Abstract
Hilar ectopic dentate granule cells (DGCs) are a salient feature of aberrant plasticity in human temporal lobe epilepsy (TLE) and most rodent models of the disease. Recent evidence from rodent TLE models suggests that hilar ectopic DGCs contribute to hyperexcitability within the epileptic hippocampal network. Here we investigate the intrinsic excitability of DGCs from humans with TLE and the rat pilocarpine TLE model with the objective of comparing the neurophysiology of hilar ectopic DGCs to their normotopic counterparts in the granule cell layer (GCL). We recorded from 36 GCL and 7 hilar DGCs from human TLE tissue. Compared with GCL DGCs, hilar DGCs in patient tissue exhibited lower action potential (AP) firing rates, more depolarized AP threshold, and differed in single AP waveform, consistent with an overall decrease in excitability. To evaluate the intrinsic neurophysiology of hilar ectopic DGCs, we made recordings from retrovirus-birthdated, adult-born DGCs 2–4 mo after pilocarpine-induced status epilepticus or sham treatment in rats. Hilar DGCs from epileptic rats exhibited higher AP firing rates than normotopic DGCs from epileptic or control animals. They also displayed more depolarized resting membrane potential and wider AP waveforms, indicating an overall increase in excitability. The contrasting findings between disease and disease model may reflect differences between the late-stage disease tissue available from human surgical specimens and the earlier disease stage examined in the rat TLE model. These data represent the first neurophysiological characterization of ectopic DGCs from human hippocampus and prospectively birthdated ectopic DGCs in a rodent TLE model.
Keywords: dentate gyrus, ectopic, intrinsic excitability, seizure, epileptogenesis
temporal lobe epilepsy (TLE) is the most common type of medically intractable epilepsy (Ramey et al. 2013). The mechanisms of TLE pathogenesis are not well understood, but the hippocampus is widely believed to be a critical structure for seizure development and progression. In some cases, patients with refractory TLE undergo surgical resection of the affected tissue, including the hippocampus, as a means of seizure suppression. This procedure provides the unique opportunity to study the neurophysiology of living human brain tissue. Previous work has demonstrated that resected hippocampal tissue from TLE patients displays physiological features consistent with hyperexcitability (Dietrich et al. 1999; Franck et al. 1995; Selke et al. 2006; Surges et al. 2012).
The hippocampal dentate gyrus is strongly implicated in epileptogenesis, largely because of its well-documented plasticity in TLE. Aberrant plasticity such as mossy fiber sprouting, persistent hilar basal dendrites, and ectopic location of adult-born dentate granule cells (DGCs) have all been implicated in hyperexcitability and have been described in both human and experimental TLE (de Lanerolle et al. 1989; Houser et al. 1990; Parent et al. 2006; Scharfman and Gray 2007; Sutula et al. 1989; von Campe et al. 1997). It has recently been suggested that, in rodent TLE models, hilar ectopic DGCs represent the subset of DGCs most predisposed to hyperexcitability in the epileptic network (Scharfman et al. 2000; Scharfman and Pierce 2012; Zhan et al. 2010). However, the intrinsic neurophysiology of hilar ectopic DGCs has not been studied in human tissue, nor has it been thoroughly characterized in rodent models. In this study, we compared intrinsic excitability of hilar ectopic DGCs to normotopic DGCs [those in the granule cell layer (GCL)] from human TLE tissue. We also compare birthdated hilar ectopic and normotopic DGCs in the rat pilocarpine status epilepticus (SE) model to assess alterations in intrinsic excitability in a more controlled experimental paradigm.
METHODS
Human tissues acquisition.
Surgical specimens were obtained from 24 patients (9 men, 15 women), ages 19–57 yr at surgery (mean 37.7 ± 11.4 yr), with mean epilepsy duration of 18.1 ± 12.1 yr. All patients had a history of failed anti-epileptic drug treatment, and 22 of the 24 were being treated by one or more of the following drugs at the time of surgery: lamotrigine, valproic acid, phenytoin, levetiracetam, zonisamide, carbamazepine, oxcarbazepine, phenobarbital, clonazepam, lorazepam, gabatril, felbamate, topiramate, lacosamide, gabapentin. Only two subjects had their anti-epileptic drugs tapered at the time of surgery. All patients participated after informed consent, in accordance with the University of Michigan Institutional Review Board.
Animals.
Animal procedures were performed using protocols approved by the University Committee on Use and Care of Animals of the University of Michigan. Animals were purchased from Charles River and kept under a constant 12:12 h light-dark cycle with access to food and water ad libitum. Epileptic animals and sham controls were generated as described previously (Kron et al. 2010). Briefly, 8-wk-old male Sprague-Dawley rats were pretreated with atropine methylbromide (5 mg/kg ip; Sigma-Aldrich, St. Louis, MO) 20 min prior to pilocarpine hydrochloride (340 mg/kg ip; Sigma-Aldrich) for epileptic animals, or an equivalent volume of 0.9% saline for sham animals. After 90 min of SE, seizures were terminated with diazepam (10 mg/kg ip; Hospira, Lake Forest, IL). In our hands, 90% (by observation) to 100% (by video/EEG recording) of animals treated according to this protocol exhibit spontaneous behavioral seizures after at least 1 wk of recovery. Sham controls were treated with diazepam 2 h after the saline injection. Four days after SE/sham treatment, animals were injected with green fluorescent protein (GFP)-expressing retrovirus (RV) bilaterally into the dentate gyrus as described previously (Kron et al. 2010). Acute slice recordings were made 2–4 mo after SE/sham treatment.
Slice preparation (human).
Tissue was acquired in the operating room from subjects undergoing anterior temporal lobectomy with amygdalo-hippocampectomy or selective amygdalo-hippocampectomy for medically refractory epilepsy. Clinical evaluation to localize the region of seizure onsets included neuroimaging and video-electroencephalographic ictal recordings with scalp and, in some cases, intracranial electrodes. During surgery, care was taken to maintain hippocampal perfusion until it was resected en bloc and immediately placed in ice cold Hibernate A (Brain Bits, Springfield, IL). All reagents for the following solutions were obtained from Fisher Scientific (Fair Lawn, NJ), unless otherwise specified. The tissue was transported on ice as quickly as possible (5–7 min) to the laboratory where it was transferred to ice-cold, oxygenated, cutting solution containing the following (mM): 206 sucrose, 2.8 KCl, 1 MgCl2·6H2O, 1.25 NaH2PO4, 1 CaCl2, 10 d-glucose, 26 NaHCO3, 0.4 ascorbic acid (pH 7.4). Slices (400 μm thickness) were cut perpendicular to the anterior-posterior axis with a vibrating blade microtome (VT1000S, Leica Microsystems, Buffalo Grove, IL), allowed to recover for 15 min in 34°C, oxygenated N-methyl-d-glutamine based solution containing (mM): 92 N-methyl-d-glutamine (Sigma Aldrich), 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate (Sigma Aldrich), 2 thiourea (Sigma, Aldrich), 3 sodium pyruvate (Gibco Life Technologies, Grand Island, NY), 10 MgSO4·7H2O (Sigma Aldrich), 0.5 CaCl2·2H2O (pH adjusted to 7.35 with 10N HCl). We find that recovery in this solution provides a significant improvement in the health of slices made from both rat and human tissue (Zhao et al. 2011). Slices were then rested for at least 1 h at room temperature in artificial cerebrospinal fluid (aCSF) containing the following (mM): 124 NaCl, 2.8 KCl, 2 MgSO4, 1.25 NaH2PO4, 2 CaCl2, 10 d-glucose, 26 NaHCO3, and 0.4 ascorbic acid (pH 7.4), before being transferred individually to the recording chamber and continuously perfused (∼1.5 ml/min) with oxygenated aCSF heated to 32°C.
