Abstract
Surround suppression is a well-known example of contextual interaction in visual cortical neurophysiology, whereby the neural response to a stimulus presented within a neuron's classical receptive field is suppressed by surrounding stimuli. Human psychophysical reports present an obvious analog to the effects seen at the single-neuron level: stimuli are perceived as lower-contrast when embedded in a surround. Here we report on a visual paradigm that provides relatively direct, straightforward indices of surround suppression in human electrophysiology, enabling us to reproduce several well-known neurophysiological and psychophysical effects, and to conduct new analyses of temporal trends and retinal location effects. Steady-state visual evoked potentials (SSVEP) elicited by flickering “foreground” stimuli were measured in the context of various static surround patterns. Early visual cortex geometry and retinotopic organization were exploited to enhance SSVEP amplitude. The foreground response was strongly suppressed as a monotonic function of surround contrast. Furthermore, suppression was stronger for surrounds of matching orientation than orthogonally-oriented ones, and stronger at peripheral than foveal locations. These patterns were reproduced in psychophysical reports of perceived contrast, and peripheral electrophysiological suppression effects correlated with psychophysical effects across subjects. Temporal analysis of SSVEP amplitude revealed short-term contrast adaptation effects that caused the foreground signal to either fall or grow over time, depending on the relative contrast of the surround, consistent with stronger adaptation of the suppressive drive. This electrophysiology paradigm has clinical potential in indexing not just visual deficits but possibly gain control deficits expressed more widely in the disordered brain.
Keywords: visual cortex, electroencephalography, steady-state visual evoked potential, inhibition, contextual interactions
neuronal responses to visual stimuli are influenced in many ways by stimulation of surrounding areas (Allman et al. 1985; Carandini 2004). Such contextual interactions have always been a pervasive topic in vision science, but more recently have also become a focus of increasing interest in clinical research, for example on schizophrenia (Dakin et al. 2005; Seymour et al. 2013), migraine (Battista et al. 2011), depression (Golomb et al. 2009) and autism (Foss-Feig et al. 2013). One particularly widely observed contextual effect is that of surround suppression, where responses to stimuli placed within a neuron's classical receptive field are inhibited by stimuli placed beyond it (Blakemore and Tobin 1972). This effect has been best characterized in single-cell visual studies in animals and tends to be strongest when surround stimulus features such as orientation and drift direction match those of the central stimulus (Cavanaugh et al. 2002a, 2002b; Deangelis et al. 1994; Levitt and Lund 1997). In human psychophysics, an analogous perceptual effect is observed, whereby a central (or “foreground”) stimulus is perceived as having lower contrast under the presence of a surround, and again the relative features of center and surround strongly influence the effect (Chubb et al. 1989; Petrov et al. 2005).
Functional magnetic resonance imaging (fMRI), whose spatial resolution enables segregation of distinct visual areas, has also revealed surround suppression effects (e.g., Tajima et al. 2010) and has been used to show that surround-induced activation modulations in primary visual cortex (V1), but not higher areas V2 or V3, can quantitatively account for psychophysical reports (Zenger-Landolt and Heeger 2003). However, not all fMRI effects mirror psychophysical effects; for example, whereas psychophysical suppression effects have been found to be stronger at peripheral than foveal locations (Xing and Heeger 2000), the opposite has been observed in fMRI (Williams et al. 2003).
A considerable number of human electroencephalography (EEG) studies have examined aspects of contextual interaction in the visual system (Appelbaum et al. 2008; Joo et al. 2012; Joo and Murray 2014; Polat and Norcia 1996; Xiao and Wade 2010; Zemon and Ratliff 1982). However, few have directly examined spatial surround suppression effects in the prototypical paradigm employed in animal neurophysiology, human psychophysics and fMRI, where luminance-modulated grating stimuli are fully embedded within spatially extended, surrounding patterns of comparable properties (e.g., Ohtani et al. 2002).
Our goal was to design and validate a visual paradigm that is based on this prototypical configuration and evokes robust indices of surround suppression from early visual cortex by exploiting anatomical and signal-summation principles (Vanegas et al. 2013), and to link the resultant modulation patterns with psychophysical reports from the same individuals. We presented flickering foreground stimuli within full-screen, static surround patterns and tested whether the suppressive response modulations exhibit orientation specificity, retinal location effects (foveal vs. peripheral), and effects of temporal adaptation. Our approach yields robust and simply-derived electrophysiological markers of surround suppression in humans that may hold promise in inferring excitatory-inhibitory imbalances in clinical populations through both temporal (contrast adaptation) and spatial (surround suppression) aspects of gain control.
MATERIALS AND METHODS
Subjects.
EEG data were recorded from 21 healthy subjects between 22 and 32 yr old (8 women). All participants reported normal or corrected-to-normal vision and no history of neurological disorders. Informed consent was obtained prior to their participation, and all experimental procedures were approved by the Institutional Review Board of The City College of New York. Three participants were excluded from analysis, one on account of trigger loss due to technical problems, one due to excessive drift related to perspiration, and one on account of excessive blinking detected in more than 40% of trials. This left a pool of 18 suitable data sets for full analysis, in each of which blinks were detected in less than 20% of trials.
Stimuli and procedure.
The procedure was conducted inside a dark, sound-proof and radio-frequency interference shielded room. Stimuli were presented dichoptically on a gamma-corrected CRT monitor (Dell M782) with a refresh rate of 100 Hz and 800 × 600 resolution, at a viewing distance of 57 cm. Stimulus presentation and psychophysics staircases were programmed in MATLAB (6.1, The MathWorks, Natick, MA, 2000), using the PsychToolbox extension (Brainard 1997; Pelli 1997). In the electrophysiological recording sessions, subjects were simply instructed to maintain fixation on a small, white, central fixation spot presented for the duration of each trial, and to try to restrict blinking to the 500-ms intertrial interval in which the fixation spot was extinguished. Eye gaze was monitored continually using an EyeLink 1000 (SR-Research) eye tracker (see Fig. 2A for fixation maps).
Fig. 2.
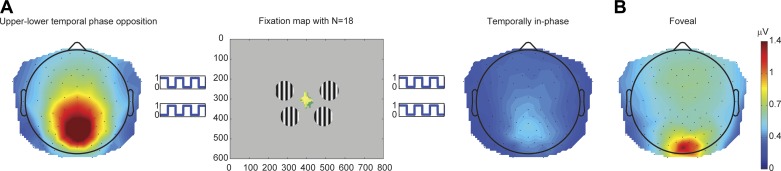
Group average topographies of 25-Hz steady-state visual evoked potential (SSVEP) amplitude for 100% FG and 0% surround. A: peripheral stimulus. Left: SSVEP amplitude topography when a temporal phase difference of 180° was applied between upper and lower flickering disks. Right: topography for the same condition but with in-phase flicker (configuration 3). Phase opposition brought about a strong enhancement for both this 25-Hz case and for 7.14-Hz flicker (configuration 5). In the center, the average fixation map for each individual is superimposed on the stimulus display schematic. B: SSVEP amplitude topography for foveal stimulation (configuration 4). Maximal SSVEP amplitude was elicited at electrode POz for peripheral stimulation and Oz for foveal. SSVEP amplitude measurements were taken from the appropriate frequency bin in a 2.24-s Fourier transform.
