Abstract
Stress activates multiple neural and endocrine systems to allow an animal to respond to and survive in a threatening environment. The corticotropin-releasing factor system is a primary initiator of this integrated response, which includes activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis. The energetic response to acute stress is determined by the nature and severity of the stressor, but a typical response to an acute stressor is inhibition of food intake, increased heat production, and increased activity with sustained changes in body weight, behavior, and HPA reactivity. The effect of chronic psychological stress is more variable. In humans, chronic stress may cause weight gain in restrained eaters who show increased HPA reactivity to acute stress. This phenotype is difficult to replicate in rodent models where chronic psychological stress is more likely to cause weight loss than weight gain. An exception may be hamsters subjected to repeated bouts of social defeat or foot shock, but the data are limited. Recent reports on the food intake and body composition of subordinate members of group-housed female monkeys indicate that these animals have a similar phenotype to human stress-induced eaters, but there are a limited number of investigators with access to the model. Few stress experiments focus on energy balance, but more information on the phenotype of both humans and animal models during and after exposure to acute or chronic stress may provide novel insight into mechanisms that normally control body weight.
Keywords: corticotropin-releasing factor system, comfort foods, restraint stress, chronic social stress
stress has been defined as a state of threatened homeostasis (208), which results in an array of physiological, neurological, and behavioral changes. Activation of the sympathetic nervous system (SNS) stimulates norepinephrine and epinephrine release from the adrenal medulla. Activation of the hypothalamic-pituitary-adrenal (HPA) axis (Fig. 1) releases glucocorticoids (GC) from the adrenal cortex (168) and other endocrine responses include suppression of growth hormone (135), thyroid-stimulating hormone (135), and reproductive hormones (111) but increased prolactin release (8).These and other physiological and behavioral responses allow an individual to respond to a change in environment but, with chronic stress, a variety of pathologies can develop that eventually become life threatening (190). Several reviews have been published in recent years addressing the function of specific aspects of the stress-responsive systems (113, 172, 203) or the effect of stress on animal behavior (12, 132, 163). By contrast, the focus of this review is to determine which animal models are appropriate for the investigation of stress-induced changes in energy balance.
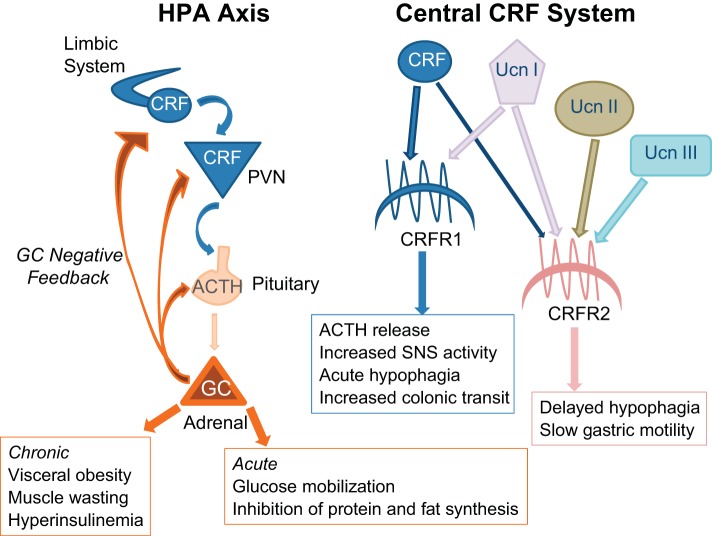
Fig. 1.
Schematic representation of the hypothalamic pituitary adrenal axis (HPA) and corticotropin-releasing factor (CRF) system and their impact on energy balance. PVN, paraventricular nucleus of the hypothalamus; UCN, urocortin; CRFR, CRF receptor; SNS, sympathetic nervous system; ACTH, adrenocorticotropic hormone.