Slice preparation (rodent).
Animals were anesthetized with isoflourane (Vet One, Boise, ID) and transcardially perfused with ice-cold cutting solution (as above) for 60 s. After decapitation, brains were rapidly removed and rested for 2 min in ice-cold, oxygenated cutting solution. Brains were then blocked to isolate the hippocampus, and 400-μm-thick sections were cut coronally with a vibrating blade microtome (VT1000S, Leica Microsystems, Buffalo Grove, IL) in ice-cold, oxygenated cutting solution, and from that point on were treated identically to human tissue slices.
Electrophysiological recordings and analysis.
Cells were visualized using epifluorescence and infrared differential interference contrast (IR-DIC) optics. For human neurons, only IR-DIC was used to visualize and patch. Rodent GFP+ DGCs were first identified under epifluorescence (525-nm emission filter), then visualized and patched using IR-DIC. Whole cell current clamp recordings were obtained using borosilicate glass electrodes (Sutter Instruments, Novato, CA) with a 4–7 MΩ open tip resistance. Pipettes contained 0.3% biocytin (Sigma Aldrich) in internal solution made with either (in mM) 120 potassium methyl sulfate (Sigma Aldrich) or 120 potassium gluconate (Sigma Aldrich), and 20 KCl, 10 HEPES, 0.2 EGTA, 2 MgCl·6H2O, 4 Na2 ATP, 0.3 Tris-GTP, and 7 phosphocreatine, pH adjusted to 7.25, with KOH. Recordings were obtained using a Dagan Cornerstone amplifier (Minneapolis, MN) in bridge mode, filtered at 2 kHz and digitized at 10 kHz. Data were acquired using pClamp 10.0. Synaptic stimuli were delivered via a glass theta electrode filled with aCSF and positioned in the molecular layer.
Seal resistances of >1 GΩ were achieved before breaking into whole cell mode. Resting membrane potential (RMP) was determined in each cell immediately after break in. Each neuron was sequenced through a series of current clamp protocols to determine input resistance, sag ratio, spike frequency accommodation (SFA), action potential (AP) properties, and postburst slow afterhyperpolarization (sAHP). Sag was determined by injecting hyperpolarizing steps up to −0.1 pA and calculated as the ratio of the steady-state response over the peak negative potential. To measure AP threshold and spike properties, short (10-ms) current steps were delivered at increasing amplitudes of 15 pA until a single AP was elicited. Threshold was defined as the membrane potential measured just before the spike upstroke. Spike height was measured from threshold to peak, and half-width was defined as the width (in ms) of the spike at half-maximal height. Input resistance and spike train firing properties were assessed by longer intracellularly injected current steps (−150 pA to 500 pA, 500 or 1,000 ms). Input resistance was determined by the slope of the linear portion of the voltage/current plot generated by all traces that did not contain AP firing. SFA index was determined in traces that contained four or more APs by dividing the interspike intervals by the interspike interval of the first two spikes in a given train. Cells were not all driven by the same levels of injected current. As a result, firing frequency of each group was determined by generating scatter plots of the data for every cell and fitting each data set with a Poisson regression line through the origin.
In a subset of cells, sAHP was measured by eliciting 5 or 6 APs in a 100-ms time period, with cells either at their resting potential or held at 5 mV below AP threshold. The magnitude of the sAHP was then determined by comparing the membrane potential immediately prior to the 100-ms current injection with the membrane potential 1 s after the end of the current step. Synaptic input was also assessed in a subset of cells by placing a stimulating electrode in the molecular layer and delivering stimulations of increasing magnitude, to elicit maximum excitatory postsynaptic potential response or AP firing in the postsynaptic cell.
Immunohistochemistry.
Following recording, slices were immediately placed in 4% paraformaldehyde (Sigma Aldrich) in phosphate-buffered saline (PBS) (pH 7.4) and refrigerated for up to 1 wk. For biocytin visualization, slices were rinsed with PBS, and endogenous peroxidase activity was quenched with 0.1% hydrogen peroxide in 10% methanol and PBS. After a second wash in PBS, slices were permeabilized with 2% Triton X-100 (Sigma Aldrich) in PBS and then incubated at room temperature in avidin/biotinylated enzyme complex (Vector Laboratories, Burlingame, CA). After 1–2 days, slices were rinsed with PBS and then reacted with 3,3′-diaminobenzidine (Invitrogen, Grand Island, NY) until the cells could be visualized. Slices were slide-mounted before cresyl violet counterstaining and coverslipping. Images were acquired on a Leica DSM-IRB inverted microscope (Leica Microsystems, Buffalo Grove, IL) connected to a SPOT Flex digital camera (SPOT Imaging Solutions, Sterling Heights, MI).
Statistics.
Off-line analysis was completed in Clampfit, and statistical comparisons were made using GraphPad Prism 6 software. RMP, input resistance, sag ratio, AP threshold, AP spike height, and AP half-width were compared between groups using one-way ANOVA with Tukey's post hoc test for multiple comparisons. Data are presented as means ± SE with the significance level set at P < 0.05. SFA distribution and single AP type distribution were compared between groups using Fisher's exact test with the significance level set at P < 0.05. Data are presented as contingency histograms. The slopes of the lines describing AP firing rate were generated using the nonlinear regression line through the origin function in Prism. The best-fit values for slope were compared using the extra sum of squares F-test with the significance level set at P < 0.05. Mean amplitudes of sAHP were compared using unpaired Student's t-test (human) or one-way ANOVA (rodent), with data presented as means ± SE, and the significance level set at P < 0.05.
RESULTS
In the present study, we investigated intrinsic neurophysiological properties of DGCs in tissue resected from subjects with intractable TLE, as well as from pilocarpine or sham-treated rats. Results from cells in human tissue are discussed first, followed by results from rodent cells.
Differentiating DGCs from interneurons.
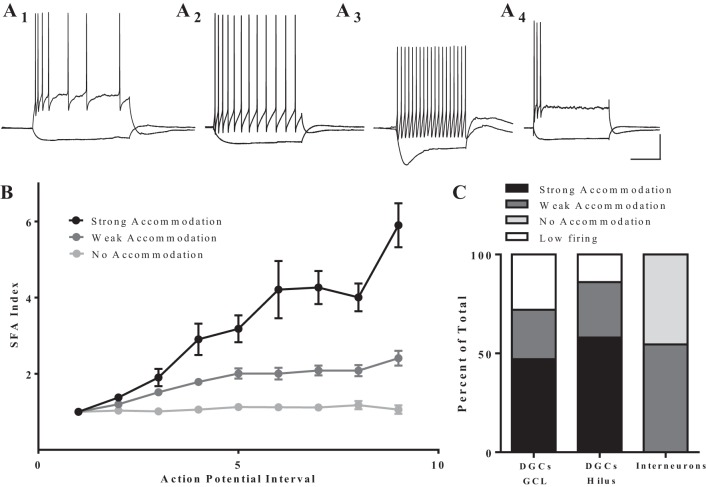
We used a set of neurophysiological criteria to distinguish DGCs from dentate interneurons, and DGC morphology and cell location were confirmed with biocytin staining in a subset of cases (Fig. 1). Intrinsic membrane properties are reported in Table 1. Neurophysiological characteristics that identify DGCs include SFA, a hyperpolarized RMP (more negative than −60 mV), and lack of sag current (Fournier and Crepel 1984; Fricke and Prince 1984; Staley et al. 1992). A SFA index value was calculated for each cell that fired at least four APs in response to depolarizing current steps (Fig. 2). This value is ∼1 for cells that do not accommodate and becomes larger as the degree of accommodation increases. In our data set, six cells showed no accommodation (SFA index 0.9–1.2) and were, therefore, classified as interneurons (Fig. 2A3). An additional five cells exhibited weak accommodation (SFA index 2.0–3.0), but had other features that did not fit the electrophysiological profile of a DGC, such as obvious sag current and depolarized RMP (less negative than −60 mV). These cells were also classified as interneurons; all 11 cells were excluded from further analysis. The remaining 43 cells in this data set fit the neurophysiological criteria for DGCs.