Our paradigm consisted of a sequence of discrete trials in which a “foreground” stimulus composed of either one or four vertically-oriented circular gratings flickered at a certain frequency within a nonflickering (full-screen) static “surround” (see Fig. 1). The flickering foreground elicits a steady-state visual evoked potential (SSVEP) in the EEG over posterior scalp at the fundamental frequency of stimulation, whose amplitude increases monotonically with foreground contrast (see, e.g., Lauritzen et al. 2010). Surround suppression was measured as a relative reduction in amplitude of the SSVEP due to surround contrast. Each trial began with the presentation of the fixation spot for 500 ms, after which the foreground and surround stimuli were simultaneously presented for 2,400 ms, with the foreground stimulus flickering on and off at either 25 or 7.14 Hz (see Table 1 for a list of all configurations), while the surround remained static. Foreground and surround patterns were sinusoidal luminance-modulated gratings with a spatial frequency of 1 cycles/° in all conditions. The average luminance of all gratings was equal to that of the blank gray, 0% contrast surround, which was 65 cd/m2 (one-half the maximum luminance of our monitor).
Fig. 1.
Example stimulus configurations and contrasts. In all configurations, the foreground (FG) stimulus flickered on and off on a static surround (SS). A: peripheral FG configuration, here shown on 0% contrast, blank midgray surround. B: peripheral FG with parallel, spatially in-phase and opposite-phase SS (configurations 1, 3, 5 and 6 in Table 1 with 100% FG and 50% SS in this example). C: same as B but with orthogonal SS (configuration 2). D: foveal FG on spatially in-phase (left) and opposite-phase (right), parallel SS (configurations 4 and 7 with 100% FG and 50% SS).
Table 1.
Settings for each stimulus configuration
| Configuration No. | FG Contrast, % | Static Surround Contrast, % | Surround Spatial Phase (Relative To FG), ° | Surround Orientation (Relative to FG), ° | Position | Upper-Lower Temporal Phase Difference, ° | Flickering Frequency, Hz |
|---|---|---|---|---|---|---|---|
| 1 | [0, 25, 50, 75, 100] | [0, 50, 100] | 0, 180 | 0 | Peripheral | 180 | 25 |
| 2 | [0, 25, 50, 75, 100] | [0, 50, 100] | 0, 180 | 90 | Peripheral | 180 | 25 |
| 3 | [50, 100] | [0, 100] | 0, 180 | 0 | Peripheral | 0 | 25 |
| 4 | [50, 100] | [0, 100] | 0, 180 | 0 | Foveal | n/a | 25 |
| 5 | [50, 100] | [0, 100] | 0, 180 | 0 | Peripheral | 180 | 7.14 |
| 6 | [50, 100] | [0, 100] | 0, 180 | 0 | Peripheral | 0 | 7.14 |
| 7 | [50, 100] | [0, 100] | 0, 180 | 0 | Foveal | n/a | 7.14 |
A total of 100 conditions with various contrast combinations, phase alignment, and frequency/position configuration were randomly interleaved. Each of seven possible configurations had a particular position (peripheral or foveal), surround orientation, temporal phase difference, and flicker frequency (7.14 or 25 Hz). FG, foreground; n/a, not applicable.
We tested the effects of surround suppression under several different stimulus configurations with the dual purpose of facilitating comparison with previous psychophysical, neurophysiology and neuroimaging studies, and determining the most effective stimulation parameters for robust quantification of visual suppression in scalp EEG (Table 1). In the main stimulus configuration in which we expected to observe maximal suppression, the foreground stimulus consisted of four peripheral disks with radius 2° each, seamlessly cut out of a full-screen vertical grating, with all aspects of the foreground and surround matching, including spatial phase and orientation (Fig. 1B).
We strategically placed the disks at four locations that, on a population-average level, produce robust “C1” components (the initial deflection of the transient visual evoked potential; Ales et al. 2013; Di Russo et al. 2002; Kelly et al. 2013), as well as SSVEPs that are inverted in polarity for the upper and lower visual fields (Di Russo et al. 2007). Consistent with previous measurements (see also Di Russo et al. 2002; Vanegas et al. 2013), these were at polar angles of 20° above (upper) and 45° below (lower) the horizontal meridian at an eccentricity of 5° of visual angle. On the basis of recent work in which we demonstrated dramatic improvements in SSVEP signal-to-noise ratio (SNR), we flickered the upper disks with opposite temporal phase relative to the lower disks in the foreground, causing oscillatory summation on the scalp because of the cortical surface orientation of early retinotopic visual areas (Vanegas et al. 2013). To confirm that this phase opposition technique provides more robust signals in the current paradigm, we also tested a version of the same disks configuration with temporally in-phase stimulation of the upper and lower locations with a parallel surround (Table 1, configurations 3 and 6). Topographies are presented in Fig. 2A, showing a striking enhancement of the SNR for the opposite-phase flicker between upper and lower disks.
To test for the influence of orientation differences between foreground and surround, we included a configuration where the surround was horizontally oriented and thus orthogonal to the foreground (configuration 2 in Table 1; Fig. 1C). To test for the influence of retinal position on suppression effects, we also included configurations in which a single, flickering foreground disk (radius 2°) was centered at fixation (“foveal” configurations 4 and 7). Finally, we additionally examined a lower temporal flicker frequency of 7.14 Hz (configurations 5–7). We tested at least two foreground (50, 100%) and two surround contrasts (0, 100%) for all configurations. In just the peripheral high-frequency configurations (both parallel and orthogonal), we tested additional foreground levels of 0, 25, and 75% and the additional surround level of 50% to generate finer-grained contrast-response functions (see Table 1).
In the parallel configurations, although the gratings are vertically oriented and thus are luminance-modulated in the horizontal dimension only, some temporal contrast modulation in the vertical dimension can also contribute to the measured SSVEP due to the seamless border between the foreground and surround. In the case of spatially in-phase foreground and surround, this additional vertical contrast modulation over time due to the border would have a contribution that tends to oppose the on-off cycle of the foreground itself, thus tending to reduce the aggregate SSVEP in the presence of a surround. To control for this potential contribution, we presented the surround pattern in all configurations at two randomized, equally likely spatial phases of 0° and 180° relative to the foreground. The vertical contrast border component for the opposite-spatial-phase surround modulates in temporal phase with the on-off cycle of the foreground, thus tending to increase the overall SSVEP amplitude. Thus, upon establishing that such a contribution was present but small with respect to the surround suppression effect in our first analysis of contrast response functions, we controlled for the potential tendency simply by averaging across spatially in-phase and opposite-phase conditions within each configuration in all further analyses.
