Abstract
Paracetamol is commonly used to control mild-to-moderate pain or to reduce opioid exposure as part of multimodal analgesia, and is the only compound recommended to treat fever in neonates.
Paracetamol clearance is lower in neonates than in children and adults. After metabolic conversion, paracetamol is subsequently eliminated by the renal route. The main metabolic conversions are conjugation with glucuronic acid and with sulphate. In the urine of neonates sulphated paracetamol concentration is higher than the glucuronidated paracetamol level, suggesting that sulfation prevails over glucuronidation in neonates. A loading dose of 20 mg/kg followed by 10 mg/kg every 6 hours of intravenous paracetamol is suggested to achieve a compartment concentration of 11 mg/L in late preterm and term neonates. Aiming for the same target concentration, oral doses are similar with rectal administration of 25 to 30 mg/kg/d in preterm neonates of 30 weeks’ gestation, 45 mg/kg/d in preterm infants of 34 weeks’ gestation, and 60 mg/kg/d in term neonates are suggested. The above-mentioned paracetamol doses for these indications (pain, fever) are well tolerated in neonates, but do not result in a significant increase in liver enzymes, and do not affect blood pressure and have limited effects on heart rate. In contrast, the higher doses suggested in extreme preterm neonates to induce closure of the patent ductus arteriosus have not yet been sufficiently evaluated regarding efficacy or safety. Moreover, focussed pharmacovigilance to explore the potential causal association between paracetamol exposure during perinatal life and infancy and subsequent atopy is warranted.
Key words: Dosing, glucuronidation, metabolism, neonate, paracetamol, sulfation
Introduction
Paracetamol, N-acetyl-p-aminophenol (also known as acetaminophen), is a readily available, over-the-counter antipyretic and analgesic compound. It is the most often prescribed drug to treat mild-to-moderate pain or fever in infants, including neonates, and can be administered by different routes (ie, oral, rectal, or intravenous). It has analgesic and antipyretic activity, but has only very modest peripheral anti-inflammatory properties.1–3 In its therapeutic concentration range, paracetamol is metabolized by the liver to paracetamol-glucuronide (47%–62%) and paracetamol-sulphate (25%–36%) as main metabolites, and subsequently eliminated by the renal route in adults. Only 1% to 4% is excreted unchanged in urine, and about 8% to 10% of paracetamol is oxidized to 3-hydroxy-paracetamol and the (hepatic) toxic metabolite N-acetyl-p-benzoquinone-imine (NAPQI).4 Maturation-related changes in paracetamol disposition, metabolic, and elimination clearance occur throughout childhood, but are most prominent in early life.5,6 Neonates have an overall lower paracetamol metabolic and elimination clearance capacity, and the between-subject variability is explained by covariates such as size or weight, organ function, or disease characteristics.7–9 Compared with other drugs, a relevant body of evidence on pharmacokinetic properties and disposition of paracetamol in term and preterm neonates has been reported following intravenous and enteral (oral, rectal) administration. Despite this, there is still relevant variability in dosing suggestions as retrieved in reference textbooks or websites (Table I).10–13
Table I.
Dosing suggestions for paracetamol for (pre)term neonates as retrieved in reference sources.
| Source | Administration route | Suggested dose |
|---|---|---|
| Neofax10 | ||
| Oral | Loading dose | 20–25 mg/kg |
| Maintenance | 12–15 mg/kg/dose | |
| Interval | q6h in term neonates | |
| q8h in preterm neonates ≥32 wk PMA | ||
| q12h in preterm neonates <32 wk PMA | ||
| Rectal | Loading dose | 30 mg/kg |
| Maintenance | 12–18 mg/kg/dose | |
| q6h in term neonates | ||
| q8h in preterm neonates ≥32 wk PMA | ||
| q12h in preterm neonates <32 wk PMA | ||
| Intravenous | No suggestions provided | |
| BNFc11 | ||
| Oral | Loading dose | 20 mg/kg |
| Maintenance | 10–15 mg/kg/dose | |
| q6–8 h in ≥32 wk | ||
| q8–12h in <32 wk PMA | ||
| ≥32 wk PMA, max 60 mg/kg/d | ||
| <32 wk PMA, max 30 mg/kg/d | ||
| Rectal | Loading dose | 30 mg/kg in ≥32 wk |
| Maintenance | 20 mg/kg in <32 wk | |
| 20 mg/kg q8h (max 60 mg/kg/d) ≥32 wk PMA | ||
| 15 mg/kg q12h (max 30 mg/kg/d) in <32 wk | ||
| Intravenous | Loading dose | No suggestions provided |
| Maintenance | 7.5 mg/kg, q4-6 h, max 30 mg/kg/d when <10 kg, and limited to term neonates | |
| Neonatal formulary12 | ||
| Oral | Loading dose | 24 mg/kg |
| Maintenance | 12 mg/kg/dose | |
| q4h in ≥32 wk PMA, q8h in <32 wk | ||
| Rectal | Loading dose | 36 mg/kg |
| Maintenance | 24 mg/kg, q8h in term neonates | |
| No advice in preterm neonates | ||
| Intravenous | Loading dose | 20 mg/kg, irrespective of age |
| Maintenance | 15 mg/kg, q6h in term cases | |
| 12.5 mg/kg, 31–36 wk PMA | ||
| 10 mg/kg, ≤30 wk PMA | ||
| Dutch formulary13 | ||
| Oral | Loading dose | Not sufficiently supported by clinical evidence |
| Maintenance | 60 mg/kg/d, >32 wk PMA | |
| 30 mg/kg/d, 28–32 wk PMA | ||
| Rectal | Loading dose | 30 mg/kg, <32 wk PMA |
| Maintenance | 20 mg/kg, 28–32 wk PMA | |
| 20 mg/kg, q8h in term neonates | ||
| 20 mg/kg, q12h in preterm neonates | ||
| Intravenous | Off label in preterm neonates | |
| Loading dose | 20 mg/kg, irrespective of age | |
| Maintenance | 10 mg/kg, max 40 mg/kg/d, in term cases | |
| 10 mg/kg, max 30 mg/kg/d, 31–36 wk PMA | ||
| 10 mg/kg, max 20 mg/kg/d, <31 wk PMA |
PMA = postmenstrual age (in weeks).
