Abstract
Conscious perception sometimes fluctuates strongly, even when the sensory input is constant. For example, in motion-induced blindness (MIB), a salient visual target surrounded by a moving pattern suddenly disappears from perception, only to reappear after some variable time. Whereas such changes of perception result from fluctuations of neural activity, mounting evidence suggests that the perceptual changes, in turn, may also cause modulations of activity in several brain areas, including visual cortex. In this study, we asked whether these latter modulations might affect the subsequent dynamics of perception. We used magnetoencephalography (MEG) to measure modulations in cortical population activity during MIB. We observed a transient, retinotopically widespread modulation of beta (12–30 Hz)-frequency power over visual cortex that was closely linked to the time of subjects' behavioral report of the target disappearance. This beta modulation was a top-down signal, decoupled from both the physical stimulus properties and the motor response but contingent on the behavioral relevance of the perceptual change. Critically, the modulation amplitude predicted the duration of the subsequent target disappearance. We propose that the transformation of the perceptual change into a report triggers a top-down mechanism that stabilizes the newly selected perceptual interpretation.
Keywords: beta oscillations, bistable perception, brain dynamics, brain state, perceptual decision-making
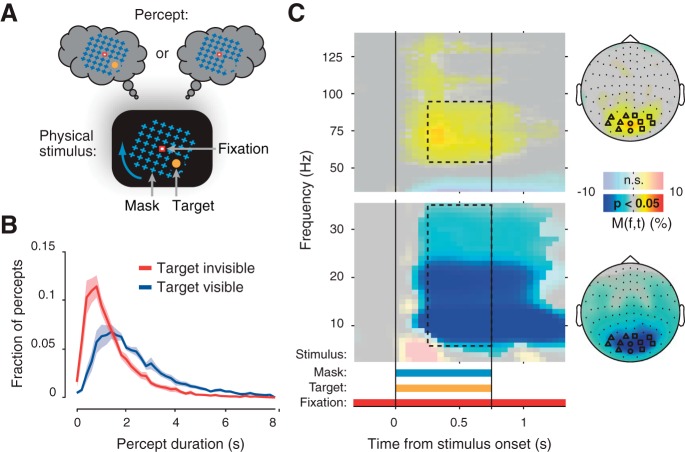
when the sensory input to the brain is ambiguous, perception often changes spontaneously, followed by periods of stable perception, a phenomenon called multistable perception (Blake and Logothetis 2002; Deco and Romo, 2008; Leopold and Logothetis 1999; Sterzer et al. 2009). For example, in an illusion dubbed “motion-induced blindness” (MIB), a salient visual target surrounded by a rotating mask suddenly disappears from perception for some time (see Fig. 1A) (Bonneh et al. 2001; Bonneh and Donner 2011). A hallmark of these MIB disappearances (as well as of other multistable illusions) is that the duration of each percept varies widely and unpredictably from one perceptual change to the next (see Fig. 1B). Intriguingly, the statistics of these perceptual dynamics correlate to the dynamics of thought and exploratory decision-making (Leopold and Logothetis 1999; Carter and Pettigrew 2003).
Fig. 1.
Motion-induced blindness (MIB) stimulus, perceptual dynamics, and cortical stimulus response. A: schematic of the MIB illusion. Bottom, stimulus configuration. The small but salient target (yellow disc) was surrounded by a large moving mask (rotating blue grid). The target was presented in different visual field quadrants for different subjects, at an eccentricity of 3°. Top, alternating perception of the target. B: group average frequency distributions of target invisible and target visible durations during magnetoencephalography (MEG; n = 11 subjects). Shaded areas indicate SE. C: cortical response to the MIB stimulus during Stimulus-on-off condition. Scalp maps show topography of 8- to 35-Hz and 60- to 90-Hz modulations (0.25–0.75 s after stimulus onset; see dashed outlines on time-frequency representations). Transparency level indicates clusters of significant modulation (P < 0.05, 2-sided permutation test across subjects, cluster-corrected; n = 10 subjects). Highlighted symbols indicate MEG sensors showing the biggest stimulus response. These sensors are used for the subsequent analyses of overall power modulations (triangles and squares, sensors used for lateralization analyses; Fig. 3). M(f,t), power modulation.
Studies of the neural basis of multistable perception have reported transient modulations of activity during the perceptual switches, in visual cortex (Donner et al. 2008; Haynes et al. 2005; Lee et al. 2007; Leopold and Logothetis 1996; Polonsky et al. 2000; Tong and Engel 2001) as well as parietal and frontal association cortex (Britz et al. 2011; Knapen et al. 2011; Lumer et al. 1998; Sterzer and Kleinschmidt 2007; Zaretskaya et al. 2010). Some of the switch-related modulations of cortical activity appear to precede the switches and are absent when switches are evoked by the physical stimulus, in line with a causal role in prompting the switch (Britz et al. 2009; Donner et al. 2008; Lumer et al. 1998; Sterzer and Kleinschmidt 2007). Others, however, occur later in time and irrespective of whether the perceptual switches emerge spontaneously or are evoked by a stimulus change (Donner et al. 2008; Frassle et al. 2014; Knapen et al. 2011). Specifically, functional magnetic resonance imaging (fMRI) studies of MIB have identified a retinotopically widespread modulation in early visual cortex, which is decoupled from the cortical target representation (Donner et al. 2008, 2013; Hsieh and Tse 2009). This modulation is contingent on the behavioral report of target disappearance and absent during passive viewing (Donner et al. 2008).
In the present study, we used whole head magnetoencephalography (MEG) recordings (Hamalainen et al. 1993) during MIB to 1) characterize the electrophysiological signatures of the report-related transient modulations in visual cortex identified with fMRI, and 2) to test whether they might shape the subsequent perceptual dynamics. Subjects' reports of perceptual switches were accompanied by a transient modulation of beta-band (12–30 Hz) activity over visual cortex, which was decoupled from both the stimulus properties and motor response but strongly linked to the behavioral relevance of target disappearance. The amplitude of this modulation predicted (in the case of MIB) the duration of the subsequent illusory target disappearance. We propose that behaviorally relevant changes of perception trigger an active top-down mechanism that stabilizes the internal state of visual cortex as well as perceptual suppression.
MATERIALS AND METHODS
We report data from two MEG experiments: a main experiment and a control experiment including high-resolution eye tracking to test for a dependence of the effects reported in this paper on microsaccades.
Main Experiment
Subjects.
MEG data were acquired at the VU University Medical Center (Amsterdam) from 11 healthy subjects with normal or corrected-to-normal vision (4 women, age range: 23–37 yr). The experiment was approved by the local ethics committee, and each subject gave written informed consent. Each subject participated in several MEG sessions on different days of about 2-h duration each.
Stimulus.
The target was a salient yellow disc (full contrast, diameter: 0.12° or 0.2° of visual angle) surrounded by a moving mask (square, equally spaced grid of 9 × 9 blue crosses, 17° width/length), both superimposed on a black background and centered on a fixation mark (red outline, white inside, 0.8° width and length) (Fig. 1A). The target was located on one of the four visual field diagonals at an eccentricity of 3°. Target size and location (visual field quadrant) were individually selected for each subject before MEG, to yield a percentage of target invisible time of at least 20%. The mask rotated around the fixation square (speed: 120°/s). The target was separated from the mask by a black “protection zone” subtending about 2° around the target (Bonneh et al. 2001).
Stimuli were presented using Presentation software (NeuroBehavioral Systems, Albany, CA) and projected via two mirrors onto the ceiling of the MEG scanner room by a liquid crystal display (LCD) projector (BarcoData 8200 LC; Barco Projection Systems, Kuurne, Belgium) with a pixel resolution of 800 × 600 and a 60-Hz refresh rate. Subjects were supine and viewed the stimuli projected onto the ceiling of the MEG room (field of view: 18° × 23°).
Behavioral tasks and design.
All subjects participated in various tasks designed to 1) determine the factors driving transient modulations of cortical activity around reports of perceptual switches and 2) identify the impact of these transient modulations on subsequent neural activity and perception.
STIMULUS-ON-OFF CONDITION.
On each trial, the complete MIB stimulus (target, mask, and fixation mark) was presented for 0.75 s, preceded and succeeded by fixation of an otherwise black screen. This stimulus duration was too short to induce MIB target disappearance but sufficiently long to measure the stimulus-induced modulation of cortical population activity (Fig. 1C). The subjects' task was to maintain stable fixation and passively view the stimulus.
MIB CONDITION.
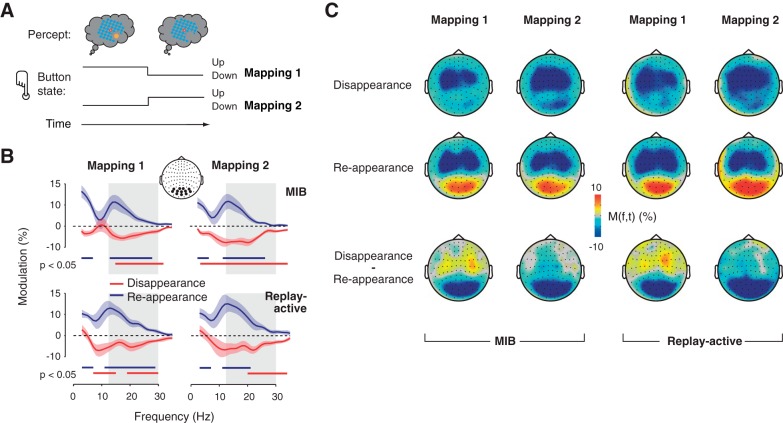
The MIB stimulus was continuously presented for several runs of 2-min duration each. The subjects' task was to maintain stable fixation and report the spontaneous disappearance and reappearance of the target by pressing or releasing a response button with their index finger (left or right, counterbalanced across subjects). The mapping between perceptual switch and motor response was flipped between the 2 recording days (see Fig. 4A): button press for indicating target disappearance (release for reappearance) on day 1, and button release for disappearance (press for reappearance) on day 2.
