Abstract
Fetal and subsequent early postnatal iron deficiency causes persistent impairments in cognitive and affective behaviors despite prompt postnatal iron repletion. The long-term cognitive impacts are accompanied by persistent downregulation of brain-derived neurotrophic factor (BDNF), a factor critical for hippocampal plasticity across the life span. This study determined whether early-life iron deficiency epigenetically modifies the Bdnf locus and whether dietary choline supplementation during late gestation reverses these modifications. DNA methylation and histone modifications were assessed at the Bdnf-IV promoter in the hippocampus of rats [at postnatal day (PND) 65] that were iron-deficient (ID) during the fetal-neonatal period. Iron deficiency was induced in rat pups by providing pregnant and nursing dams an ID diet (4 mg/kg Fe) from gestational day (G) 2 through PND7, after which iron deficiency was treated with an iron-sufficient (IS) diet (200 mg/kg Fe). This paradigm resulted in about 60% hippocampal iron loss on PND15 with complete recovery by PND65. For choline supplementation, pregnant rat dams were given dietary choline (5 g/kg) from G11 through G18. DNA methylation was determined by quantitative sequencing of bisulfite-treated DNA, revealing a small alteration at the Bdnf-IV promoter. Chromatin immunoprecipitation analysis showed increased HDAC1 binding accompanied by reduced binding of RNA polymerase II and USF1 at the Bdnf-IV promoter in formerly ID rats. These changes were correlated with altered histone methylations. Prenatal choline supplementation reverses these epigenetic modifications. Collectively, the findings identify epigenetic modifications as a potential mechanism to explicate the long-term repression of Bdnf following fetal and early postnatal iron deficiency.
Keywords: hippocampus, DNA methylation, iron deficiency, epigenetics, histone methylation
early-life micronutrient deficiencies profoundly affect brain development and function, leading to reduced educational and job potentials (41). Among these, iron deficiency anemia is the most prevalent, affecting 20–30% of pregnant women and their offspring (38). Early-life iron deficiency anemia causes negative and long-lasting effects on learning and memory, emotion, and social behavior (31). Of great concern is the finding that these learning and memory deficits in humans persist into adulthood despite prompt iron treatment (6, 29, 49).
The hippocampus is an important component of the neural circuit responsible for learning and memory (36, 56). During late fetal and early neonatal life, the rapidly developing hippocampus demands substantial iron import (48, 59) and is vulnerable to iron deficiency. Indeed, animal models of fetal-neonatal iron deficiency anemia show behavioral abnormalities analogous to those in humans that are accompanied by changes in neurotransmission, neuronal structure, and neurochemistry (14, 16, 30). These changes include long-term dysregulation of molecules critical for synaptic plasticity (44, 61). Moreover, similar hippocampal pathologies are observed in the nonanemic genetic models of hippocampus-specific iron deficiency, suggesting that these effects are specific to the loss of neuronal iron (7, 15), which underscores the critical requirement for adequate iron during hippocampal development.
Consistent with learning and memory deficits, early-life iron deficiency causes persistent downregulation of Bdnf, a gene critical for hippocampal plasticity (62, 66). The Bdnf gene consists of eight 5′-noncoding exons, a single 3′-coding exon, and two differential poly-A tail signals, all of which potentially generate 16 transcript variants (1). The Bdnf-IV transcript variant is robustly expressed in the postnatal rat hippocampus (1). The long-term downregulation of Bdnf resulting from early-life iron deficiency potentially implicates an epigenetic mechanism, such as changes in DNA methylation, histone modifications, or chromatin protein binding (25). Bdnf is epigenetically regulated in the hippocampus in a number of contexts, including memory formation, environmental enrichment, perinatal exposure to methylmercury, and seizures (24, 32, 34, 43). Moreover, repression of hippocampal Bdnf by early-life iron deficiency is reversed with choline supplementation during late gestation (23). As a methyl donor, choline supplementation can epigenetically alter gene transcription (26, 42) and may improve Bdnf expression by reversing the epigenetic modifications caused by early-life iron deficiency. However, whether early-life iron deficiency induces chromatin remodeling at the Bdnf locus has yet to be determined. Thus, we examined the specific effects of early-life iron deficiency on DNA and histone methylation at the Bdnf-IV promoter.
MATERIALS AND METHODS
Animals.