Although intravenous paracetamol administration remains off label for specific subpopulations (eg, limited to term neonates, or children younger than age 2 years in the United States) in many countries, these formulations are increasingly used in neonates.8,14,15 The registered dose is 7.5 mg/kg q6h for term neonates up to infants weighing 10 kg. A dose of 15 mg/kg q6h (max daily dose 60 mg/kg) is recommended between 10 and 40 kg body weight. In clinical practice, a loading dose (20 mg/kg) and higher maintenance doses are suggested (Table I) and have been evaluated in regard to efficacy and safety.14,15
Effective and safe drug administration in neonates should consider the evolving physiologic characteristics (eg, maturation and disease) of a newborn who will receive the drug and pharmacokinetic and pharmacodynamic properties of a given drug. Consequently, drug disposition in neonates is as diverse as the neonates who are admitted to our neonatal intensive care units.16,17 This is also true for paracetamol. Using a systematic bibliographic search strategy, we aim to provide an overview on the pharmacokinetic and pharmacodynamic properties of paracetamol in neonates. This will be followed by a discussion with specific emphasis on newly emerging issues related to potential effects (patent ductus arteriosus [PDA]) and side effects (atopy and emerging biomarkers).
Literature Search
Our literature search was performed using PubMed and EMBASE databases as search engines. The following key words were used: pharmacokinetics paracetamol/acetaminophen neonate, metabolism paracetamol/acetaminophen neonate, and effects paracetamol/acetaminophen neonate. The reference list of each article was read carefully, and the selected references were examined.
Results
Pharmacokinetic properties and metabolism of paracetamol in neonates
Intravenous
The most recently reported pooled study on intravenous paracetamol pharmacokinetic properties was based on a population pharmacokinetic analysis of 3 published studies, resulting in 943 paracetamol observations in 158 neonates (27–45 weeks postmenstrual age [PMA]). There were only 58 preterm neonates, of whom 21 were extreme preterm, 19 had a birth weight lower than 1500 g, and 31 were small for gestational age.8 A 2-compartment linear disposition model with first-order elimination fitted best to analyse time-concentration points. The volume of distribution was 70.4 L/70 kg and the clearance increased from 2.85 L/h per 70 kg at 27 weeks to reach 7.05 L/h per 70 kg by 42 weeks PMA.8 Weight was the major covariate (57.5% of variance). Clearance expressed as milligrams per kilogram per hour increased only slightly with PMA (0.138 L/kg/h at 28 weeks PMA to 0.167 L/kg/h at 44 weeks PMA), and contributes to only 2.2% of variance. High unconjugated bilirubin levels only contributed an additional 1.2%. Based on this pooled analysis, it was concluded that size (predicted by weight) was the major covariate of clearance. We hereby mainly confirmed earlier clearance estimates in a further extended cohort of (pre)term neonates. Paracetamol clearance in neonates—described using allometric scaling—was one-third of the mature value reported in adults (16.2 L/h/70 kg).6,8 Clearance maturation is slow before age 40 weeks PMA and subsequently matures rapidly to reach 90% of adult capacity at 1 year of life.6,8 In addition, the distribution volume was higher in early infancy when compared with other pediatric populations.6,8 The volume of distribution decreased from 27 weeks PMA (0.64 L/kg) to reach a mature value from 6 months of age (0.4–0.45 L/kg) onward. The increased volume of distribution in neonates supports the use of a larger initial dose (loading dose) of intravenous paracetamol in neonates if one aims to attain a given paracetamol threshold concentration sooner (ie, >10–11 mg/L6,8) because a higher distribution volume results in a proportionally lower peak concentration,8,16 as reflected in Table I.
Finally, based on the pooled analysis, a mean paracetamol serum concentration of 11 mg/L was predicted in neonates aged 28 to 44 weeks PMA given a standard dose of 10 mg/kg/6 h intravenous paracetamol. In Figure 1, all pooled time-concentration observations collected in the Leuven cohorts are provided.8,18,19 However, data of this drug in extreme preterm neonates were still limited. It is encouraging that since this pooled analysis, additional data have been reported or have been collected. This includes observations in a cohort of very preterm infants (<32 weeks gestational age) (N = 15). Repeated dosing (7.5 mg/kg q6h) resulted in median paracetamol levels of 10 mg/L at steady state, quite similar to the levels aimed for in the pooled analysis.20 A preliminary analysis of a repeated intravenous paracetamol (15 mg/kg) pharmacokinetic study (89 samples in 10 patients) conducted in the United States (National Institute of Child Health and Human Development [No. R01HD060543]) resulted in a median clearance estimate of 0.151 L/kg/h and a median distribution volume of 1.21 L/kg with weight as the most relevant covariate.21
Figure 1.
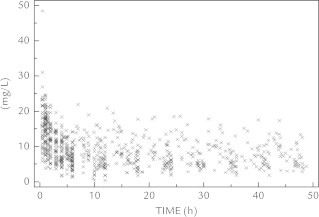
Time-concentration points for paracetamol as pooled time-concentration observations as collected in the Leuven cohorts,8,18,19 reflecting a median paracetamol concentration of about 10 mg/L using the dosing regimens as suggested in the literature13,36 (ie, loading dose of 20 mg/kg irrespective of postmenstrual age, maintenance dose of 10 mg/kg, q6-8-12 h).
Paracetamol either undergoes sulfation and glucuronidation,22,23 mainly by the UDP-glucuronosyltransferase 1A6 and to lesser extent the UDP-glucuronosyltransferase 1A9 isoenzyme. A progressive increase in the contribution of glucuronidation to paracetamol elimination with increasing age throughout childhood has been described.22–24 Maturation-related aspects of intravenous paracetamol metabolism in neonates were explored based on quantification of paracetamol-glucuronide (APAP-G), paracetamol-sulfate (APAP-S), and free paracetamol in 147 urine samples of 23 neonates during repeated administration of intravenous propacetamol. The median molar contribution of APAP-G to overall urine paracetamol (APAP-G + APAP-S + free paracetamol) elimination was 14% (range = 1%–53%). Covariates of this APAP-G/total amount of paracetamol (APAP-T) (APAP-G + APAP-S + free paracetamol) (G/T) ratio were postnatal age, postmenstrual age, and repeated administration.23 The median urinary APAP-G/APAP-S (G/S) ratio was 0.27.23 van Ganzewinkel et al20 also generated data on paracetamol metabolism. Based on urine collections, it was confirmed that both glucuronidation and sulfation elimination increased with repeated administration. The G/S ratio in this preterm cohort was 0.08, reflecting the influence of prematurity on paracetamol glucuronidation capacity. Moreover, plasma glutathione remained stable during repeated exposure.20 This suggests that repeated paracetamol administration had no effect on the glutathione plasma levels in the preterm neonate, potentially reflecting glutathione stores
Enteral
A pooled analysis on the developmental pharmacokinetic properties of enteral (ie, oral and rectal) paracetamol in premature neonates and infants was performed by Anderson et al.5 A population pharmacokinetic analysis of paracetamol time-concentration profiles was studied in 283 children (n = 124 aged ≤6 months). Neonates and infants were given either single or multiple doses of 4 different formulations: oral elixir, rectal solutions, or triglyceride or capsular suppository. The median postnatal age of children younger than age 6 months was 1 day (range = birth–6 months), median postmenstrual age was 40 weeks (range = 28–64 weeks), and median weight was 3.1 kg (range = 1.2–9.0 kg).