Fig. 1.
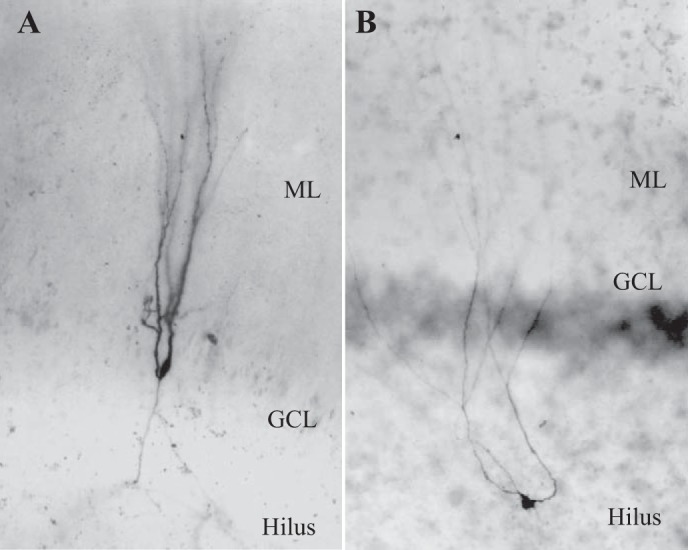
Biocytin filled cells display dentate granule cell (DGC) morphology. A: typical human DGC with soma in the granule cell layer (GCL), dendrites in the molecular layer (ML), and axon in the hilus. B: physiologically identified human DGC with hilar ectopic soma.
Table 1.
Passive membrane properties used to differentiate DGCs from interneurons and to investigate differences between hilar DGCs and those in the GCL
| GCL DGCs | Hilar DGCs | Interneurons | |
|---|---|---|---|
| n | 36 | 7 | 11 |
| Resting membrane potential*, mV | −70.47 ± 0.82 | −68.81 ± 1.96 | −64.18 ± 1.23‡ |
| Input resistance*, MΩ | 198.58 ± 15.43 | 153.32 ± 16.9 | 142.07 ± 57.04 |
| Sag ratio* | 1.12 ± 0.01 | 1.07 ± 0.04 | 1.25 ± 0.08‡ |
| Action potential threshold*, mV | −42.98 ± 1.45 | −22.87 ± 8.14‡ | −42.47 ± 2.51† |
| Action potential spike height*, mV | 79.69 ± 2.21 | 53.72 ± 11.37‡ | 69.96 ± 4.01 |
| Action potential half-width, ms | 0.99 ± 0.05 | 0.92 ± 0.13 | 0.77 ± 0.09 |
Values are means ± SE; n, no. of cells.
GCL, granule cell layer; DGC, dentate granule cells. Analyses were done using one-way ANOVA with Tukey's post hoc test for multiple comparisons. Action potential characteristics were measured from single-spike protocol.
Main effect determined by one-way ANOVA.
Significantly different from GCL with post hoc analysis.
Significantly different from hilus with post hoc analysis.
Fig. 2.
Cells display distinct spike frequency accommodation (SFA) patterns, which help to distinguish DGCs from interneurons. A: representative traces of firing pattern in response to a depolarizing step (500 ms). A1: strong accommodation (n = 21). A2: weak accommodation (n = 11). A3: no accommodation (n = 6). A4: low firing [did not fire more than 3 action potentials (APs) in a spike train at the highest level of stimulation]. For such cells (n = 11), SFA index could not be calculated. Scale bar: 200 ms, 25 mV. B: plot of SFA index vs AP interval. Strongly accommodating cells reached an SFA index value of at least 3 by the end of the spike train, while the value for weakly accommodating cells never exceeded 2.5, and the value for nonaccommodating cells never exceeded 1.2. Values are means ± SE. C: distribution of SFA types among DGCs in the GCL, DGCs cells in the hilus, and cells that do not fit physiological characterization of DGCs (putative interneurons). There was no significant difference in the distribution of firing patterns between DGCs in the hilus or the GCL (P = 0.75, Fisher's exact test).
SFA.
Proexcitatory changes in SFA have been reported in DGCs from human TLE tissue (Dietrich et al. 1999). In fact, it has been suggested that DGCs with a reduced SFA are an aberrant subpopulation of DGCs in TLE (Selke et al. 2006). Since ectopic location also defines an aberrant subpopulation of DGCs that is thought to be proexcitatory (Hester and Danzer 2013; Scharfman et al. 2000; Zhan et al. 2010), we asked whether ectopic DGCs show reduced SFA compared with normotopic DGCs in the GCL. We calculated SFA index values for all cells that fired 4 or more APs within a single current step; 17 GCL and 4 hilar neurons displayed strong accommodation (index value ≥ 3.0: Fig. 2, A1 and B), 9 GCL and 2 hilar neurons displayed weak accommodation (index value 2.0–3.0: Fig. 2, A2 and B). However, SFA index could not be calculated for 10 GCL and 1 hilar cells, which are called “low-firing” neurons because they could not be induced to fire more than 3 APs in response to prolonged injected current (Fig. 2A4). We observed no significant difference (P = 0.75, Fisher's exact test) in the distribution of firing patterns between DGCs in the hilus or the GCL (Fig. 2C).
Firing frequency.
A common method of assessing intrinsic excitability is to measure the number of APs fired by a cell in response to somatic current injection. We delivered current steps of increasing magnitude to DGCs in the hilus and GCL and compared their responses. There was considerable variation in the AP firing response and amount of injected current required to drive AP firing in individual DGCs, regardless of cell location. Poisson regression lines through the data from each group revealed that DGCs in the hilus exhibited a modest but statistically significant reduction in firing rates compare with DGCs in the GCL (slope of 0.01 ± 0.0014 vs. 0.023 ± 0.0012; P < 0.0001, extra sum of squares F-test; Fig. 3).
Fig. 3.
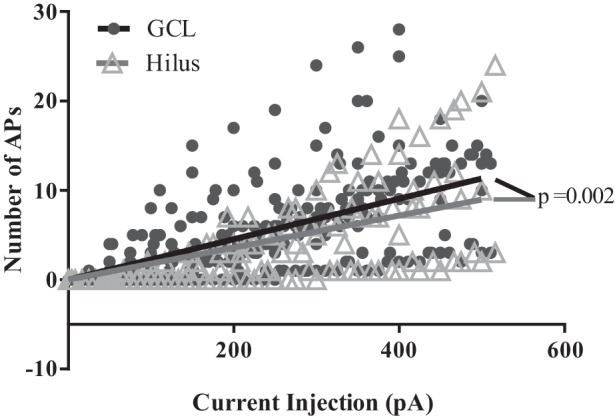
Hilar DGCs are slightly, but significantly, less excitable than DGCs in the GCL. All cells fire at least one AP in response to ≥300 pA of injected current. The solid black and gray lines were generated by fitting the respective scatter plot data with a Poisson regression line that passes through the origin. The slope of the regression line for hilar cells (0.01794 ± 0.001277) is significantly different from the slope of the line for GCL cells (0.02266 ± 0.0015, P = 0.0019, extra sum of squares F-test).
Postburst afterhyperpolarization.