Fig. 4.
Beta power over motor cortex does not encode report. A and B: same as Fig. 3, A and B, but for sensors overlying motor cortex during MIB and Replay-active. A: frequency spectra of MEG power modulation. B: reappearance − disappearance difference for overall power modulation and lateralization contralateral vs. ipsilateral (contra − ipsi) with respect to hand used for report.
REPLAY CONDITIONS.
In the three different Replay conditions, the target was physically removed from the screen in the same temporal sequence as it had previously disappeared during one of several previous MIB runs completed by the corresponding subject. The physical offsets and onsets of the target were always instantaneous, mimicking the typically abrupt quality of the perceptual switches in MIB for the small target used in this study. The general purpose of the Replay conditions was to test whether report-related, transient modulations in cortical activity during MIB may have prompted the spontaneous perceptual switch (then they should be specific to MIB) or were driven by the perceptual switch (then they may also occur during Replay-active). On the basis of previous fMRI results (Donner et al. 2008), we expected a retinotopically widespread modulation in visual cortex around behavioral report of target disappearance and reappearance, in both MIB and Replay.
Replay-active: this condition was identical to MIB in all respects except for the changes in the physical presence of the target.
Replay-no-mask: this condition was identical to Replay-active, except that the target was presented without mask. The purpose of this condition was to test whether potential report-related modulations during MIB and Replay-active may have reflected a contextual effect in the sensory processing of the mask stimulus: it is conceivable that the sensory processing of the mask differed between the (perceived or physical) presence and the (perceived or physical) absence of the target. The Replay-no-mask condition allowed us to differentiate between 1) contextual modulations of the sensory processing of the mask and 2) report-related, nonsensory modulations that were decoupled from the physical MIB stimulus: modulations should disappear during Replay-no-mask in the former case but should persist in the latter case.
Replay-passive: this condition was identical to Replay-active, except that subjects did not report the target on- and offsets and that target present and absent durations were drawn from Gaussian distributions (mean: 2.2 s for target on, 2 s for target off; SD: ± 0.184 s). The purpose of this condition was to test whether activity modulations during MIB and Replay-active depended on the need for behavioral report of the target disappearance and reappearance events. We ensured that subjects attended to the stimuli by excluding all trials in which subjects broke fixation or made eye blinks.
General design.
All 11 subjects participated in the main experiment consisting of the MIB, Replay-active, and Replay-no-mask conditions. Across 2 recording sessions (days 1 and 2), subjects performed 32 MIB runs, 24 Replay-active runs, and 8 Replay-no-mask runs. In each session, the experimental conditions were presented in blocks of four 2-min runs, each preceded and succeeded by a 30-s blank fixation period. Conditions were presented in pseudorandom order. The first four runs of each session were MIB. In the remaining runs, MIB, Replay-active, and Replay-no-mask conditions were randomly selected, under the constraint that the total number of runs per session would be 16, 12, and 4 for MIB, Replay-active, and Replay-no-mask, respectively. Ten subjects participated in two further scanning sessions, during which they performed the Stimulus-on-off (300–600 trials) and Replay-passive conditions (12–20 runs).
MEG data acquisition.
Whole head MEG data were acquired at 1,250 Hz in a magnetically shielded room on a 306-channel whole head neuromagnetometer (Elekta Neuromag, Helsinki, Finland) for several recording blocks. During each recording block, subjects performed four of the 2-min runs of the different experimental conditions described above. Each of the 102 sensor units consisted of two orthogonal planar gradiometers and one magnetometer. An anti-aliasing filter and a high-pass filter of 410 and 0.1 Hz, respectively, were applied online. The electrooculogram (EOG; measured from the upper right eye canthi) and electrocardiogram (ECG) were simultaneously recorded. Before MEG recordings, the positions of four head localization coils and the outline of the subjects' scalp (500 points) were digitized with a three-dimensional (3D) digitizer. During each 10-min recording block, the relative position of the coils to the MEG sensors was continuously monitored. For all blocks, head motion was <5 mm (Euclidian distance).
Preprocessing of MEG data.
The MEG data were analyzed using MaxFilter software (Elekta Neuromag, version 2.10) (version 5.10.2) and the FieldTrip (Oostenveld et al. 2011) and custom-made MATLAB software (The MathWorks, Natick, MA). The following preprocessing steps were performed: signal space separation, trial extraction, muscle and eye artifact rejection, and resampling to 500 Hz. All sensors were used for signal space separation. Only planar gradiometers were included in muscle artifact rejection and further analyses of the experimental effects of interest. MEG signals measured by these planar gradiometers peak primarily above the source (Hamalainen et al. 1993).
NOISE REMOVAL.
Background noise in the MEG data was removed using the temporal extension of Signal Space Separation (tSSS) technique within the MaxFilter software (Taulu et al. 2004). To this end, we used a time window of 8 s and a correlation limit of 0.9.
TRIAL EXTRACTION.
For the Stimulus-on-off condition, we extracted trials of fixed durations, ranging from 0.5 s before to 1.75 s after stimulus onset (i.e., 1 s after stimulus offset). For the MIB and Replay conditions involving subjects' reports, we extracted trials of variable duration, centered on subjects' button presses or releases, from the 2-min runs of continuous stimulation. Thus, in the case of MIB, the term “trial” refers to an epoch of constant stimulation and is solely defined on the basis of subjects' subjective reports of target disappearance and reappearance. The following constraints were used to avoid mixing data segments from different percepts when averaging across trials: 1) the maximum trial duration ranged from −1.5 to 1.5 s relative to report; 2) when another report occurred within this interval, the trial was terminated 0.5 s from this report; 3) when two reports succeeded one another within 0.5 s, no trial was defined; and 4) for the analysis of Replay-active and Replay-no-mask, we included only those reports that were preceded by a physical change of the target stimulus within 0.2 to 1 s, thus discarding reports following illusory target disappearances. In an alternative analysis of all Replay conditions, trials were defined in the same way as described above but aligned to physical target on- and offsets, eliminating the need to discard illusory target disappearances.
TRIAL REJECTION AND LINE NOISE REMOVAL.
Trials containing eye blinks, saccades, or muscle artifacts were rejected from further analysis using standard automatic methods. The signal time courses were bandpass filtered in the specific frequency range that contained most of the artifact. These ranges were as follows: 1–15 Hz (EOG channel only) for blinks and 110–140 Hz (MEG gradiometers only) for muscle activity. Filtering was followed by z-transformation. Trials exceeding a predefined threshold z-score were removed completely from analysis. We used the following thresholds: blinks, z = 4 for all subjects; muscle, z = 7.5 for 9 subjects and z = 10 for 2 subjects. Line noise was removed by subtracting the 50-, 100-, 150-, and 200-Hz Fourier components from the raw MEG time course of each trial.
Analysis of MEG power modulations.
SPECTRAL ANALYSIS OF MEG POWER.
We used sliding window Fourier transform (Mitra and Pesaran 1999) (window length: 400 ms, step size: 50 ms) to calculate time-frequency representations of the MEG power (spectrograms) for the two gradiometers of each sensor and each single trial. We used a single Hanning taper for the frequency range 3–35 Hz (frequency resolution: 2.5 Hz, bin size: 1 Hz) and the multi-taper technique for the frequency range 36–140 Hz (spectral smoothing: 8 Hz, bin size: 2 Hz, 5 tapers). After time-frequency analysis, the two orthogonal planar gradiometers of each sensor were combined by taking the sum of their power values.
STIMULUS-INDUCED POWER MODULATIONS.
For Stimulus-on-off, spectrograms were averaged aligned to MIB stimulus onsets. For MIB and Replay conditions, spectrograms were averaged aligned to perceptual reports of target disappearances/reappearances (MIB and Replay) or to the corresponding target off/onsets (Replay).
SWITCH-RELATED POWER MODULATIONS.
Power modulations [denoted as M(f,t) in Figs. 1–3, 5, and 7] were characterized as the percentage of power change at a given time and frequency bin, relative to a “baseline” power value for that frequency bin. For Stimulus-on-off, the baseline was computed as the mean power across the prestimulus blank fixation interval (from −0.25 to 0 s relative to stimulus onset). For MIB and Replay, the baseline was computed by averaging the power values across all time points within each trial (maximum time range: −1.5 to 1.5 s around the event) and then across all trials from a given recording session, pooling across disappearance and reappearance trials. We computed separate baselines for each experimental condition (i.e., MIB, Replay-active, etc.) and each recording session. Consequently, the M(f,t) values around perceptual reports represent the modulation of MEG power relative to the mean power across all artifact-free epochs from all runs of a given condition (MIB, Replay-active, etc.).
Fig. 3.
