |
| RF time, min, median (Q1–Q3) | 36 (27–45) | 31 (18–41) | 36 (13–50) | 28 (19–37) | 34 (23–43) | 0.142 |
| Procedure time, min, median (Q1–Q3) | 302 (242–345) | 339 (260–417) | 291 (247–346) | 379 (328–433) | 327 (253–410) | 0.185 |
| Ventilation time, h, median (Q1–Q3) | 16 (12–25) | 12 (6–24) | 6 (5–29) | 20 (12–24) | 15 (8–24) | 0.121 |
IABP indicates intra-aortic balloon pump; RF: Radiofrequency; and VT, ventricular tachycardia.
Statistically significant.
Mean number of VTs induced and ablated per patient in the entire cohort was 3.17±1.8 and 2.20±1.2, respectively, with no significant differences between both the groups. More patients in the non-IABP group could undergo entrainment/activation mapping when compared with those in the IABP group (59% versus 82%; P=0.046). The number of unstable VTs mapped and ablated per patient was more in non-IABP group compared with the IABP group (0.32±0.48 versus 1.05±0.78; P<0.001). The number of VTs terminated by ablation was more in the non-IABP group compared with that in the IABP group (0.91±0.81 versus 1.59±1.01 per patient; P=0.007). IABP group needed more rescue shocks per patient when compared with the non-IABP group (3.0±1.5 versus 1.9±2.2; P=0.049) to terminate unstable VT during the procedure. The lower rescue shock rate in the non-IABP group was primarily driven by the lower shock rate in the Impella group (1.6±2.8 per patient). There were no differences in the fluoroscopy time, ablation time, and the procedural time between both the groups.
Complications and Outcomes
Acute procedural success as defined by the noninducibility of clinical VT was achieved in 58 (88%) patients with no difference between both the groups (Table 3). Mean duration of stay in the hospital was 8±6 days with no significant difference between both the groups. The mean duration of postprocedural pLVAD support was 4±12 hours (range, 2–36 hours). At the time of discharge, 43 of 55 patients (78%) were on antiarrhythmics with no significant difference between both the groups (15; 88% in IABP versus 28; 74% in non-IABP; P=0.227).
Table 3.
Comparison of Baseline Characteristics of Patients Undergoing Ventricular Tachycardia Ablation With Different Percutaneous Left Ventricular Assist Devices
| IABP (N=22) | Non-IABP Combined (N=44) |
Non-IABP Subgroups | Total (N=66) |
P Value (IABP vs Non-IABP) |
||
|---|---|---|---|---|---|---|
| Impella (N=25) | TandemHeart (N=19) | |||||
| Inducible VTs at the end of the procedure, mean±SD | 0.32±0.48 | 0.32±0.46 | 0.35±0.47 | 0.26±0.45 | 0.31±0.46 | 0.853 |
| Acute success, % | 19 (86) | 39 (89) | 21 (84) | 18 (95) | 58 (88) | 0.790 |
| Major complications, % | 3 (14) | 14 (32) | 9 (36) | 5 (19) | 17 (26) | 0.143 |
| Hematoma | 2 (10) | 6 (14) | 4 (16) | 2 (11) | 8 (12) | |
| Pericardial tamponade | 1 (5) | 5 (11) | 4 (16) | 1 (5) | 6 (9) | |
| Arteriovenous fistula | 0 | 1 (2) | 0 | 1 (5) | 1 (2) | |
| Myocardial infarction | 0 | 1 (2) | 1 (5) | 0 | 1 (2) | |
| Death in the EP laboratory | 0 | 1 (2) | 0 | 1 (5) | 1 (2) | |
| Days in the hospital, mean±SD | 7.2±3.9 | 8.2±7.7 | 8.1±9.2 | 8.4±5.3 | 7.9±6.7 | 0.561 |
| Death in the hospital, % | 5 (23) | 6 (14) | 3 (12) | 3 (16) | 11 (17) | 0.350 |
| Recurrence of VT, % | 11 (50) | 18 (42) | 11 (46) | 7 (37) | 29 (45) | 0.532 |
| Repeat VT ablation, % | 7 (32) | 7 (16) | 4 (16) | 3 (16) | 14 (21) | 0.136 |
| Death in 12 mo, % | 8 (36) | 12 (36) | 8 (32) | 4 (21) | 20 (30) | 0.449 |
IABP indicates intra-aortic balloon pump; EP, electrophysiology; and VT, ventricular tachycardia.