Dentate granule neurons exhibit a postburst afterhyperpolarization (AHP) (Podlogar and Dietrich 2006; Tanner et al. 2011), and the slow component of the postburst AHP (sAHP) is thought to regulate firing rate and SFA in neurons (Faber and Sah 2003). Therefore, we hypothesized that the sAHP would be larger in ectopic relative to normotopic DGCs, and that this might account for the decreased firing frequency observed in ectopic DGCs. Subsets of cells (6 in the hilus and 30 in the GCL) were driven to fire at 50 Hz for 100 ms. The sAHP was measured at 1,000 ms after the offset of the current step. Notably, it has been suggested that DGCs in human tissue from TLE patients do not exhibit a sAHP (Dietrich et al. 1999). While we did not observe sAHP in 30% of cells tested, we did record a sAHP of at least −0.27-mV amplitude in the majority of cells. However, there was no difference in the mean sAHP amplitude between the two groups (P = 0.76, unpaired t-test) (Fig. 4). We observed some heterogeneity in the postburst AHP waveform among DGCs, but there was no difference in the amplitude of the medium AHP (mAHP) (measured at 250 ms after the current offset) between the two groups (P = 0.83, unpaired t-test) (Fig. 4).
Fig. 4.
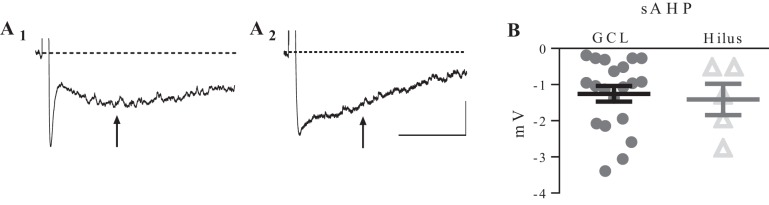
Cells in GCL and hilus exhibited a prominent postburst after hyperpolarizaion. A1: sample trace from a cell in the GCL. A2: trace from a cell in the hilus. Arrows indicate when the slow afterhyperpolarization (sAHP) component was measured. B: the average amplitude of the sAHP recorded from cells in the GCL (n = 30) and the hilus (n = 6) was not significantly different (P = 0.48 unpaired t-test). Values are means ± SE. Scale bars: 1 s, 2 mV.
Individual AP waveform.
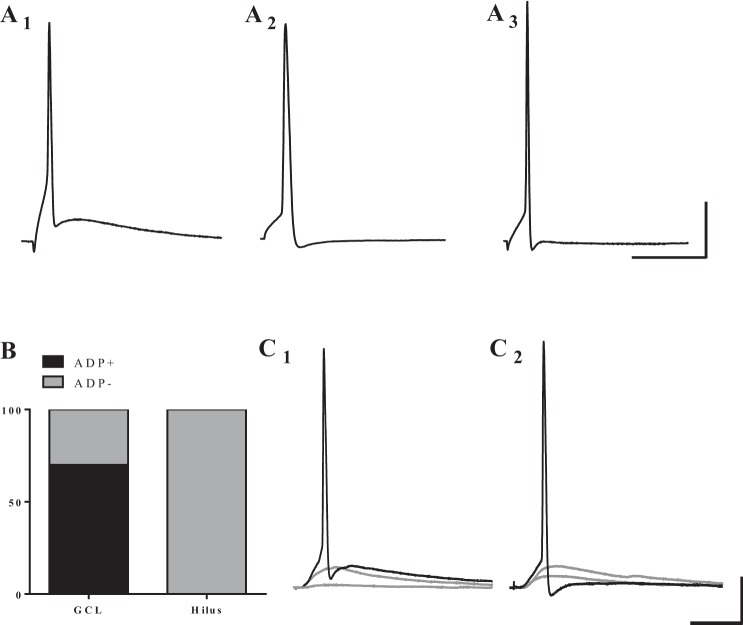
Although several reports have described the repetitive firing properties of human DGCs, there is relatively little data carefully evaluating single AP waveforms. Therefore, we investigated the properties of individual APs by eliciting a single spike with a 10-ms current injection at the AP threshold (Fig. 5). We observed two distinct shapes of AP traces: APs with (Fig. 5A1) or without (Fig. 5, A2 and A3) an afterdepolarization (ADP). Both AP waveforms were observed in cells in the GCL, with a majority of GCL cells exhibiting an ADP. However, AP traces from cells recorded in the hilus (Fig. 5B) all lacked ADP. In a subset of cells in both the hilus and GCL, molecular layer stimulation could drive a cell to fire a single AP. Interestingly, we observed distinct AP shapes with this type of stimulation as well (Fig. 5, C1 and C2).
Fig. 5.
Cells in the GCL exhibit two distinct single AP waveforms in response to short depolarizing pulses, while hilar DGCs all exhibit similar waveforms. A: example traces of APs elicited by direct, somatic current injection. Cells in the GCL display AP traces: with an afterdepolarization (ADP; A1), or without an ADP (A2). A3: hilar cells only exhibit APs without an ADP. B: distribution of cells that fired each type of AP classified by location. The majority of cells in the GCL fired APs with an ADP (25/36), while none of the 7 hilar cells exhibited an ADP. C: a subset of cells in both the GCL and hilus could be driven synaptically to fire single APs. Of these cells, some fired an AP with ADP (C1), and others fired an AP without ADP (C2). Scale bars: 25 ms and 20 mV.
Neurophysiology of ectopic DGCs in a rodent TLE model.
Hilar ectopic DGCs have been shown in multiple animal models of TLE to exhibit anatomical and physiological features of disease-related plasticity (Althaus and Parent 2014; Parent and Murphy 2008). By comparing recordings from ectopic DGCs to normotopic DGCs, we identified neurophysiological features that we believe are likely to represent disease-related plasticity in human TLE. These features include reduced firing frequency in response to injected current, a higher AP threshold, and an AP waveform without an ADP. Since the tissue included in this study came from patients with a wide range of disease duration (time from clinical diagnosis of TLE to surgical resection) (18.78 ± 12.10 yr), we examined whether disease duration correlated with differences in these neurophysiological features. Using Pearson correlation analysis, we found no correlation between the patients' disease duration and sAHP amplitude (R2 = 0.033, P = 0.29), firing rate (R2 = 0.039, P = 0.40), SFA index (R2 = 0.00057, P = 0.91) or ADP amplitude (R2 = 0.040, P = 0.24). However, in the absence of control tissue (e.g., from patients with extrahippocampal, nonepileptic lesions), we have limited ability to determine whether the observed differences are caused by disease-related plasticity in a subpopulation of DGCs, normal biological variability, or uncontrolled variables in our sample population.
To address these questions directly, we turned to the pilocarpine-induced SE model in adult male rats. This model allows comparison to nonseizure controls and avoids confounds present for human tissue. These confounds include variability in age of seizure onset and severity, medication history, sex, and birthdates of individual DGCs. DGCs are generated in the human and rodent brain throughout adulthood and into senescence (Eriksson et al. 1998; Kuhn et al. 1996). In rodent models of TLE, DGC age at the onset of epileptogenesis is a critical factor in determining the cell's response to the insult. Cells that are born after the epileptogenic insult show the greatest degree of morphological disease-related plasticity and are the only DGCs that migrate ectopically (Jessberger et al. 2007; Kron et al. 2010; Walter et al. 2007). Using the rodent model and retroviral reporter labeling to birthdate and prospectively identify specific DGC cohorts, we directly compared hilar ectopic DGCs with normotopic DGCs that were the same age with respect to SE, as well as to similar aged, birthdated cells in intact tissue.
Confirming DGC identity.
In animals that underwent SE, GFP-labeled cells (Kron et al. 2010) were patched from throughout the GCL and the hilus. In sham-treated animals, fluorescent cells were only present in the GCL. In a subset of experiments, DGC morphology and location was confirmed with biocytin staining (Fig. 6, A and B). All cells that were patched in rodent tissue exhibited neurophysiological features characteristic of DGCs, including SFA, hyperpolarized RMP, and lack of sag current (Table 2). In a subset of sham-treated animals, GFP-negative cells were patched from the GCL to determine whether the RV labeling affected the cells' intrinsic properties. We found that GFP-negative cells (n = 13) exhibited RMP of −75.79 ± 1.340, IR of 302.57 ± 33.30, were mostly low firing (10 out of 13), and had a prominent ADP. The values for GFP-negative cells were not significantly different from those of GFP-positive cells from sham animals (P > 0.05 for RMP and P > 0.05 for input resistance, Student's t-test). These data suggest that the RV-GFP does not alter the intrinsic neurophysiological properties of DGCs that were measured in this study.