Corticotropin-Releasing Factor System and Energy Balance
The corticotropin-releasing factor (CRF) system (Fig. 1) is the principal initiator of endocrine and behavioral responses to stress. In rodents, the CRF system includes the neurotransmitters CRF, urocortin (Ucn I), Ucn II, and Ucn III (128, 171, 233, 236). There are two subtypes of G protein-coupled CRF receptors (CRFR1 and CRFR2) each with multiple isoforms and different tissue distributions (94, 101, 203, 234). CRF binding protein (CRFBP) sequesters CRF and Ucn I (100) but not Ucn II or Ucn III (128). CRF and Ucn I are widely distributed in neurons across the brain (213) and both bind to CRFR1 and CRFR2, but CRF has a much greater affinity for CRFR1 than CRFR2 (23), whereas Ucn, which has 45% homology to CRF, has high affinity for both receptors (78, 236). Ucn II and III are selective ligands for CRFR2 (78, 128, 171) and have a more limited distribution than CRF or Ucn (128, 171), but both are expressed in areas of the forebrain including the hypothalamus.
The brain stem, limbic, and hypothalamic pathways that are involved in the initiation and coordination of a stress response have been reviewed in detail by Ulrich-Lai and Herman (231). Activated SNS afferents directly target peripheral organs and stimulate the adrenal medulla to increase circulating catecholamines, allowing an immediate “fight-or-flight” response to the threat. Activation of the HPA increases circulating concentrations of GC (corticosterone in most rodents and cortisol in humans and nonhuman primates), which modify tissue metabolism to inhibit energy storage and increase glucose availability to muscle and brain. GC exert negative fast feedback to downregulate stress-induced GC and catecholamine release by inhibiting expression of CRF mRNA in the hypothalamus (64, 97), but not the midbrain or brain stem (96), and by inhibiting activation of the SNS and pituitary gland (30, 249). Pituitary adrenocorticotropic hormone (ACTH) also inhibits HPA activity at the level of the paraventricular nucleus of the hypothalamus (PVH), reducing the amount of CRF protein present without changing CRF mRNA expression (186). Thus, in combination with negative feedback from the limbic system (185), GCs inhibit HPA activity and end the endocrine response to an acute stress (Fig. 1). For an insightful discussion of the specific and temporal aspects of different levels of HPA feedback control see the review by Watts (246).
There is diurnal rhythmicity to ACTH and ACTH-dependent GC release, which are secreted in a pulsatile manner with brief bursts of release at approximately hourly intervals (241). In people, plasma ACTH and cortisol levels peak in the morning and reach a nadir in the late evening (89). This pattern is reversed in nocturnally active animals (102). In conditions of acute stress ACTH concentrations reach a peak within about 5 min (63) and GC peak within 30 min and then start to decline (87). Chronic stress may increase hormone concentration at the nadir and reduce the amplitude of the diurnal cycle (58).
Central and peripheral (217) injections of CRF (62), Ucn I (202), Ucn II (98), and Ucn III (68) suppress food intake. Ucn I has the longest half-life and Ucn III has the shortest. A single intraperitoneal injection of Ucn I may inhibit food intake and weight gain of mice for up to 48 h, whereas a similar dose of Ucn III is effective for <2 h (217). The potency of Ucn I is likely associated with its affinity for both CRFR1 and CRFR2 compared with the selective affinity of other endogenous CRFR ligands. Peripheral administration of Ucn I decreases both the number and size of meals, whereas Ucn II decreases meal frequency, but not meal size (69). When Ucn II is given centrally the inhibition of food intake is delayed by several hours (98), whereas central injection of CRF produces an immediate, but transient, inhibition of food intake (115) that is due in part to an inhibition of noradrenergic activity in the PVH (114). In the periphery, CRFR1 activation stimulates colonic transit, whereas CRFR2 activation slows gastric motility and stomach emptying (136), potentially inhibiting food intake through peripheral sensory systems independent of direct central modulation of anorexigenic or orexigenic factors.