Our choice to use surround patterns that were static rather than flickering, phase-reversing or drifting, was based on practical considerations of 1) the obtrusiveness of the full-screen surround pattern for patient populations in potential follow-up work; 2) the avoidance of a lure to make eye movements; and 3) the idea that a continuously present surround may exert a stronger influence than one that is present on a fraction of display frames during flicker. Since we were primarily interested in how foreground SSVEP modulated as a function of surround contrast, and not on how the foreground and surround interacted in any other more complex way, we had no reason to flicker or phase-reverse the surround at different frequencies to derive separable responses to it. It is worth mentioning, however, that we conducted post hoc additional tests in one subject who showed robust suppression effects using our main configuration, to briefly explore alternative stimulation configurations for both the foreground and surround. We found that the SSVEP itself, as well as the surround-induced modulation of the SSVEP, were weaker and inconsistent for phase-reversed foregrounds, regardless of the type of surround stimulation (static, pattern-pulse flicker, phase-reversed or drifted), despite the fact that we phase-opposed the upper and lower phase-reversing stimuli in the appropriate way analogous to the pattern-pulse case. In addition, for pattern-pulse foreground stimulation, we found that, while the effects were similarly robust to the original settings for drifting surround, the SSVEP was greatly attenuated and surround-modulation was apparently absent when we flickered the surround at a different frequency. This preliminary investigation indicates that these stimulation configurations may have a strong influence on the measurement of contextual interactions from early visual cortex. However, these issues were not the focus of the present study, and a systematic study with more subjects will be necessary to determine the influence of these stimulation configurations more definitively.
A full listing of the stimulation configurations, including the contrasts tested for each, is provided in Table 1. A total of 100 conditions with 6 trials per condition were presented, randomly interleaved and split into 3 blocks of 170 trials each, leading to a total testing time of ∼35 min.
Data acquisition and analysis.
EEG data were recorded at a sample rate of 500 Hz from a 97-channel montage of electrodes with an online reference at standard site FCz, using Brain Products DC amps and the actiCAP system (Oostenveld and Praamstra 2001). We applied an online notch filter at 60 Hz, and impedances were stable below 25 kΩ.
Data were analyzed offline in Matlab using in-house scripts in conjunction with data reading routines and topographic mapping functions of EEGLAB (an open source toolbox for EEG analysis; Delorme and Makeig 2004). Individual epochs were extracted in the interval [−600, 2,600] ms relative to the flicker stimulus onset at 0 ms. We baseline-corrected each trial epoch by subtracting the mean value over the points [−200, 0] ms relative to stimulus onset and re-referenced all channels to average mastoids by simple subtraction. To detect blinks, we used an in-house blink detection script based on the frontal electrodes (FP1 and FP2) with a threshold of 40 μV.
To derive contrast response functions and examine the influence of surround patterns, we computed a fast Fourier transform for a 2240-ms window beginning 160 ms after stimulus onset (avoiding most of the early transient evoked potential) and extracted the single amplitude value at the frequency of stimulation (25 or 7.14 Hz). In this analysis, we discarded all trials in which more than one blink occurred in the 2,240-ms epoch, resulting in an average trial count greater than 5.9 (out of a maximum of 6) across subjects. Topographies showed that SSVEP amplitude was at electrode “POz” and “Oz” for peripheral and foveal stimulus, respectively (Fig. 2, A, left, and B).
To examine changes in the pattern of surround suppression over time due to adaptation to the foreground and surround stimuli, we additionally carried out time-frequency analyses. Specifically, a short-time Fourier transform was computed on sliding windows of 560 ms with an overlap of 75% (140-ms step) over the 2.4-s trial length, starting from 280 ms prior to stimulus onset. Individual time segments were artifact-rejected based on blink detection, resulting in an average of at least 5.8 trials for each 560 ms sliding window beyond time zero. To illustrate how the pattern of time-resolved SSVEP changes can be explained by differences in the strength of adaptation of the foreground and surround, we fitted the grand-average, time-resolved contrast response functions with a model that accounts for variations over time as well as foreground and surround contrast. First, the basic increase in amplitude as a function of foreground contrast was captured by the standard Naka-Rushton function (Naka and Rushton 1966; Peirce 2007). Second, the influence of the spatial surround was encapsulated in an additive term in the denominator. Third, the temporal adaptation of both the foreground and surround drives was described by decaying exponentials with the same time constant but asymptotes that were permitted to differ. The complete model is thus described by the relation:
where r is the SSVEP amplitude at time t, Rm is the maximal response, R0 is the spectral baseline noise, σ is the contrast at which one-half of the maximum response is achieved, β is the coefficient of suppression, which scales the influence of surround contrast in the denominator, and n is the exponent that accounts for nonlinearity of the function. The time-dependent foreground drive Df(t) and suppressive drive Ds(t) were modeled as decaying exponential functions beginning at the veridical physical contrast of the stimulus and asymptotically tending toward a fraction of that value, captured in the relations:
where τ is the mutual time constant for adaptation of foreground and surround drives, and Cf and Cs are the foreground and surround contrasts, respectively. K∞,f and K∞,s represent factors by which the foreground and suppressive drive are asymptotically reduced relative to the initial value, respectively. The fit was carried out on the data over the interval 420 to 2,240 ms so that fast Fourier transform windows stayed within the bounds of the stimulation period, and using the method of least squares.
Psychophysical task.
In a separate session, we asked the same subjects to perform a psychophysical contrast: matching task to estimate their perceived foreground contrast for varying levels of surround contrast. All but two subjects were able to return for this session. A one-up, one-down staircase procedure (Levitt 1971) was run concurrently for each of six conditions: 1) peripheral foreground disks with no surround (0% surround); 2) peripheral foreground disks with parallel, spatially in-phase surround at 100% contrast; 3) peripheral foreground disks with parallel, spatially opposite-phase surround at 100% contrast; 4) peripheral foreground disks with orthogonal surround at 100% contrast; 5) foveal foreground disks with no surround (0% surround); and 6) foveal foreground disks with parallel, spatially in-phase surround at 100% contrast.
We used the same stimulus configuration as in the SSVEP recordings (see Fig. 1); this time only with a flicker frequency of 25 Hz. In each two-interval forced-choice trial, we presented an isolated foreground (match, varying) on a midgray surround and an embedded foreground (test, constant at 50% contrast) one after the other, in randomized order. The subjects were asked to indicate whether the foreground disks in the first or second interval had higher contrast. Two blocks were recorded for each subject, starting with a matching contrast of either 0.6 or 0.2, counterbalanced across subjects. Twenty trials for each of the six interleaved conditions were run in each block. We used a contrast step size of 5% (both up and down) on the first 10 trials to reach a point closer to the asymptote more quickly, and from trial 11 to trial 20 reduced the step size to 2.5% for finer adjustments. The point of subjective equality (PSE) was computed by taking the mean of the last four matching contrast levels in each respective condition, at which point the staircase had typically reached its asymptotic level.
Statistical analyses.
Analysis of effects on SSVEP amplitude were carried out for a population of 18 subjects. Three repeated-measures analyses of variance (ANOVA) were carried out, each including the two factors of foreground and surround contrast at the levels that were in common among all configurations (50 and 100% foreground, 0 and 100% surround). The first ANOVA additionally included the factor of surround orientation. The second one included the factors of flickering frequency (25 Hz, 7.14 Hz) and spatial position of foreground stimuli (foveal, peripheral). The third one was carried out on the time-frequency data and included the additional factor of time with two levels targeting the beginning and end of the stimulation epoch (the 560-ms window centered at 420 ms and 2,100 ms). To ensure that results are not disproportionately biased by individuals with larger spectral amplitudes at the flicker frequency, we recomputed all statistical tests for SNR measures of SSVEP amplitude, in which the amplitude at the SSVEP frequency is divided by the mean amplitude in the immediately adjacent frequency bins (see e.g., Kim and Verghese 2012) and additionally log-transformed to ensure suitability for parametric tests. For the sake of conservative reporting, we only list main effects and interactions that were significant both for raw amplitude and for this SNR measure.