Fig. 6.
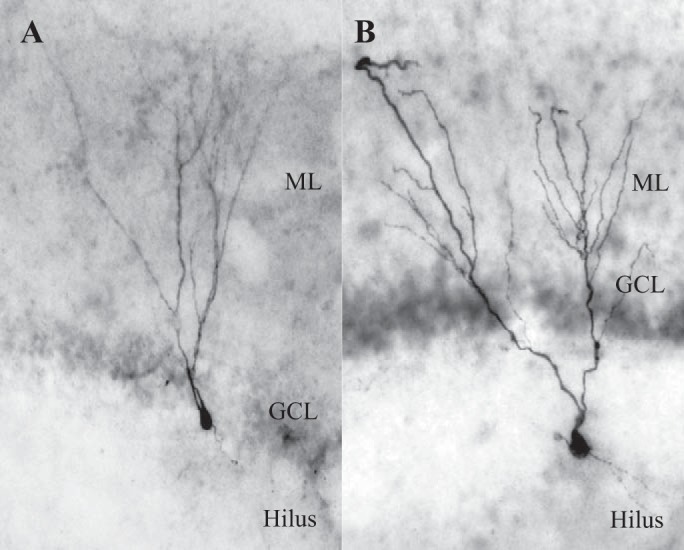
Biocytin filled cells display DGC morphology. A: typical rat DGC with soma in the GCL and dendrites in the ML. B: rat DGC with hilar ectopic soma.
Table 2.
Passive membrane properties of all cells recorded from rodents displayed features of DGCs
| Normotopic SE | Ectopic SE | Normotopic Sham | |
|---|---|---|---|
| n | 38 | 7 | 6 |
| Resting membrane potential*, mV | −71.62 ± 0.65 | −65.22 ± 3.15† | −71.57 ± 1.37 |
| Input resistance, MΩ | 282.50 ± 31.65 | 273.45 ± 31.75 | 226.0 ± 32.71 |
| Sag ratio | 1.005 ± 0.001 | 0.999 ± 0.002 | 1.003 ± 0.001 |
| Action potential threshold, mV | −40.79 ± 1.20 | −41.07 ± 2.59 | −37.87 ± 2.29 |
| Action potential spike height, mV | 72.04 ± 2.93 | 63.48 ± 4.84 | 67.49 ± 6.53 |
| Action potential half-width (ms)* | 0.99 ± 0.03 | 1.3 ± 0.14†‡ | 0.95 ± 0.07 |
Values are means ± SE; n, no. of cells. SE, status epilepticus. Ectopic DGCs had depolarized resting membrane potential and wider action potential than normotopic or sham DGCs, respectively. Analyses were done using one-way ANOVA with Tukey's post hoc test for multiple comparisons.
Main effect determined by one-way ANOVA.
Significantly different from normotopic SE with post hoc analysis.
Significantly different from sham with post hoc analysis.
Firing behavior in response to injected current steps.
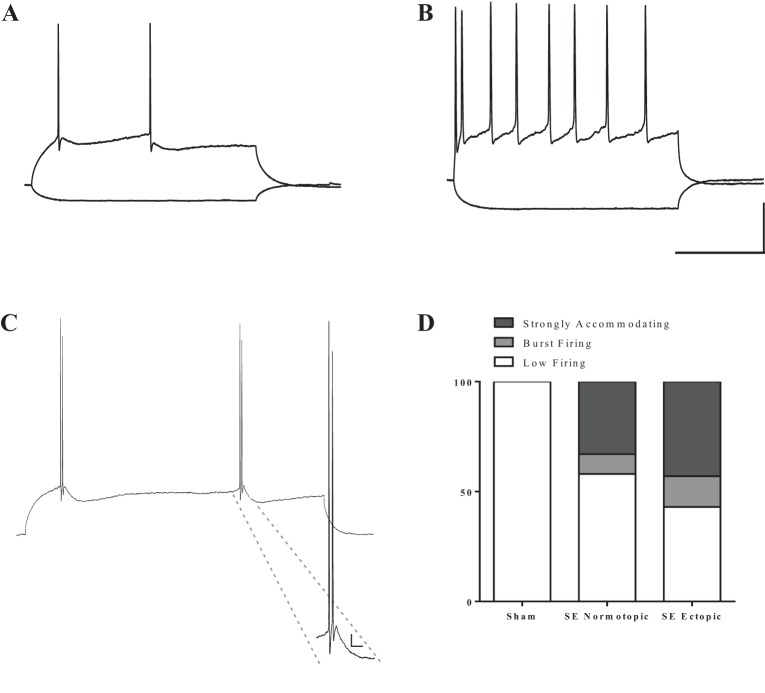
In total, we made successful recordings from 45 RV-GFP-labeled DGCs in SE rats and 6 labeled cells from sham-treated rats. Most DGCs (n = 30) from all groups were low firing (i.e., fired 3 AP or less even at the maximal amplitude of injected current). All adult-born DGCs from control tissue fired less than three AP in response to this stimulation intensity (Fig. 7, A and D). The DGCs from SE tissue that fired four or more APs in response to prolonged somatic current injection (n = 15) all exhibited strong accommodation, with a SFA index of at least 3.0 (Fig. 7B). Interestingly, a subset of both normotopic and ectopic cells from pilocarpine-treated animals fired unique AP doublets (Fig. 7C). These cells were classified as burst firing because the two APs were within 10 ms of one another and on the same depolarizing envelope (Metz et al. 2005). There was no significant difference in the distribution of low-firing, strongly accommodating, or burst firing cells between normotopic and ectopic DGCs in pilocarpine-treated animals (Fig. 7D, P = 0.73 Fisher's exact test).
Fig. 7.
All cells from rodent tissue display firing behavior characteristic of DGCs. A: sample trace from a low firing cell. These cells only fired up to 3 APs in response to injected current. B: sample trace from a cell with sustained firing in response to injected current. Cells that fired 4 or more APs always exhibited strong SFA (an index value of at least 3.0). Scale bars: 200 ms, 25 mV. C: a small subset of cells from epileptic tissue fired AP doublets, even in response to low levels of current injection. Inset presents a larger image of the doublet. These were classified as burst firing cells. Scale bar: 20 ms, 5 mV. D: distribution of firing patterns among cells in each group. There was no significant difference between the firing pattern distributions of normotopic vs. ectopic DGCs (P = 0.73 Fisher's exact test). SE, status epilepticus.
Firing frequency in response to somatic current injection.
We were surprised to find that ectopic DGCs in human tissue tended to fire fewer APs in response to injected current than normotopic DGCs, although there has been some recent evidence in rats that some cells born after an epileptogenic insult show an overall reduction in excitability (Jakubs et al. 2006). To determine whether hilar DGCs have lower firing rates than their age-matched normotopic counterparts, we compared the firing frequency of hilar ectopic and normotopic DGCs in tissue from rats that experienced SE (Fig. 8). DGCs from sham-treated animals were excluded from this analysis because none of these cells fired APs in response to current levels ≤ 130 pA. For each cell, we plotted the number of APs evoked by current injections of increasing amplitudes and fit the data from each group with Poisson regression lines through the origin. In contrast to the data from human TLE patients, ectopic DGCs displayed modest but significant increased firing rates compared with their normotopic counterparts (slope 0.032 ± 0.0038 vs 0.017 ± 0.0014; P < 0.0001, extra sum of squares F-test).
