Abstract
This study tested the hypothesis that sweat rate during passive heat stress is limited by baroreceptor unloading associated with heat stress. Two protocols were performed in which healthy subjects underwent passive heat stress that elicited an increase in intestinal temperature of ∼1.8°C. Upon attaining this level of hyperthermia, in protocol 1 (n = 10, 3 females) a bolus (19 ml/kg) of warm (∼38°C) isotonic saline was rapidly (5–10 min) infused intravenously to elevate central venous pressure (CVP), while in protocol 2 (n = 11, 5 females) phenylephrine was infused intravenously (60–120 μg/min) to return mean arterial pressure (MAP) to normothermic levels. In protocol 1, heat stress reduced CVP from 3.9 ± 1.9 mmHg (normothermia) to −0.6 ± 1.4 mmHg (P < 0.001), while saline infusion returned CVP to normothermic levels (5.1 ± 1.7 mmHg; P > 0.999). Sweat rate was elevated by heat stress (1.21 ± 0.44 mg·cm−2·min−1) but remained unchanged during rapid saline infusion (1.26 ± 0.47 mg·cm−2·min−1, P = 0.5), whereas cutaneous vascular conductance increased from 77 ± 10 to 101 ± 20% of local heating max (P = 0.029). In protocol 2, MAP was reduced with heat stress from 85 ± 7 mmHg to 76 ± 8 mmHg (P = 0.048). Although phenylephrine infusion returned MAP to normothermic levels (88 ± 7 mmHg; P > 0.999), sweat rate remained unchanged during phenylephrine infusion (1.39 ± 0.22 vs. 1.41 ± 0.24 mg·cm−2·min−1; P > 0.999). These data indicate that both cardiopulmonary and arterial baroreceptor unloading do not limit increases in sweat rate during passive heat stress.
Keywords: arterial baroreceptors, cardiopulmonary baroreceptors, hyperthermia, central venous pressure, skin blood flow, sweat rate
sweating and cutaneous vasodilation are elicited not only by increases in core and skin temperatures, but also by nonthermal factors. For instance, central command, metaboreceptors, mechanoreceptors, and osmoreceptors all independently affect both sweating and cutaneous vasodilation (20, 23). In contrast, baroreceptors have long been known to influence cutaneous vasodilation (11), but their influence upon sweat rate remains debatable (31).
Passive heat stress reduces cardiac filling pressures (38) and arterial blood pressure (7), unloading both cardiopulmonary and arterial baroreceptors, respectively. The vast majority of studies examining a potential role for baroreceptors in the control of sweating during passive heat stress have done so using perturbations that further unload baroreceptors. Such studies, using passive heat stress in combination with either head-up tilt (37), pharmacological agents (36), or during lower-body negative pressure (1, 24), have consistently shown that further baroreceptor unloading does not affect sweat rate or its thermal sensitivity. In contrast, baroreceptor unloading modulates sweat rate during the recovery from dynamic exercise (19), which is also characterized by a state of arterial and cardiopulmonary baroreceptor unloading, as cardiac filling and arterial blood pressures are reduced (9, 10). Rather than eliciting further baroreceptor unloading, however, postexercise studies have shown that arterial and/or cardiopulmonary baroreceptor “reloading” maintains sweat rate at elevated levels, particularly during the early stages of recovery following dynamic exercise (13–15, 17, 28). It is therefore conceivable that the discrepancy between passive heat stress and postexercise studies regarding the potential role of baroreceptors in modulating sweat rate results from the former relying on procedures that cause further baroreceptor unloading (e.g., lower-body negative pressure, head-up tilt, pharmacological reductions in blood pressure), whereas the latter relied upon maneuvers that cause baroreceptor reloading (e.g., lower-body positive pressure, head-down tilt, supine posture).
Few studies have attempted to examine whether baroreceptor reloading can influence sweat rate during passive heat stress. Our laboratory has previously shown that brief sequential decreases and increases in mean arterial pressure do not cause parallel changes in sweat rate in passively heat-stressed subjects (36). Furthermore, sweat rate is unaffected during passive heating performed in a head-down tilt position relative to the upright seated posture (18). However, findings from both studies are limited in that arterial baroreceptor reloading was very brief (<1 min) and immediately followed sodium nitroprussside infusion and subsequent arterial baroreceptor unloading (36), while head-down tilt did not fully offset baroreceptor unloading associated with heat stress (18). As such, it remains to be determined whether baroreceptor unloading during heat stress limits the increase in sweat rate and if so, whether one baroreceptor population (arterial vs. cardiopulmonary) is primarily responsible. Our laboratory has previously shown that cardiopulmonary baroreceptor reloading via rapid saline infusion allows for greater increases in cutaneous vasodilation during passive heat stress (3). These findings suggest that cardiopulmonary baroreceptor unloading limits the increase in cutaneous vasodilation during passive heat stress in humans. Therefore, the purpose of this study was to examine whether baroreceptor unloading also limits the increase in sweat rate during passive heat stress. We also examined potential separate influences of cardiopulmonary and arterial baroreceptors by performing procedures that primarily targeted each population. We tested the hypothesis that both cardiopulmonary and arterial baroreceptor unloading limit the increase in sweat rate during passive heat stress, such that reloading both baroreceptor populations would further elevate sweat rate.