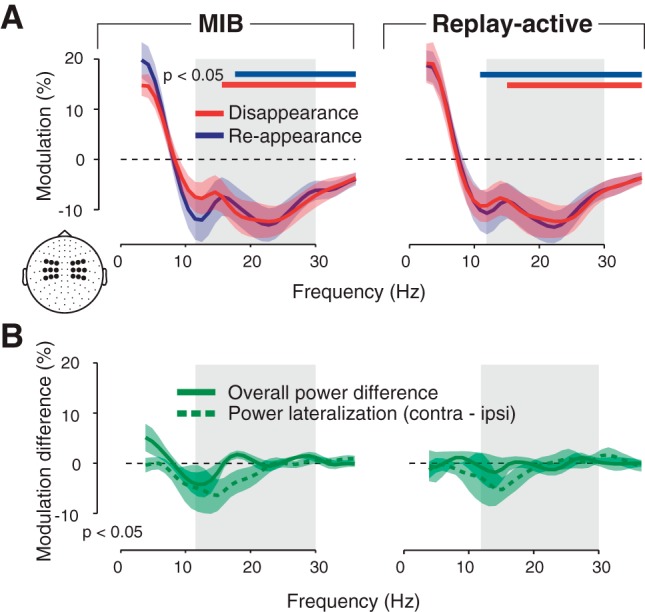
Widespread beta-power transient over visual cortex encodes report. In A and B, graphs show spectra of mean low-frequency (<35 Hz) power modulation during transient time window (−0.3 to 0.3 s; see Fig. 2A) around report. Colored bars indicate clusters of significant modulation (P < 0.05, 2-sided permutation test, cluster-corrected). A: MIB, Replay-active, and difference (MIB − Replay-active). Inset scalp map represents the sensor group used for this analysis. B: solid line indicates the reappearance − disappearance difference for overall power modulation (i.e., difference between blue and red line in A). Dashed line indicates lateralization contralateral vs. ipsilateral with respect to target hemifield. Triangles and squares in scalp map represent the sensors used for lateralization analyses. Green solid bar represents clusters of significant overall modulation; black bar represents clusters of significant difference between overall modulation and lateralization (P < 0.05, 2-sided permutation test, cluster-corrected). There is no significant frequency cluster for the lateralization. Shaded colored areas indicate SE (n = 11 subjects); shaded gray areas indicate the range of beta-band modulation during MIB (12–30 Hz). C: topographical maps of transient 12- to 30-Hz modulations and disappearance − reappearance differences.
Fig. 5.
Beta-power transient over visual cortex is unrelated to motor act. A: opposite mappings of perceptual event to motor act on days 1 and 2. See main text for details. B and C: same as Fig. 3, A and C, but separately for both mappings (i.e., recording days).
Fig. 7.
Beta-band MEG power modulation during MIB is not due to microsaccades. A: modulations of microsaccade rate for MIB target disappearance and reappearance. Gray shaded areas represent the intervals used for computing the difference between the number of microsaccades before and around report (rate change). Error bars indicate SE across subjects. B. time-frequency representation of the correlation between microsaccade rate change and MEG power modulation around MIB disappearance and reappearance reports. Transparency level highlights clusters of significant modulation (P < 0.05, 2-sided permutation test, cluster-corrected). Inset scalp map represents the sensor group used for this analysis. C: time-frequency representation of MEG power modulation, selectively averaged across trials without microsaccades between −0.35 and 0.25 s relative to report (dotted outline). Transparency level highlights clusters of significant modulation (P < 0.05, 2-sided permutation test, cluster-corrected). D: frequency spectra of power modulation for disappearance and reappearance in the transient time window for trials without microsaccades in this time window. Red solid bar indicates clusters of significant modulation (P < 0.05, 2-sided permutation test, cluster-corrected).
We focused our analysis of MEG power modulations around MIB reports on those cortical regions that also processed the physical MIB stimulus (i.e., visual cortex). Therefore, before performing the statistical tests described in this and the following section, we averaged all power modulations across the 12 occipital sensors exhibiting the biggest stimulus-induced (high and low frequency) response during Stimulus-on-off (marked in Fig. 1C).
STATISTICAL TESTS OF POWER MODULATIONS.
We used a two-tailed permutation test (1,000 permutations) (Efron and Tibshirani 1998) to test the significance of the overall power modulations of this sensor group and of the difference in power modulation between the sensors contralateral and ipsilateral to the target location (i.e., power lateralization). We tested the overall power modulation and power lateralization for 1) significant deviations from zero, 2) significant differences between disappearance and reappearance, and 3) differences between MIB and Replay-active. For all these tests, we used a cluster-based procedure (Oostenveld et al. 2011) to correct for multiple comparisons. For time-frequency representations of power modulations (see Figs. 1, 2, and 7), this was done across all time-frequency bins. Because the focus of the current study was on transient modulations of cortical activity around the perceptual switches, we averaged power modulations across the time window −0.3 to 0.3 s around report (“transient” time window indicated by black bar in Fig. 2A) for all subsequent analyses before performing statistical tests (see Figs. 3–7). For statistical tests of the spectra of transient power modulations around report (see Figs. 3–5 and 7), we performed the permutation test with cluster-based multiple comparison correction across frequency bins. Because our focus was on the MIB illusion (with the Replay conditions as controls), we defined our frequency band for further analyses on the basis of the significant cluster around disappearance reports during MIB (12–30 Hz; see Figs. 2A and 3A).
Fig. 2.
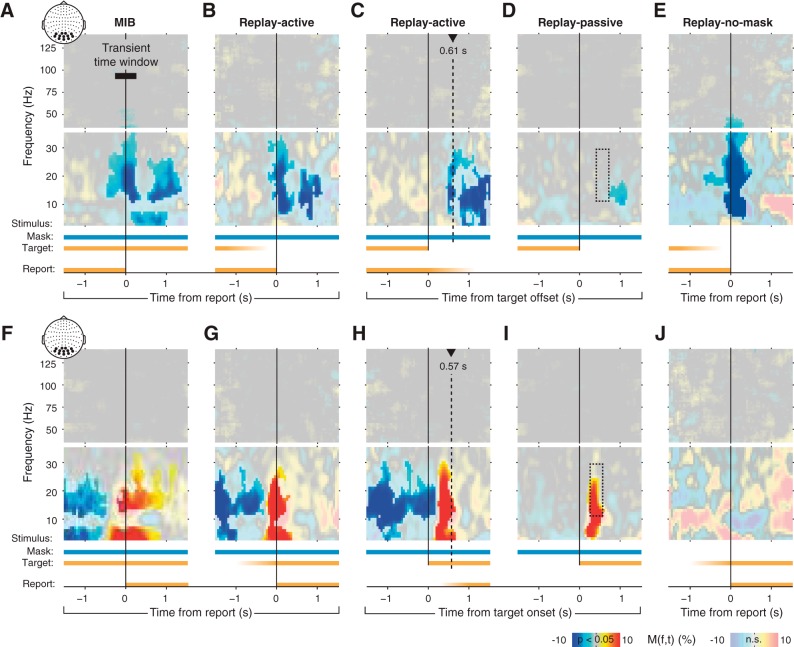
Switch-related power modulations over visual cortex. MEG power modulations are shown as time-frequency representations, averaged across trials and subjects. Transparency level highlights clusters of significant modulation (P < 0.05, 2-sided permutation test, cluster-corrected). In A–J, graphs at top and middle correspond to high- and low-frequency ranges, respectively; colored bars at bottom correspond to time course of stimulus components and subjects' reports. Fading indicates variable timing of (instantaneous) stimulus changes with respect to the trigger. Different panels correspond to different experimental conditions and/or different trigger events. Inset scalp maps represent the sensor group used for this analysis. A: MIB, aligned to report. Black bar represents transient time window used for subsequent analyses. B: Replay-active condition, aligned to report. C: Replay-active condition, aligned to stimulus change. Dotted vertical line indicates median reaction time. D: Replay-passive condition, aligned to stimulus change. E: Replay-no-mask condition, aligned to report. F–J: same as A–E, but for target reappearance. Dotted rectangle in D and I shows time-frequency window for Table 1.
Correlation analysis.
We intended to test whether the overall power modulation in the beta band (12–30 Hz) that we observed during the transient time window around subjects' reports (−0.3 to 0.3 s from report) predicted the duration (i.e., stability) of the perceptual suppression. To this end, we collapsed the single-trial power modulation values across the transient time window around report and the beta band. This yielded one scalar modulation value per trial. These scalar modulation values were then concatenated into a new (across trial) time series, which was submitted to piecewise linear detrending to remove slow intrinsic dynamics from the cortical power that are unrelated to perceptual or cognitive processing (Donner et al. 2009; Leopold et al. 2003). On the basis of the amplitude of the detrended single-trial modulation values, we then grouped trials (within subjects) into 10 (or other numbers for control, see below) percentile bins (see Linkenkaer-Hansen et al. 2004 for an analogous procedure).
Each trial was then associated with a transient beta-band modulation and with the duration of the preceding percept (target visible) and succeeding percept (target invisible). After normalizing individual percept durations by each subject's median percept duration (separately for both percepts), we collapsed the normalized percept durations per bin (based on transient beta modulation within subjects; see above) and, finally, computed the mean and normalized duration per bin across subjects. The normalization compensated for substantial interindividual variability in the perceptual durations, thereby isolating the trial-to-trial variability of percept durations. However, the results reported in this article were qualitatively identical without this normalization (data not shown). We then correlated the bin rank number to normalized percept duration.
We used a permutation (shuffling) procedure (1,000 permutations) to test the significance of the correlations (Efron and Tibshirani 1998). Mapping the individual power modulation values into a common range of percentile bins before averaging them across subjects compensated for the interindividual variability in the power modulation values, which is caused by many factors other than neural activity (e.g., individual differences in head geometry and brain anatomy). To assess the robustness of the observed correlations, we repeated these analyses for several different bin numbers (15, 30, and 60 bins).
A stabilizing effect of the transient beta-band modulation on the duration of perceptual suppression would predict 1) a significant and strong correlation with the duration of the succeeding (target invisible) percept during MIB, 2) no correlation with the duration of the preceding (target visible) percept during MIB, and 3) no correlation with the duration of the succeeding (target invisible) percept during Replay, where the percept durations are largely determined by the physical target on- and offsets. Thus we predicted significant differences in the correlation coefficients for prediction 1 vs. prediction 2 and for prediction 1 vs. prediction 3. To test for these two predicted differences in correlation, we again used a permutation procedure (1,000 permutations). Here, we permuted the labels (e.g., “preceding” vs. “succeeding”) of pairs of bin rank number and corresponding percept durations.