Fig. 8.
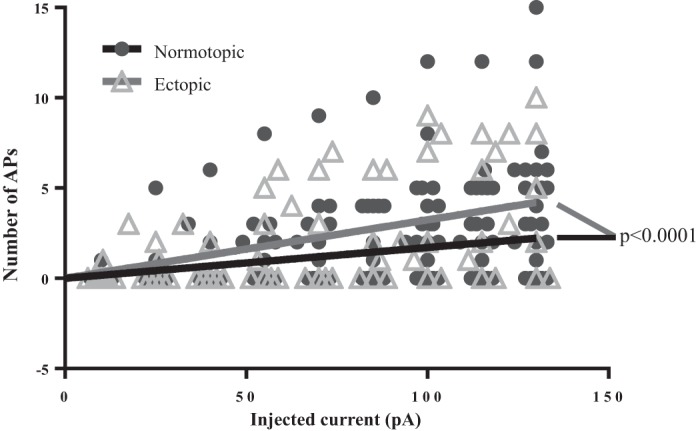
Ectopic DGCs from rodent SE tissue exhibit a moderate, but significant, increase in excitability compared with normotopic DGCs. Both normotopic and ectopic DGCs born after SE exhibit more excitability than age-matched DGCs from sham-treated animals. All cells in the sham group were low-firing cells, so only cells from SE-treated animals were included for comparison of firing frequency. The solid black and gray lines were generated by fitting the respective scatter plot data with a Poisson regression line that passes through the origin. The slope of the line for ectopic DGC firing (0.032 ± 0.0038) is significantly different from the slope of the line for normotopic DGC firing (0.017 ± 0.0014; P < 0.0001, extra sum of squares F-test).
Postburst sAHP.
Although sAHP was not a primary determinant of cell firing frequency in human DGCs, we wanted to assess whether the more controlled environment of the rodent tissue might reveal a relationship between sAHP and firing frequency. Postburst sAHP was recorded in a subset of cells from each group (3 sham, 27 normotopic, 6 ectopic; Fig. 9A). The waveform of the postburst AHP in rodent DGCs differed somewhat from that recorded in most human cells, but DGCs in both sham and SE-treated rats exhibited a prominent sAHP. In the rodent DGCs, as in human, the mean amplitudes of the sAHP were not significantly different between any of the groups (Fig. 9B; P = 0.31, one-way ANOVA).
Fig. 9.
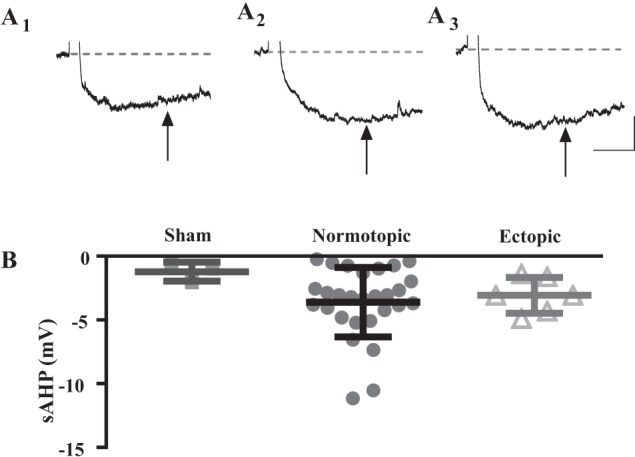
A subset of cells from each group exhibited postburst sAHP. Representative traces are from a cell from a sham-treated animal (A1), a normotopic cell from an SE-treated animal (A2), and an ectopic cell from an SE-treated animal (A3). B: there was no significant difference in the mean size of the postburst sAHP between any of the groups (P = 0.311, one-way ANOVA). All values are means ± SE. Scale bars: 500 ms, 2 mV.
Individual AP waveform.
When individual APs were evoked with brief current injections, we observed two distinct AP shapes in the recordings from rodent cells (Fig. 10, A1 and A2). However, in contrast to what was observed from human DGCs, cells without an ADP were much rarer in the rodent tissue. Only 1 in 6 sham cells, 4 in 38 normotopic, and 1 in 7 hilar cells lacked ADP. There was no difference between ectopic and normotopic DGCs, or between these groups and sham-treated DGCs, in the distribution of cells where the AP lacked an ADP (Fig. 10B) (P = 0.91, Fisher's exact test). Because ADP size is correlated with burst firing in other cell types (Metz et al. 2005), we measured the amplitude of the ADP, relative to RMP at 5 ms after the fast repolarization phase to determine whether there was a relationship in our data set between ADP size and burst firing. Interestingly, the mean amplitude of ADP for burst firing cells (25.11 ± 0.84) was significantly larger than the mean ADP amplitude for nonburst firing cells (15.39 ± 1.1) from SE tissue (P < 0.0001, unpaired t-test).
Fig. 10.
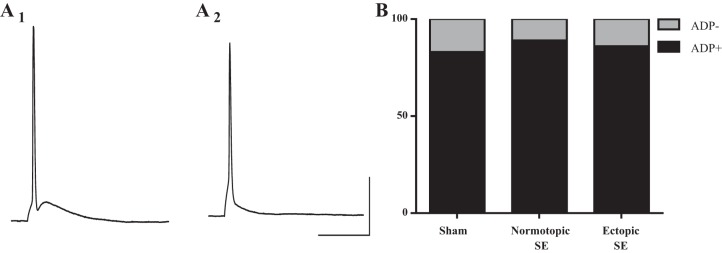
Two distinct shapes of single AP traces were observed in all three groups of rodent DGCs. Example trace showing AP: with a prominent ADP (A1) and without an ADP (A2). B: graph showing the distribution of single-spike AP type across all groups. There was no significant difference in the percentage of cells that fired each type of AP across any of the three groups (P = 0.91, Fisher's exact test). Scale bars: 20 ms, 25 mV.
DISCUSSION
The goal of this study was to determine whether hilar ectopic DGCs in TLE display altered intrinsic neurophysiological features compared with normotopic DGCs in the GCL. We examined ectopic DGCs from resected human TLE tissue and from pilocarpine rat TLE model tissue. The recordings from human neurons provided powerful data because they allowed us rare insight into living, diseased human brain function. However, the lack of human control tissue and uncontrolled variables somewhat limited the ability to draw definitive conclusions about the aberrant nature of neurophysiological differences observed among human DGCs. Combining human and rodent studies provided greater insight into the likely relationship between intrinsic neurophysiology in human DGCs and epileptogenesis.
The primary findings are that hilar DGCs in hippocampal tissue from epileptic patients tend to fire fewer APs in response to somatic current injection, have a more depolarized AP threshold, and lack an ADP after a single AP. These features all suggest decreased excitability. While this may seem counterintuitive, since disease-related plasticity is often thought of as being proepileptogenic, and therefore proexcitatory, there is evidence from multiple rodent models for the development of compensatory mechanisms that may reduce excitability after epileptogenesis (Jakubs et al. 2006; Peng et al. 2013; Zhan and Nadler 2009; Zhang et al. 2009). One possible explanation for the lower firing frequency in the human tissue is that ectopic DGCs represent a subset of DGCs that develop compensatory mechanisms to reduce excitability. However, our recordings from rat tissue suggest that hilar DGCs are actually more excitable than their normotopic counterparts, and normotopic DGCs in an SE animal are more excitable than their sham control counterparts. These results are consistent with previous reports suggesting ectopic DGCs from rodent tissue are more active (Dashtipour et al. 2001; Scharfman et al. 2000; Zhan and Nadler 2009).