Major complications included pericardial tamponade/effusion requiring drainage, vascular complications requiring intervention, stroke, and intraprocedural death. Seventeen (26%) patients had ≥1 major complication during the hospitalization. There were numerically more complications in the non-IABP group when compared with those in the IABP group (32% versus 14%; P=0.143); however, it did not reach statistical significance.(Table 3) In-hospital death occurred in 11 (17%) patients with no significant difference between both the groups. Of the patients who died in the hospital, 9 (14%) patients had LVEF ≤15%. Patients with LVEF ≤15% were much more likely to die in the hospital compared with those with LVEF >15% (53% versus 4%; P<0.001). The only independent predictor of in-hospital mortality was low LVEF (P=0.003).
After a mean follow-up of 12±5 months, 29 (45%) patients had ≥1 VT recurrence, and repeat VT ablation was performed in 14 (21%) patients. Among the patients with recurrence, the mean time to recurrence was 19±22 days in the IABP group versus 25±45 days in the non-IABP group (P=0.703). During the follow-up time, 20 (30%) patients died. There were no significant differences in mortality or VT recurrence between both the groups. The predictors of mortality are shown in Table 4. The only independent predictors of long-term mortality were presence of cardiac resynchronization therapy (odds ratio, 13; P=0.028) and intensive care unit status at the time of VT ablation (odds ratio, 21; P=0.023).
Table 4.
Predictors of Long-Term Mortality (Mean Follow-Up 12 Months) After Ventricular Tachycardia Ablation Using a Percutaneous Left Ventricular Assist Device
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Alive at Last Follow-Up (N=46) |
Dead by Last Follow-Up (N=20) |
P Value | Odds Ratio | 95% CI | P Value | |
| Age, y, mean±SD | 65±12 | 71±10 | 0.052 | |||
| Prior VT ablation, % | 11 (24) | 11 (55) | 0.014 | |||
| CRT-D device, % | 13 (28)* | 13 (65)* | 0.005* | 12.9* | 1.3–126.4* | 0.028* |
| Atrial fibrillation, % | 15 (33) | 12 (60) | 0.038 | |||
| Amiodarone, % | 25 (54) | 18 (90) | 0.005 | |||
| LVEF, mean±SD | 32±13 | 17±7 | <0.001 | |||
| LVEF≤15% | 6 (13) | 11 (55) | <0.001 | 0.2–54.2 | 0.442 | |
| In ICU before the ablation, % | 16 (35)* | 16 (80)* | 0.001* | 21.3* | 1.5–298.6* | 0.023* |
| No. of days in the hospital, mean±SD | 6.1±4.8 | 11.9±8.5 | 0.001 | |||
| No. of rescue shocks, mean±SD | 1.96±1.5 | 3.05±3.0 | 0.050 | |||
CI indicates confidence interval; CRT-D, cardiac resynchronization therapy-defibrillator; ICU, intensive care unit; LVEF, left ventricular ejection fraction; and VT, ventricular tachycardia.
Statistically significant.
Discussion
This is the largest study to date reporting the role of pLVADs in VT ablation. In our multicenter, observational study, we found that when compared with IABP, the use of TandemHeart or Impella for hemodynamic support during VT ablation is associated with (1) performing activation/entrainment mapping of more number of VTs, (2) successful mapping and ablation of a higher number of unstable VTs, (3) terminating VT with ablation possible in more patients, and (4) having fewer rescue shocks to terminate VT. However, this does not translate into improved success rate, either short or long term. Low LVEF is an independent predictor of in-hospital mortality.