Standardized to a 70-kg person using allometric “1/4 power” models, median clearance was 12.5 L/h (44%), and the volume of distribution was 66.6 L (20%). Paracetamol clearance increased from 28 weeks PMA (0.74 L/hour/70 kg) with a maturation half-life of 11.3 weeks to reach 10.8 L/h/70 kg at 60 weeks PMA. The absorption half-life for the oral elixir was 0.21 hours (120%) with a lag time of 0.42 hours (70%), but absorption was further delayed (2 hours) in premature neonates during the first days of life. Absorption lag time was negligible by rectal route for all 3 formulations. The bioavailability of the capsule suppository relative to elixir decreased with age from 0.92 (22%) at age 28 weeks to 0.86 at age 2 years, whereas the triglyceride base formulation decreased from 0.86 (35%) at age 28 weeks to 0.5 at age 2 years. The relative bioavailability of the rectal solution was 0.66. Based on these estimates, it was concluded that a mean steady state target concentration ~10 mg/L at trough can be achieved by an oral dose of 25 mg/kg/d in premature neonates at age 30 weeks PMA, 45 mg/kg/d at age 34 weeks PMA, and 60 mg/kg/d at term age.5 Similar to the estimates for intravenous pharmacokinetic properties, the volume of distribution decreased exponentially with a maturation half-life of 11.5 weeks from 109.7 L/70 kg at age 28 weeks after conception to 72.9 L/70 kg by age 60 weeks PMA. Finally, Van Lingen et al9 also generated data on paracetamol metabolism using urinary metabolite excretion. Similar to the observations following intravenous administration, paracetamol sulphate was the main metabolite, with a mean molar G/S ratio of 0.12 (28–32 weeks PMA) and 0.28 (32–36 weeks PMA), respectively.9,23 To further reflect the influence of age on glucuronidation capacity, the G/S ratio in paracetamol metabolites retrieved in urine in term neonates was 0.2722 and 0.344 following a single oral paracetamol administration.
Clinical indications for paracetamol
Fever
There are few trials that have directly compared the antipyretic properties of paracetamol against placebo or physical methods.25 Although fever is 1 of the most common manifestations of illness in children, this symptom is much less common in newborns. Consequently, specific reports in newborns (ie, first 28 days of life) on temperature reduction after paracetamol administration are very limited, whereas reports from early infancy onward are much more common, often in the setting of postimmunization fever.1 As part of a study on thermodynamics in 99 neonates exposed to intravenous paracetamol, 6 cases with fever were included. In neonates with fever (>37.8°C), the median decrease (–0.8°C) was more prominent during the first 2 hours after administration. Individual trends over time after intravenous paracetamol (20 mg/kg) administration are provided in Figure 2.26 In neonates (n = 93) with normothermia, paracetamol administration had no effect on the body temperature and hypothermia was not observed in this cohort, but has been reported in both children27 and adults.1,3,28
Figure 2.
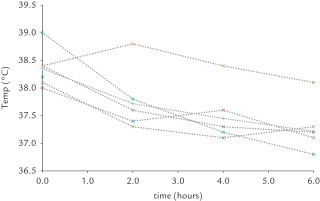
Individual trends in body temperature (°C) over time (hours) after intravenous paracetamol (20 mg/kg) administration.26
Pain
Adequate management of pain in neonates is a major issue in contemporary neonatal care. In an attempt to avoid opioids, there is an emerging use of paracetamol.29 However, we should be aware of the difference in currently available evidence to support the use of paracetamol for either procedural versus posttraumatic or postsurgery pain in neonates. In essence, the data on paracetamol analgesia during procedures are limited, but suggest an overall poor analgesic effect for procedural pain relief. In a randomized, placebo-controlled study, Shah et al30 documented that paracetamol administration (oral, 20 mg/kg; n = 75) was not effective to blunt pain expression related to heel lancing. Paracetamol administration (oral, 15 mg/kg) was not found to ameliorate the immediate postoperative pain of circumcision, either, although it provided some pain reduction afterward.31 The effect of paracetamol administration (rectal, 20 mg/kg; n = 122) in neonates following vacuum extraction was documented by Van Lingen et al32 in a randomized, placebo-controlled trial study design. A single dose of paracetamol significantly improved drinking behavior, but did not result in a significant change in objective pain scores and there were no positive effects following repeated administration. Using a preemptive approach in 123 term neonates after assisted delivery, infants had low pain scores immediately after birth, irrespective of paracetamol administration. However, paracetamol administration (rectal, 20–25 mg/kg; n = 123) was associated with a more pronounced stress response during heel lancing on Day 2 to 3.33
Although effective analgesia in neonates is still in part hampered by a paucity of pharmacodynamic data, there are published data on both a pharmacokinetic/pharmacodynamic model (monotherapy or mild-to-moderate pain) as well as the morphine-sparing effect of coadministration of paracetamol in neonates and young infants following noncardiac surgery. In an attempt to provide evidence for analgesic effectiveness of intravenous paracetamol (loading dose = 20 mg/kg) in neonates, pain scores after an intravenous paracetamol loading dose (www.ClinicalTrials.gov identifier: NCT00969176) were analyzed using repeated measures ANOVA and an Emax model with a delayed response compartment.34 Using repeated measures ANOVA, there was a trend (P = 0.02) for lower pain scores within 30 minutes after administration, with a slight increase in pain scores from 5 hours onward (Figure 3).34 An Emax model had a maximum effect of 4.15 out of 14 pain units, an effective concentration to have 50 % of the maximal effect (EC50) of 2.07 mg/L, and the equilibration half time between plasma and effect site was 1.58 hours. Based on these observations, it was concluded that intravenous paracetamol is effective for moderate pain.33 An effect compartment concentration of 10 mg/L (loading dose = 20 mg/kg) is associated with a pain score reduction of 3.4 units, suggesting similarities in the paracetamol effect compartment concentration in neonates compared with children.34–36 Further studies should focus on both pharmacokinetic and pharmacodynamic data in different pain models to unveil correct dosing and correct indications.