Microsaccade Control Experiment
Subjects.
MEG data and high-resolution (infrared) eye data were simultaneously acquired at the Universitätsklinikum, Hamburg-Eppendorf, from 22 healthy subjects with normal or corrected-to-normal vision. The experiment was approved by the local ethics committee, and each subject gave written informed consent. One subject failed to complete the full experiment, and one subject had poor eye tracking data quality. Both subjects were excluded. Thus 20 subjects (11 women, age range: 20–54 yr) were included in the analysis.
Stimulus and behavioral task.
The target was a full-contrast Gabor patch (diameter: 2°, 2 cycles) surrounded by a rotating mask (17° × 17° grid of white crosses), both superimposed on a gray background and centered on a fixation mark (red outline, white inside, 0.8° width and length) in the middle of the screen. The target was located in either the lower left or lower right visual field quadrant (eccentricity: 5°, counterbalanced between subjects). The mask rotated at a speed of 160°/s and was separated from the target by a protection zone of 2°. We used a Gabor target on a gray background in this experiment to eliminate any changes in perceived brightness during MIB for analyses of changes in pupil diameter that will be the focus of a separate report.
Stimuli were presented using Presentation software (NeuroBehavioral Systems). Stimuli were back-projected on a transparent screen using a Sanyo PLC-XP51 projector with a resolution of 1,024 × 768 pixels at 60 Hz.
Subjects were seated 58 cm from the screen in a whole head MEG scanner setup in a dimly lit room. The stimulus was continuously presented for 6 runs of 3-min duration each. During that time, subjects kept their gaze on the fixation mark and reported the spontaneous target disappearance and reappearance by pressing a response button with their right index finger and right middle finger, respectively. To select the 25 sensors overlying visual cortex that showed the biggest stimulus-induced response, subjects additionally performed the Stimulus-on-off condition described above for the main experiment, but with the grayscale MIB stimulus (see Stimulus-on-off condition).
Data acquisition.
MEG data were acquired at 1,200 Hz on a 275-channel whole head neuromagnetometer (CTF 275; VSM/CTF Systems, Port Coquitlam, BC, Canada). Subjects were placed in a seated position inside the scanner. The location of the subjects' head was measured in real time using three fiducial markers placed in the both ears and on the nasal bridge to control for excessive movement. The EOG and ECG were recorded to aid artifact rejection.
Concurrently with the MEG recordings, the position of the left eye's pupil was sampled at 1,000 Hz with an average spatial resolution of 15–30′ arc, using an MEG-compatible EyeLink 1000 Long Range Mount system (SR Research, Osgoode, ON, Canada) placed on a table under the stimulus presentation screen. The eye tracker was calibrated before every block of four runs.
MEG and eye tracking data analysis.
PREPROCESSING.
Both the MEG and eye tracking data were analyzed using the FieldTrip (Oostenveld et al. 2011) and custom-made MATLAB software (The MathWorks). The following preprocessing steps of the MEG data were performed as described for the main experiment above: trial extraction, muscle and eye artifact rejection, and resampling to 500 Hz.
To align the eye and MEG data, the continuous eye data was first upsampled to 1,200 Hz to match the MEG sampling rate. The triggers of perceptual reports in the MEG and eye data were then used to align the two data sets. Periods of blinks in the eye data were detected using the EyeLink's standard algorithms with default settings. Blinks were removed by linear interpolation of values measured just before and after each identified blink (interpolation time window: from 0.1 s before until 0.1 s after blink). Trials in which the gaze was more than 100 pixels from the fixation cross for more than 10% of the trial's duration were excluded from further analysis. Finally, the eye tracking data was downsampled to 250 Hz.
SPECTRAL ANALYSIS OF MEG POWER.
Spectral analysis of MEG power modulations was performed as described for the main experiment above. We used the 25 sensors over visual cortex showing the biggest increase in gamma power during the Stimulus-on-off condition for the analyses of power modulation during MIB (see Fig. 7, scalp map insets).
ANALYSIS OF MODULATIONS OF MICROSACCADE RATE.
Microsaccades were detected using the algorithm developed by Engbert and colleagues (Engbert and Kliegl 2003, Engbert and Mergenthaler 2006). Preprocessed eye data were first smoothed with a window of 20 ms to optimize microsaccade extraction. The permitted amplitude range was 0.08°–2° of visual angle, with minimum duration of 16 ms; microsaccades outside these ranges were rejected. Microsaccades that occurred within 20 ms of one another were merged into one saccade. The algorithm yielded a binary time course of saccade occurrences (i.e., 1's embedded in a stream of 0's) for each trial. These time courses were convolved with a Gaussian window (σ = 0.1 s) (Bonneh et al. 2010; Martinez-Conde et al. 2004) and averaged across trials (separately for target disappearance and reappearance) and subjects.
CORRELATION BETWEEN MEG POWER AND MICROSACCADE RATE CHANGE.
One analysis tested whether changes in microsaccade rate around perceptual switches in MIB (Bonneh et al. 2010) cause modulations of MEG power over visual cortex. To this end, we correlated the microsaccade rate change around perceptual report to the MEG power modulation time courses M(f,t) on a trial-by-trial basis. We calculated the microsaccade rate change for each trial by subtracting the number of microsaccades during a time window showing the transient MEG power modulations (−0.35 to 0.25 s relative to report) from the number of saccades in the preceding window (−0.95 to −0.35 s relative to report). Because responses in visual cortex that are evoked in visual cortex must occur only after some delay, we shifted the first time window by 50 ms backward in time relative to the transient time window used to extract the transient MEG modulations analyzed in this study (−0.3 to 0.3 relative to report). The presently reported results are robust with respect to this choice and also occurred for other delays (including 0 ms; data not shown).
We then correlated this rate change with MEG power in target disappearance trials, separately for each time-frequency bin (interval −1 to 1 s around report in the 3- to 35-Hz frequency range). The resulting time-frequency representations of correlation values were then tested for significant clusters across subjects by using the cluster-based permutation procedure described above (see Statistical tests of power modulations).
ASSESSING MEG POWER MODULATIONS IN THE ABSENCE OF MICROSACCADES.
In a complementary analysis, we tested whether the transient beta-band modulation was also evident in the absence of any microsaccades during the critical time window (−0.35 to 0.25 s relative to report) in which microsaccades could have evoked modulations in MEG power. Longer time windows would have yielded an insufficient number of microsaccade-free trials (zero for most subjects). Only subjects who had more than 15 microsaccade-free trials per condition were included in this analysis (n = 11 for disappearance and n = 6 for reappearance). The results were qualitatively similar when an inclusion threshold of 5, 10, 20, or 25 trials was used (data not shown).
RESULTS
Eleven healthy subjects viewed the continuous presentation of the MIB stimulus (Fig. 1A) during 32 runs (2 min each) while MEG activity was recorded (see materials and methods). The durations of MIB target disappearances and reappearances varied widely (Fig. 1B). In several control conditions, perceptual switches similar to MIB target disappearances and reappearances were exogenously triggered by physically removing the target from the screen in the same temporal sequence as MIB disappearances in that same subject, thus “replaying” the subjective MIB illusion to the subject. We therefore collectively refer to these control conditions as Replay. We used three different Replay conditions to disentangle the relationships between physical stimulus components, perceptual switches, subjects' behavioral reports, and the measured modulations of cortical activity (see below). During the Replay-active condition, subjects reported target disappearance and reappearance as during MIB. The Replay-passive condition was identical to Replay-active, except that subjects did not report the target disappearances. The Replay-no-mask condition was identical to Replay-active, except that the target was presented without the surrounding mask (see materials and methods and below for the motivation behind each condition).
We focused our analyses on MEG sensors overlying occipital cortex that were most strongly driven by the MIB stimulus when presented for 0.75 s in separate runs (Fig. 1C). Consistent with previous studies (Donner and Siegel 2011; Fries 2009; Jensen and Mazaheri 2011), sensors overlying visual cortex showed the strongest stimulus-induced enhancement of gamma-band (60–90 Hz) and suppression of low-frequency (8–35 Hz) power. We computed the overall power across 12 sensors with maximum stimulus-induced responses, as well as the lateralization (contralateral or ipsilateral) with respect to the variable (left or right) visual hemifield position of the target. The corresponding sensors are highlighted on the topographical maps in Fig. 1C.
Top-Down Modulation in Visual Cortex During Report of Perceptual Change
We analyzed the switch-related modulations in these 12 visually responsive sensors during the continuous viewing of the stimulus in the MIB illusion and the three Replay conditions described above. This was done to test four predictions about the switch-related modulations, which were derived from previous studies (Donner et al. 2008; Wilke et al. 2006): there should be a widespread modulation over visual cortex that 1) exhibits opposite polarity for MIB target disappearance and re-appearance, 2) occurs also during Replay-active, 3) is contingent on the need for behavioral report of the perceptual changes (i.e., absent during Replay-passive), and 4) is decoupled from the MIB stimulus components (target, mask). More specifically, regarding prediction 4, we expected that the modulation would occur also in the absence of the mask (Replay-no-mask). The tests of these predictions are described in the following and shown in Figs. 2 (all conditions) and 3 (focusing on MIB and Replay-active). A qualitative summary of the power modulations in all conditions is provided in Table 1.
Table 1.
Sign of the beta-band power modulation (12–30 Hz) around report (−0.3 to 0.3 s)
| MIB | Replay-Active | Replay-Passive | Replay-No-Mask | |
|---|---|---|---|---|
| Disappearance | ↓ | ↓ | n.s. | ↓ |
| Reappearance | ↑ | ↑ | ↑ | n.s. |
Arrows indicate significant changes in power (12–30 Hz) in the time window from −0.3 to 0.3 s relative to report during motion-induced blindness (MIB) and the Replay conditions (see text for details). ↓, Power suppression; ↑, power enhancement; n.s., not significant.