METHODS
Two protocols were undertaken. Ten subjects (3 women) participated in protocol 1, while 11 subjects (5 women) participated in protocol 2. Subject characteristics (mean ± SD) for protocol 1 were age, 29 ± 5 yr; height, 177 ± 10 cm; weight, 75.5 ± 12.2 kg; and for protocol 2 were age, 26 ± 5 yr; height, 178 ± 12 cm; weight, 71.3 ± 14.9 kg. Subjects were nonsmokers, not taking medications, and were free of any known cardiovascular, metabolic, neurological, or psychological diseases. Since only within-subject comparisons were performed in the present study (see Data and Statistical Analyses), menstrual cycle phase was recorded, but not controlled for in female subjects. Each subject was fully informed of the experimental procedures and possible risks before giving informed, written consent. Both protocols and the informed consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital of Dallas, and all procedures conformed to the standards set by the Declaration of Helsinki. Subjects arrived at the laboratory euhydrated, confirmed via urine specific gravity (protocol 1: 1.015 ± 0.008; protocol 2: 1.012 ± 0.008), and were asked to refrain from strenuous exercise, alcohol, and caffeine for a period of 24 h prior to their arrival.
Instrumentation
Both protocols.
Approximately 120 min prior to experimental testing, each subject swallowed a telemetry pill (HQ, Palmetto, FL) for the measurement of intestinal temperature. Mean skin temperature was measured as the weighted average of six thermocouples (abdomen, calf, chest, lower back, thigh, upper back) attached to the skin (34). Mean body temperature was subsequently calculated as intestinal temperature × 0.8 + mean skin temperature × 0.2. Body temperature was controlled via a water-perfused tube lined suit (Med-Eng, Ottawa, ON, Canada) that covered the entire body except the head, hands, one forearm, and the feet. Blankets were placed on the subjects to minimize any potential heat loss from sweat evaporation. Heart rate was continuously recorded from an electrocardiogram (HP Patient Monitor, Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Beat-to-beat blood pressure was measured continuously via the Penaz method (Finometer Pro, FMS, Amsterdam, The Netherlands), which was confirmed intermittently via auscultation of the brachial artery by electrosphygmomanometry (Tango+, SunTech, Raleigh, NC). Forearm (local) sweat rate was measured by placing a plastic capsule covering 2.83 cm2 of skin on the ventral surface of the exposed forearm. This capsule was perfused with dry nitrogen at a flow rate of 0.3 l/min. Water vapor in the gas exiting the capsule was measured by capacitance hygrometry (Vaisala, Woburn, WA), and forearm sweat rate was calculated by multiplying the humidity output (in g/m3) by flow rate and dividing that value by the surface area of the capsule.
Instrumentation unique to protocol 1.
Skin blood flow was measured via laser-Doppler flowmetry (Periflux System 5000, Perimed, Stockholm, Sweden) from the ventral surface of the uncovered forearm. At the end of the experimental protocol, the area surrounding the laser-Doppler probe was heated to 44°C for 30 min to produce a locally induced maximum skin blood flow (16). Cutaneous vascular conductance was calculated as laser-Doppler flux divided by mean arterial pressure and expressed as a percentage of maximum. In 8 of 10 subjects, a peripherally inserted central venous catheter was advanced into the superior vena cava via the basilic vein. Positioning of the central venous catheter was confirmed by 1) the distance that the catheter was advanced, 2) observation of adequate pressure waveforms, and 3) an appropriate rapid rise and fall in pressure during a Valsalva and Mueller maneuver, respectively. The central venous catheter was connected to a pressure transducer and zeroed at the position of the midaxillary line. This catheter was used for continuous measurement of central venous pressure (CVP).