To test for effects in the psychophysical data, we submitted the PSE for each subject to a one-way repeated-measures ANOVA with stimulation condition as the sole factor. Contingent on this reaching significance, we conducted planned comparisons (paired t-tests) to confirm that peripheral parallel, orthogonal and foveal PSEs were significantly lower with than without a surround, and to test whether orthogonal-surround suppression was weaker than parallel and whether foveal suppression differed from peripheral.
RESULTS
Our first practical goal was to demonstrate that, by phase-opposing the temporal flicker of upper and lower foreground gratings, thus activating neighboring but oppositely-oriented regions in the calcarine sulcus in alternation (see Vanegas et al. 2013), we could enhance SSVEP amplitude far above the noise level and thus facilitate robust measurement of suppression effects. For 100% contrast, peripheral foreground stimuli flickering at 25 Hz in the absence of a surround, we measured almost a quadrupling (×3.9) in amplitude relative to the same configuration with in-phase flickering (see scalp topographies in Fig. 2A), and amplitude was more than doubled (×2.3) for the 7.14-Hz flicker. Aside from such issues of robust measurement, our primary goal was to measure SSVEP modulations under several contextual configurations, varying factors that have been found to influence surround suppression effects in animal electrophysiology studies, human psychophysics and functional imaging, and to link the neurophysiological effects to psychophysical reports in the same subjects.
Surround suppression of foreground SSVEP: influences of relative orientation, retinal location, flicker frequency and time.
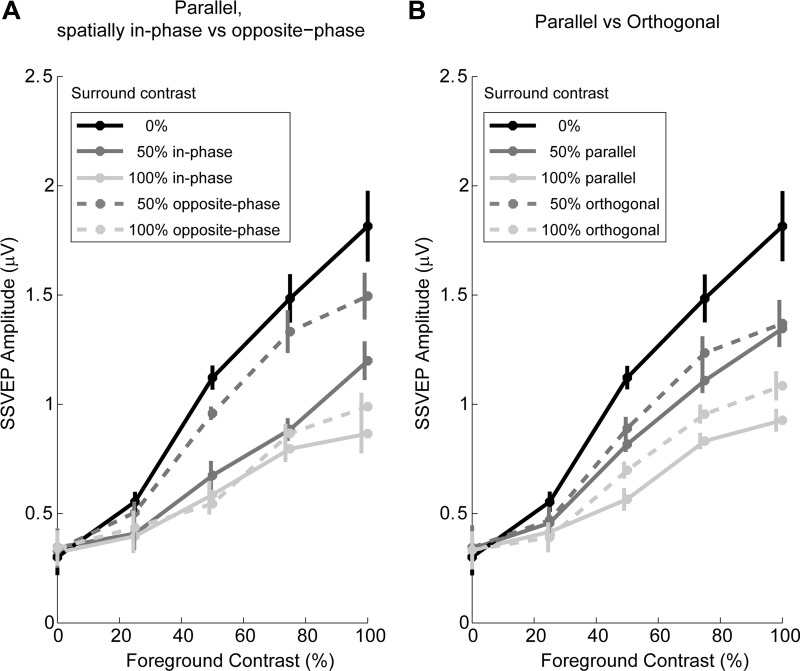
In Fig. 3, we plot the contrast response functions (SSVEP amplitude as a function of foreground stimulus contrast) for the first two peripheral stimulus configurations, which included five foreground contrasts and three surround contrasts (see Table 1). Figure 3A compares the two spatial phases for parallel surrounds, while Fig. 3B compares parallel to orthogonal surrounds with spatial phase collapsed. Note that, although there were two spatial phases of the surround in the orthogonal case, they do not differ in a meaningful way in terms of phase alignment with the foreground gratings. SSVEP amplitude clearly increases as a function of foreground contrast in all configurations, and the entire contrast response function is markedly reduced with increasing surround contrast. Spatial phase had the expected effect of increasing the SSVEP in the spatially opposite-phase setting relative to in-phase, particularly in the 50% surround condition (dark gray trace in Fig. 3), but the primary effect of surround suppression was clearly evident for both spatial phases, indicating that the border contribution was not so large that it could explain the in-phase suppression effect. Because the spatially in- and opposite-phase surrounds tend to bias the SSVEP amplitude in opposite directions, we collapse across spatial phase in all of the following analyses and figures.
Fig. 3.
Average SSVEP contrast response functions across 2.24 s of peripheral stimulation at 25 Hz. A: parallel surround configuration, comparing spatially in-phase (solid lines) and opposite-phase (dashed lines) surrounds (configuration 1). B: comparison of parallel (solid lines; configuration 1) vs. orthogonal (dashed lines; configuration 2) surround, collapsed across surround spatial phase. Error bars represent the standard error of the mean after subtracting intersubject variance not relevant to repeated-measures comparisons (Cousineau 2005). A single 0% surround trace is shown because surround spatial phase and orientation are meaningless in this case. Note that contrast is plotted on a linear rather than logarithmic scale as is typically done, as our FG contrasts were spaced linearly.
A 2 × 2 × 2 ANOVA with the factors of surround orientation relative to foreground (parallel, orthogonal), foreground contrast (50, 100%), and surround contrast (0, 100%) revealed a significant main effect of foreground contrast (F1,17 = 29.8, P = 4.23 × 10−5), and of surround contrast (F1,17 = 33.1, P = 2.32 × 10−5). Furthermore, there was a significant main effect of surround orientation (F1,17 = 9.28, P = 0.00729), and an interaction between surround orientation and surround contrast (F1,17 = 7.22, P = 0.0156), reflecting a stronger suppressive effect when surround orientation matched that of the foreground stimulus.