This discrepancy between results from human and rodent cells may be due to species-related differences, but it could also stem from qualitative differences between the disease and the disease model. The pilocarpine SE model mimics severe epilepsy, but our recordings are made 2–4 mo post-SE, which is early in the chronic stage. Conversely, the patients who participated in this study had experienced seizures for 18.1 ± 12.3 yr and were refractory to multiple anti-seizure medications. Their tissue is likely reflective of the late stage of the disease. Perhaps hilar DGCs exhibit increased excitability early in the course of epilepsy, but over time develop compensatory or homeostatic mechanisms to intrinsically reduce their activity levels. One such mechanism might be a shift toward more depolarized AP threshold, which we observed in this population. Alternatively, hyperexcitable ectopic DGCs may initially be present during early epileptogenic stages, but might be selectively eliminated over the course of the disease, biasing the surviving population toward hypoexcitability. To our knowledge the intrinsic excitability of hilar ectopic DGCs in late-stage rodent model tissue remains unknown, and we believe this is an important area for further research. However, it is important to acknowledge the variability in firing rate among individual cells. In this data set from human tissue, DGCs in the GCL have the capacity to be more excitable than DGCs in the hilus. However, this does not necessarily mean that GCL DGCs increase overall network excitability and hilar cells decrease network excitability. Likewise, it is not necessarily true that only ectopic DGCs from rodent tissue contribute to increased network excitability. Rather, there may be cell autonomous factors, in addition to location, that contribute to the overall role of individual cells in modulating network properties.
The size of the sAHP is thought to play a role in regulating firing frequency in some neurons (Faber and Sah 2003). However, we found no difference in the sAHP amplitude between hilar and GCL DGCs in human, suggesting that the lower firing frequency is not correlated with a larger sAHP. Similarly, we found no relationship between sAHP amplitude and firing frequency in rodent DGCs: there was no significant difference in sAHP amplitude among control, normotopic-SE or ectopic-SE groups, and the sAHP amplitude did not correlate with number of APs generated, regardless of cell location.
We observed considerable qualitative variability in the shape of the AHP waveform among human DGCs, which was far less prevalent among rodent DGCs. The AHP of most human DGCs had a fast peak (as seen in Fig. 4, A1 and A2), some had a prominent medium peak (as seen in Fig. 4A1), and 70% had a slow component. In contrast, the AHP of nearly all rodent DGCs examined lacked discernible fast and middle peaks, but had a slow component (as seen in Fig. 9, A1–A3). Our data suggest the possibility of calcium dysregulation as an underlying factor in the observed physiological variability and uncoupling of AHP and firing frequency. Calcium and calcium-activated potassium conductances regulate numerous aspects of neuronal firing, including the postburst AHP (Faber and Sah 2003). However, the relative heterogeneity among human DGCs, which is not present among rodent DGCs, suggests that these cells experience dysregulated calcium homeostasis in a variety of cell autonomous ways. For example, small-conductance Ca2+-activated K (SK) channels are important in some cell types for producing the postburst mAHP (Faber and Sah 2003), which varies considerably among cells in our data set. Large-conductance Ca2+-activated K (BK) channels, which also contribute to the AHP (Faber and Sah 2003), are altered in a rodent model of epilepsy (Pacheco Otalora et al. 2008). Therefore, it is reasonable to hypothesize that SK and BK channels could both be differentially expressed among individual DGCs.
Previous reports using tissue from human TLE patients have implicated changes in SFA as a feature of disease-related plasticity (Dietrich et al. 1999; Selke et al. 2006). Nonepileptic rodent and human DGCs (DGCs from tissue that was resected due to tumors) typically exhibit strong accommodation (Selke et al. 2006; Staley et al. 1992). Dietrich et al. and Selke et al. found that, in human TLE tissue, some DGCs exhibited weak or no SFA, which they attributed to loss of interspike mAHP. We also found that some human DGCs were weakly accommodating; however, we rarely observed interspike mAHPs, even when cells exhibited strong accommodation. Interestingly, there was no difference in distribution of weakly and strongly accommodating cells between hilar and GCL DGCs, suggesting that a weak SFA may not be an identifying feature of the most aberrant subset of DGCs from human TLE tissue. We did, however, find a trend toward a positive correlation (R2 = 0.115, P = 0.078, Pearson correlation analysis) when we examined the relationship between sAHP and SFA index for individual cells, irrespective of cell location. This suggests that the postburst sAHP may contribute to regulating SFA in human DGCs in TLE. Interestingly, SFA of rodent DGCs was less heterogeneous; all exhibited strong accommodation or low firing activity. This suggests that a change in SFA is not a necessary feature of an epileptic network. However, it is also possible that altered SFA is an important feature of TLE in humans that the rat pilocarpine model fails to recapitulate.
Hilar DGCs from human TLE hippocampal specimens all exhibited a single AP waveform that lacked a prominent ADP, while the majority of GCL DGCs exhibited an AP waveform with an ADP. Previous work characterizing the AP of DGCs from other species suggested that, under normal conditions, AP waveforms from nearly all DGCs contain a prominent ADP (Aradi and Holmes 1999; Dudek et al. 1976; Zhang et al. 1993). As such, an AP waveform that lacks ADP may represent a shift from normal neurophysiology. In sham-treated rats, the majority of cells exhibited an AP waveform with a prominent ADP. Interestingly, a comparable percentage of both normotopic and ectopic DGCs from epileptic rodents also exhibited an ADP+ AP waveform. Therefore, the chronic epilepsy phase in rodents does not appear to be associated with an altered DGC ADP following a single AP.
It is important to note that ADP+ and ADP− denote only the most obvious differences in the AP waveforms that we observed. There was considerable qualitative variability in the shape of the AP waveforms within each ADP-defined group, which was most pronounced among DGCs from human tissue. Similar to the variability observed in the human AHP waveforms, we propose that dysregulation of calcium conductances underlies the heterogeneity in AP waveforms from human DGCs. In intact rodents, the ADP is primarily generated by T-type calcium channels (Zhang et al. 1993). However, the shape of the ADP can be influenced by other calcium and calcium-activated channels. Using computational modeling to study the complement of calcium and calcium-activated currents that could produce DGC firing behavior, Aradi and Holmes (1999) confirmed that T-type channels are critical for generating an ADP+ AP waveform, but also found that other combinations of L-type, N-type, BK and SK channels regulated ADP waveforms in ways that closely mimic many of the shapes we observed in the human DGCs. Importantly, calcium is not alone in regulating the ADP. Persistent sodium current can influence the ADP in some cell types, and it is altered in both clinical and experimental TLE (Agrawal et al. 2003; Azouz et al. 1996; Chen et al. 2011; Vreugdenhil et al. 2004).
In DGCs from rodent tissue, epilepsy-related changes in ADP could be driving the burst firing that we observed in a subset of neurons. About 15% of ectopic and normotopic cells from pilocarpine-treated rat tissue fired AP doublets (in some cases triplets). This type of burst firing has been described previously, but was only found in hilar ectopic DGCs and was thought to be related to dendritic morphology or the age of the individual cell (Zhan and Nadler 2009). Cells born after SE in the rodent model are the most likely to develop aberrant features and are the only group susceptible to ectopic migration (Kron et al. 2010). As a result, the ectopic cells from the prior study would all have been born after SE, while the normotopic cells could have been born well before SE. Our method of birthdating adult-born DGCs provides high confidence that they were all generated after the onset of epileptogenesis and were at least 8 wk old and functionally mature at the time of recording. This suggests that the age of the cell with respect to SE, rather than the cell's location, may be the more important factor in its propensity for burst firing. This propensity for burst firing might be driven by an increase in T-type calcium channels, similar to what has been reported for CA1 pyramidal cells in a rat model of TLE (Su et al. 2002).