Gestational day 2 (G2) pregnant Sprague-Dawley rat dams were obtained from Charles River (Wilmington, MA) and kept on a 12:12-h light-dark cycle with ad libitum access to food and water. Fetal-neonatal iron deficiency was induced by dietary manipulation, as described previously (46) with purified diets. In brief, pregnant dams were given a purified iron-deficient (ID) diet (4 mg/kg Fe, 1.1 g/kg choline chloride, TD 80396; Harlan Teklad, Madison, WI) from G2 to postnatal day (PND) 7; thereafter, nursing dams were given a purified iron-sufficient (IS) diet (200 mg/kg Fe, 1.1 g/kg choline chloride, TD 01583; Harlan Teklad). Iron treatment commenced at PND7 to mimic the full-term human neonate in terms of hippocampal development (http://www.translatingtime.net). This dietary manipulation generated iron-deficient (ID) pups with ∼60% hippocampal iron loss at PND15 (20), a time when rapid dendritic arborization and synaptogenesis commence in CA1 (45), and the hippocampus is iron-repleted by PND65 (formerly iron-deficient, FID) (21). Control iron-sufficient rats (IS) were generated from pregnant dams maintained on a purified IS diet. To supplement the choline-fortified diet, pregnant rat dams were given ID+choline (4 mg/kg Fe, 5.0 g/kg choline chloride) or IS+choline (200 mg/kg Fe, 5.0 g/kg choline chloride) diets during gestational day 11 through 18, as previously described (23). All litters were culled to eight pups (six males and two females) at PND0 and weaned at PND21. Same sex littermates were housed together with 4 rats/cage. Only male offspring were used in the experiments. The University of Minnesota Institutional Animal Care and Use Committee approved all experiments in this study.
Hippocampal dissection.
PND65 rats were killed by an intraperitoneal injection of pentobarbital sodium (100 mg/kg). Brains were removed from the cranium and bisected along the midline on an ice-cold metal block. Both left and right hippocampi were dissected separately and immediately flash-frozen in liquid nitrogen and stored individually at −80°C.
Bisulfite-conversion and pyrosequencing.
DNA was extracted using the standard phenol chloroform method, and the amount was measured using a NanoDrop spectrophotometer. One microgram of DNA was bisulfite-treated using the EpiTect Bisulfite Kit (Qiagen, Valencia, CA) following the manufacturer's protocol. Pyrosequencing was performed to analyze the methylation profiles at Bdnf using custom-designed oligonucleotides that target the rat Bdnf-IV (NW_047763: 251886–252199) promoter (Bdnf-P4). The PyroMark PCR kit (Qiagen) was used in a 25-μl reaction, according to the manufacturer's protocol. PCR conditions were 95°C for 15 min followed by 45 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 15 s. Ten microliters of the biotinylated PCR product was used for each sequencing assay. Pyrosequencing was done using the PyroMark Q96MD (Qiagen) system following the manufacturer's protocol and the PyroMark Gold 96 reagents kit (Qiagen). Methylation was analyzed using Qiagen's Pyro Q-CpG software. 7 CpG sites were analyzed for Bdnf-P4.
Chromatin immunoprecipitation assay.
Chromatin was prepared from hippocampal tissue following the manufacturer's recommendation (Millipore, Temecula, CA). All left hippocampi were homogenized in ice-cold PBS (500 μl) using a motorized plastic pestle and pelleted by centrifugation at 13,000 rpm (30 s). The pellets were resuspended in PBS and fixed in 1% formaldehyde solution with intermittent mixing at 37°C (5 min) and room temperature (5 min). Formaldehyde fixative was removed with a PBS (1 ml) rinse by pelleting suspension. Pellets were then resuspended in 500-μl lysis buffer [1% SDS, 10 mM EDTA, 50 mM Tris pH 8.1, 1 mM PMSF, 10 μl 10× protease inhibitor cocktails (Roche, Indianapolis, IN)] and cooled in an ice bath for 10 min. Lysates were then sonicated (Branson Sonifier 250; VWR Scientific, Batavia, IL) to shear DNA (200–1,000 bp), 1/10 of which was used for validation by agarose gel electrophoresis following a cross-linking reversal (0.2 M NaCl, 65°C overnight). Sonicated lysates were diluted 10-fold with a chromatin immunoprecipitation assay (ChIP) dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-pH 8.1, 167 mM NaCl) and precleared with 75 μl of protein A agarose/salmon sperm DNA (50% slurry; Millipore). Following removal of Protein A agarose, precleared lysate was immunoprecipitated by ChIP-grade antibody with an end-over-end rotation (4°C, overnight). The antibody-histone complex was collected by the addition of 50 μl of protein A agarose/salmon sperm DNA slurry with mixing (4°C, 1 h). Following washes (per manufacturer's protocol; Millipore), immune-histone complex was eluted in 200 μl of elution buffer (1% SDS, 0.1 M NaHCO3). Reverse cross-linking was achieved by incubation in NaCl (0.2 M, 65°C, overnight). A protease digestion (20 μg proteinase K, 20 mM EDTA, 100 mM Tris-pH 6.5, 45°C, 1 h) was performed to recover DNA, which was further purified using ChIP DNA clean and concentration kit (Zymo Research, Irvine, CA). Antibodies used in this study included anti-H4ac (GAM-0202; SA Biosciences, Frederick, MD), anti-H3K4me3 (ab8580; Abcam, Cambridge, MA), anti-H3K4me1 (ab8895; Abcam), anti-H3K27me3 (ab6002; Abcam), anti-USF1 (sc-229X; Santa Cruz Biotechnology, Santa Cruz, CA), anti-HDAC1 (CS200577; Millipore, Billerica, MA), and anti-RNA pol II (8WG16; Pierce, Rockford, IL). Normal IgG was used as a negative control. Levels of precipitated GAPDH (active) and MyoD1 (inactive) loci were used to validate the ChIP experiments.