Experimental Protocol 1
This protocol was performed to determine whether reloading of primarily the cardiopulmonary baroreceptors, via restoring hyperthermia-induced reductions in CVP to normothermic levels, increased sweat rate during passive heat stress. Following instrumentation, subjects rested in the supine position for a minimum of 30 min, while 34°C water circulated through the suit, after which baseline normothermic thermal and hemodynamic data were obtained. Subjects were then passively heated by circulating ∼49°C water through the suit. After intestinal temperature increased ∼1.8°C, warmed (∼38°C) isotonic saline was rapidly administered over 6.9 ± 2.1 min through a catheter placed in an antecubital vein (different from the one used to place the CVP catheter) sufficient to return CVP to pre-heat stress levels (2, 3). After the infusion, mean skin temperature was returned to pre-heat stress levels by perfusing the suit with cool (∼20°C) water, during which time maximum skin blood flow was determined by local heating.
Experimental Protocol 2
This protocol was performed to determine whether reloading of primarily arterial baroreceptors, via restoring hyperthermia-induced reductions in mean arterial pressure (MAP) to normothermic levels, increased sweat rate during passive heat stress. Similar to protocol 1, subjects rested in the supine position following instrumentation, but their lower legs were suspended below heart level (legs were bent at knees at ∼73° angle), promoting reductions in MAP during passive heat stress. Following a minimum of 30 min, during which time 34°C water circulated through the suit, baseline normothermic thermal and hemodynamic data were obtained. Subjects were then passively heated by circulating ∼49°C water through the suit. After intestinal temperature increased ∼1.8°C, phenylephrine (60–120 μg/min) was intravenously titrated for 5 min to increase MAP 12 ± 3 mmHg, restoring MAP to normothermic levels. Skin blood flow was not measured in this protocol as phenylephrine acts directly on the cutaneous vasculature, independent of neural control.
Data and Statistical Analyses
Data were acquired continuously at 50 Hz throughout the experiment (Biopac, Santa Barbara, CA). Data are presented as a 1-min average immediately before whole body heating (normothermia) and immediately before rapid saline (protocol 1) or phenylephrine (protocol 2) infusion (hyperthermia), while data throughout the saline and phenylephrine infusions are presented as minute averages. The duration of the saline infusion differed between subjects (range: 5–10 min). Therefore, the first 4 min of these data, and data during the final minute of the infusion, are presented. All data were statistically analyzed using one-way repeated-measures analysis of variance with the repeated factor of time (normothermia, hyperthermia, as well as minutes 1–4 and the final minute, or minutes 1–5 of rapid saline and phenylephrine infusions, respectively), and where appropriate, post hoc Bonferroni pairwise comparisons were made. Data were analyzed using GraphPad Prism (version 6, GraphPad Software, La Jolla, CA) with a priori statistical significance set at P ≤ 0.05. All data are reported as means ± SD.
RESULTS
Protocol 1
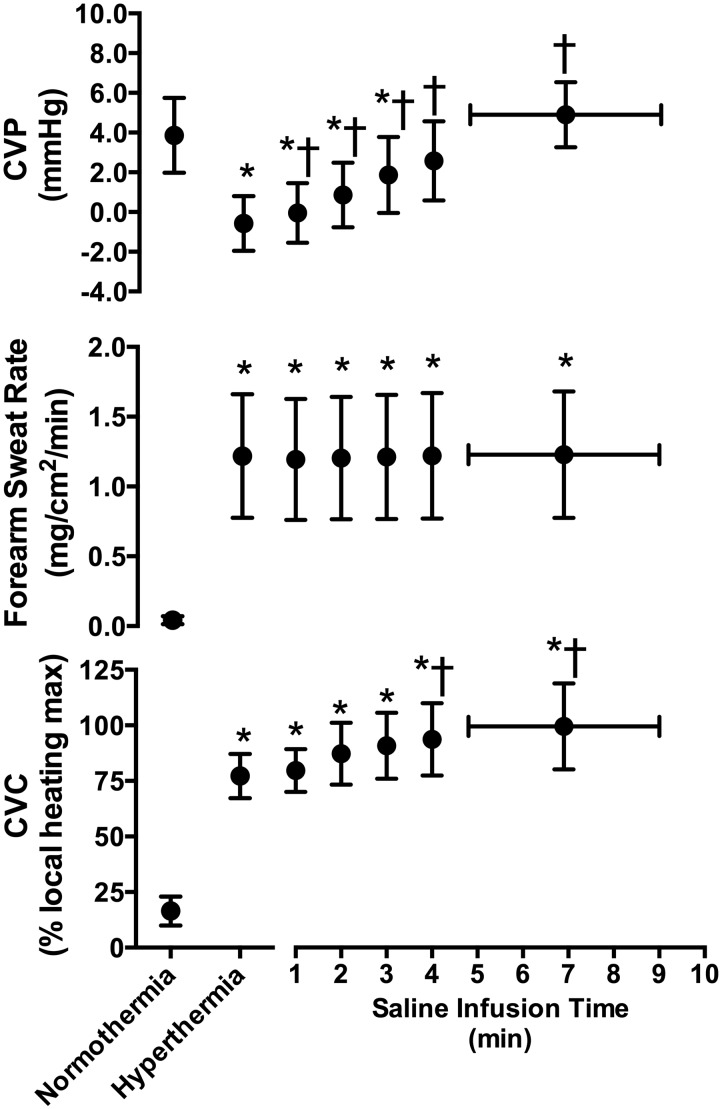
Passive heat stress increased intestinal temperature, mean skin temperature, and heart rate (all P < 0.001), while MAP was unchanged (P = 0.765, Table 1). CVP decreased (P < 0.001) with heat stress, while forearm sweat rate and CVC were increased (P < 0.001, Fig. 1). Rapid infusion of 19 ± 2 ml/kg of saline raised CVP (P ≤ 0.019), returning it to normothermic values (P > 0.999, Fig. 1). This saline infusion caused further increases in CVC (P ≤ 0.051) but did not cause any further changes in forearm sweat rate (P ≥ 0.439, Fig. 1). By the end of the saline infusion, intestinal temperature had further increased by 0.2 ± 0.1°C (P = 0.005), while MAP decreased to values that were lower than during normothermia (P = 0.029, Table 1).