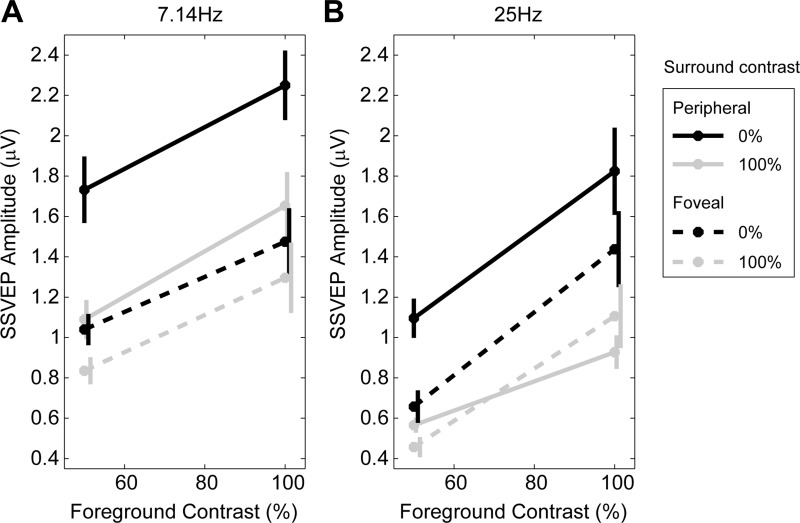
Figure 4 plots SSVEP amplitude as a function of retinal location and flicker frequency, as well as the two foreground and two background contrast levels. A 2 × 2 × 2 × 2 ANOVA with the factors of stimulus position (peripheral, foveal), flicker frequency (25, 7.14 Hz), foreground contrast (50, 100%), and surround contrast (0, 100%) revealed significant main effects of surround contrast (F1,17 = 36.4, P = 1.34 × 10−5), foreground contrast (F1,17 = 50.7, P = 1.71 × 10−6) and flicker frequency (F1,17 = 13.6, P = 0.00185), the latter reflecting greater SSVEP amplitude for lower flicker frequencies. A significant interaction of surround contrast and location (F1,17 = 24.2, P = 1.28 × 10−4) indicated that suppression was stronger for peripheral stimuli compared with foveal (Fig. 4, continuous vs. dashed lines). Although flicker frequency did not appear to have an influence on the strength of suppression measured at the tested contrasts (i.e., no surround contrast × flicker frequency interaction), we carried out additional tests to compare the SNR across the two frequencies to assess signal robustness more generally. SNR was measured as the amplitude at each discrete flicker frequency divided by the mean of the two neighboring frequency bins for 100% foreground and 0% surround. Wilcoxon signed-rank tests revealed that the 25-Hz SSVEPs had significantly higher SNRs than 7.14 Hz at both peripheral (medians 7.91 vs. 4.39, P = 6.29 × 10−4) and foveal (7.17 vs. 4.44, P = 0.0139) locations.
Fig. 4.
Effects of retinal location and flicker frequency. Peripheral stimuli (solid lines) were more strongly suppressed by the high-contrast surround than foveal stimuli (dashed lines) at both flicker frequencies: 7.14 Hz (configurations 5 and 7, Table 1; A) and 25 Hz (configurations 1 and 4; B).
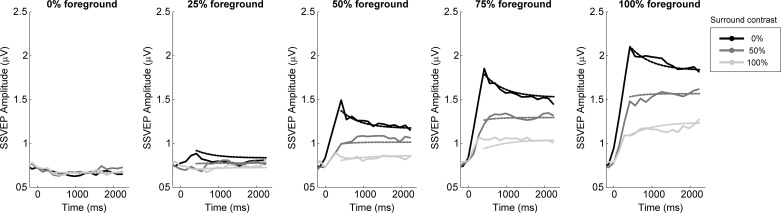
Figure 5 shows the time course of SSVEP amplitude changes over the 2.4-s stimulation interval for peripheral, 25-Hz, parallel surround patterns (configuration 1 in Table 1). The smoother curves overlaid on the real data represent the model fit. After a transient response peak at 400 ms post-flicker onset, the no-surround conditions exhibit an approximately exponential decrease over time as expected, due to contrast adaptation. In the presence of the surround, however, the foreground signal appeared to grow over time in many cases rather than drop. Confirming this, a 2 × 2 × 2 ANOVA, including the factors of time (420 ms, 2,100 ms), surround contrast (0, 100%) and foreground contrast (50, 100%), revealed a significant interaction between surround contrast and time (F1,17 = 27.5, P = 6.54 × 10−5). In addition, the ANOVA revealed the expected main effect of surround contrast (F1,17 = 27.1, P = 7.08 × 10−5), foreground contrast (F1,17 = 26.6, P = 7.82 × 10−5), and time (F1,17 = 5.7, P = 0.0288).
Fig. 5.
Temporal analysis. The grand-average SSVEP amplitude traced over the duration of the flicker stimulation. Increasing FG contrasts are plotted from left to right (0, 25, 50, 75, 100%), and each of the three surround contrasts are superimposed in each. The smoother dashed lines represent a model fit to the data, in which FG and divisive surround drives each exponentially decay (i.e., adapt) but to varying degrees.
The increase of the foreground signal embedded in a surround over the short timeframe of 2.4 s is consistent with patterns observed in monkey neurophysiology (Cavanaugh et al. 2002a, see their Figs. 11 and 12), where it was inferred that a stronger decrease in gain of the surround suppressive influence compared with the decreasing foreground gain may explain the effect. To illustrate how this explanation could also apply in our data, we fitted the grand-average, time-resolved contrast response functions with a model. In this model, the suppressive influence of the surround drive is incorporated as an additive term in the denominator of a Naka-Rushton function, and the foreground and suppressive drives are described by decaying exponential functions of time with the same decay rate but different asymptotic levels (see materials and methods). The best fitting parameters were calculated for the main configuration of stimulation (configuration 1 in Table 1), including all levels of foreground and surround contrasts, after averaging across subjects. All eight parameters of the model were free to vary in the least-squares fit, and, when optimized, took the following values: Rm = 2.45 μV, R0 = 0.67 μV, n = 1.81, σ = 0.82%, β = 2.13, τ = 503.6 ms, K∞,f = 0.7825, and K∞,s = 0.5633. The lower asymptote K∞,s relative to K∞,f indicates that the suppressive drive Ds(t) adapts more strongly than the foreground drive Df(t), with the result that, on balance, foreground SSVEP amplitude grows over time as it is released from suppression.
Psychophysical effects and neurophysiology-behavior correlations.
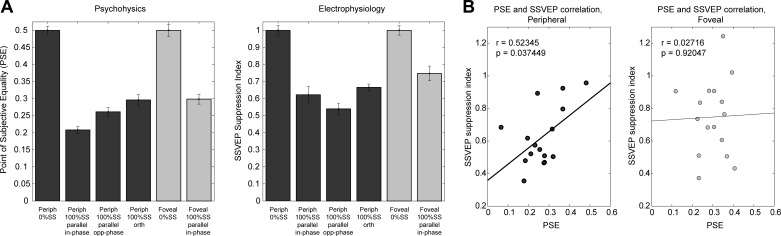
Using a two-interval forced-choice matching task governed by adaptive staircases, PSE of grating stimuli presented alone with respect to surround-embedded stimuli were estimated for six stimulation conditions (see materials and methods). Figure 6A shows the mean PSE values for each condition (left) alongside SSVEP suppression indices for the corresponding conditions (right). The suppression index was computed as the ratio of SSVEP amplitude for 50% foreground contrast (i.e., the test contrast in the psychophysics sessions) with 100% relative to 0% surround contrast. Therefore, the values for the 0% surround conditions are necessarily equal to 1, but are nevertheless presented in the figure to provide the scale against which suppression is measured.
Fig. 6.
Psychophysical measures. A, left: psychophysical measures of point of subjective equality (PSE). Right: SSVEP suppression index for the same conditions as those run in the psychophysics experiment. Both bar plots show average for 16 subjects on each condition. B: correlation between PSE and SSVEP amplitude collapsed across peripheral stimulus conditions (parallel spatially in-phase and opposite-phase, and orthogonal SS). Subjects who showed stronger psychophysical effects of suppression in the periphery (left) tended to also show stronger SSVEP suppression effects, but this was not the case for foveal FG (right).