Of note, there are no data supporting the superiority of activation/entrainment mapping over substrate mapping. Activation mapping is probably a more desirable method by many electrophysiologists. It enables to accurately define the VT circuit, identify the critical isthmus, and perform limited ablation to terminate VT. Unfortunately, the majority of patients have ≥1 hemodynamically unstable VT, making it difficult to perform activation mapping.2,3,7 In addition, VT ablation in the setting of ICD storms is often a challenge because of more unstable nature of such patients. In such patients, pLVADs is increasingly used in many centers for hemodynamic support during VT ablation.
Few studies have evaluated the safety and efficacy of pLVADs in supporting unstable VT ablation procedures.5–8,12,13 In the first reported case of VT ablation with a pLVAD, Friedman et al13 used the TandemHeart to perform successful endocardial and epicardial mapping and ablation of a previously unstable VT. Recently, the use of an Impella for hemodynamic support of unstable VT ablations was first reported in a case series by Abuissa et al.8 In this case series, they successfully performed activation and entrainment mapping of unstable VTs in all 3 patients and terminated VT during ablation in 1 patient. Carbucicchio et al7 reported the largest series to date on the use of pLVADs in VT ablation. They reported their experience with percutaneous complete cardiopulmonary support in 19 patients with severely depressed LVEF and recurrent unstable VTs.
More recently, Miller et al5 reported a prospective, observational study evaluating the role of pLVADs in unstable VT ablation. Of the 23 procedures included in the study, Impella was used in 10, IABP was used in 6, and the remaining 7 did not have a mechanical support device during VT ablation. Patients with Impella could be maintained in VT for longer time enabling more activation and entrainment mapping when compared with those without an Impella. Also, more VTs could be terminated with ablation and fewer rescue shocks were needed in the Impella group when compared with those without an Impella. The outcomes of ablation did not differ between both the groups. Our findings are largely in line with their study findings. We recently reported a retrospective, matched, observational study comparing 13 patients undergoing TandemHeart-assisted VT ablation with 18 matched patients undergoing conventional substrate-map–based ablation for unstable VT. Despite having a higher intraprocedural burden of VT in patients with pLVAD, the immediate and long-term success rates did not differ between both groups.6
More entrainment mapping and better targeting of VT did not translate to better long-term success. This potentially could be a reflection of sicker patients receiving pLVADs. However, in this series and an earlier one by Bunch et al,6 there was no difference in the baseline characteristics between the groups. Alternatively, it is possible that substrate ablation alone may be equal (or even superior) to the use of entrainment mapping. Substrate mapping in sinus rhythm has been validated in multiple small trials and is the basis for a completed Federal Drug Administration approval for VT ablation. Also, in our study, the radiofrequency ablation time did not differ between both the groups, despite more activation mapping in the non-pLVAD group. This is likely because of the additional substrate modification performed in many patients after the initial activation mapping and ablation. Future, larger trials are needed to determine whether adding activation and entrainment mapping to substrate mapping is helpful.
Procedural complexity and complication rate should be taken into account while considering pLVAD for supporting VT ablation. Seventeen (26%) of our patients had ≥1 major complication. This is probably also a reflection of the multiple comorbidities in our cohort. In accordance with a previous multicenter study, poor LVEF was an independent and a powerful predictor of in-hospital mortality.14 Unfortunately, outcomes after VT ablation remain suboptimal. In our high-risk population, the recurrence of VT (46% at 12-month follow-up) is similar to previously published rate in a large multicenter study.14 Considering the higher complication rate, the cost of pLVAD implantation and intermediate success rates, careful and thorough risk–benefit assessment should be performed, especially in patients with low LVEF.