Table 1.
Thermal and hemodynamic data during normothermia, hyperthermia (pre-saline infusion), and during the last minute of rapid saline infusion
| Normothermia | Hyperthermia | Saline Infusion | |
|---|---|---|---|
| Intestinal temperature, °C | 37.0 ± 0.3 | 38.9 ± 0.4* | 39.1 ± 0.5*† |
| Mean skin temperature, °C | 34.7 ± 0.2 | 39.3 ± 0.8* | 39.2 ± 0.8* |
| Mean body temperature, °C | 36.6 ± 0.3 | 39.0 ± 0.5* | 39.1 ± 0.5*† |
| MAP, mmHg | 82 ± 8 | 79 ± 11 | 74 ± 8* |
| Heart rate, beats/min | 59 ± 10 | 113 ± 17* | 112 ± 14* |
Values are means ± SD. MAP, mean arterial pressure;
Different from normothermia (P ≤ 0.023).
Different from hyperthermia (P = 0.005).
Fig. 1.
Central venous pressure (CVP, top, n = 8), forearm sweat rate (middle, n = 10), and cutaneous vascular conductance (CVC, bottom, n = 10) immediately prior to passive heat stress (normothermia), during hyperthermia, and throughout the rapid saline infusion (means ± SD). Data during the infusion are presented until the last common time point of saline infusion (4 min), while the last data point represents the mean infusion time and associated response across all subjects. *Different from normothermia (P ≤ 0.028). †Different from hyperthermia (P ≤ 0.051).
Protocol 2
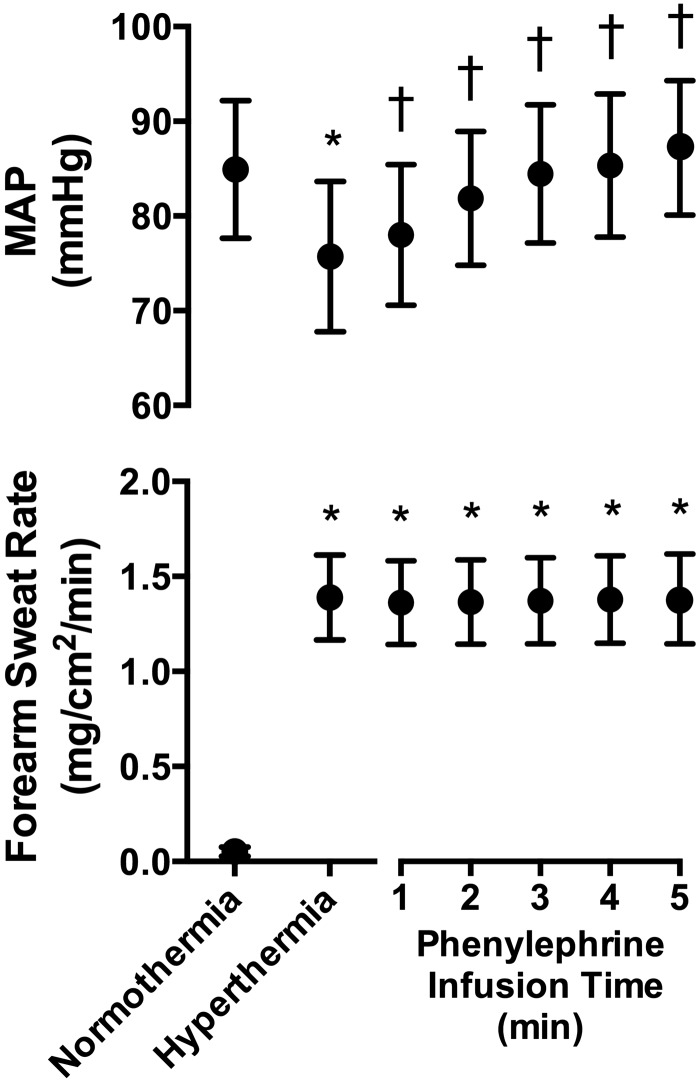
Passive heat stress increased (P < 0.001) intestinal temperature, mean skin temperature, heart rate, and forearm sweat rate (Table 2, Fig. 2), while MAP decreased by 9 ± 7 mmHg (P = 0.048, Fig. 2). Phenylephrine infusion increased MAP (P < 0.001), restoring it to normothermic values (P > 0.999) but did not affect forearm sweat rate (P > 0.999, Fig. 2). By the end of the phenylephrine infusion, intestinal temperature had further increased by 0.2 ± 0.1°C (P < 0.001), while heart rate decreased (P < 0.001) relative to that occurring during hyperthermia (Table 2).