A one-way ANOVA, including all six conditions, revealed a significant effect (F5,75 = 66.2, P = 8.55 × 10−15). Follow-up planned comparisons showed significant psychophysical suppression in the parallel spatially in-phase (t15 = 14.9994, P = 1.942 × 10−10), parallel spatially opposite-phase (t15 = 10.606, P = 2.293 × 10−8), orthogonal (t15 = 7.669, P = 1.443 × 10−6) and foveal (t15 = 8.595, P = 3.521 × 10−7) surround conditions when each was compared with its corresponding no-surround condition. In addition, spatially in-phase PSEs were lower (i.e., more suppression) than spatially opposite-phase (t15 = 3.981, P = 0.0012), orthogonal PSEs were significantly higher (i.e., less suppression) than parallel (collapsing across spatial phase, t15 = 4.879, P = 0.0002), and significantly higher in foveal than peripheral for spatially in-phase stimuli (t15 = 4.117, P = 0.0009). These effects are all in line with the above-mentioned SSVEP effects. However, the effects of spatial phase warrants cautious interpretation, since the same considerations of border effects in SSVEP-generation (see discussion in materials and methods) may not apply to perception, and, although the relative elevation of SSVEP amplitude in the presence of a spatially opposite-phase surround was strong for most contrast combinations (see Fig. 3), it was not apparent in the particular condition of 50% foreground and 100% surround, which was used to derive the SSVEP suppression indices in Fig. 6A (right). Moreover, while the psychophysical effect of spatial phase is in line with other studies that, similar to ours, imposed no gap between the foreground and surround stimuli (Ejima and Takahashi 1985; Olzak and Laurinen 1999), this effect is absent in studies where there is a gap (Cannon and Fullenkamp 1991; Petrov and McKee 2006; Xing and Heeger 2001), suggesting the potential influence of a brightness induction effect at the border (see Snowden and Hammett 1998).
To assess whether there is a relationship between the degree of electrophysiological surround suppression and the reduction in PSE due to the surround in psychophysical tests, we computed the correlation between the SSVEP suppression index and the PSE (Fig. 6B). A significant correlation was found for peripheral stimuli (r = 0.523, P = 0.0375), but not for foveal stimuli (P = 0.9).
DISCUSSION
The phenomenon of surround suppression has been studied using a wide range of techniques. The dominant stimulation paradigm has been a simple one, wherein foreground pattern stimuli are fully embedded in a more extended surround of comparable properties (e.g., Cavanaugh et al. 2002a, 2002b; Petrov et al. 2005; Zenger-Landolt and Heeger 2003), but such a paradigm has not yet been applied in human neurophysiology, despite obvious clinical potential in light of recent psychophysics (e.g., Dakin et al. 2005) and neuroimaging (e.g., Seymour et al. 2013) work. In the present study, we measured steady-state responses (SSVEPs) to flickering foreground stimuli embedded in static surrounds in human subjects, and found dramatic suppression effects, which depended on surround orientation and retinal location, as well as stimulation parameters such as frequency and temporal phase-offsets. Moreover, the magnitude of suppression at peripheral locations correlated with the perceived contrast reduction and also interacted with mechanisms of adaptation over time.
Robust measurement of contextual interactions in the visual system in human electrophysiology has long been recognized as a challenge due to the noninvasive nature of the technique (e.g., Zemon and Ratliff 1982). Here we have demonstrated that by using upper-lower phase-opposition, which exploits human visual cortical geometry and organization to boost SSVEP SNR (Vanegas et al. 2013), a reliable neurophysiological signal can be obtained upon which suppressive influences are robustly resolved. A key factor in robust measurement of these contextual modulatory effects is whether visual responses, even when strongly suppressed, remain clear of the background EEG noise level, as reflected in spectral amplitude in neighboring frequencies. For 25-Hz peripheral stimulation, we measured a median SNR of 7.91 for 100% contrast foregrounds with no surround, i.e., SSVEP amplitude exceeded noise levels by almost a factor of 8, and even for 50% foregrounds in the presence of 100% contrast surround, this factor decreased only to a median value of 2.95, still well clear of the noise. Even though suppressive effects were significant for low (7.14 Hz) as well as high (25 Hz) flicker frequencies, SNRs for 25 Hz were almost double those for 7.14 Hz, on account of the higher background spectral noise level at low frequencies. The ability to obtain high-SNR responses at high frequencies also means that measurements can be made well away from lower-frequency endogenous brain rhythms such as alpha (8–14 Hz) that are known to be reactive to visual factors. In terms of observer comfort, higher-frequency flicker is far less obtrusive to watch over extended periods of time. Finally, previous psychophysics work has shown a greater confounding influence of the foreground-surround border when using frequencies below 10 Hz (Kelly 1969), which may be a critical factor in correlating perceptual effects with neurophysiological measures.
Our findings add to a considerable amount of previous neurophysiology research in cats (e.g., Deangelis et al. 1994) and monkeys (e.g., Cavanaugh et al. 2002b), psychophysics in humans (e.g., Petrov et al. 2005; Xing and Heeger 2000) and human fMRI (e.g., Williams et al. 2003), in showing stronger suppressive effects when surround orientation matched the foreground stimulus. Although reliable, the size of the orientation effect on either the SSVEP suppression or psychophysical PSE reduction was not large in our data. This may be due to the fact that the orientation effects were measured only at peripheral locations, which have been shown to exhibit much less orientation-specificity in psychophysical contrast-matching suppression effects than at the fovea, where very little suppression is seen for orthogonal surrounds (Xing and Heeger 2000).
In comparing foveal to peripheral locations for iso-oriented surrounds, we found that suppression effects were greater in the periphery for both SSVEP and PSE indices. This is in line with previous psychophysical results (Xing and Heeger 2000) and opposes findings of one fMRI study examining the issue (Williams et al. 2003). In the latter study, the surround covered a smaller display area relative to the foreground compared with many studies showing strong suppression in the periphery (Xing and Heeger 2000; Zenger-Landolt and Heeger 2003), and it has been inferred from psychophysical results that suppressive neuronal interactions occur over a more extended spatial area in the periphery, commensurate with cortical magnification (Petrov and McKee 2006), which may offer an explanation for the discrepancy. The difference between suppressive effects at peripheral and foveal locations has been shown to be particularly dramatic when measured in terms of psychophysical contrast detection (as opposed to contrast matching), in which case no suppression at all has been reported at the fovea (Petrov et al. 2005). These differences have been considered to highlight important functional distinctions between visual processing at the fovea vs. in the periphery (Petrov et al. 2005).