Limitations
The biggest limitation to our study is the relatively small patient population and its observational nature. Another limitation is that the selection of the pLVAD and the ablation techniques were primarily operator dependent. The LVEF assessment was made just before the procedure and may be a reflection of acute impairment because of recurrent ICD shocks or some other precipitating acute event. However, it is still a useful and easily available predictor of mortality. Termination of VT was based on the arterial blood pressure monitoring, and cerebral oximetry or transcranial Doppler was not used for determining the hemodynamic instability. Access to the LV was primarily driven by the type of pLVAD used and might have potentially affected the results of the procedures. Despite the above limitations, it is the largest study to date on the use of pLVAD during VT ablation and does lay a foundation for a large prospective study to identify patient populations who would benefit the most from these devices.
Conclusions
In the largest study to date of pLVAD use in VT ablation, we found that patients who underwent Impella or TandemHeart implantation had more number of unstable VTs ablated, more number of activation/entrainment mapping of VTs, more number of VTs terminated with ablation, and needed fewer rescue shocks when compared with those who underwent IABP implantation. However, this did not translate to improved long- term freedom from VT. Low LVEF is an independent and powerful predictor of in-hospital mortality after VT ablation.
Supplementary Material
Figure 1.
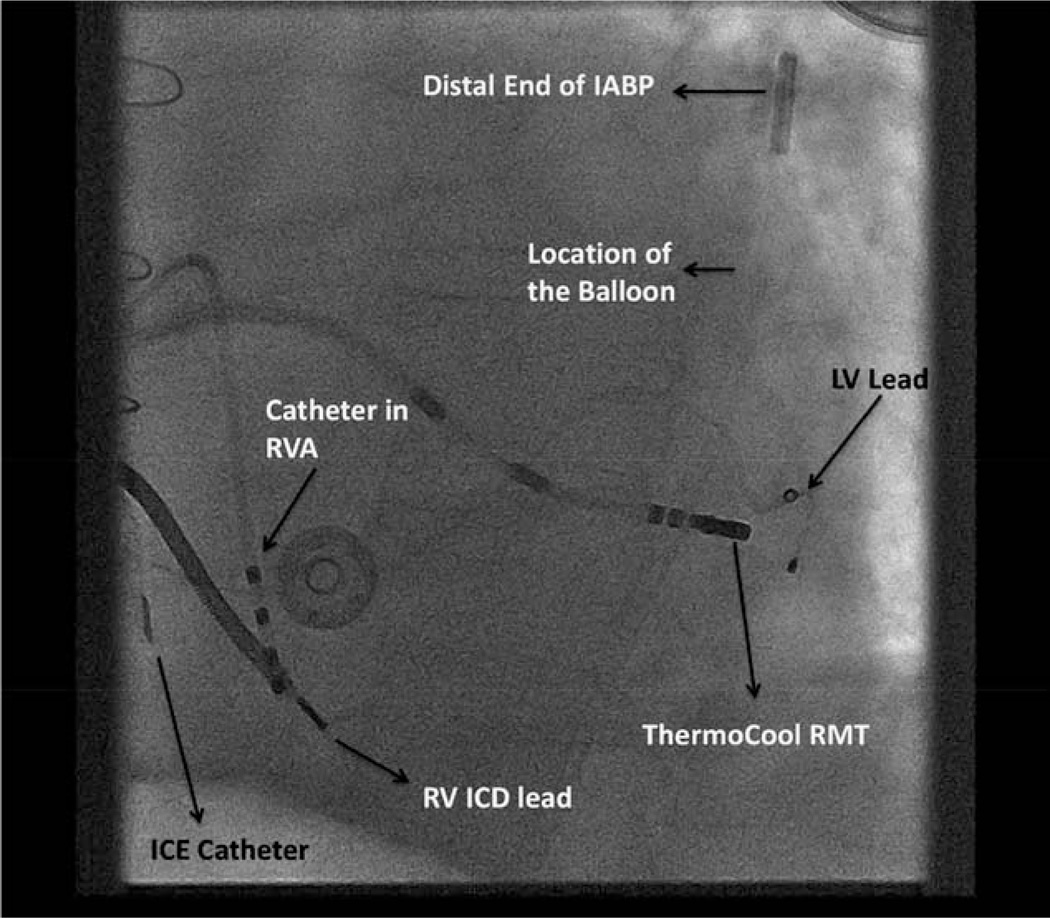
Patient with an intra-aortic balloon pump (IABP) in place undergoing ventricular tachycardia ablation with Stereotaxis remote navigation system. A left anterior oblique view of the chest with the IABP balloon in the descending aorta. ICD indicates implantable cardiac defibrillator; ICE, intracardiac echocardiography; LV, left ventricle; RMT, remote magnetic technology; RV, right ventricle; and RVA, right ventricular apex.