Table 2.
Thermal and hemodynamic data during normothermia, hyperthermia (pre-phenylephrine infusion), and during the last minute of phenylephrine infusion
| Normothermia | Hyperthermia | Phenylephrine Infusion | |
|---|---|---|---|
| Intestinal temperature, °C | 36.9 ± 0.2 | 38.7 ± 0.4* | 38.9 ± 0.4*† |
| Mean skin temperature, °C | 33.9 ± 0.5 | 39.9 ± 0.5* | 39.9 ± 0.4* |
| Mean body temperature, °C | 36.3 ± 0.2 | 39.0 ± 0.4* | 39.1 ± 0.4*† |
| Heart rate, beats/min | 56 ± 9 | 107 ± 16* | 95 ± 16*† |
Values are means ± SD.
Different from normothermia (P < 0.001);
Different from hyperthermia (P < 0.001).
Fig. 2.
Mean arterial pressure (MAP, top) and forearm sweat rate (bottom) immediately prior to passive heat stress (normothermia), during hyperthermia, and throughout the phenylephrine infusion (means ± SD, n = 11). *Different from normothermia (P ≤ 0.048). †Different from hyperthermia (P ≤ 0.007).
DISCUSSION
This study tested the hypothesis that baroreceptor unloading associated with passive heat stress limits the increase in sweat rate. Contrary to our hypothesis, reloading of primarily cardiopulmonary (via rapid saline infusion) and primarily arterial (via phenylephrine infusion) baroreceptors did not elicit further increases in sweat rate (Figs. 1 and 2). Therefore, the present results suggest that baroreceptor unloading coincident with severe passive heat stress does not limit increases in sweat rate during this exposure in humans.
A number of studies have examined a potential role for baroreceptors in modulating sweat rate during exercise, passive heat stress, and during the recovery from dynamic exercise. Findings from early studies comparing thermoregulatory responses during supine (i.e., baroreceptor loading) and upright (i.e., baroreceptor unloading) exercise suggest that baroreceptor unloading during exercise does not affect sweat production (8, 21), but that upright exercise delays the onset threshold for cutaneous vasodilation (12, 29). Subsequently, studies using lower-body negative pressure established a potential role for cardiopulmonary baroreceptor unloading in altering the neural drive (i.e., skin sympathetic nerve activity) for sweating (5) as well as both the onset threshold for sweat production (27) and its sensitivity to changes in core temperature (26, 32). These latter findings, however, were largely attributed to potential reductions in skin temperature that accompany the use of lower-body negative pressure (35), which can decrease sweat rate independent of baroreceptor unloading (39). Consequently, a number of studies using alternative methods to unload baroreceptors, while minimizing decreases in skin temperature, were undertaken. In these studies, passive heat stress in combination with either head-up tilt (37), pharmacological agents (36), or during lower-body negative pressure with or without small decreases in skin temperature (1, 4, 24) have consistently shown that further baroreceptor unloading (relative to that which occurs during heat stress itself) does not affect skin sympathetic nerve activity, sweat rate, or its thermal sensitivity.