A recent fMRI study has shown that, although suppression is stronger in extrastriate areas, the degree of suppression in V1 agrees best with psychophysical reports, indicating that V1 may be site of readout for decisions based on contrast (Zenger-Landolt and Heeger 2003). That study placed foreground stimuli in the periphery at around the same eccentricity as in our study, and we also found a link between contrast perceptual reports and neural measures of suppression, albeit a cruder one in the form of a correlation across subjects. We did not, however, find a significant correlation in the fovea. While it is tempting to take this as further demonstration of the qualitative differences between foveal and peripheral vision, it could more simply be a matter of measurement: foveal stimuli project to the human occipital pole which is less reliable in surface orientation so that variation in SSVEP across subjects may reflect variations in geometry more than variations in underlying signal strength. Moreover, the foveal SSVEP amplitudes for the 50% foreground, 100% surround conditions used in the neurophysiology-behavior correlation tended to lie closer to the noise floor in most subjects than did the equivalent peripheral signals (medians 1.85 μV for foveal compared with 2.95 μV for peripheral, signed-rank test marginal at P = 0.0707), suggesting that interindividual variation may not have been expressed to its full extent in the foveal configuration, precluding the detection of a correlation with behavior. This will need further work to resolve, however.
In humans, the time course of adaptation has been examined using counterphase sinusoidal grating stimuli, under steady-state and transient stimulation, resembling an exponential decay that can be fitted in responses recorded on a 40-s time lapse, and whose time constant depends upon the spatial frequency and contrast of the adapting grating (Heinrich and Bach 2001; Ho and Berkley 1988). Our temporal analysis of SSVEP amplitude provided a similar view on the time course of adaptation, but additionally revealed the interesting effect whereby, in the presence of a suppressive surround, the response grows rather than drops over time. The effects of adaptation on neuronal suppression effects have been studied also in animal neurophysiology (Cavanaugh et al. 2002a; Knierim and Vanessen 1992; Webb et al. 2005). Our results are consistent with those of Cavanaugh et al. (2002a), who showed that, with adaptation over the course of 1.5–6 s, neurons responded as if with a larger effective receptive field, i.e., they preferred larger stimuli. This meant that, whereas responses to small stimuli quickly and steeply declined over time, for certain larger stimulus sizes the response actually grew over time. Cavanaugh et al. explained this effect in terms of a ratio-of-Gaussians model in which the gain of the suppressive component decreased more strongly than the center component. We mirrored this mechanism in a simple model fit to our data incorporating divisive suppression and exponential decay to different levels of the foreground and surround drives. It should be noted, however, that the stronger adaptation of the surround drive in our paradigm may be partly due to the fact that the surround was static, whereas the foreground was dynamic, flickering on and off. Our purpose in this model fit was not to establish or validate a new model, and we did not make comparisons with alternative models; rather, the model fit provided a way to capture the salient aspects of the data in interpretable parameters. While low per-subject trial numbers precluded individual fits in these data, such a fitting procedure has clear potential in providing parameterized metrics of temporal (K∞,f, K∞,s and τ) as well as spatial (β) gain control mechanisms in clinical settings.
Surround stimuli do not always cause suppression of foreground responses, in particular, when the foreground contrast is high and the surround contrast is low, psychophysical reports are of greater perceived contrast than what is physically presented. We found no facilitatory effects of the surround in our electrophysiological indices, most likely because we did not measure conditions where the foreground contrast was much higher than the surround. The one condition that could be argued meets this criterion is the foreground-100% and surround-50% condition, which does show strong suppression here, but it should be noted that the flickering of the foreground means that it has a lower effective contrast perceptually.
Several aspects of visual contextual interaction have been examined using human neurophysiology (EEG/MEG). These studies have examined issues such as nonlinear interactions due to colinearity (Polat and Norcia 1996), figure-background border interactions (Appelbaum et al. 2008), interactions across chromatic and achromatic channels (Xiao and Wade 2010), cross-orientation overlay suppression (Mannion et al. 2011), differences among steady-state response harmonics (Zemon and Ratliff 1982), and the influence of surface structure (Joo and Murray 2014). Of those that specifically looked at spatial surround suppression, most examined transient evoked potentials rather than steady-state. For example, Ohtani et al. (2002) measured transient neuromagnetic responses to stimuli appearing within a preexisting surround and found suppression effects of the very earliest component whose generator was estimated as lying close to the occipital pole in presumed V1. Haynes et al. (2003) further found that such suppression of the early neuromagnetic response correlated with perception. Interocular suppression has also been characterized using visual evoked potentials and was found to be size specific and luminance and orientation dependent (Harter et al. 1976, 1980). Using steady-state responses to phase-reversing luminance vs. chromatically modulated patterns, Xiao and Wade (2010) were able to show that there is barely any suppression across chromatic and achromatic channels compared with within, and through source-imaging could localize these patterns to V1. Relative orientation appeared not to influence the interactions, but this was attributed to the low spatial frequencies used (Xiao and Wade 2010). Source-estimated steady-state responses have also been employed to examine the selective routing of figure vs. ground-related information through separable networks in the visual system (Appelbaum et al. 2006). Our paradigm highlights alternative stimulation parameter choices that could be used in combination with more complex analytic approaches, such as these in further basic research on vision, but in addition provides robust and straight-forward indices with minimal signal analysis, making it highly suitable for wide applicability in the clinical domain. Behavioral indices of contextual interactions such as surround suppression have been highly informative in clinical studies implicating inhibitory dysfunction in schizophrenia (Dakin et al. 2005), aging (Karas and McKendrick 2012), migraine (Battista et al. 2011), depression (Golomb et al. 2009) and autism (Foss-Feig et al. 2013). Such behavioral indexes, which have been used in the majority of clinical investigations so far, can be challenging to measure with precision in low-functioning neurological and psychiatric populations, thus highlighting the importance of direct neurophysiological indices. Our ongoing investigations will attempt to realize this clinical potential.
GRANTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award SC2-GM-099626.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.I.V., A.B., and S.P.K. conception and design of research; M.I.V. performed experiments; M.I.V. and S.P.K. analyzed data; M.I.V., A.B., and S.P.K. interpreted results of experiments; M.I.V. prepared figures; M.I.V. drafted manuscript; M.I.V., A.B., and S.P.K. edited and revised manuscript; M.I.V., A.B., and S.P.K. approved final version of manuscript.