Real-time PCR.
For analysis of precipitated DNA from ChIP, SYBR-Green PCR (Fast SYBR Green master mix; Applied Biosystems, Foster City, CA) was used to amplify Bdnf-P4 using the following oligonucleotides: Bdnf-IV forward (GATGAAAGGTTTGGCTTCTGTG) and reverse (TCGGTGAATGGGAAAGTGG). Oligonucleotides were validated for amplification efficiency (−3.2 slope). These oligonucleotides amplify a 250-bp Bdnf-IV (NW_047673:251697–251947) promoter. Input DNA (10%) was used as a normalizer to account for input amount (ΔCt). Data were expressed as a ratio to IS control (2−ΔΔCt) using one of the IS sample as a calibrator (ΔΔCt). For Bdnf mRNA quantitation, TaqMan PCR was used with primers and probes obtained from Applied Biosystems (Foster City, CA). Real-time PCR was performed with Stratagene 3000P instrument.
Western blot analysis.
To validate the mRNA data, we used Western blot to quantify levels of BDNF protein using a previously described protocol (63). In brief, 30 μg of hippocampal protein lysate was separated using a 4–20% SDS-PAGE gel (Novex, Life Technologies, Carlsbad, CA). Proteins were blotted onto a nitrocellulose membrane (Pierce), blocked with blocking buffer for fluorescent Western blotting (Rockland, Gilbertsville, PA), and incubated with antibodies against BDNF (rabbit IgG; Abcam), and β-actin (mouse monoclonal; Sigma, St. Louis, MO). Following PBS ± 0.1% Tween-20 washes, blots were incubated in Alexa-Fluor 700 anti-mouse (Invitrogen, Grand Island, NY) and IR Dye-800 anti-rabbit (Rockland), and analyzed by Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE). Integrated intensity of BDNF normalized to β-actin was determined using Photoshop CS5.1 (Adobe, San Jose, CA).
Statistics.
Group differences were analyzed by unpaired t-tests with alpha set at 0.05. Data were graphed and statistically analyzed using Prism 5 GraphPad (GraphPad Software, San Diego, CA).
RESULTS
Early-life iron deficiency induces changes in DNA methylation at Bdnf-IV promoter.
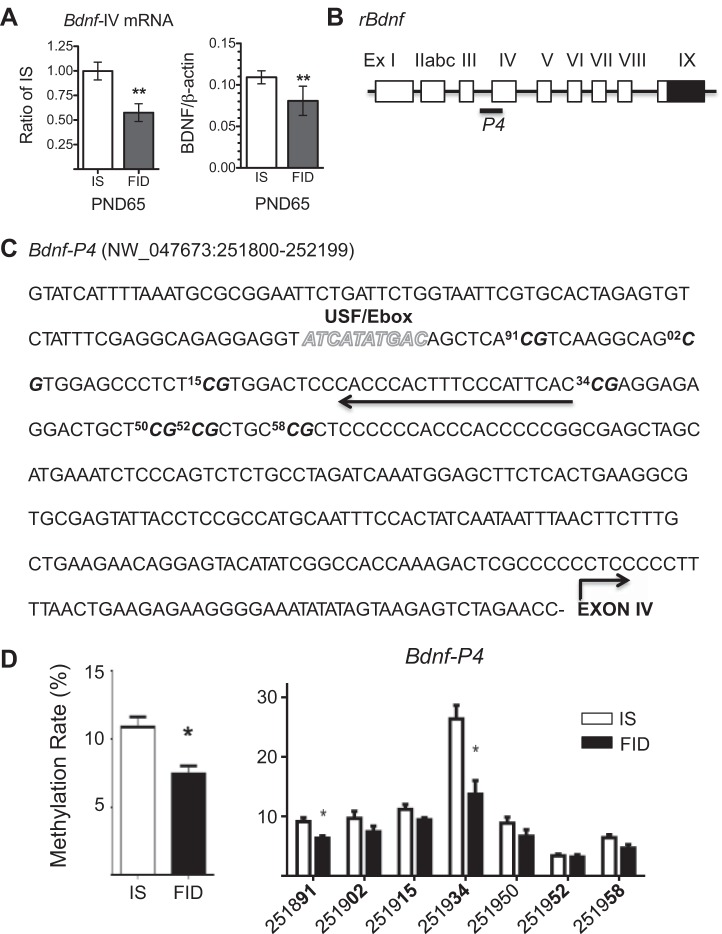
Consistent with our previous findings (4, 62), early-life iron deficiency caused a persistent decrease in hippocampal Bdnf-IV (previous nomenclature BDNF-III) mRNA and BDNF protein (Fig. 1A). To determine whether decreased expression was associated with changes in DNA methylation, we assessed DNA methylation at the promoter region proximal to the transcription start site of this transcript variant (Fig. 1B). Within this promoter region, there are multiple CG sites (Fig. 1C) with methylation levels that are differentially affected by early-life iron deficiency (Fig. 1D). Overall, the PND65 FID hippocampus showed a small reduction in percentage of DNA methylation at the Bdnf-IV (P4) promoter (Fig. 1D). To determine whether early-life iron deficiency affects expression of DNA methyltransferases, we measured hippocampal Dnmt1 and Dnmt3a mRNA and found no difference in either ID PND15 or FID PND65 hippocampus compared with IS controls (data not shown).