Figure 3.
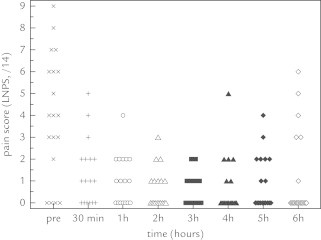
Individual pain scores following intravenous paracetamol administration (loading dose, 20 mg/kg).34 LNPS = Leuven neonatal pain scale.
It has recently been documented that paracetamol does result in a clinically relevant reduction in morphine consumption (–66%) when integrated in multimodal analgesia (morphine plus paracetamol).37 The cumulative median morphine dose in the first 48 hours postoperatively after an initial loading dose (100 μg/kg) in all neonates at the end of surgery was 121 μg/kg (interquartile range = 99–264 μg/kg) in the paracetamol group (n = 33; 4 × 7.5 mg/kg/d) and 357 μg/kg (interquartile range = 220–605 μg/kg) in the morphine group (n = 38). The between-group difference was 66% (95% CI, 34%–109%) lower in the paracetamol group, whereas pain scores and adverse effects were not significantly different between both groups.36 The morphine-sparing effect of paracetamol, initially described in children and adults, has hereby been confirmed in neonates.3,37,38
Observations on safety and tolerance of paracetamol in neonates
Issues on safety and tolerance of paracetamol mainly relate to either hepatotoxicity or hemodynamic effects. For both, data on neonates are available in the public domain. The hepatoxicity is not a direct effect from paracetamol itself, but relates to 1 of its metabolites, NAPQI. NAPQI depletes the liver from glutathione that acts as antioxidant, and directly damages cells in the liver at the mitochondrial level, subsequently resulting in liver failure.1,39 NAPQI itself is generated in the liver through cytochrome P450 2E1 activity, an isoenzyme assumed to be less active in early infancy.16,17
There is some literature on the systematic evaluation of liver enzymes in cohorts of neonates exposed to paracetamol that suggest overall good tolerance.40 The hepatic tolerance in neonates was investigated as part of prospective studies on the pharmacokinetic and pharmacodynamic properties of intravenous paracetamol in neonates.39 Hepatic enzyme profiles were retrieved from 2 days before until 2 days after intravenous paracetamol administration in 189 infants. There was no significant increase in alanine aminotransferase, aspartate aminotransferase, or gamma-glutamyl transferase when pretreatment observations (n = 310) were compared with observations during (n = 649) or during with after (n = 173) treatment, nor was there a significant increase during administration of paracetamol. This study on hepatic tolerance provides evidence on safety aspects of intravenous paracetamol in neonates. This has been further confirmed by the absence of changes in plasma glutathione levels as reported by van Ganzewinkel et al.20
Despite the safety pattern of these prospective data, individual cases with potential relevant hepatic toxicity related to paracetamol in newborns have been reported and are summarized in Table II.41–48 Overall, the number of cases reported remains very limited, and observed in-hospital 10-fold drug errors do not systematically result in significant morbidity or mortality.36,45
Table II.
Overview on the cases of newborns exposed to potential toxic doses of paracetamol as published in the literature41–48
| Author | Clinical characteristics and dose | Management and outcome |
|---|---|---|
| Isbister et al, 200141 | Former preterm newborn, 37 wk PMA | N-acetyl cysteine IV + activated charcoal transient increase in prothrombin time, no hepatic enzymatic abnormalities, full recovery |
| 55 days postnatal age, 2.2 kg | ||
| Oral paracetamol, 136 mg/kg | ||
| de la Pintiére et al, 200342 | Term newborn, in early neonatal life | N-acetyl cysteine IV |
| 2 x intravenous (pro)pacetamol, 307 mg/kg | No adverse effects were observed, Discharge Day 7 | |
| Walls et al, 200843 | Term newborn, early neonatal life (Day 4) | N-acetyl cysteine IV, renal and hepatic failure (liver enzymes, INR abnormalities, hypoglycemia) |
| Following circumcision, emesis and lethargic | ||
| Oral paracetamol, 156 + 78 + 78 mg/kg/d | Full recovery, with discharge after 1 wk | |
| Nevin et al, 200944 | preterm newborn, 35 weeks PMA | N-acetyl cysteine IV, normal liver enzymes |
| 7 weeks postnatal age, 2.6 kg | Transient INR abnormalities (1.27–1.04), vitamin K | |
| Intravenous paracetamol, 146 mg/kg | Full recovery, discharge on Day 5 | |
| MHRA, 201045 | 23 cases (worldwide) <1 jr | No data on management reported |
| Intravenous paracetamol overdose | one case (1 out of 23) died | |
| Most common error: 10-fold error | ||
| Porta et al, 201246 | Extreme preterm newborn, 27 weeks PMA | N-acetyl cysteine IV. No changes in liver enzymes, bilirubin, or prothrombin time. Full recovery |
| 12 d postnatal age, 940 g | ||
| Indication: Abdominal distention, sepsis | ||
| Intravenous paracetamol, 446 mg/kg | ||
| Campbell et al, 201347 | Former preterm newborn, 40 wk PMA | N-acetyl cysteine IV. No changes in liver enzymes, bilirubin, or prothrombin time. Full recovery |
| Postnatal age 3 mo, 2.3 kg | ||
| Indication: retinal laser surgery | ||
| Intravenous paracetamol, 75 mg/kg | ||
| Bucaretchi et al, 201448 | 26 days old, term newborn, 3.125 kg | Volume replacement, plasma, inotropics, and ventilation |
| Indication: “feverish and weepy” | N-acetyl cysteine IV. 34 days of hospitalization | |
| Oral, 180 mg/kg (3 d 10 mg/kg, q4h) | ||
| Not yet reported, Leuven, 2014 | 3-wk old term newborn, 3.5 kg | N-acetyl cysteine IV. No changes in liver enzymes, bilirubin, or prothrombin time. Full recovery |
| Indication: pain, posttraffic accident (cranial, thoracic injuries) | ||
| Intravenous paracetamol: 200 mg/kg |
IV = intravenous, PMA = postmenstrual age; INR = intranational normalized ratio; blood clotting test.