In contrast to passive heat stress studies, baroreceptor modulation of sweating has consistently been demonstrated during the recovery from dynamic exercise. Rather than eliciting further baroreceptor unloading, as in the aforementioned passive heat stress studies, most of these studies reversed the cardiopulmonary and/or arterial baroreceptor unloading that typically occurs postexercise (9, 10). For example, Journeay et al. (14) used lower-body positive pressure to reload cardiopulmonary and arterial baroreceptors following dynamic exercise and observed elevated sweat rates that ultimately resulted in a faster return of core temperature to baseline compared with a control (i.e., no positive pressure) condition. Similarly, a number of other postexercise studies have shown that baroreceptor reloading maintains sweat rate at elevated levels compared with when baroreceptors are naturally unloaded during inactive recovery following dynamic exercise (13, 15, 17, 28). In light of the findings from these studies, we hypothesized that the cardiopulmonary and arterial baroreceptor unloading which occurs during passive heat stress places a “brake” on sweat rate. If so, then releasing that figurative brake, by returning the loading status of these baroreceptors to pre-heat stress levels, would elicit further increases in sweat rate. Contrary to this hypothesis, however, sweat rate remained unchanged during both rapid saline (Fig. 1) and phenylephrine (Fig. 2) infusions. In contrast, and as previously shown (3), cardiopulmonary baroreceptor reloading via rapid saline infusion elicited large increases in cutaneous vascular conductance (upwards of 25%; Fig. 1). It should be considered that small decreases in MAP (i.e., arterial baroreceptor unloading) during rapid saline infusion may have negated potential increases in sweat rate during reloading of cardiopulmonary baroreceptors. However, a targeted reloading of arterial baroreceptors via phenylephrine infusion had no effect on sweat rate during protocol 2 (Fig. 2), suggesting that the lack of increase in sweat rate during rapid saline infusion is not confounded by small decreases in MAP. Overall, the current data suggest that while cardiopulmonary baroreceptor unloading may limit increases in cutaneous vasodilation during passive heating in humans, they do not appear to limit the increase in sweat rate. Furthermore, these observations support prior observations of differential baroreceptor control of skin blood flow and sweating in the passively heat stressed human (12).
It should be noted that at least two previous studies have examined the effect of baroreceptor loading on sweat rate during passive heat stress, although definite conclusions as to a potential modulation of sweat rate by baroreceptors could not be reached. Our laboratory previously reported that sweat rate during passive heat stress was unaffected by bolus infusions of sodium nitroprusside immediately followed by phenylephrine that elicited sequential decreases and increases in blood pressure (36). Although these findings suggested that arterial baroreceptor loading does not affect sweat rate during passive heat stress, no insight into a potential role for cardiopulmonary baroreceptor loading could be assessed. Moreover, conclusions regarding arterial baroreceptor control of sweating from that study are clouded by 1) the fact that in all cases, blood pressure was first reduced by sodium nitroprusside, which likely affected the extent of arterial baroreceptor reloading during subsequent phenylephrine administration; and 2) the short period of time blood pressure was elevated (typically <1 min) as a result of the bolus approach, which may have been insufficient to observe any effects upon sweat rate. Furthermore, Kenny et al. (18) compared sweating and cutaneous vascular conductance during passive heat stress in the upright seated position (baroreceptor unloading) and during 15° head-down tilt (baroreceptor loading). Although they did not observe any differences in either sweating or cutaneous vascular conductance between conditions, it is possible that 15° head-down tilt was not sufficient to fully reload baroreceptors, as it did not return blood pressure to normothermic levels during the passive heat stress period. Furthermore, 15° head-down tilt did not increase cutaneous vascular conductance, despite cardiopulmonary baroreceptor reloading being clearly capable of eliciting further increases in cutaneous vascular conductance [Ref. 3, and Fig. 1]. Overall, however, the findings of the present study support these previous observations, and extend them by providing conclusive evidence that reloading of either cardiopulmonary or arterial baroreceptors does not affect sweat rate during passive heat stress in humans.
Considerations
The findings of the present study are partly dependent upon the assumption that sweat rate could be further elevated during rapid saline and phenylephrine infusion periods. Considering that core temperature increased ∼1.8°C during passive heating, it is possible that any influence of baroreceptor unloading may have been overridden by the high thermal drive. Indeed, previous studies have shown that the relative influence of nonthermal factors, including baroreceptors, is attenuated when thermal drive is elevated (6, 22). Furthermore, maximal levels of forearm sweat rate may have been attained during the heating period. Both possibilities would have created a “ceiling effect” thus limiting any further increases in sweat rate during baroreceptor reloading. While these considerations may have limited the potential to observe further increases in forearm sweat rate during the baroreceptor reloading periods, their possible occurrence would nonetheless suggest that baroreceptor unloading did not limit the increase in sweat rate during severe passive heating in the present study. If baroreceptor unloading associated with heat stress modulates sweat rate, we would have expected the thermal drive to be insufficient to override this baroreflex influence, which would have effectively limited the increase in sweat rate. This scenario would have provided a background against which to observe further increases in forearm sweat rate during baroreceptor reloading. Therefore, the lack of increase in forearm sweat rate during both rapid saline and phenylephrine infusions indicates that baroreceptor unloading during severe passive heating did not impede increases in forearm sweat rate. That said, although forearm sweat rate is representative of other nonglabrous areas during passive heating (33) and it responds to nonthermal stimuli similarly to other nonglabrous skin sites (25), we cannot rule out the possibility that sweat rate in other areas of the body increased during the baroreceptor reloading interventions.