Although some of the intrinsic properties of DGCs in the rodent epileptic tissue were less heterogeneous than DGCs from human tissue, important epilepsy-related changes were present among rodent DGCs. Recordings from the rodent hippocampus revealed that the birthdated DGCs all displayed strong SFA or low firing activity, similar size and shape of postburst AHP, and most exhibited a single AP waveform that included a large ADP. However, notable differences between DGCs of sham-treated and SE animals were an increase in firing rate, the emergence of burst firing, and a more depolarized RMP. These differences all point to an increase in intrinsic excitability of DGCs born after SE compared with adult-born DGCs in a sham-treated animal.
In summary, we have found that hilar ectopic DGCs from human TLE tissue were less excitable than normotopic DGCs in the GCL. Interestingly, we found the opposite relationship in pilocarpine-treated rat tissue; hilar ectopic DGCs were more excitable than normotopic DGCs, and, furthermore, normotopic DGCs were more excitable than DGCs from sham-treated rats. We believe the discrepancies between results from the human disease and the disease model are related, at least in part, to the different stages of the disease. Human tissue was obtained from patients who had experienced epilepsy for many years, sometimes decades, and represents the late stage of disease. The rat model is designed to mimic severe epilepsy, but recordings were made early in the chronic stage of the disease. Unfortunately, due to the relatively small sample size, our human tissue data set was not amenable to a subgroup analysis of subjects with the shortest epilepsy durations. Furthermore, it is not clear how disease progression in rodent models compares to that in humans.
The discrepancies may also result from unresolved species-related differences or limitations of the animal model. Perhaps DGCs from healthy human tissue are naturally more heterogeneous than DGCs from rodent tissue. Alternatively, human DGCs may exhibit features of disease-related plasticity that rodent DGCs would not exhibit, regardless of the stage of disease. Rodent models are more homogenous by design, but there is still heterogeneity present among individual animals within any given model. More data from later stage epileptic rodents and from patients earlier in the disease course would help determine whether animal models can recapitulate the same level of heterogeneity seen in the human tissue.
GRANTS
The project described was supported by the National Center for Research Resources, Grant UL1-RR-024986, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000433 [the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH)] through the Michigan Institute for Clinical and Health Research, CURE (Citizens United for Research in Epilepsy), and NIH NS-058585. A. L. Althaus was supported by the Training Program in Organogenesis T32-HD-007505, the National Institute of Mental Health Research Training-Biological Sciences T32-MH014279, and the Epilepsy Foundation Predoctoral Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A. and G.G.M. performed experiments; A.A. and G.G.M. analyzed data; A.A., J.M.P., and G.G.M. interpreted results of experiments; A.A. prepared figures; A.A. and G.G.M. drafted manuscript; A.A., O.S., J.M.P., and G.G.M. edited and revised manuscript; A.A., O.S., J.M.P., and G.G.M. approved final version of manuscript; O.S., J.M.P., and G.G.M. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Helen Zhang for the production of high-titer GFP retrovirus, without which the rat experiments would not have been possible. The authors also express our deepest gratitude to the patients who consented to allow us to use their tissue for these experiments.
REFERENCES
- Agrawal N, Alonso A, Ragsdale DS. Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia 44: 1601–1604, 2003. [DOI] [PubMed] [Google Scholar]
- Althaus AL, Parent JM. Role of adult neurogenesis in seizure-induced hippocampal remodeling and epilepsy. In: Endogenous Stem Cell-Based Brain Remodeling in Mammals, edited by Junier M.-P., Kernie SG. Boston, MA: Springer, 2014, p. 87–104. [Google Scholar]
- Aradi I, Holmes WR. Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. J Comput Neurosci 6: 215–235, 1999. [DOI] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol 492: 211–223, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Su H, Yue C, Remy S, Royeck M Sochivko D, Opitz T, Beck H, Yaari Y. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J Neurophysiol 105: 117–129, 2011. [DOI] [PubMed] [Google Scholar]
- Dashtipour K, Tran PH, Okazaki MM, Nadler JV, Ribak CE. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res 890: 261–271, 2001. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 495: 387–395, 1989. [DOI] [PubMed] [Google Scholar]
- Dietrich D, Clusmann H, Kral T, Steinhauser C, Blumcke I, Heinemann U, Schramm J. Two electrophysiologically distinct types of granule cells in epileptic human hippocampus. Neuroscience 90: 1197–1206, 1999. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Deadwyler SA, Cotman CW, Lynch G. Intracellular responses from granule cell layer in slices of rat hippocampus: perforant path synapse. J Neurophysiol 39: 384–393, 1976. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317, 1998. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 9: 181–194, 2003. [DOI] [PubMed] [Google Scholar]
- Fournier E, Crepel F. Electrophysiological properties of dentate granule cells in mouse hippocampal slices maintained in vitro. Brain Res 311: 75–86, 1984. [DOI] [PubMed] [Google Scholar]
- Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia 36: 543–558, 1995. [DOI] [PubMed] [Google Scholar]
- Fricke RA, Prince DA. Electrophysiology of dentate gyrus granule cells. J Neurophysiol 51: 195–209, 1984. [DOI] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci 33: 8926–8936, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci 10: 267–282, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52: 1047–1059, 2006. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci 27: 9400–9407, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci 30: 2051–2059, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz AE, Jarsky T, Martina M, Spruston N. R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons. J Neurosci 25: 5763–5773, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Hernandez EF, Arshadmansab MF, Francisco S, Willis M, Ermolinsky B, Zarei M, Knaus HG, Garrido-Sanabria ER. Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res 1200: 116–131, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol 59: 81–91, 2006. [DOI] [PubMed] [Google Scholar]
- Parent JM, Murphy GG. Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia 49, Suppl 5: 19–25, 2008. [DOI] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci 33: 14392–14405, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlogar M, Dietrich D. Firing pattern of rat hippocampal neurons: a perforated patch clamp study. Brain Res 1085: 95–101, 2006. [DOI] [PubMed] [Google Scholar]
- Ramey WL, Martirosyan NL, Lieu CM, Hasham HA, Lemole GM Jr, Weinand ME. Current management and surgical outcomes of medically intractable epilepsy. Clin Neurol Neurosurg 115: 2411–2418, 2013. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 20: 6144–6158, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia 48, Suppl 2: 33–41, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Pierce JP. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia 53, Suppl 1: 109–115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selke K, Muller A, Kukley M, Schramm J, Dietrich D. Firing pattern and calbindin-D28k content of human epileptic granule cells. Brain Res 1120: 191–201, 2006. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Otis TS, Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol 67: 1346–1358, 1992. [DOI] [PubMed] [Google Scholar]
- Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, Beck H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci 22: 3645–3655, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges R, Kukley M, Brewster A, Ruschenschmidt C, Schramm J, Baram TZ, Beck H, Dietrich D. Hyperpolarization-activated cation current Ih of dentate gyrus granule cells is upregulated in human and rat temporal lobe epilepsy. Biochem Biophys Res Commun 420: 156–160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 26: 321–330, 1989. [DOI] [PubMed] [Google Scholar]
- Tanner GR, Lutas A, Martinez-Francois JR, Yellen G. Single K ATP channel opening in response to action potential firing in mouse dentate granule neurons. J Neurosci 31: 8689–8696, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Campe G, Spencer DD, de Lanerolle NC. Morphology of dentate granule cells in the human epileptogenic hippocampus. Hippocampus 7: 472–488, 1997. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Hoogland G, van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci 19: 2769–2778, 2004. [DOI] [PubMed] [Google Scholar]
- Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci 27: 7541–7552, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol 102: 670–681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Timofeeva O, Nadler JV. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol 104: 3293–3304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Valiante TA, Carlen PL. Contribution of the low-threshold T-type calcium current in generating the post-spike depolarizing afterpotential in dentate granule neurons of immature rats. J Neurophysiol 70: 223–231, 1993. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci 29: 14247–14256, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 8: 745–752, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]