de Maat et al3,28 reported on a cohort of adult medium-care and intensive-care patients with intravenous paracetamol-induced hypotension. They hereby confirmed earlier reports on hemodynamic (side) effects of paracetamol in critically ill adults.3,28 Based on prospectively collected observations in 72 neonates, only a very modest decrease in heart rate (7 bpm) and mean arterial blood pressure (3 mm Hg) following intravenous paracetamol administration was observed. A minority (9%) of neonates developed hypotension (mean arterial blood pressure < postmenstrual age in weeks, mm Hg).49 Interestingly, these neonates already had significantly lower blood pressure before paracetamol administration. Consequently, it was concluded that in a setting of open label administration to alleviate pain, hemodynamic effects of intravenous paracetamol administration in neonates remained modest with the suggestion to be more careful in the specific setting of impaired hemodynamic properties in neonates.
General Discussion and New Aspects of an Old Drug
Data on paracetamol pharmacokinetic/pharmacodynamic properties in neonates are available, and suggest that the same effect compartment concentration (10 mg/L) should be aimed for.34,35 This means that a loading dose should be considered (intravenous or oral 20 mg/kg, rectal 30–40 mg/kg), followed by maintenance (intravenous or oral 10 mg/kg, rectal 1–18 mg/kg) doses (in term neonates q6h, in preterm [<32 weeks] neonates q8h) to compensate for differences in distribution volume and clearance, respectively (Table I).5,8 Similar to children and adults, paracetamol has opioid sparing (–66%) effects in neonates after major noncardiac surgery.37 In contrast, the currently available data on paracetamol routes and doses suggest that paracetamol is only a poor analgesic for procedural pain relief.30,31 Data on safety suggest that paracetamol has a good safety profile in neonates when administered for a limited time (48–72 hours).40,49
We also would like to explore some novelties related to either tolerance/safety (assessment of short-term toxicity by new biomarkers and emerging issues related to long-term safety) or to new indications (eg, PDA).
The mechanism of paracetamol-induced liver injury involves mitochondrial dysfunction and oxidative stress.1,40 Besides the commonly used markers of liver necrosis, newer and likely more sensitive biomarkers of mitochondrial damage have been suggested.40 These include plasma glutamate dehydrogenase activity, mitochondrial DNA concentration, but also long-chain acylcarnitines or paracetamol protein adducts have been postulated to be sensitive biomarkers.40,50–53 Perturbations in long-chain acylcarnitines in children with paracetamol exposure or toxicity suggest that mitochrondrial injury and associated impairment in the β-oxidation of fatty acids are clinically relevant as biomarkers of paracetamol toxicity.50 Paracetamol protein adducts are a biomarker of paracetamol metabolism, reflecting oxidation of paracetamol and generation of the reactive metabolite N-acetyl-p-benzoquinone imine.51,52 Similarly, higher levels of paracetamol protein adducts correspond to liver toxicity in patients with paracetamol-related acute liver failure. The available observations in pediatric and adolescent patients following paracetamol overdose support the need for a further examination of the role of these protein adducts as clinically relevant and specific biomarkers of paracetamol toxicity.52 To the very best of our knowledge, none of these biomarkers has been evaluated in newborns or young infants.
Besides novelties related to short-term toxicity, there are also epidemiologic studies that suggest a link between paracetamol exposure in early infancy and the risk to develop asthma or other atopy-related diseases similar to the link between fetal/maternal exposure and atopy in early infancy, although not all studies come to the same conclusions.54–57 There may be a polymorphisms-related link between exposure and side effect. This is because maternal antioxidant gene polymorphisms (eg, nuclear erythroid 2 p45-related factor 2 polymorphism and glutathione S-transferase) may modify this relationship between prenatal paracetamol exposure and childhood asthma, strengthening the evidence for causality.58 The same association and similar polymorphisms have been documented for postnatal exposure. The association seems to be more significant in genetically susceptible children, related to antioxidant genes (eg, N-actyl transferase 2, nuclear erythroid 2 p45-related factor 2 polymorphism, and glutathione S-transferase polymorphisms), and the effect may be mediated by eosinophilic inflammation.59 Focussed pharmacovigilance may be needed to further unveil the complex, potentially causal association between paracetamol exposure and subsequent increase risk to develop atopy.60
Symptomatic PDA is a common condition in (extreme) preterm infants. The most frequently administrated drugs to treat this indication are cyclooxygenase inhibitors (ie, ibuprofen and indomethacin) to block prostaglandin synthesis and to induce muscular constriction at the level of the ductus arteriosus. Unfortunately, the use of these drugs is associated with relevant adverse effects, such as renal dysfunction, intestinal bleeding, and perforation or dysfunction of platelet aggregation.61,62 Consequently, there remains a need for alternative treatments that result in better closure rates or fewer adverse effects. A serendipity observation of Hammerman et al63 linked paracetamol exposure with PDA closure.
Since that article, case reports and case series have described the use of oral or intravenous paracetamol in patients with contraindications to or who had previously failed nonsteroidal anti-inflammatory drug therapy for PDA.64 There seems to be a publication bias, because the number of negative observations is very limited.65 Two clinical trials compared the efficacy of oral paracetamol (15 mg/kg q6h for 3 days) versus oral ibuprofen in 90 (<30 weeks gestational age) and 160 preterm infants (<34 weeks gestational age), respectively.66,67 Paracetamol was not inferior to ibuprofen, with closure rates from 72.5% to 81.2%.