Fig. 1.
Early-life iron deficiency decreased BDNF expression and altered DNA methylation at the Bdnf-IV promoter in PND65 rat hippocampus. A: reduced Bdnf-IV mRNA and BDNF protein levels in PND65 formerly iron-deficient (FID) hippocampus compared with iron sufficient (IS) control. B: schematic representation of the rat Bdnf locus with multiple noncoding 5′-exons. C: selected sequence of Bdnf-IV promoter (P4) is shown with CpG islands (bold and italic), qPCR oligonucleotide (arrow), and identified USF1 binding site (italicized and stylized letters). D: quantitative analysis of methylated CpG showed a lower overall methylation rate, particularly at two CpG islands within promoter P4 of FID hippocampus. Values are expressed as means ± SD; n = 5 or 6/group. *P < 0.05 and **P < 0.01, using an unpaired t-test.
Increased presence of HDAC1 at the Bdnf-IV promoter.
Given the implication of HDAC1 in modulating Bdnf-IV expression (24, 65), we quantified the presence of HDAC1 at the Bdnf-P4. At PND65, levels of HDAC1 were increased in the FID hippocampus compared with IS controls (Fig. 2A). Consistent with previous findings relating HDAC1, histone H4 acetylation, and RNA polymerase II in modulating expression of genes with multiple splice variants (17), the PND65 FID hippocampus showed a decrease in H4ac at the Bdnf-P4 (Fig. 2B). We further analyzed the binding of RNA polymerase II (pol II) and found a decrease in pol II at the Bdnf-P4 in FID hippocampus (Fig. 2C). Finally, binding of the activity-dependent transcription factor USF1 at the Bdnf-P4 (8, 68) was similarly decreased in the adult FID hippocampus (Fig. 2D).
Fig. 2.
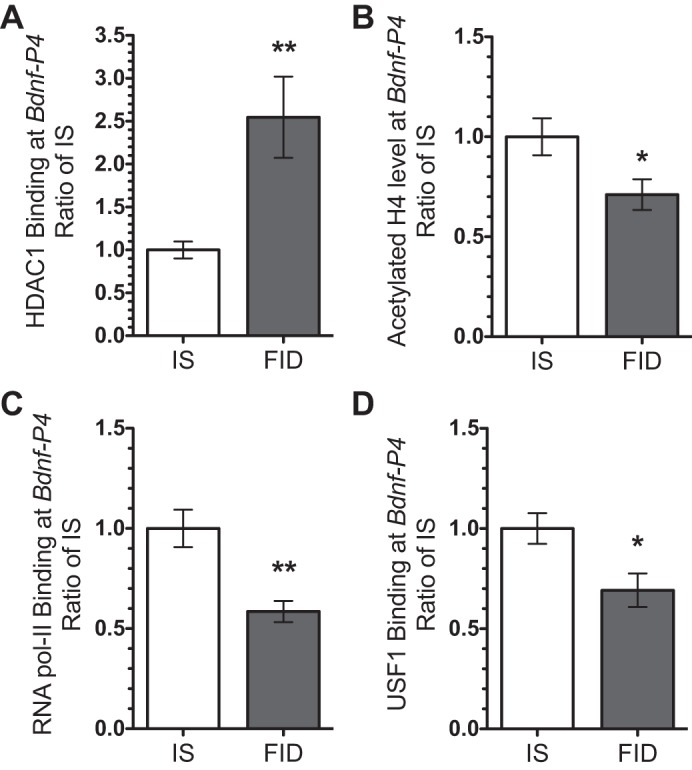
Early-life iron deficiency reduced histone acetylation and binding of transcription regulators at the Bdnf-IV promoter in PND65 FID hippocampus. ChIP analyses showed an increased HDAC1 binding (A), decreased histone H4 acetylation (B), decreased binding of RNA polymerase II (C), and USF1 (D). Values are expressed as means ± SE; n = 4–8/group. *P < 0.05 and **P < 0.01, using an unpaired t-test.
Abnormal histone H3 methylation.
To identify a possible mechanism behind the increased HDAC1 concomitant with a decreased USF1 binding at the Bdnf-IV promoter in the FID hippocampus, we analyzed histone H3 modifications (marks) that modify chromatin structure and influence transcriptional activity. The adult FID hippocampus showed a significant increase in K27me3 and K4me1 marks accompanied by a decrease in the K4me3 mark (Fig. 3).