Figure 2.
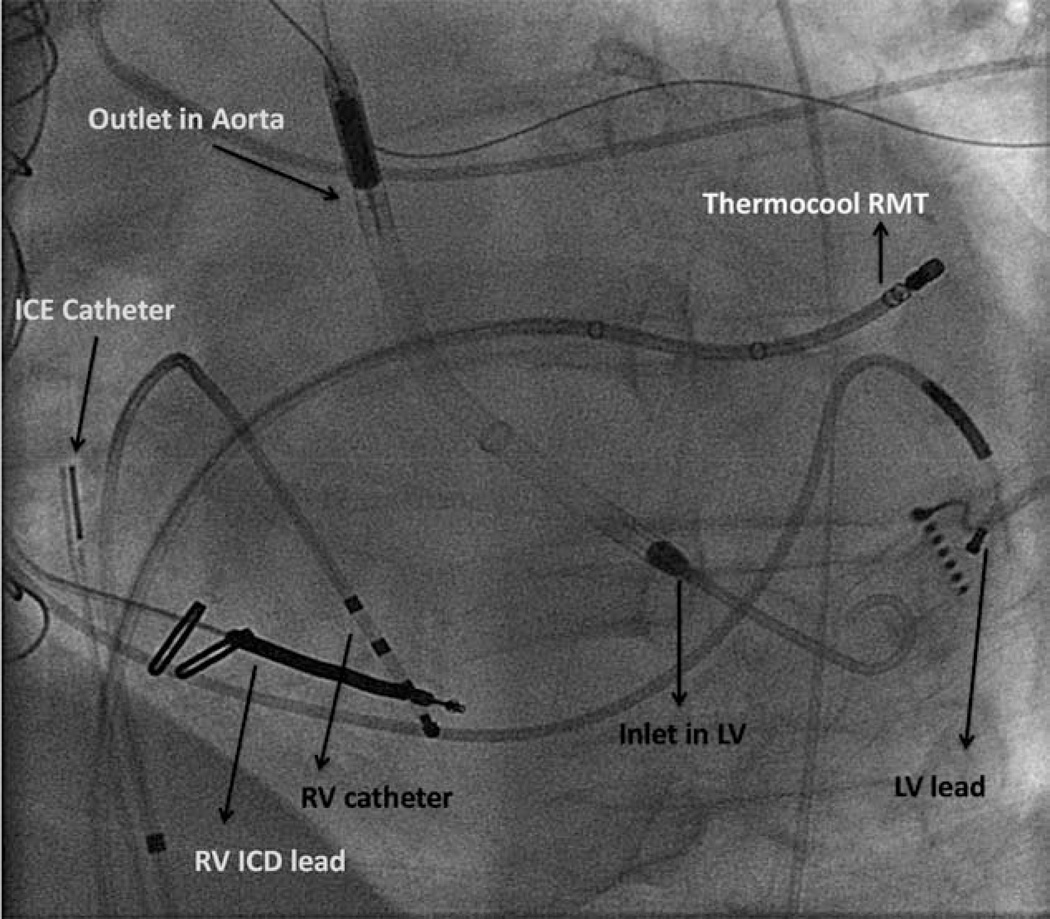
Patient with an Impella in place undergoing ventricular tachycardia ablation with Stereotaxis remote navigation system. ICD indicates implantable cardiac defibrillator; ICE, intracardiac echocardiography; LV, left ventricle; RMT, remote navigation technology; and RV, right ventricle.