REFERENCES
- Ales JM, Yates JL, Norcia AM. On determining the intracranial sources of visual evoked potentials from scalp topography: a reply to Kelly et al. Neuroimage 64: 703–711, 2013. [DOI] [PubMed] [Google Scholar]
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive-field–neurophysiological mechanisms for local global comparisons in visual neurons. Annu Rev Neurosci 8: 407–430, 1985. [DOI] [PubMed] [Google Scholar]
- Appelbaum LG, Wade AR, Pettet MW, Vildavski VY, Norcia AM. Figure-ground interaction in the human visual cortex. J Vis 8: 81–19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum LG, Wade AR, Vildavski VY, Pettet MW, Norcia AM. Cue-invariant networks for figure and background processing in human visual cortex. J Neurosci 26: 11695–11708, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista J, Badcock DR, McKendrick AM. Migraine increases centre-surround suppression for drifting visual stimuli. PLoS One 6: e18211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Tobin EA. Lateral inhibition between orientation detectors in cats visual-cortex. Exp Brain Res 15: 439–440, 1972. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Cannon MW, Fullenkamp SC. Spatial interactions in apparent contrast–inhibitory effects among grating patterns of different spatial-frequencies, spatial positions and orientations. Vision Res 31: 1985–1998, 1991. [DOI] [PubMed] [Google Scholar]
- Carandini M. Receptive fields and suppressive fields in the early visual system. Cogn Neurosci 3: 313–326, 2004. [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol 88: 2530–2546, 2002a. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Selectivity and spatial distribution of signals from the receptive field surround in macaque V1 neurons. J Neurophysiol 88: 2547–2556, 2002b. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G, Solomon JA. Texture interactions determine perceived contrast. Proc Natl Acad Sci U S A 86: 9631–9635, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson's method. Tutor Quant Methods Psychol 1: 42–45, 2005. [Google Scholar]
- Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol 15: R822–R824, 2005. [DOI] [PubMed] [Google Scholar]
- Deangelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cats primary visual-cortex. J Neurophysiol 71: 347–374, 1994. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp 15: 95–111, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, Spinelli D, Hillyard SA. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum Brain Mapp 28: 323–334, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima Y, Takahashi S. Apparent contrast of a sinusoidal grating in the simultaneous presence of peripheral gratings. Vision Res 25: 1223–1232, 1985. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci 33: 8243–8249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb JD, McDavitt JRB, Ruf BM, Chen JI, Saricicek A, Maloney KH, Hu J, Chun MM, Bhagwagar Z. Enhanced visual motion perception in major depressive disorder. J Neurosci 29: 9072–9077, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter MR, Conder ES, Towle VL. Orientation-specific and luminance effects–inter-ocular suppression of visual evoked-potentials in man. Psychophysiology 17: 141–145, 1980. [DOI] [PubMed] [Google Scholar]
- Harter MR, Towle VL, Musso MF. Size specificity and interocular suppression–monocular evoked-potentials and reaction-times. Vision Res 16: 1111–1117, 1976. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Roth G, Stadler M, Heinze HJ. Neuromagnetic correlates of perceived contrast in primary visual cortex. J Neurophysiol 89: 2655–2666, 2003. [DOI] [PubMed] [Google Scholar]
- Heinrich SP, Bach M. Adaptation dynamics in pattern-reversal visual evoked potentials. Doc Ophtalmol 102: 141–156, 2001. [DOI] [PubMed] [Google Scholar]
- Ho WA, Berkley MA. Evoked-potential estimates of the time course of adaptation and recovery to counterphase gratings. Vision Res 28: 1287–1296, 1988. [DOI] [PubMed] [Google Scholar]
- Joo SJ, Boynton GM, Murray SO. Long-range, pattern-dependent contextual effects in early human visual cortex. Curr Biol 22: 781–786, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SJ, Murray SO. Contextual effects in human visual cortex depend on surface structure. J Neurophysiol 111: 1783–1791, 2014. [DOI] [PubMed] [Google Scholar]
- Karas R, McKendrick AM. Age related changes to perceptual surround suppression of moving stimuli. Seeing Perceiving 25: 409–424, 2012. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Flickering patterns and lateral inhibition. J Opt Soc Am 59: 1361–1368, 1969. [Google Scholar]
- Kelly SP, Vanegas MI, Schroeder CE, Lalor EC. The cruciform model of striate generation of the early VEP, re-illustrated, not revoked: a reply to Ales et al. (2013). Neuroimage 82: 154–159, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Verghese P. The selectivity of task-dependent attention varies with surrounding context. J Neurosci 32: 12180–12191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Vanessen DC. Neuronal responses to static texture patterns in area-V1 of the alert macaque monkey. J Neurophysiol 67: 961–980, 1992. [DOI] [PubMed] [Google Scholar]
- Lauritzen TZ, Ales JM, Wade AR. The effects of visuospatial attention measured across visual cortex using source-imaged, steady-state EEG. J Vis 10: 39, 2010. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am 49: 467, 1971. [PubMed] [Google Scholar]
- Levitt JB, Lund JS. Contrast dependence of contextual effects in primate visual cortex. Nature 387: 73–76, 1997. [DOI] [PubMed] [Google Scholar]
- Mannion D, Tsai J, Wade A. The source of overlay masking in the human visual system. J Vis 11: 49, 2011. [Google Scholar]
- Naka KI, Rushton WAH. S-potentials from luminosity units in retina of fish (Cyprinidae). J Physiol 185: 587–599, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani Y, Okamura S, Yoshida Y, Toyama K, Ejima Y. Surround suppression in the human visual cortex: an analysis using magneto encephalography. Vision Res 42: 1825–1835, 2002. [DOI] [PubMed] [Google Scholar]
- Olzak LA, Laurinen PI. Multiple gain control processes in contrast-contrast phenomena. Vision Res 39: 3983–3987, 1999. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 112: 713–719, 2001. [DOI] [PubMed] [Google Scholar]
- Peirce JW. The potential importance of saturating and supersaturating contrast response functions in visual cortex. J Vis 7: 13, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Petrov Y, Carandini M, McKee S. Two distinct mechanisms of suppression in human vision. J Neurosci 25: 8704–8707, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y, McKee SP. The effect of spatial configuration on surround suppression of contrast sensitivity. J Vis 6: 224–238, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Norcia AM. Neurophysiological evidence for contrast dependent long-range facilitation and suppression in the human visual cortex. Vision Res 36: 2099–2109, 1996. [DOI] [PubMed] [Google Scholar]
- Seymour K, Stein T, Sanders LLO, Guggenmos M, Theophil I, Sterzer P. Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacology 38: 2607–2612, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden RJ, Hammett ST. The effects of surround contrast on contrast thresholds, perceived contrast and contrast discrimination. Vision Res 38: 1935–1945, 1998. [DOI] [PubMed] [Google Scholar]
- Tajima S, Watanabe M, Imai C, Ueno K, Asamizuya T, Sun P, Tanaka K, Cheng K. Opposing effects of contextual surround in human early visual cortex revealed by functional magnetic resonance imaging with continuously modulated visual stimuli. J Neurosci 30: 3264–3270, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas MI, Blangero A, Kelly SP. Exploiting individual primary visual cortex geometry to boost steady state visual evoked potentials. J Neural Eng 10: 036003, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BS, Dhruv NT, Solomon SG, Tailby C, Lennie P. Early and late mechanisms of surround suppression in striate cortex of macaque. J Neurosci 25: 11666–11675, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AL, Singh KD, Smith AT. Surround modulation measured with functional MRI in the human visual cortex. J Neurophysiol 89: 525–533, 2003. [DOI] [PubMed] [Google Scholar]
- Xiao B, Wade AR. Measurements of long-range suppression in human opponent S-cone and achromatic luminance channels. J Vis 10: 19, 2010. [DOI] [PubMed] [Google Scholar]
- Xing J, Heeger DJ. Center-surround interactions in foveal and peripheral vision. Vision Res 40: 3065–3072, 2000. [DOI] [PubMed] [Google Scholar]
- Xing J, Heeger DJ. Measurement and modeling of center-surround suppression and enhancement. Vision Res 41: 571–583, 2001. [DOI] [PubMed] [Google Scholar]
- Zemon V, Ratliff F. Visual evoked potentials–evidence for lateral interactions. Proc Natl Acad Sci U S A 79: 5723–5726, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenger-Landolt B, Heeger DJ. Response suppression in V1 agrees with psychophysics of surround masking. J Neurosci 23: 6884–6893, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]