Perspectives
The findings from the present study add to a growing body of recent literature indicating that baroreceptors do not influence sweat production during passive heat stress (1, 4, 24, 36, 37). A consistent lack of baroreceptor modulation of sweat production during passive heat stress is puzzling compared with baroreceptor modulation of sweating during (26, 27) and following (13–15, 17, 28) dynamic exercise. Although speculative, it is possible that factors specific to dynamic exercise engage baroreceptors in the control of sweating. Future studies should consider examining this possibility. Determining whether baroreceptors modulate sweat rate during passive heat stress is important as, from a practical perspective, prolonged sweating in hot environments often leads to dehydration (30), exacerbating baroreceptor unloading. Thus it is perhaps unsurprising that a number of studies have examined a potential role for baroreceptor unloading in eliciting volume preservation responses, such as reducing sweat production. Such a role for baroreceptors (should it exist) would be advantageous for individuals whose occupational or recreational pursuits render them passively hyperthermic, dehydrated, and at an elevated risk of a hemorrhagic injury that would induce further volume loss (e.g., soldiers, firefighters, miners, etc.). The findings of the present study not only suggest that baroreceptor unloading during such scenarios does not preserve fluid status secondary to decreasing sweat production, but also that reloading of the baroreceptors is unlikely to improve sweat production and therefore contribute to temperature regulation.
Conclusions
The present study examined whether baroreceptor unloading limits the increase in forearm sweat rate and cutaneous vascular conductance during severe passive heat stress. The results show that reloading of cardiopulmonary baroreceptors (via rapid saline infusion) elicits large increases in cutaneous vascular conductance, consistent with prior observations (3), without any effect upon forearm sweat rate. Furthermore, infusion of phenylephrine to reload arterial baroreceptors did not elicit further increases in forearm sweat production. These findings suggest that cardiopulmonary and arterial baroreceptor unloading during severe passive heat stress do not limit increases in sweat rate.
GRANTS
This project was funded in part by National Institutes of Health Grants R01-HL-61388 and F32-AG-04328 and Department of Defense Grant W81XWH-12–1-0152.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.J.S., R.A.I.L., J.P., and C.G.C. conception and design of research; Z.J.S., R.A.I.L., and J.P. performed experiments; Z.J.S. analyzed data; Z.J.S., D.G., and C.G.C. interpreted results of experiments; Z.J.S. prepared figures; Z.J.S., D.G., R.A.I.L., J.P., and C.G.C. edited and revised manuscript; Z.J.S., D.G., R.A.I.L., J.P., and C.G.C. approved final version of manuscript; D.G. drafted manuscript.
ACKNOWLEDGMENTS
We thank the subjects for participating in this study. We also thank J. Kern, R.N., and N. Kennedy, R.N., for technical assistance.
REFERENCES
- 1.Binder K, Lynn AG, Gagnon D, Kondo N, Kenny GP. Hyperthermia modifies muscle metaboreceptor and baroreceptor modulation of heat loss in humans. Am J Physiol Regul Integr Comp Physiol 302: R417–R423, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 277: H2348–H2352, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol 86: 605–610, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Dodt C, Gunnarsson T, Elam M, Karlsson T, Wallin BG. Central blood volume influences sympathetic sudomotor nerve traffic in warm humans. Acta Physiol Scand 155: 41–51, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon D, Jay O, Reardon FD, Journeay WS, Kenny GP. Hyperthermia modifies the nonthermal contribution to postexercise heat loss responses. Med Sci Sports Exerc 40: 512–522, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Ganio MS, Brothers RM, Lucas RA, Hastings JL, Crandall CG. Validity of auscultatory and Penaz blood pressure measurements during profound heat stress alone and with an orthostatic challenge. Am J Physiol Regul Integr Comp Physiol 301: R1510–R1516, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenleaf JE, van Kessel AL, Ruff W, Card DH, Rapport M. Exercise temperature regulation in man in the upright and supine positions. Med Sci Sports 3: 175–182, 1971. [PubMed] [Google Scholar]
- 9.Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev 29: 65–70, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol 89: 1830–1836, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JM. Nonthermoregulatory control of human skin blood flow. J Appl Physiol 61: 1613–1622, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol 50: 814–818, 1981. [DOI] [PubMed] [Google Scholar]