The paracetamol dose used in most case series and trials was a 15 mg/kg dose q6h for 3 days, much higher than commonly used in extreme preterm neonates (Table I). Paracetamol therapy was reported to be well tolerated, with only a few reported incidents of elevated liver enzymes. However it should not be taken for granted yet that paracetamol induces PDA closure. If closure is driven by prostaglandin reduction, we should be aware that paracetamol exerts only very modest peripheral prostaglandin-related effects and exerts its effects mainly through the central nervous system. Related to prostaglandin synthesis, paracetamol inhibits peroxidase (prostaglandin G2 to prostaglandin H2 conversion) as 1 of its mechanisms of action, but this inhibition is competitive with the prostaglandin G2 concentration itself and peroxides.62 Potential advantages of paracetamol may relate to the preservation of the aggregation capacity of platelets.68
Related to paracetamol for PDA closure, we need a shift toward a more extensive research program. Taking all the above-mentioned results into account, this will include pharmacokinetic properties, effectiveness, and safety. There are data on paracetamol pharmacokinetic properties and safety, but these data were based on lower dosing regimens (20–40 mg/kg/24 h, 1–2 days) and were collected in more mature neonates. Consequently, we do not have subpopulation-specific pharmacokinetic properties or safety data. Some new concepts to assess paracetamol toxicity (acylcartinitines and paracetamol protein adducts) mentioned earlier in the Discussion should be integrated in such studies.
Finally, we are unaware of any median paracetamol concentration to aim for to induce closure of the ductus. Consequently, dose-seeking studies and in vitro studies are needed to guide dosing. A relevant in vitro observation was recently published by El-Khuffash et al.69 Using in vitro contractibility studies, these authors illustrated a concentration/effect profile depending on maturation and concentration. On pressure myography, paracetamol induced a concentration-dependent constriction of the ductus arteriosus in term mice, but only up to 30% of baseline, and this required concentrations >1 µmol/L (~100 mg/L, 10-fold higher when compared with the median level for analgesia).
Conclusions
We summarized the currently available information on paracetamol use in neonates. For pain and fever the long-needed data on the pharmacokinetic and pharmacodynamic properties in (pre)term neonates finally have been generated. In contrast, we still need data in extreme preterm neonates. We suggest that emerging novel evaluation tools related to safety (short- and long-term) and to potential new indications (eg, PDA) for its use in neonates need further studies.
Conflicts of Interest
Academic research, not supported by external sponsors.
Acknowledgments
This work was supported by the Ministry of the University and Scientific and Technical Research (Rome, Italy). Karel Allegaert is supported by the Fund for Scientific Research of Flanders, Belgium (Fundamental Clinical Investigatorship 1800214N).
Both authors contributed equally.
References
- 1.Prescott L.F. A critical bibliographic review. Second edition. Taylor and Francis publishers; London: 2001. Paracetamol. [Google Scholar]
- 2.Cuzzolin L., Antonucci R., Fanos V. Paracetamol (acetaminophen) efficacy and safety in the newborn. Curr Drug Metab. 2013;14:178–185. [PubMed] [Google Scholar]
- 3.Duggan S.T., Scott L.J. Intravenous paracetamol (acetaminophen) Drugs. 2009;69:101–113. doi: 10.2165/00003495-200969010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Miller R.P., Roberts R.J., Fisher L.J. Acetaminophen elimination kinetics in neonates, children and adults. Clin Pharmacol Ther. 1976;19:284–294. doi: 10.1002/cpt1976193284. [DOI] [PubMed] [Google Scholar]
- 5.Anderson B.J., van Lingen R.A., Hansen T.G., Lin Y.C., Holford N.H. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology. 2002;96:1336–1345. doi: 10.1097/00000542-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Anderson B.J., Pons G., Autret-Leca E., Allegaert K., Boccard E. Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatr Anaesth. 2005;15:282–292. doi: 10.1111/j.1460-9592.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- 7.Allegaert K., Vanhaesebrouck S., Verbesselt R., van den Anker J.N. In vivo glucuronidation activity of drugs in neonates: extensive interindividual variability despite their young age. Ther Drug Monit. 2009;31:411–415. doi: 10.1097/FTD.0b013e3181a8cc0a. [DOI] [PubMed] [Google Scholar]
- 8.Allegaert K., Palmer G.M., Anderson B.J. The pharmacokinetics of intravenous paracetamol in neonates: size matters most. Arch Dis Child. 2011;96:575–580. doi: 10.1136/adc.2010.204552. [DOI] [PubMed] [Google Scholar]
- 9.vanLingen R.A., Deinum J.T., Quak J.M., Kuizenga A.J., vanDam J.G., Anand K.J., Tibboel D., Okken A. Pharmacokinetics and metabolism of rectally administered paracetamol in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1999;80:F59–F63. doi: 10.1136/fn.80.1.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young T.E. Twenty-fourth Edition. Thomson Reuters; Montvale, NJ, USA: 2011. Neofax. [Google Scholar]
- 11.BMJ group . The Royal Pharmaceutical Society of Great Britain; London, UK: 2011. British National Formulary for children, 2011-2012. [Google Scholar]
- 12.Neonatal Formulary. Drug use in pregnancy and the first year of life. 6th Edition, BMJ books, Wiley-Blackwell, 2011.
- 13.Nederlands Kenniscentrum Farmacotherapie bij kinderen. Kinderformularium. http://www.kinderformularium.nl. Accessed November 01, 2014.
- 14.Allegaert K., Murat I., Anderson B.J. Not all intravenous paracetamol formulations are created equal. Paediatr Anaesth. 2007;17:811–812. doi: 10.1111/j.1460-9592.2007.02227.x. [DOI] [PubMed] [Google Scholar]
- 15.Bartocci M., Lundeberg S. Intravenous paracetamol: the ‘Stockholm protocol’ for postoperative analgesia of term and preterm neonates. Paediatr Anaesth. 2007;17:1120–1121. doi: 10.1111/j.1460-9592.2007.02322.x. [DOI] [PubMed] [Google Scholar]
- 16.Allegaert K., Verbesselt R., Naulaers G. Developmental pharmacology: neonates are not just small adult. Acta Clin Belg. 2008;63:16–24. doi: 10.1179/acb.2008.003. [DOI] [PubMed] [Google Scholar]
- 17.Kearns G.L., Abdel-Rahman S.M., Alander S.W., Blowey D.L., Leeder J.S., Kauffman R.E. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 18.Allegaert K., Van der Marel C.D., Debeer A., Pluim M.A., Van Lingen R.A., Vanhole C., Tibboel D., Devlieger H. Pharmacokinetics of single dose intravenous propacetamol in neonates: effect of gestational age. Arch Dis Child Fetal Neonatal Ed. 2004;89:F25–F28. doi: 10.1136/fn.89.1.F25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allegaert K., Anderson B.J., Naulaers G. Intravenous paracetamol (propacetamol) pharmacokinetics in term and preterm neonates. Eur J Clin Pharmacol. 2004;60:191–197. doi: 10.1007/s00228-004-0756-x. [DOI] [PubMed] [Google Scholar]
- 20.Van Ganzewinkel C., Derijks L., Anand K.J. Multiple intravenous doses of paracetamol result in a predictable pharmacokinetic profile in very preterm infants. Acta Paediatr. 2014;103:612–617. doi: 10.1111/apa.12638. [DOI] [PubMed] [Google Scholar]
- 21.Cook SF, Sherwin CM, Williams EF, et al. Population pharmacokinetics of intravenous acetaminophen in preterm and term neonates [abstract]. American Conference on Pharmacometrics, annual meeting, Las Vegas, Nevada, 11-17 October 2014.