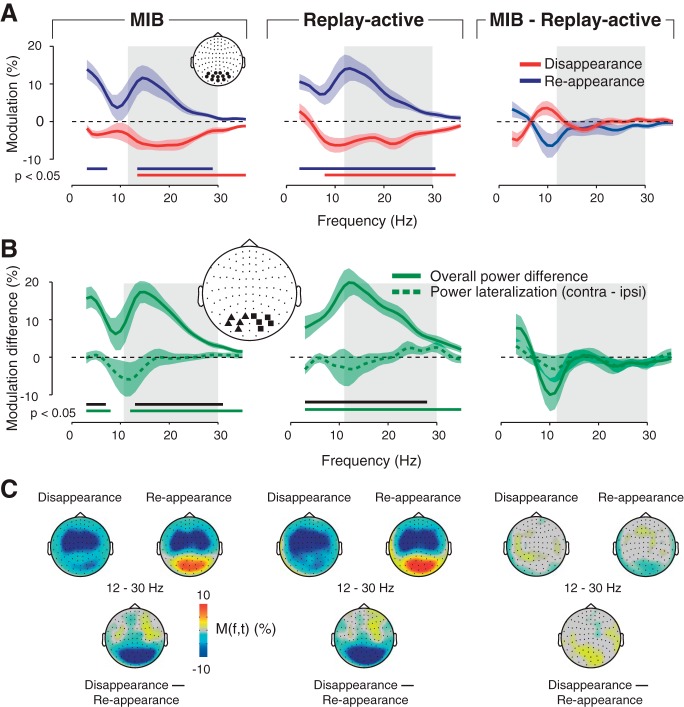
As expected, we observed MEG power modulations over visual cortex with opposite polarity for MIB target disappearance (Fig. 2A) and reappearance (Fig. 2F). Specifically, around target disappearance, power was transiently suppressed in the beta band (12–30 Hz; Figs. 2A and 3A, left, red line). This transient suppression was followed by more sustained suppression, first in the <8-Hz range (from about 0.25 s after report) and then again in the beta band (from about 0.5 s after report). The modulation profile was nearly inverted for reappearance, with an enhancement of beta power around report (Figs. 2F and 3A, left, blue line). Notably, all these modulations around report excluded the gamma and alpha bands that are associated with bottom-up stimulus processing and attentional modulation in visual cortex (Donner and Siegel 2011; Fries 2009; Jensen and Mazaheri 2011). Furthermore, the modulations seemed widespread across the visual cortex (Fig. 3C; see Top-Down Modulation is Decoupled From Target Stimulus and Motor Act).
Previous fMRI results indicate that rather than prompting the perceptual switch, retinotopically global modulations in early visual cortex (in particular V1) are a consequence of the switch, occurring in both MIB and a replay of MIB, provided that these perceptual changes are actively reported (Donner et al. 2008). We therefore expected similar modulations during MIB and the Replay-active condition. Indeed, we found a similar beta-band modulation (with the same opposite polarity for disappearance and reappearance) also in Replay-active (Figs. 2, B and G, and 3A, middle). Here, the modulation peaked closely around subjects' median reaction time relative to the physical target offsets or onsets in Replay-active (dashed vertical lines in Fig. 2, C and H). There was no significant difference between transient power modulations during MIB and Replay-active across the beta-frequency range (Fig. 3A, right). The only difference between MIB and Replay-activity was a stronger modulation of alpha-band (8–12 Hz) power in Replay-active (P = 0.026, permutation test after collapsing across the 9- to 12-Hz range). This enhanced alpha modulation during Replay-active is consistent with attention capture by the transient change of the physical stimulus. The similarity in the spectral profile and amplitude of the power modulations during MIB and Replay-active is consistent with the beta-band modulation being a consequence, not the cause, of the perceptual switches during MIB.
Previous fMRI results showed that the global modulations in early visual cortex occurred only when the target disappearance and reappearances were actively reported, whereas they were absent during passive viewing (Donner et al. 2008). Similar observations were made for modulations of local field potential power in monkey cortex and thalamus (Wilke et al. 2006, 2009). Thus we expected the beta-band modulation to be absent in the Replay-passive condition. Indeed, in line with this prediction, there was no transient beta-power suppression during disappearance (Fig. 2D). The dashed rectangle in Fig. 2D indicates the interval containing the significant transient modulation in Replay-active (compare with Fig. 2C). During target reappearance in Replay-passive, however, the power was enhanced across a wider range including the beta band (Fig. 2H). Thus the beta suppression during target disappearance (but not the beta enhancement during target reappearance) critically depended on the behavioral relevance of the perceptual change, possibly combined with the unpredictable timing of the changes.
Finally, we tested whether the beta-band modulation was independent of the presence of the mask. Indeed, in the Replay-no-mask condition the beta suppression during target disappearance was robust (Fig. 2E), and at least as strong as in MIB and Replay-active (with mask), implying that it did not require the presence of the mask. By contrast, there was no significant power enhancement during target reappearance (Fig. 2J). This might indicate that the power enhancement seen in the other conditions (Fig. 2, F–I) might reflect a “contextual modulation” of the visual response to the mask by the reappearance of the target (Zipser et al. 1996). Such a contextual modulation might occur, for example, because the target might interfere with the representation of the mask as a coherent surface.
There was a double dissociation between beta-power modulations during Replay-passive and Replay-no-mask conditions (cf. Fig. 2, D and E vs. I and J, and columns 3 vs. 4 in Table 1). This indicates that the beta suppression during target disappearance and the power enhancement during target reappearance are functionally distinct. The former, but not the latter, is consistent with a nonsensory modulation of population activity. This modulation does not reflect the cortical response to the MIB stimulus, but rather an endogenous signal originating within the brain. In the following, we therefore refer to this signal during target disappearance as a “top-down modulation.”
Top-Down Modulation is Decoupled From Target Stimulus and Motor Act
One well-characterized source of top-down modulation in visual cortex is spatial attention (Kastner and Ungerleider 2000). Spatial attention to visual targets contained in one visual hemifield induces a selective lateralization of MEG power, relative to the target, in different frequency bands (Jensen and Mazaheri 2011; Siegel et al. 2008; Wyart and Tallon-Baudry 2008). During MIB, power modulations reflecting spatial attention might be selective for the target, the perception of which changes over time, as shown in an MIB study using one target per hemifield (Händel and Jensen 2014). In contrast to the spatial attention prediction, we found that the beta-band modulation was not selective for target location during MIB (Fig. 3B, left) and Replay-active (Fig. 3B, middle). There was no evidence for lateralization in the beta range, even for the most sensitive quantification of the beta-band modulation (disappearance − reappearance difference; dotted lines). Second, the overall power modulation (i.e., ipsilateral and contralateral sensors pooled; solid lines) was significantly stronger than the lateralization. Note that power suppression in the alpha band (around 10 Hz), in contrast, exhibited a trend to significant lateralization, in particular during MIB, in line with the results of Händel and Jensen (2014). In sum, the beta-band modulation during perceptual changes is widespread across visual cortex, distinct from spatially selective attention signals that have been measured with the use of similar techniques in other studies. Consequently, collapsing the beta-band modulation across subjects irrespectively of target location yielded a robust modulation (Figs. 2 and 3).
Given the strong link of the beta-band modulation to behavioral report, another possible source of the top-down modulation in visual cortex is the motor act (button presses/releases) used for report. Motor movements are commonly associated with a suppression of beta-band oscillations in the motor system (Donner et al. 2009; Pfurtscheller and Lopes da Silva 1999). Indeed, we observed strong beta-power modulation over left and right motor cortices during report (Figs. 3C and 4A). However, in line with previous studies (Donner et al. 2009; Pfurtscheller and Lopes da Silva 1999), this motor beta-power modulation was stereotypically negative, irrespective of the type of report, in sharp contrast to the beta modulation over visual cortex (Fig. 3C). Furthermore, the amplitude of the motor beta suppression did not differ between disappearance and reappearance reports (Fig. 4), again in sharp contrast to the visual cortex beta modulation (Fig. 3).
The dissociation between the visual beta-band modulation and motor act was also evident in a separate analysis of the two recording sessions, in which the mapping between perceptual switch and motor response was flipped (Fig. 5). On day 1, subjects pressed the response button to indicate target disappearance and released the button to indicate target reappearance; on day 2, this mapping was reversed (Fig. 5A). The beta-band modulation in visual cortex was qualitatively identical for both mappings, with a significant suppression of beta power for disappearance and an enhancement for reappearance (Fig. 5, B and C).
In sum, the beta-band modulation in visual cortex was decoupled from the target, and thus unlikely to reflect spatial attention, and it was also decoupled from activity in the motor cortex that was related to the behavioral report. A third possibility, which is consistent with all results presented in this report, is that the beta-power suppression during target disappearance is driven by the central process that transforms the perceptual change into behavioral report: the beta-band suppression 1) is closely linked in time to the behavioral report, 2) occurs irrespective of whether this perceptual change is spontaneous (MIB) or stimulus-evoked (Replay), and 3) is unaffected by large changes in the stimulus configuration (Replay-no-mask), but 4) is strongly affected by eliminating the need for behavioral report (Replay-passive).
Top-Down Modulation Predicts Duration of Perceptual Illusion
We next tested for a possible functional role of the transient modulation in visual cortex: stabilization of the subsequent MIB illusion. To this end, we correlated the amplitude of the transient beta suppression to the duration of the subsequent MIB target disappearance (i.e., using MIB duration as an index of perceptual stability; see materials and methods). Indeed, stronger beta suppressions (i.e., populating the lower rank bins) were followed by longer MIB durations (Fig. 6A, right). This correlation was highly significant across a range of different bin sizes as well as without normalizing individual MIB durations by the median per subject (data not shown).