Figure 3.
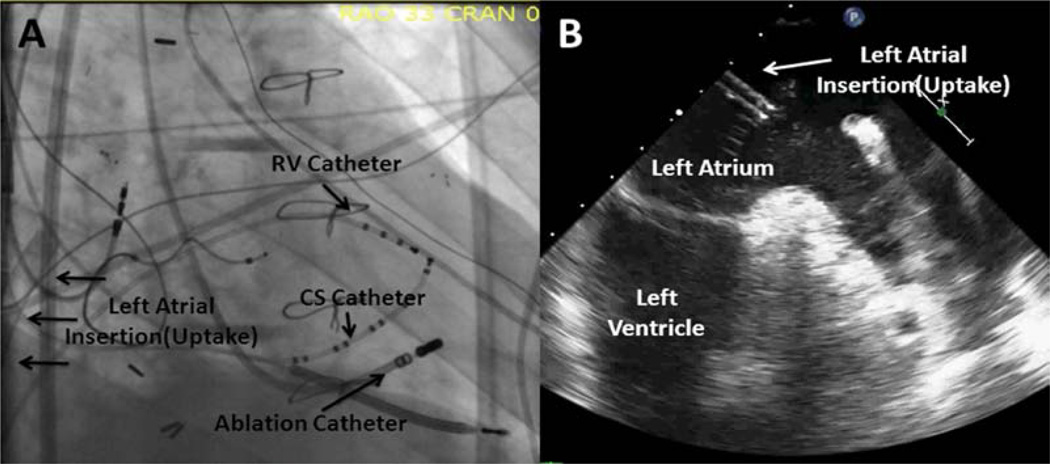
Patient with a TandemHeart device in place. A, Is a right anterior oblique fluoroscopic imaged with the intake cannula (labeled with arrows) inserted into the left atrium. B, Is an image of the corresponded transesophageal echocardiogram image with the intake cannula inserted in the left atrium and labeled for reference. CS indicates coronary sinus; and RV, right ventricle.
CLINICAL PERSPECTIVE.
Ventricular tachycardia (VT) ablation in patients with hemodynamically unstable VT is challenging. One of the well-accepted strategies of VT ablation requires detailed activation mapping during VT. Hemodynamic stability during these procedures, independent of whether the patient is in VT or not, can be challenging because of the prolonged procedural times, general anesthesia, and coexisting severe impairment of left ventricular function. Percutaneous left ventricular assist devices during these procedures can aid in improving hemodynamic status, sustaining VT for longer durations for more accurate mapping, terminating VT with ablation, and minimizing rescue defibrillation shocks. Nevertheless, the use of percutaneous left ventricular assist devices during VT ablation does not improve the success of the procedure (VT recurrence and mortality). The risk and cost of percutaneous left ventricular assist device implantation should be taken into consideration while choosing the most appropriate VT ablation candidates who would benefit from it.
Acknowledgments
Dr Bunch received modest speaker’s honorarium from St. Jude Medical, Sanofi Aventis, and Biosense Webster and is a consultant for Boston Scientific. Dr Di Biase served as a consultant for Hansen Medical and Biosense Webster. Dr Lakkireddy received modest speaker’s honorarium from Boehringer Ingelheim, Jansen, St. Jude Medical. Dr Mahapatra is an employee of St. Jude Medical. Dr Natale served as a consultant and received speaker honoraria from Biosense Webster, Medtronic, Biotronik, Boston Scientific, and Life Watch.
Footnotes
The Data Supplement is available at http://circep.ahajournals.org/lookup/suppl/doi:10.1161/CIRCEP.113.000548/-/DC1.
Disclosures
The other authors report no conflicts.