- 13.Journeay WS, Jay O, McInnis NH, Leclair E, Kenny GP. Postexercise heat loss and hemodynamic responses during head-down tilt are similar between genders. Med Sci Sports Exerc 39: 1308–1314, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Journeay WS, Reardon FD, Jean-Gilles S, Martin CR, Kenny GP. Lower body positive and negative pressure alter thermal and hemodynamic responses after exercise. Aviat Space Environ Med 75: 841–849, 2004. [PubMed] [Google Scholar]
- 15.Journeay WS, Reardon FD, Martin CR, Kenny GP. Control of cutaneous vascular conductance and sweating during recovery from dynamic exercise in humans. J Appl Physiol 96: 2207–2212, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Kellogg DL Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Kenny GP, Gagnon D, Jay O, McInnis NH, Journeay WS, Reardon FD. Can supine recovery mitigate the exercise intensity dependent attenuation of post-exercise heat loss responses? Appl Physiol Nutr Metab 33: 682–689, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Kenny GP, Gagnon D, Shiff D, Armstrong R, Journeay WS, Kilby D. Influence of nonthermal baroreceptor modulation of heat loss responses during uncompensable heat stress. Eur J Appl Physiol 108: 541–548, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Kenny GP, Jay O, Journeay WS. Disturbance of thermal homeostasis following dynamic exercise. Appl Physiol Nutr Metab 32: 818–831, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kenny GP, Journeay WS. Human thermoregulation: separating thermal and nonthermal effects on heat loss. Front Biosci 15: 259–290, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Horvath SM, Diaz FJ, Bransford DR, Drinkwater BL. Thermoregulation during rest and exercise in different postures in a hot humid environment. J Appl Physiol 48: 999–1007, 1980. [DOI] [PubMed] [Google Scholar]
- 22.Kondo N, Horikawa N, Aoki K, Shibasaki M, Inoue Y, Nishiyasu T, Crandall CG. Sweating responses to a sustained static exercise is dependent on thermal load in humans. Acta Physiol Scand 175: 289–295, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kondo N, Nishiyasu T, Inoue Y, Koga S. Non-thermal modification of heat-loss responses during exercise in humans. Eur J Appl Physiol 110: 447–458, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Lynn AG, Gagnon D, Binder K, Boushel RC, Kenny GP. Divergent roles of plasma osmolality and the baroreflex on sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol 302: R417–R423, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Machado-Moreira CA, McLennan PL, Lillioja S, van Dijk W, Caldwell JN, Taylor NA. The cholinergic blockade of both thermally and non-thermally induced human eccrine sweating. Exp Physiol 97: 930–942, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Mack G, Nishiyasu T, Shi X. Baroreceptor modulation of cutaneous vasodilator and sudomotor responses to thermal stress in humans. J Physiol 483: 537–547, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack GW, Cordero D, Peters J. Baroreceptor modulation of active cutaneous vasodilation during dynamic exercise in humans. J Appl Physiol 90: 1464–1473, 2001. [DOI] [PubMed] [Google Scholar]
- 28.McInnis NH, Journeay WS, Jay O, Leclair E, Kenny GP. 15 degrees head-down tilt attenuates the postexercise reduction in cutaneous vascular conductance and sweating and decreases esophageal temperature recovery time. J Appl Physiol 101: 840–847, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Roberts MF, Wenger CB. Control of skin blood flow during exercise by thermal reflexes and baroreflexes. J Appl Physiol 48: 717–723, 1980. [DOI] [PubMed] [Google Scholar]
- 30.Sawka MN. Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc 24: 657–670, 1992. [PubMed] [Google Scholar]
- 31.Shibasaki M, Crandall CG. Mechanisms and controllers of eccrine sweating in humans. Front Biosci 2: 685–696, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solack SD, Brengelmann GL, Freund PR. Sweat rate vs. forearm blood flow during lower body negative pressure. J Appl Physiol 58: 1546–1552, 1985. [DOI] [PubMed] [Google Scholar]
- 33.Taylor NA, Machado-Moreira CA. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem Physiol Med 2: 4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Vissing SF. Differential activation of sympathetic discharge to skin and skeletal muscle in humans. Acta Physiol Scand Suppl 639: 1–32, 1997. [PubMed] [Google Scholar]
- 36.Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol 536: 615–623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson TE, Cui J, Crandall CG. Mean body temperature does not modulate eccrine sweat rate during upright tilt. J Appl Physiol 98: 1207–1212, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol 585: 279–285, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M, Crandall CG. Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans. J Appl Physiol 109: 1301–1306, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]