Fig. 6.
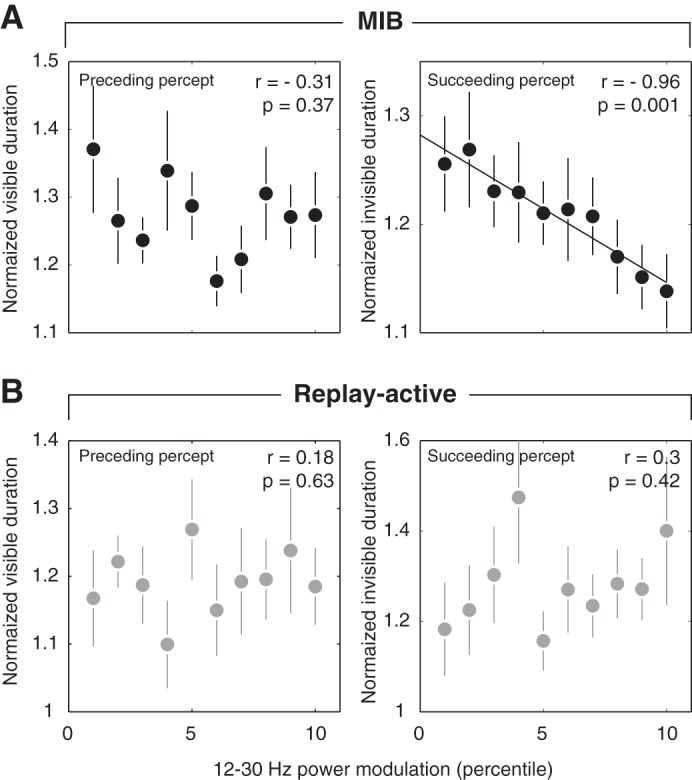
Beta-power transient predicts stability of MIB illusion. Pearson correlation between beta-power suppression during disappearance report and the duration of the “preceding percept” (i.e., target visible, left) or “succeeding percept” (i.e., target invisible, right). Single-trial percept durations were normalized by each subject's median percept duration (see main text for details). A: MIB. B: Replay-active. Error bars indicate SE (n = 11 subjects).
There was no significant correlation to the preceding target visible duration (Fig. 6A, left) and a significant difference in the correlations for succeeding vs. preceding percept duration (P = 0.03, permutation test). Thus, as for the correlation to variability of cortical activity, the correlation to MIB duration was directed in time, specific for the succeeding target disappearance.
As shown in the previous sections, the beta suppression (and its relation to trial-to-trial variability) was indistinguishable between MIB and Replay-active. The key difference between both conditions was the stability of perception: Target perception was bistable in MIB (i.e., percept durations governed by cortical interactions) but stable during Replay (i.e., percept durations governed by the physical on- and offsets of the target). Accordingly, as expected, there was no significant correlation during Replay-active (Fig. 6B), and the correlation was significantly smaller (i.e., less negative) than during MIB (P = 0.002, permutation test).
Finally, the association between beta-band activity and MIB disappearance duration was specific for the sensors overlying visual cortex. There was no significant correlation between the beta-power suppression over motor cortex (which was even stronger than that over visual cortex) during target disappearance and the subsequent MIB duration (r = −0.34, P = 0.34, permutation test; data not shown). In line with the findings reported above, this indicates that the beta suppression during target disappearance reports reflects a top-down process confined to visual cortex and distinct from the process suppressing beta-band power over motor cortex.
Modulation is Not Due to Microsaccades
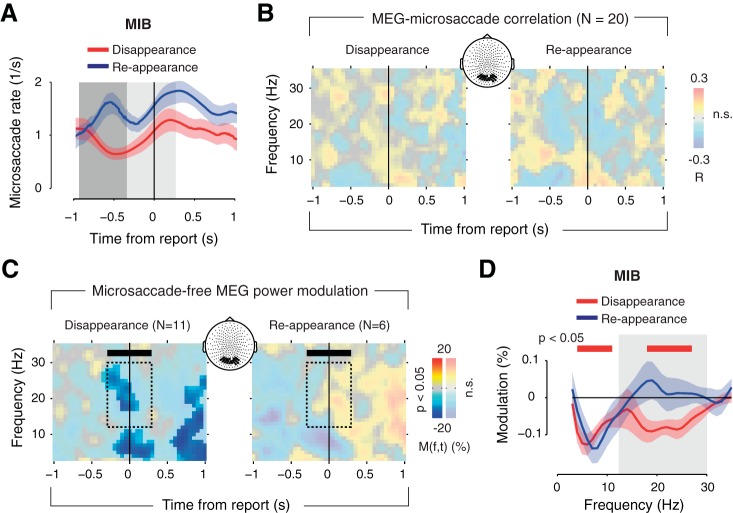
One potential concern is that the power modulation in visual cortex may have been due to subtle changes in fixational eye movements during the perceptual switches (Bonneh et al. 2010; Hsieh and Tse 2009). Specifically, target disappearance reports during MIB and Replay are accompanied by a reduction in the rate of microsaccades (Bonneh et al. 2010). This reduction may have been associated with MEG power suppression in the beta range.
This concern seems unlikely for two reasons. First, our analysis excluded all trials containing blinks and saccades that were detectable with EOG. Second, smaller microsaccades during sustained visual stimulation induce broadband (from low to high frequency) local field potential power enhancements in visual cortex (Bosman et al. 2009) and associated eye movement artifacts in the extracranial EEG (Yuval-Greenberg et al. 2008). A modulation of microsaccades, therefore, predicts broadband power modulations, whereas the power modulation reported here was confined to the beta band.
To conclusively rule out this concern, we performed an additional control experiment in which we again measured MEG power modulations over visual cortex while simultaneously monitoring microsaccades with a high-resolution infrared eye tracker (see materials and methods). The microsaccade rate exhibited similar modulations as previously reported (Bonneh et al. 2010), decreasing before MIB target disappearance and increasing before reappearance (Fig. 7A). However, during both target disappearance and reappearance, no time-frequency cluster of the MEG power modulation was significantly correlated with this change in microsaccade rate (Fig. 7B).
Finally, we tested whether the transient beta-band modulation was also evident in the absence of any microsaccades. We selectively averaged MIB disappearance and reappearance trials that contained no microsaccades (specifically in the time window from −0.35 to 0.25 s relative to report in which microsaccades could have evoked MEG power modulations) and found robust, statistically significant transient beta-band suppression for target disappearance (Fig. 7C, left) with a frequency profile similar to that in the main experiment (Fig. 7D). Although beta-band modulation for reappearance was again enhanced (Fig. 7C, right), it did not reach statistical significance, presumably because of the lower number of subjects that had a sufficient number of microsaccade-free trials to be included in this analysis (see materials and methods). These findings replicate the main neurophysiological signature reported in this work in an independent group of subjects (on a different MEG system) and further rule out the concern that this signature may be due to microsaccades.
DISCUSSION
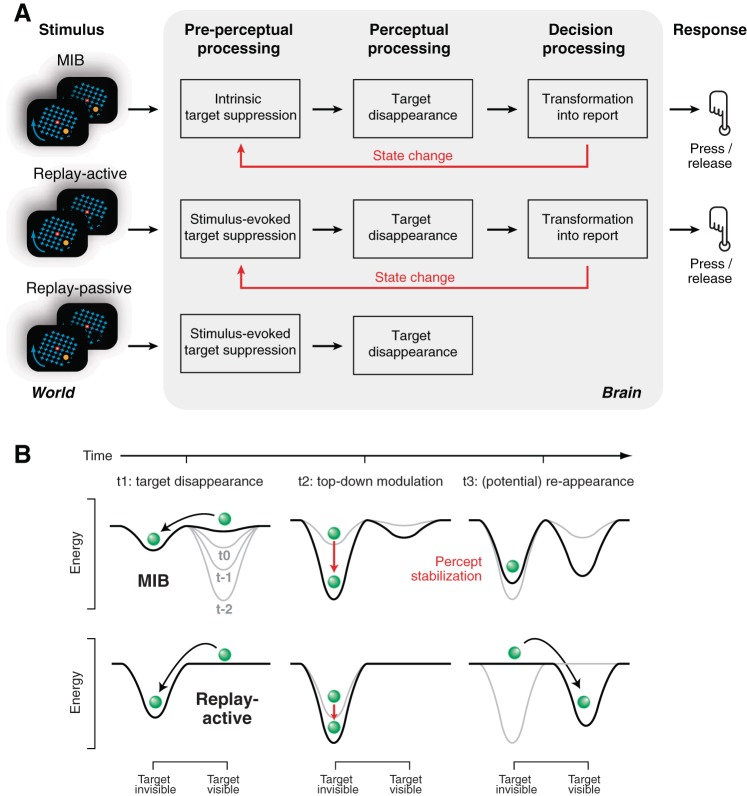
We examined whether transient top-down modulations in visual cortex during perceptual changes in a multistable illusion may have an impact on the subsequent perceptual state. Although some previous indirect evidence points to the existence of an active stabilization mechanism during continuous (Einhauser et al. 2008) or intermittent viewing (Leopold et al. 2002) of ambiguous stimuli, a neural signature of such a mechanism has not yet been observed. We reasoned that an active stabilization mechanism should be evident as a modulation of neural activity in visual cortex, which predicts the stability of the subsequent cortical state and the perceptual interpretation of the ambiguous stimulus. Consistent with this idea, we found a transient modulation of beta-band activity in visual cortex right around reports of MIB target disappearances. The amplitude of this modulation predicts the duration of the subsequent perceptual suppression of the target. This modulation was 1) closely coupled to subjects' behavioral reports of the perceptual changes and 2) independent of whether the perceptual change was initiated endogenously (MIB) or by an external stimulus change (Replay), but 3) contingent on the changes' behavioral relevance and/or unpredictable timing and 4) decoupled from the components of the MIB stimulus (target and mask), as well as from 5) the motor cortical activity leading to the final button press reports.