References
- 1.Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, Della Bella P, Hindricks G, Jaïs P, Josephson ME, Kautzner J, Kay GN, Kuck KH, Lerman BB, Marchlinski F, Reddy V, Schalij MJ, Schilling R, Soejima K, Wilber D, European Heart Rhythm Association (EHRA) European Heart Rhythm Association (EHRA); Registered Branch of the European Society of Cardiology (ESC); Heart Rhythm Society (HRS); American College of Cardiology (ACC); American Heart Association (AHA) EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6:886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 2.Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, Riva S, Moltrasio M, Cireddu M, Veglia F, Della Bella P. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117:462–469. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 3.Soejima K, Suzuki M, Maisel WH, Brunckhorst CB, Delacretaz E, Blier L, Tung S, Khan H, Stevenson WG. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104:664–669. doi: 10.1161/hc3101.093764. [DOI] [PubMed] [Google Scholar]
- 4.Jared Bunch T, Mahapatra S, Madhu Reddy Y, Lakkireddy D. The role of percutaneous left ventricular assist devices during ventricular tachycardia ablation. Europace. 2012;14(Suppl 2):ii26–ii32. doi: 10.1093/europace/eus210. [DOI] [PubMed] [Google Scholar]
- 5.Miller MA, Dukkipati SR, Mittnacht AJ, Chinitz JS, Belliveau L, Koruth JS, Gomes JA, d’Avila A, Reddy VY. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J Am Coll Cardiol. 2011;58:1363–1371. doi: 10.1016/j.jacc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Bunch TJ, Darby A, May HT, Ragosta M, Lim DS, Taylor AM, DiMarco JP, Ailawadi G, Revenaugh JR, Weiss JP, Mahapatra S. Efficacy and safety of ventricular tachycardia ablation with mechanical circulatory support compared with substrate-based ablation techniques. Europace. 2012;14:709–714. doi: 10.1093/europace/eur347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbucicchio C, Della Bella P, Fassini G, Trevisi N, Riva S, Giraldi F, Baratto F, Marenzi G, Sisillo E, Bartorelli A, Alamanni F. Percutaneous cardiopulmonary support for catheter ablation of unstable ventricular arrhythmias in high-risk patients. Herz. 2009;34:545–552. doi: 10.1007/s00059-009-3289-3. [DOI] [PubMed] [Google Scholar]
- 8.Abuissa H, Roshan J, Lim B, Asirvatham SJ. Use of the Impella micro-axial blood pump for ablation of hemodynamically unstable ventricular tachycardia. J Cardiovasc Electrophysiol. 2010;21:458–461. doi: 10.1111/j.1540-8167.2009.01673.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar K, Kini AS. Percutaneous left ventricular support devices. Cardiol Clin. 2010;28:169–184. doi: 10.1016/j.ccl.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Miller MA, Dukkipati SR, Koruth JS, d’Avila A, Reddy VY. How to perform ventricular tachycardia ablation with a percutaneous left ventricular assist device. Heart Rhythm. 2012;9:1168–1176. doi: 10.1016/j.hrthm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Sosa E, Scanavacca M, d’Avila A, Oliveira F, Ramires JA. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000;35:1442–1449. doi: 10.1016/s0735-1097(00)00606-9. [DOI] [PubMed] [Google Scholar]
- 12.Cesario DA, Saxon LA, Cao MK, Bowdish M, Cunningham M. Ventricular tachycardia in the era of ventricular assist devices. J Cardiovasc Electrophysiol. 2011;22:359–363. doi: 10.1111/j.1540-8167.2010.01911.x. [DOI] [PubMed] [Google Scholar]
- 13.Friedman PA, Munger TM, Torres N, Rihal C. Percutaneous endocardial and epicardial ablation of hypotensive ventricular tachycardia with percutaneous left ventricular assist in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 2007;18:106–109. doi: 10.1111/j.1540-8167.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, Gonzalez MD, Worley SJ, Daoud EG, Hwang C, Schuger C, Bump TE, Jazayeri M, Tomassoni GF, Kopelman HA, Soejima K, Nakagawa H Multicenter Thermocool VT Ablation Trial Investigators. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.