Fig. 3.
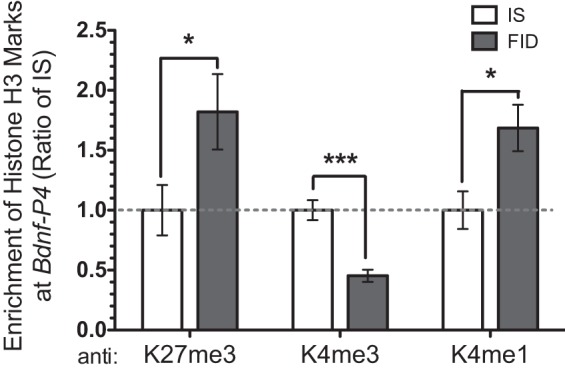
Early-life iron deficiency altered histone H3 methylation at the Bdnf-IV promoter in PND65 hippocampus. ChIP analyses showed an increased enrichment of K27me3 and K4me1 and a decreased enrichment of K4me3. Data were normalized by 10% input and calibrated to IS control. Values are expressed as means ± SE; n = 6–8/group. *P < 0.05, ***P < 0.001, using an unpaired t-test.
Dietary choline supplementation during late gestation reverses early-life iron deficiency-induced epigenetic modifications.
We have previously shown that dietary choline supplementation during late gestation normalizes hippocampal Bdnf-IV expression in adult FID rats (23). This transcriptional recovery is accompanied by a partial recovery of hippocampal BDNF protein (Fig. 4). To determine whether this reversal is associated with remodeling of chromatin structures, we analyzed histone modifications at the Bdnf-IV promoter following prenatal choline supplementation. Consistent with the findings presented in the Figs. 2 and 3, HDAC1 binding and enrichment of the K27me3 mark were increased in the FID hippocampus (Fig. 5, A and B), while USF1 binding and enrichment of the K4me3 mark were decreased (Fig. 5, C and D). These epigenetic modifications induced by early-life iron deficiency were partially or completely reversed by prenatal dietary choline supplementation (Fig. 5, FID-C group).
Fig. 4.
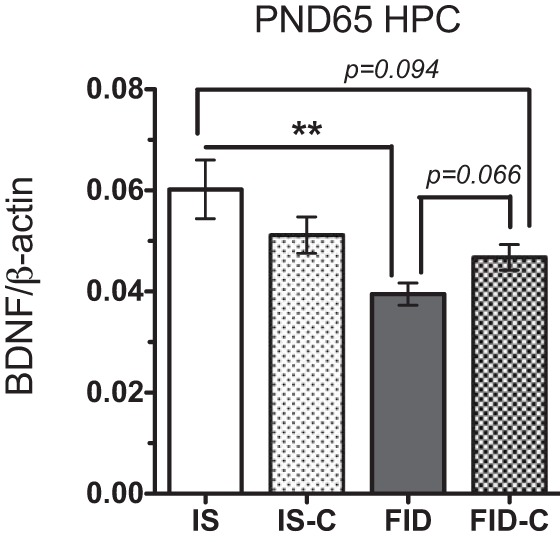
Western blot analysis showed a partial recovery of BDNF protein level in PND65 FID hippocampus following prenatal choline supplementation. Values are expressed as means ± SE; n = 4–6/group. **P < 0.01, using an unpaired t-test.
Fig. 5.
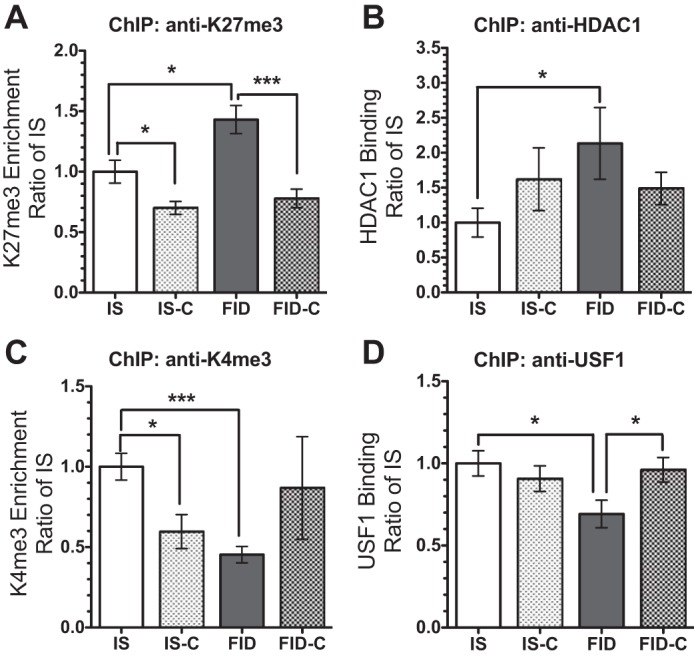
Dietary choline supplementation during late gestation reverses early-life iron deficiency induced epigenetic modifications at the Bdnf-IV promoter. ChIP analyses of K27me3 enrichment (A), HDAC1 binding (B), K4me3 enrichment (C), and USF1 binding (D) showed consistent changes induced by ID, which recovered with choline supplementation. Values are expressed as means ± SE; n = 6–8/group. *P < 0.05, and ***P < 0.001, using an unpaired t-test.