Figure 8 illustrates our interpretation of the origin and functional impact of the transient beta-band suppression during disappearance reports. Our results suggest that this signal is of top-down origin, triggered by the process that transforms the perceptual change into a behavioral report (Fig. 8A). We refer to this transformation as the perceptual decision, acknowledging that our task did not entail a choice between two options. Because the decision is independent of the cause of the perceptual change (intrinsic or stimulus evoked), the beta-band suppression is evident during MIB and its replay. However, when there is no need for reporting the perceptual change (Replay-passive), no beta suppression is observed.
Fig. 8.
Conceptual model of beta-power transient. A: schematic of the hypothetical process driving the report-related beta-band modulation in visual cortex. During MIB, Replay-active, and Replay-passive, the cortical representation of the target is suppressed, leading to the target's disappearance. The suppression occurs either spontaneously (i.e., due to intrinsic cortical processing in MIB) or in response to the physical target offset (Replay). If the perceptual disappearance is task-relevant (MIB and Replay-active, but not Replay-passive), a central decision process transforms it into a behavioral report. This transformation induces the top-down modulation in visual cortex. The top-down modulation, in turn, alters the state of visual cortex and, hence, the target representation. B: schematic of dynamical algorithm for cortical state change and perceptual stabilization. In both MIB (top) and Replay-active (bottom), the percept (green “ball”) is in the “target visible” valley. Adaption gradually flattens this valley before target disappearance (sequence: t−2, t−1, t0). The ball then hops into the “target invisible” valley (perceptual switch, t1). Behavioral report of this perceptual event induces a state change that deepens the target invisible valley (t2; red arrows). This, in turn, stabilizes perception during MIB (t3, top right): When the state change is strong, the percept variable is less likely to move back to the visible valley some time after the switch (t3). By contrast, during Replay (t3, bottom right), the physical target reappearance alters the energy landscape (i.e., eliminates the target invisible valley) and thereby prevents the state change at t2 from affecting the percept duration.
Whatever the exact nature of this top-down signal, it seems to alter (stabilize) the internal state of visual cortex (Fig. 8B). The movement of a “percept variable” (green “ball” in Fig. 8B) (Braun and Mattia 2010; Moreno-Bote et al. 2007) across an energy landscape with two valleys (basins of attraction; in the case of MIB, corresponding to target visible and invisible) provides a useful metaphor for understanding this effect (Deco and Romo 2008). In this scheme, the stabilizing state change can be conceived as an active force (red arrow) transiently deepening the valleys. Only if the sensory input is ambiguous and, consequently, the perceptual interpretation meta-stable (i.e., during MIB) does this transient state change culminate in a perceptual stabilization: the stronger the state change (i.e., longer red arrow) during a perceptual transition, the longer the subsequent perceptual illusion. During Replay, the physical removal of the target stimulus instantaneously alters the energy landscape, thus overriding the effect of the internal state change and precluding a link to percept duration. Note that we use the term “state change” to refer to a change in the dynamics of cortical activity (i.e., the shape of the energy landscapes in Fig. 8B) and not perception. The changes in perceptual state (i.e., target disappearances and reappearances) experienced by the subject correspond to the hopping of the percept variable from one valley of this landscape to the other.
Different from other multistable illusions, the MIB illusion is asymmetric in the sense that it only entails a single nonveridical percept (target invisible). This might explain why the top-down modulation is limited to the target disappearances. The power enhancement differed functionally from the target reappearance in that it also occurred during passive viewing of target stimulus onsets and depended on the presence of the mask. It seems likely that these functional differences are due to the inherent asymmetry of MIB and would not occur in symmetric bistable phenomena, such as binocular rivalry (Brascamp et al. 2006) or 3D structure from motion (Klink et al. 2008). Despite the differences in symmetry, recent psychophysical work (Bonneh et al. 2014) establishes analogous dynamical properties for MIB as for the above two phenomena. We thus hypothesize that analogous beta suppression effects as observed during MIB target disappearance will occur during all switches in these illusions. Future work should test this hypothesis.
Mounting evidence suggests that the widespread state change in visual cortex characterized in this work might be a general phenomenon. Several studies reported modulations of population activity in human and monkey early visual cortex, which were largely decoupled from cortical stimulus representations but linked to behaviorally relevant events (Cardoso et al. 2012; Choe et al. 2014; de-Wit et al. 2012; Donner et al. 2008, 2013; Jack et al. 2006; Hsieh and Tse 2009; Sirotin and Das 2009; Swallow et al. 2012; Wilke et al. 2006). Specifically, several human fMRI studies reported retinotopically widespread modulations in V1 during perceptual reports in MIB (Donner et al. 2008; Hsieh and Tse 2009), bistable motion binding (de-Wit et al. 2012), and visual discrimination tasks (Choe et al. 2014). Local field potential results from monkey visual cortex and thalamus (Gail et al. 2004; Wilke et al. 2006, 2009) point to modulations in the alpha and beta bands as electrophysiological underpinning of the widespread fMRI signal modulations associated with bistable perceptual dynamics. By establishing the retinotopically widespread nature and functional consequences of the switch-related beta-band modulations in the human brain, our current MEG results add critical new information to this emerging body of evidence.
What is the source of the beta-band modulation in visual cortex? At the functional level, the modulation could be a phasic arousal signal depending on the task demands (thus absent during passive viewing) and the timing of events that drive the modulation. It could also indicate that a response has been made. Although these possibilities should be addressed in future work, we find that the modulation is specific for the type of the perceptual change (occurring specifically during target disappearance) and that it is dissociated from activity evident over motor cortex. Thus our observations are inconsistent with a nonspecific task- or response-related mechanism, or a direct copy of activity from the motor cortex. At the neural level, the beta-band modulation in visual cortex might originate from higher cortical areas (Nienborg and Cumming 2009; Siegel et al. 2012), the thalamus (Wilke et al. 2009), or neuromodulatory brainstem centers (Aston-Jones and Cohen 2005; de Gee et al. 2014; Einhauser et al. 2008; Hupe et al. 2009; Parikh et al. 2007) or from a combination of cortical feedback and neuromodulation (Noudoost and Moore 2011).
Indeed, neuromodulatory brainstem systems, such as the noradrenergic locus coeruleus and the cholinergic basal forebrain systems, also exhibit transient activity during perceptual reports, which can reflect the content of the report (Aston-Jones and Cohen 2005; de Gee et al. 2014; Einhauser et al. 2008; Hupe et al. 2009; Parikh et al. 2007; also Kloosterman NA, Meindertsma T, van Loon A, Bonneh Y, Lamme VA, and Donner TH, unpublished observations). Further evidence suggests that beta-band power modulations in visual cortex during visual stimulation might index changes in neuromodulatory state (Belitski et al. 2008; Donner and Siegel 2011). If such beta-band modulation emerges from the interaction between neuromodulation and the bottom-up stimulus drive, this would explain why the beta-band modulation colocalizes with the response to the MIB stimulus, despite the more widespread neuromodulatory projections to the cortex. Finally, neuromodulatory brain stem systems are in a position to stabilize the perceptual dynamics because they can dynamically alter key cortical circuit parameters in profound ways. In particular, neuromodulators suppress cortical variability (Polack et al. 2013) and may amplify inhibitory interactions in cortical circuits (Haider et al. 2013). In competitive networks underlying multistable perception (Braun and Mattia 2010; Moreno-Bote et al. 2007), a transient boost of mutual inhibition is equivalent into the deepening of valleys shown in Fig. 7B.
The cerebral cortex continuously undergoes changes in internal state (Harris and Thiele 2011; Lee and Dan 2012; Steriade 2000). Whereas these state changes have traditionally been associated with slow fluctuations of arousal level (Haider et al. 2013; Harris and Thiele 2011; Steriade 2000), some of these state changes co-occur with rapid cognitive processes (Aston-Jones and Cohen 2005; Gilbert and Sigman 2007; Parikh et al. 2007). Our current results are consistent with the idea that the active report of perceptual changes triggers a cortical state change, which can stabilize an illusory percept. Future work should address whether the effect we have identified here for the MIB illusion generalizes to other perceptual phenomena (Fischer and Whitney 2014; Jazayeri and Movshon 2007; Stocker and Simoncelli 2008), as well as to more complex decisions beyond the domain of perception (Festinger 1957).
GRANTS
This work was supported by the European Research Council DEFCON1 Grant (to V. A. F. Lamme). T. H. Donner is supported by grants from the Netherlands Organization for Scientific Research (NWO; Dossier no. 406-14-016), the European Union 7th Framework Programme (FP7/2007-2013) under Grant Agreement no. 604102 (Human Brain Project), and the Amsterdam Brain and Cognition (ABC) Priority Program (ABC2014-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.A.K., V.A.L., and T.H.D. conception and design of research; N.A.K., T.M., A.H., and B.W.v.D. performed experiments; N.A.K., T.M., A.H., and T.H.D. analyzed data; N.A.K., T.M., V.A.L., and T.H.D. interpreted results of experiments; N.A.K., T.M., and T.H.D. prepared figures; N.A.K. and T.H.D. drafted manuscript; N.A.K., T.M., and T.H.D. edited and revised manuscript; N.A.K., T.M., A.H., B.W.v.D., V.A.L., and T.H.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Heeger, Tomas Knapen, Mike X. Cohen, Simon van Gaal, Tomas Knapen, Anouk van Loon, Sander Nieuwenhuis, Jan Brascamp, and all members of the Donner laboratory for comments on the manuscript.
