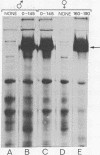
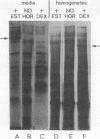
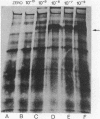
Abstract
Using the frog Xenopus laevis, we show that the addition of physiological concentrations of estradiol to cultures of liver from untreated males rapidly induces the synthesis of large amounts of vitellogenin. Sustained synthesis of vitellogenin requires continuous exposure to estradiol. A nonestrogenic steroid, dexamethasone, does not induce vitellogenin synthesis but does induce increased synthesis of a different protein in liver cultures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Q., Dolphin P. J., Lazier C. B., Munday K. A., Akhtar M. Chemical composition of an oestrogen-induced calcium-binding glycolipophosphoprotein in Xenopus laevis. Biochem J. 1971 Mar;122(1):107–113. doi: 10.1042/bj1220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink E. W., Wallace R. A. Precursor-product relationship between amphibian vitellogenin and the yolk proteins, lipovitellin and phosvitin. J Biol Chem. 1974 May 10;249(9):2897–2903. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Lofthouse R., Tata J. R. Sequential changes in the protein synthetic activity of male Xenopus laevis liver following induction of egg-yolk proteins by Estradiol-17 beta. J Biol Chem. 1975 Mar 25;250(6):2213–2218. [PubMed] [Google Scholar]
- Follett B. K., Redshaw M. R. The effects of oestrogen and gonadotrophins on lipid and protein metabolism in Xenopus laevis Daudin. J Endocrinol. 1968 Apr;40(4):439–456. doi: 10.1677/joe.0.0400439. [DOI] [PubMed] [Google Scholar]
- GALLIEN L., CHALUMEAU-LE FOULGOC M. [Demonstration of estrogenic steroid in the immature ovary of Xenopus laevis Daudin, and the estrogen cycle during laying]. C R Seances Soc Biol Fil. 1960 Jul 18;251:460–462. [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. Specific binding of estradiol to the liver chromatin of estrogenized roosters. Biochim Biophys Acta. 1974 Aug 15;361(1):84–96. doi: 10.1016/0005-2787(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Knowland J. Protein synthesis directed by the RNA from a plant virus in a normal animal cell. Genetics. 1974 Sep;78(1):383–394. doi: 10.1093/genetics/78.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Baulieu E. E. Extraction, partial purification and characterization of 'the insoluble estrogen receptor' from chick liver nuclei. FEBS Lett. 1974 Jul 1;43(1):107–111. doi: 10.1016/0014-5793(74)81117-8. [DOI] [PubMed] [Google Scholar]
- Marot J., Ozon R. Competitive protein binding method using an amphibian sex binding protein. Measurement of estradiol-17 in Pleurodeles waltlii Michah. plasma. Comp Biochem Physiol B. 1973 Apr 15;44(4):1103–1111. doi: 10.1016/0305-0491(73)90263-0. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Ozon R., Bellé R. Récepteurs de l'oestradiol-17 dans le foie de poule et de l'amphibien Discoglossus pictus. Biochim Biophys Acta. 1973 Jan 24;297(1):155–163. [PubMed] [Google Scholar]
- Redshaw M. R., Follett B. K., Nichollis T. J. Comparative effects of the oestrogens and other steroid hormones on serum lipids and proteins in Xenopus laevis Daudin. J Endocrinol. 1969 Jan;43(1):47–53. doi: 10.1677/joe.0.0430047. [DOI] [PubMed] [Google Scholar]
- Schirm J., Gruber M., Ab G. Post-translational phosphorylation of phosvitin. FEBS Lett. 1973 Mar 1;30(2):167–169. doi: 10.1016/0014-5793(73)80643-x. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Isolation of lymphoma cell variants resistant to killing by glucocorticoids. Cell. 1974 Aug;2(4):213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. 8. The estrogen-induced hepatic synthesis of a serum lipophosphoprotein and its selective uptake by the ovary and trasformation into yolk platelet proteins in Xenopus laevis. Dev Biol. 1969 May;19(5):498–526. doi: 10.1016/0012-1606(69)90085-2. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Resolution and isolation of avian and amphibian yolk-granule proteins using TEAE-cellulose. Anal Biochem. 1965 May;11(2):297–311. doi: 10.1016/0003-2697(65)90018-7. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Studies on amphibian yolk. IX. Xenopus vitellogenin. Biochim Biophys Acta. 1970 Jul 21;215(1):176–183. doi: 10.1016/0304-4165(70)90400-9. [DOI] [PubMed] [Google Scholar]