DISCUSSION
The long-lasting impairments in cognition and socio-emotional behaviors induced by early-life iron deficiency can be ascribed, in part, to the persistent dysregulation of hippocampal Bdnf (30, 62). Consistent with this notion, we have previously documented evidence that altered BDNF is associated with abnormal CA1 dendritic growth and maturation and BDNF-mediated MAPK signaling in the PND65 FID hippocampus (5, 21, 47, 61). Furthermore, there are strong links between BDNF signaling and learning and memory, which are known to be sensitive to epigenetic modifications (35, 64). However, the possibility of an epigenetic mechanism underlying the early-life iron deficiency induced long-term gene repression has not been investigated. The present study shows that early-life iron deficiency induced stable histone modifications at the Bdnf promoter. The findings reveal a potential epigenetic mechanism by which iron deficiency induces chromatin remodeling and, thereby, renders genes such as Bdnf-IV less transcriptionally responsive.
Chromatin remodeling is a dynamic process involving multiple modifications of DNA and histones (25) and is regulated, in part, by nutrient availability (50, 60). Since iron is a necessary cofactor for multiple JmjC ARID domain-containing (JARID) histone demethylases (9, 11, 54, 55, 58), a loss of iron during hippocampal development would likely affect chromatin structures. It has not previously been demonstrated whether iron deficiency affects chromatin remodeling. Our findings that early-life iron deficiency induced significant histone modifications and small changes in DNA methylation at the Bdnf locus in the adult rat hippocampus suggest that histone modifications likely play a key role in the long-term Bdnf repression. This observation differs from other models of early-life insults (e.g., stress and neglect), which demonstrate that increased DNA methylation at CpG islands around the Bdnf-IV promoter is associated with long-term repression of hippocampal Bdnf (51, 52). The disparate outcomes likely relate to a different mode of early-life insults. Our early-life iron deficiency model is a metabolic insult. Thus, our findings may uncover a dynamic interaction between iron and histone modifications in mounting an adaptive response to early-life iron deficiency.
Methylation and acetylation of histones are interdependent histone modifications. K4me3 enrichment is associated with increased histone acetylation and recruitment of histone acetyltransferases (HATs) at active loci (2, 19). In contrast, K27me3 enrichment is associated with reduced histone acetylation and increased histone deacetylases (HDACs) binding at inactive loci (2, 19, 22, 25, 27, 37). Thus, increased HDAC1 binding concomitant with decreased H4ac enrichment at the Bdnf-IV promoter in the FID hippocampus is in line with increased enrichment of K27me3 mark. Increased HDAC1 binding may lead to a lower enrichment of H4 acetylation, since hippocampal mRNA levels of HATs or HDACs were not different between IS and FID groups (transcriptomic data not shown), suggesting an unaltered de novo histone H4 acetylation. Alternatively, the impaired hippocampal learning and memory in the FID group may cause a lower H4ac enrichment at the Bdnf-IV promoter given that learning and memory regulates histone acetylation (12, 57). Moreover, neural stimulation can mediate changes in H4 acetylation and USF1 binding (8, 13). Abnormal hippocampal neurotransmission (20) may cause the lower H4ac enrichment and USF1 binding at the Bdnf-IV promoter in FID hippocampus. Collectively, these chromatin modifications likely contribute to the repression of hippocampal Bdnf.
Enrichment of the K27me3 mark is associated with repressive promoters, whereas enrichment of the K4me3 mark is associated with active promoters (2). The increased enrichment of the K27me3 mark and decreased enrichment of the K4me3 mark at the Bdnf-P4 in the FID hippocampus provides evidence that early-life iron deficiency reduces the transcriptional activity of Bdnf-P4. These changes in histone methylation marks may directly link to our previous observation of dysregulated iron-dependent JARIDs in the adult FID hippocampus (4). Analysis of JARID function in the context of early-life iron deficiency will be important in establishing a link among iron deficiency, epigenetic modifications, and transcriptional regulation. Interestingly, we also found increased enrichment of the K4me1 mark at the Bdnf-P4 of FID hippocampus. This finding appears counterintuitive because the enrichment of this mark is typically found at active promoters (2). However, emerging evidence suggests that increased enrichment of the K4me1 mark and reduced recruitment of RNA pol II at the promoter regions are associated with repression of genes that are inducible (10). In the context of early-life iron deficiency, our finding of lowered pol II binding at the Bdnf-P4 further corroborates this mechanism of Bdnf repression.