REFERENCES
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450, 2005. [DOI] [PubMed] [Google Scholar]
- Belitski A, Gretton A, Magri C, Murayama Y, Montemurro MA, Logothetis NK, Panzeri S. Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information. J Neurosci 28: 5696–5709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci 3: 13–21, 2002. [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Cooperman A, Sagi D. Motion-induced blindness in normal observers. Nature 411: 798–801, 2001. [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Donner TH. Motion induced blindness. Scholarpedia 6: 3321, 2011. [Google Scholar]
- Bonneh YS, Donner TH, Sagi D, Fried M, Cooperman A, Heeger DJ, Arieli A. Motion-induced blindness and microsaccades: cause and effect. J Vis 10: 22, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh YS, Donner TH, Cooperman A, Heeger DJ, Sagi D. Motion-induced blindness and Troxler fading: common and different mechanisms. PLoS One 9: e92894, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Womelsdorf T, Desimone R, Fries P. A microsaccadic rhythm modulates gamma-band synchronization and behavior. J Neurosci 29: 9471–9480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brascamp JW, van Ee R, Noest AJ, Jacobs RH, van den Berg AV. The time course of binocular rivalry reveals a fundamental role of noise. J Vis 6: 1244–1256, 2006. [DOI] [PubMed] [Google Scholar]
- Braun J, Mattia M. Attractors and noise: twin drivers of decisions and multistability. Neuroimage 52: 740–751, 2010. [DOI] [PubMed] [Google Scholar]
- Britz J, Landis T, Michel CM. Right parietal brain activity precedes perceptual alternation of bistable stimuli. Cereb Cortex 19: 55–65, 2009. [DOI] [PubMed] [Google Scholar]
- Britz J, Pitts MA, Michel CM. Right parietal brain activity precedes perceptual alternation during binocular rivalry. Hum Brain Mapp 32: 1432–1442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso MM, Sirotin YB, Lima B, Glushenkova E, Das A. The neuroimaging signal is a linear sum of neurally distinct stimulus- and task-related components. Nat Neurosci 15: 1298–1306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Pettigrew JD. A common oscillator for perceptual rivalries? Perception 32: 295–305, 2003. [DOI] [PubMed] [Google Scholar]
- Choe KW, Blake R, Lee SH. Dissociation between neural signatures of stimulus and choice in population activity of human V1 during perceptual decision-making. J Neurosci 34: 2725–2743, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, Bradley DC, Smith MA, Kohn A, Movshon JA, Armstrong KM, Moore T, Chang SW, Snyder LH, Lisberger SG, Priebe NJ, Finn IM, Ferster D, Ryu SI, Santhanam G, Sahani M, Shenoy KV. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci 13: 369–378, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gee JW, Knapen T, Donner TH. Decision-related pupil dilation reflects upcoming choice and individual bias. Proc Natl Acad Sci USA 111: E618–E625, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Romo R. The role of fluctuations in perception. Trends Neurosci 31: 591–598, 2008. [DOI] [PubMed] [Google Scholar]
- de-Wit LH, Kubilius J, Wagemans J, Op de Beeck HP. Bistable Gestalts reduce activity in the whole of V1, not just the retinotopically predicted parts. J Vis 12: 12, 2012. [DOI] [PubMed] [Google Scholar]
- Donner TH, Sagi D, Bonneh YS, Heeger DJ. Opposite neural signatures of motion-induced blindness in human dorsal and ventral visual cortex. J Neurosci 28: 10298–10310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Sagi D, Bonneh YS, Heeger DJ. Retinotopic patterns of correlated fluctuations in visual cortex reflect the dynamics of spontaneous perceptual suppression. J Neurosci 33: 2188–2198, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Siegel M, Fries P, Engel AK. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr Biol 19: 1581–1585, 2009. [DOI] [PubMed] [Google Scholar]
- Donner TH, Siegel M. A framework for local cortical oscillation patterns. Trends Cogn Sci 15: 191–199, 2011. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC, 1998. [Google Scholar]
- Einhauser W, Stout J, Koch C, Carter O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc Natl Acad Sci USA 105: 1704–1709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res 43: 1035–1045, 2003. [DOI] [PubMed] [Google Scholar]
- Engbert R, Mergenthaler K. Microsaccades are triggered by low retinal image slip. Proc Natl Acad Sci USA 103: 7192–7197, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger LA. A Theory of Cognitive Dissonance Stanford, CA: Stanford University Press, 1957. [Google Scholar]
- Fischer J, Whitney D. Serial dependence in visual perception. Nat Neurosci 17: 738–743, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassle S, Sommer J, Jansen A, Naber M, Einhauser W. Binocular rivalry: frontal activity relates to introspection and action but not to perception. J Neurosci 34: 1738–1747, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224, 2009. [DOI] [PubMed] [Google Scholar]
- Gail A, Brinksmeyer HJ, Eckhorn R. Perception-related modulations of local field potential power and coherence in primary visual cortex of awake monkey during binocular rivalry. Cereb Cortex 14: 300–313, 2004. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron 54: 677–696, 2007. [DOI] [PubMed] [Google Scholar]
- Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature 493: 97–100, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography – theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65: 413–497, 1993. [Google Scholar]
- Händel BF, Jensen O. Spontaneous local alpha oscillations predict motion-induced blindness. Eur J Neurosci 40: 3371–3379, 2014. [DOI] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nat Rev Neurosci 12: 509–523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature 438: 496–499, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh PJ, Tse PU. Microsaccade rate varies with subjective visibility during motion-induced blindness. PLoS One 4: e5163, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe JM, Lamirel C, Lorenceau J. Pupil dynamics during bistable motion perception. J Vis 9: 10, 2009. [DOI] [PubMed] [Google Scholar]
- Jack AI, Shulman GL, Snyder AZ, McAvoy M, Corbetta M. Separate modulations of human V1 associated with spatial attention and task structure. Neuron 51: 135–147, 2006. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, Movshon JA. A new perceptual illusion reveals mechanisms of sensory decoding. Nature 446: 912–915, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4: 186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23: 315–341, 2000. [DOI] [PubMed] [Google Scholar]
- Klink PC, van Ee R, van Wezel RJ. General validity of Levelt's propositions reveals common computational mechanisms for visual rivalry. PLoS One 3: e3473, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen T, Brascamp J, Pearson J, van Ee R, Blake R. The role of frontal and parietal brain areas in bistable perception. J Neurosci 31: 10293–10301, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Blake R, Heeger DJ. Hierarchy of cortical responses underlying binocular rivalry. Nat Neurosci 10: 1048–1054, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Dan Y. Neuromodulation of brain states. Neuron 76: 209–222, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys' percepts during binocular rivalry. Nature 379: 549–553, 1996. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Multistable phenomena: changing views in perception. Trends Cogn Sci 3: 254–264, 1999. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex 13: 422–433, 2003. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Wilke M, Maier A, Logothetis NK. Stable perception of visually ambiguous patterns. Nat Neurosci 5: 605–609, 2002. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikulin VV, Palva S, Ilmoniemi RJ, Palva JM. Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci 24: 10186–10190, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumer ED, Friston KJ, Rees G. Neural correlates of perceptual rivalry in the human brain. Science 280: 1930–1934, 1998. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nat Rev Neurosci 5: 229–240, 2004. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J 76: 691–708, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Bote R, Rinzel J, Rubin N. Noise-induced alternations in an attractor network model of perceptual bistability. J Neurophysiol 98: 1125–1139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg H, Cumming BG. Decision-related activity in sensory neurons reflects more than a neuron's causal effect. Nature 459: 89–92, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature 474: 372–375, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56: 141–154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857, 1999. [DOI] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16: 1331–1339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci 3: 1153–1159, 2000. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci 13: 121–134, 2012. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 60: 709–719, 2008. [DOI] [PubMed] [Google Scholar]
- Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 457: 475–479, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience 101: 243–276, 2000. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A. A neural basis for inference in perceptual ambiguity. Proc Natl Acad Sci USA 104: 323–328, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A, Rees G. The neural bases of multistable perception. Trends Cogn Sci 13: 310–318, 2009. [DOI] [PubMed] [Google Scholar]
- Stocker AA, Simoncelli EP. A Bayesian model of conditioned perception. Adv Neural Inf Process Syst 20: 1409–1416, 2008. [PMC free article] [PubMed] [Google Scholar]
- Swallow KM, Makovski T, Jiang YV. Selection of events in time enhances activity throughout early visual cortex. J Neurophysiol 108: 3239–3252, 2012. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M, Simola J. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr 16: 269–275, 2004. [DOI] [PubMed] [Google Scholar]
- Tong F, Engel SA. Interocular rivalry revealed in the human cortical blind-spot representation. Nature 411: 195–199, 2001. [DOI] [PubMed] [Google Scholar]
- Wilke M, Logothetis NK, Leopold DA. Local field potential reflects perceptual suppression in monkey visual cortex. Proc Natl Acad Sci USA 103: 17507–17512, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Mueller KM, Leopold DA. Neural activity in the visual thalamus reflects perceptual suppression. Proc Natl Acad Sci USA 106: 9465–9470, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J Neurosci 28: 2667–2679, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron 58: 429–441, 2008. [DOI] [PubMed] [Google Scholar]
- Zaretskaya N, Thielscher A, Logothetis NK, Bartels A. Disrupting parietal function prolongs dominance durations in binocular rivalry. Curr Biol 20: 2106–2111, 2010. [DOI] [PubMed] [Google Scholar]
- Zipser K, Lamme VA, Schiller PH. Contextual modulation in primary visual cortex. J Neurosci 16: 7376–7389, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]