- 22.Levy G., Khanna N.N., Soda D.M., Tsuzuki O., Stern L. Pharmacokinetics of acetaminophen in the human neonate: formation of acetaminophen glucuronide and sulfate in relation to plasma bilirubin concentration and D-glucaric acid excretion. Pediatrics. 1975;55:818–825. [PubMed] [Google Scholar]
- 23.Allegaert K., de Hoon J., Verbesselt R., Vanhole C., Devlieger H., Tibboel D. Intra- and interindividual variability of glucuronidation of paracetamol during repeated administration of propacetamol in neonates. Acta Paediatr. 2005;94:1273–1279. doi: 10.1111/j.1651-2227.2005.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 24.van der Marel C.D., Anderson B.J., van Lingen R.A., Holford N.H., Pluim M.A., Jansman F.G., van den Anker J.N., Tibboel D. Paracetamol and metabolite pharmacokinetics in infants. Eur J Clin Pharmacol. 2003;59(3):243–251. doi: 10.1007/s00228-003-0608-0. [DOI] [PubMed] [Google Scholar]
- 25.Meremikwu U., Oyo-Ita A. Paracetamol for treating fever in children. Cochrane Database Syst Rev. 2002:CD003676. doi: 10.1002/14651858.CD003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopchet L., Kulo A., Rayyan M. Does intravenous paracetamol administration affect body temperature in neonates ? Arch Dis Child. 2011;96:301–304. doi: 10.1136/adc.2010.203778. [DOI] [PubMed] [Google Scholar]
- 27.Richardson J., Sills J. Hypothermia following fever. Arch Dis Child. 2004;89:1177. doi: 10.1136/adc.2004.055376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Maat M.M., Tijssen T.A., Brüggemann R.J., Ponssen H.H. Paracetamol for intravenous use in medium- and intensive care patients: pharmacokinetics and tolerance. Eur J Clin Parmacol. 2010;66:713–719. doi: 10.1007/s00228-010-0806-5. [DOI] [PubMed] [Google Scholar]
- 29.Van den Anker J.N., Tibboel D. Pain relief in neonates: when to use intravenous paracetamol? Arch Dis Child. 2011;96:573–574. doi: 10.1136/adc.2011.211060. [DOI] [PubMed] [Google Scholar]
- 30.Shah V., Taddio A., Ohlsson A. Randomised controlled trial of paracetamol for heel prick pain in neonates. Arch Dis Child Fetal Neonatal Ed. 1998;79:F209–F211. doi: 10.1136/fn.79.3.f209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard C.R., Howard F.M., Weitzman M.L. Acetaminophen analgesia in neonatal circumcision: the effect on pain. Pediatrics. 1994;93:641–646. [PubMed] [Google Scholar]
- 32.Van Lingen R.A., Quak C.M., Deinum H.T. Effects of rectally administered paracetamol on infants delivered by vacuum extraction. Eur J Obstet Gynecol Reprod Biol. 2001;94:73–78. doi: 10.1016/s0301-2115(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 33.Tinner E.M., Hoesli I., Jost K. Rectal paracetamol in newborn infants after assisted vaginal delivery may increase pain response. J Pediatr. 2013;162:62–66. doi: 10.1016/j.jpeds.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Allegaert K., Naulaers G., Vanhaesebrouck S., Anderson B.J. The paracetamol concentration-effect relation in neonates. Paediatr Anaesth. 2013;23:45–50. doi: 10.1111/pan.12076. [DOI] [PubMed] [Google Scholar]
- 35.Anderson B.J., Woollard G.A., Holford N.H. Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol. 2001;57:559–569. doi: 10.1007/s002280100367. [DOI] [PubMed] [Google Scholar]
- 36.Veyckemans F., Anderson B.J., Wolf A.R., Allegaert K. Intravenous paracetamol dosage in the neonate and small infant. Br J Anaesth. 2014;112:380–381. doi: 10.1093/bja/aet559. [DOI] [PubMed] [Google Scholar]
- 37.Ceelie I., de Wildt S.N., van Dijk M. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309:149–154. doi: 10.1001/jama.2012.148050. [DOI] [PubMed] [Google Scholar]
- 38.Remy C., Marret E., Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505–513. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- 39.McGill M.R., Jaeschke H. Mechanistic biomarkers in acetaminophen-induced hepatotoxity and acute liver failure: from preclinical models to patients. Expert Opin Drug Metab Toxicol. 2014;10:1005–1017. doi: 10.1517/17425255.2014.920823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allegaert K., Rayyan M., De Rijdt T., Van Beek F., Naulaers G. Hepatic tolerance of repeated intravenous paracetamol administration in neonates. Paediatr Anaesth. 2008;18:388–392. doi: 10.1111/j.1460-9592.2008.02535.x. [DOI] [PubMed] [Google Scholar]
- 41.Isbister G.K., Bucens I.K., Whyte I.M. Paracetamol overdose in a preterm neonate. Arch Dis Child Fetal Neonatal Ed. 2001;85:F70–F72. doi: 10.1136/fn.85.1.F70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De la Pintiére A., Beuchée A., Bétrémieux P.E. Intravenous propacetamol overdose in a term newborn. Arch Dis Child Fetal Neonatal Ed. 2003;88:F351–F352. doi: 10.1136/fn.88.4.F351-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walls L., Baker C.F., Sarkar S. Acetaminophen-induced hepatic failure with encephalopathy in a newborn. J Perinatol. 2007;27:133–135. doi: 10.1038/sj.jp.7211641. [DOI] [PubMed] [Google Scholar]
- 44.Nevin D.G., Shung J. Intravenous paracetamol overdose in a preterm infant during anesthesia. Paediatr Anaesth. 2010;20:105–107. doi: 10.1111/j.1460-9592.2009.03210.x. [DOI] [PubMed] [Google Scholar]
- 45.Medicines and Healthcare products regulatory agency. Drug Safety Update. 2010;3:1-4.