Finally, methyl diets (e.g., choline, betaine) are known to influence epigenetic modifications (26, 42, 67). Choline supplementation during the critical period of hippocampal development has been shown to improve hippocampal function (28, 39, 40). Importantly, it diminishes neurobehavioral abnormalities induced by early-life insults, including early-life iron deficiency (23, 53). The reversal of early-life iron deficiency induced epigenetic modifications by prenatal choline supplementation supports the concept that epigenetics is one mechanism by which iron deficiency causes long-term gene dysregulation in the adult rat hippocampus. Whether provision of dietary choline during an early period of hippocampal development resolves the abnormal CA1 dendritic morphogenesis and BDNF-mediated MAPK signaling in the adult PND65 hippocampus remains to be determined in a future study. We predict that the results are likely affirmative given the evidence of recovered learning behavior and BDNF expression (23). The findings in the present study form a mechanistic basis for the use of dietary choline as an adjunctive therapy to complement iron treatment for early-life iron deficiency. Interestingly, significant reductions in repressive (K27me3) and active (K4me3) marks were detected in the control group supplemented with choline (Fig. 5, A and C; ISC group). Additional study is needed to determine whether these changes underlie the reduced Bdnf-IV expression in this group (23). The finding implicates a potential negative impact of too much choline.
Perspectives and Significance
Our data provide evidence that early-life iron deficiency remodels chromatin structure to alter gene expression. This reprogramming may reflect a cellular adaptation to a metabolic insult during development and may play a key role in iron deficiency-induced long-term gene dysregulation. Previous investigations into the mechanistic underpinnings, which focused on the concept of form and function, postulated that altered neural circuitry development is a basis for impaired hippocampal function in adulthood (3, 5, 20). Here, we demonstrated that early-life iron deficiency induces epigenetic modifications at a key gene locus critical for regulation of hippocampal plasticity, providing an additional mechanism by which iron modulates hippocampal development and function. The continued deficits in cognitive function and emotional behaviors, as well as increased risk of adulthood schizophrenia and depression, are concerning in spite of prompt iron treatment of early-life iron deficiency (18, 30, 33). These long-term negative effects illustrate a need to identify adjunctive remedial therapy. Our findings form a molecular basis to test the use of methyl diets, such as choline, as a potential adjunctive therapy.
GRANTS
This study was supported by National Institute of Child Health and Human Development Grant R01-HD29421–17 to M. K. Georgieff.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.V.T., R.A.S., and M.K.G. conception and design of research; P.V.T., B.C.K., and Y.-C.L. performed experiments; P.V.T., B.C.K., Y.-C.L., and R.A.S. analyzed data; P.V.T., B.C.K., Y.-C.L., R.A.S., and M.K.G. interpreted results of experiments; P.V.T. and Y.-C.L. prepared figures; P.V.T. drafted manuscript; P.V.T., B.C.K., R.A.S., and M.K.G. edited and revised manuscript; P.V.T., B.C.K., Y.-C.L., R.A.S., and M.K.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Diana Wallin for editorial assistance.
REFERENCES
- 1.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 85: 525–535, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr 133: 1174–1179, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Blegen MB, Kennedy BC, Thibert KA, Gewirtz JC, Tran PV, Georgieff MK. Multigenerational effects of fetal-neonatal iron deficiency on hippocampal BDNF signaling. Physiol Rep 1: e00096, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci 32: 238–248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics 120: e336–e345, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr 139: 672–679, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen WG, West AE, Tao X, Corfas G, Szentirmay MN, Sawadogo M, Vinson C, Greenberg ME. Upstream stimulatory factors are mediators of Ca2+-responsive transcription in neurons. J Neurosci 23: 2572–2581, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Zang J, Whetstine J, Hong X, Davrazou F, Kutateladze TG, Simpson M, Mao Q, Pan CH, Dai S, Hagman J, Hansen K, Shi Y, Zhang G. Structural insights into histone demethylation by JMJD2 family members. Cell 125: 691–702, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Cheng J, Blum R, Bowman C, Hu D, Shilatifard A, Shen S, Dynlacht BD. A role for H3K4 monomethylation in gene repression and partitioning of chromatin readers. Mol Cell 53: 979–992, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell 128: 1063–1076, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dagnas M, Mons N. Region- and age-specific patterns of histone acetylation related to spatial and cued learning in the water maze. Hippocampus 23: 581–591, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Dyrvig M, Hansen HH, Christiansen SH, Woldbye DP, Mikkelsen JD, Lichota J. Epigenetic regulation of Arc and c-Fos in the hippocampus after acute electroconvulsive stimulation in the rat. Brain Res Bull 88: 507–513, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr 2: 112–121, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fretham SJ, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus 22: 1691–1702, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golub MS. Recent studies of iron deficiency during brain development in nonhuman primates. Biofactors 36: 111–116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hnilicova J, Hozeifi S, Duskova E, Icha J, Tomankova T, Stanek D. Histone deacetylase activity modulates alternative splicing. PloS One 6: e16727, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry 65: 1136–1144, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam AB, Richter WF, Jacobs LA, Lopez-Bigas N, Benevolenskaya EV. Co-regulation of histone-modifying enzymes in cancer. PLoS One 6: e24023, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus 15: 1094–1102, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci 25: 412–420, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RD, Cowley SM. The physiological roles of histone deacetylase (HDAC) 1 and 2: complex co-stars with multiple leading parts. Biochem Soc Trans 41: 741–749, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy BC, Dimova JG, Siddappa AJ, Tran PV, Gewirtz JC, Georgieff MK. Prenatal choline supplementation ameliorates the long-term neurobehavioral effects of fetal-neonatal iron deficiency rats. J Nutr 144: 1858–1865, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koppel I, Timmusk T. Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology 75: 106–115, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Kouzarides T. Chromatin modifications and their function. Cell 128: 693–705, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem 282: 31,777–31,788, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol 8: 284–295, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Guo-Ross S, Lewis DV, Turner D, White AM, Wilson WA, Swartzwelder HS. Dietary prenatal choline supplementation alters postnatal hippocampal structure and function. J Neurophysiol 91: 1545–1555, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr 141: 740S–746S, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64: S34–S43; discussion S72–S91, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol 13: 158–165, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Lubin FD. Epigenetic gene regulation in the adult mammalian brain: multiple roles in memory formation. Neurobiol Learn Mem 96: 68–78, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci 13: 54–70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddox SA, Schafe GE, Ressler KJ. Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder. Front Psychiatry 4: 62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahan AL, Mou L, Shah N, Hu JH, Worley PF, Ressler KJ. Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with Pavlovian fear conditioning. J Neurosci 32: 4651–4659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science 285: 1870–1874, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev 11: 155–161, 2001. [DOI] [PubMed] [Google Scholar]
- 38.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 12: 444–454, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci 1: 7, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montoya D, Swartzwelder HS. Prenatal choline supplementation alters hippocampal N-methyl-d-aspartate receptor-mediated neurotransmission in adult rats. Neurosci Lett 296: 85–88, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Morris SS, Cogill B, Uauy R. Effective international action against undernutrition: why has it proven so difficult and what can be done to accelerate progress? Lancet 371: 608–621, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J 20: 43–49, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onishchenko N, Karpova N, Sabri F, Castren E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem 106: 1378–1387, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Patton SM, Coe CL, Lubach GR, Connor JR. Quantitative proteomic analyses of cerebrospinal fluid using iTRAQ in a primate model of iron deficiency anemia. Dev Neurosci 34: 354–365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull 7: 113–120, 1981. [DOI] [PubMed] [Google Scholar]
- 46.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr 133: 3215–3221, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, Erne B, Sendtner M, Schaeren-Wiemers N, Korte M, Barde YA. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci 30: 1739–1749, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice D, Barone S Jr.. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108Suppl 3: 511–533, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol 34: 762–779, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20: 1405–1428, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychol 65: 760–769, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roth TL, Sweatt JD. Epigenetic marking of the BDNF gene by early-life adverse experiences. Horm Behav 59: 315–320, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res 1237: 91–100, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitz SU, Albert M, Malatesta M, Morey L, Johansen JV, Bak M, Tommerup N, Abarrategui I, Helin K. Jarid1b targets genes regulating development and is involved in neural differentiation. EMBO J 30: 4586–4600, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sengoku T, Yokoyama S. Structural basis for histone H3 Lys 27 demethylation by UTX/KDM6A. Genes Dev 25: 2266–2277, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231, 1992. [DOI] [PubMed] [Google Scholar]
- 57.Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J Psychiatr Res 45: 460–468, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi T, Watanabe Y, Takano-Shimizu T, Kondo S. Roles of jumonji and jumonji family genes in chromatin regulation and development. Dev Dyn 235: 2449–2459, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res 55: 35–42, 1990. [DOI] [PubMed] [Google Scholar]
- 60.Teperino R, Schoonjans K, Auwerx J. Histone methyl transferases and demethylases; can they link metabolism and transcription? Cell Metab 12: 321–327, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran PV, Dakoji S, Reise KH, Storey KK, Georgieff MK. Fetal iron deficiency alters the proteome of adult rat hippocampal synaptosomes. Am J Physiol Regul Integr Comp Physiol 305: R1297–R1306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tran PV, Fretham SJ, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res 65: 493–498, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran PV, Fretham SJ, Wobken J, Miller BS, Georgieff MK. Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am J Physiol Endocrinol Metab 302: E316–E324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang WS, Kang S, Liu WT, Li M, Liu Y, Yu C, Chen J, Chi ZQ, He L, Liu JG. Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci 32: 13,763–13,775, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 14: 51–59, 2009. [DOI] [PubMed] [Google Scholar]
- 66.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 76: 628–638, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr 89: 1488S–1493S, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng F, Zhou X, Luo Y, Xiao H, Wayman G, Wang H. Regulation of brain-derived neurotrophic factor exon IV transcription through calcium-responsive elements in cortical neurons. PloS One 6: e28441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]