- 46.Porta R., Sanchez L., Nicolas M., Garcia C., Martinez M. Lack of toxicity after paracetamol overdose in a extremely preterm neonate. Eur J Clin Pharmacol. 2012;68:901–902. doi: 10.1007/s00228-011-1165-6. [DOI] [PubMed] [Google Scholar]
- 47.Campbell S., Engelhardt T., McLay J., Anderson B. Potentially life-threatening intravenous acetaminophen overdose in a 3 month old (40 weeks’ postmenstrual age) 2.3 kg baby girl. Open J Pediatr. 2013:186–187. [Google Scholar]
- 48.Bucaretchi F., Fernandes C.B., Branco M.M. Acute liver failure in a term neonate after repeated paracetamol administration. Rev Paul Pediatr. 2014;32:144–148. doi: 10.1590/S0103-05822014000100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allegaert K., Naulaers G. Haemodynamics of intravenous paracetamol in neonates. Eur J Clin Pharmacol. 2010;66:855–858. doi: 10.1007/s00228-010-0860-z. [DOI] [PubMed] [Google Scholar]
- 50.Bhattacharyya S., Yan K., Pence L. Targeted liquid chromatography-mass spectrometry analysis of serum acylcarnitines in acetaminophen toxicity in children. Biomark Med. 2014;8:147–159. doi: 10.2217/bmm.13.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James L.P., Chiew A., Abdel-Rahman S.M. Acetaminophen protein adduct formation following low-dose acetaminophen exposure: comparison of immediate-release vs extended-release formulations. Eur J Clin Pharmacol. 2013;69:851–857. doi: 10.1007/s00228-012-1410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James L.P., Alonso E.M., Hynan L.S. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–e681. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 53.James L.P., Capparelli E.V., Simpson P.M. Acetaminophen-associated hepatic injury: evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84:684–690. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lowe A.J., Carlin J.B., Bennett C.M. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ. 2010;341:c4616. doi: 10.1136/bmj.c4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farquhar H., Crane J., Mitchell E.A., Eyers S., Beasley R. The acetaminophen and asthma hypothesis 10 years on: a case to answer. J Allergy Clin Immunol. 2009;124:649–651. doi: 10.1016/j.jaci.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 56.Wickens K., Beasley R., Town I. The effects of early and late paracetamol exposure on asthma and atopy: a birth cohort. Clin Exp Allergy. 2011;41:399–406. doi: 10.1111/j.1365-2222.2010.03610.x. [DOI] [PubMed] [Google Scholar]
- 57.Holgate S.T. The acetaminophen enigma in asthma. Am J Respir Crit Care Med. 2011;183:147–148. doi: 10.1164/rccm.201007-1135ED. [DOI] [PubMed] [Google Scholar]
- 58.Shaheen S.O., Newson R.B., Ring S.M. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allergy Clin Immunol. 2010;126 doi: 10.1016/j.jaci.2010.08.047. 1141-8.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang S.H., Jung Y.H., Kim H.Y. Effect of paracetamol use on the modification of the development of asthma by reactive oxygen species genes. Ann Allergy Asthma Immunol. 2013;111:580. doi: 10.1016/j.anai.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Riley J., Braithwaite I., Shirtcliffe P. Randomized controlled trial of asthma risk with paracetamol use in infancy – a feasibility study. Clin Exp Allergy. 2014 doi: 10.1111/cea.12433. [DOI] [PubMed] [Google Scholar]
- 61.Abdel-Hady J., Nasef N., Shabaan A.E., Nour I. Patent ductus arteriosus in preterm infants: do we have the right answers? Biomed Res Int. 2013;2013:676192. doi: 10.1155/2013/676192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allegaert K., Anderson B., Simons S., van Overmeire B. Paracetamol to induce ductus arteriosus closure: is it valid ? Arch Dis Child. 2013;98:462–466. doi: 10.1136/archdischild-2013-303688. [DOI] [PubMed] [Google Scholar]
- 63.Hammerman C., Bin-Nun A., Markovitch E., Schimmel M.S., Kaplan M., Fink D. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128:e1617–e1621. doi: 10.1542/peds.2011-0359. [DOI] [PubMed] [Google Scholar]
- 64.Le J., Gales M.A., Gales B.J. Acetaminophen for patent ductus arteriosus. Ann Pharmacother. 2014 doi: 10.1177/1060028014557564. (pii: 1060028014557564) [DOI] [PubMed] [Google Scholar]
- 65.Roofthooft D.W., van Beynem I.M., Helbing W.A., Reiss I.K., Simons S.H. Paracetamol for ductus arteriosus closure: not always a success story. Concerning the article by M.Y. Oncel et al: intravenous paracetamol treatment in the management of patent ductus arteriosus in extremely low birth weight infants. Neonatology. 2013;104:170. doi: 10.1159/000353451. [DOI] [PubMed] [Google Scholar]
- 66.Oncel M.Y., Yurttutan S., Erdeve O. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164 doi: 10.1016/j.jpeds.2013.11.008. 510-514.e1. [DOI] [PubMed] [Google Scholar]
- 67.Dang D., Wang D., Zhang C., Zhou W., Zhou Q., Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS One. 2013;8:e77888. doi: 10.1371/journal.pone.0077888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sallmon H., Weber S.C., Hüning B. Thrombocytopenia in the first 24 hours after birth and incidence of patent ductus arteriosus. Pediatrics. 2012;130:e623–e630. doi: 10.1542/peds.2012-0499. [DOI] [PubMed] [Google Scholar]
- 69.El-Khuffash A., Jain A., Corcoran D. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence form human and murine studies. Pediatr Res. 2014;76:238–244. doi: 10.1038/pr.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]