In addition to the effects of CRF-related ligands on food intake, the simultaneous release of GC also has a potential to modulate food intake. Low doses of GC stimulate food intake by facilitating orexigenic activity of neuropeptide Y (NPY) and norepinephrine in the PVH (222). In addition, the diurnal rhythm of corticosterone (CORT) release has been shown to drive hypothalamic expression of agouti-related protein (AgRP) (131), which is orexigenic and coexpressed with NPY. By contrast, high doses of CORT suppress food intake, possibly through CORT-induced insulin release, which inhibits NPY expression (207). This interaction between GC and insulin has been hypothesized to contribute to development of visceral obesity in conditions of chronic stress (59) and is discussed in more detail below. The importance of normal HPA axis function in maintenance of homeostasis in general and energy balance specifically is demonstrated by weight loss, hypophagia, a reduced preference for dietary fat, hypoinsulinemia, and loss of body fat in adrenalectomized (ADX) animals (44, 125). Conversely, chronic hypercortisolemia, or Cushings syndrome, is associated with obesity, an exaggerated visceral fat accumulation, insulin resistance, and an increased preference for high-fat foods (9, 42, 46). The clinical manifestations of a “buffalo hump,” a “moon face,” and muscle-wasting separates visceral obesity caused by hypercortisolemia from central obesity that develops independent of a primary hormonal disturbance.
In addition to inhibiting food intake, acute activation of the CRF system increases energy expenditure (209) due to CRF and/or Ucn I stimulation of SNS outflow to brown adipose tissue (BAT) (151) and increased locomotor activity. The hypophagia produced by a single injection of CRF is greatly attenuated during chronic central CRF infusion, but weight loss is sustained, leading to the suggestion that thermogenesis is the primary cause of negative energy balance with chronically elevated CRF (5). The increase in expenditure is initiated in the forebrain because Ucn I infused into the lateral ventricle causes the same reduction in food intake but a greater weight loss than a similar dose of Ucn I infused into the fourth ventricle (80). A CRF-inducible SNS pathway that involves the preoptic area, the dorsomedial hypothalamus (DMH), and the raphe pallidus has been identified (45). Thus animal models in which the CRF system is acutely stimulated lose weight because food intake is inhibited while thermogenesis and locomotor activity are increased. The CRF system is activated by both physical and psychological stressors, but this review will focus on stressors that are primarily psychological because physical stressors, such as cold exposure and exercise, induce changes in energy balance independent of activation of the CRF system.
One of the less commonly monitored responses is stress-induced hyperthermia. In laboratory rats stress can either increase or decrease body temperature depending on the type of stress that is applied (237), but psychological or mixed stressors tend to increase core body temperature in multiple species (56, 110, 133, 260). This has been described as emotional hyperthermia because it can be blocked by anxiolytic drugs (1, 156, 225), and changes in core temperature have been proposed as a valid screen for the anxiolytic effect of drugs (156). In experimental animals the degree of hyperthermia is proportional to the severity of stress. Body temperature will normalize even while a mild stress continues to be applied, whereas temperature remains elevated for the duration of more severe stressors (107). A chronic 10-day central infusion of CRF produces similar changes in body temperature as those induced by chronic stress, such that body temperature is increased during both the light and dark phase and the amplitude of the diurnal rhythm is blunted (32). The thermogenic response is slow to habituate and the rats remain hyperthermic during the first 7 days of infusion. By contrast, motor activity is increased only during the first 24 h of CRF infusion (32). Chronic peripheral infusions of CORT do not disrupt diurnal cycles of activity or body temperature (65), and it is clear that the hyperthermia involves centrally mediated SNS stimulation of BAT thermogenesis (194) and constriction of skin blood vessels to inhibit heat loss (157). Stress activates neurons in the medullary raphe region (130), which contains sympathetic glutamatergic and tryptophan hydroxylase-positive serotoninergic premotor neurons that project to BAT (152). Blockade of β3-adrenergic receptors or suppression of activity in glutamatergic neurons each attenuate the stress-induced hyperthermia, but neither fully blocks the response (130). Indomethacin, an anti-inflammatory agent, has no effect on the thermogenic response to social stress in mice (130), confirming previous reports that separate emotional thermogenesis from fever (156, 238). There is little information on the effect of psychological stress on human core temperature (239). Because the response is dependent on activation of BAT, it will be proportional to the amount of BAT available for activation. Rodents are usually housed below thermoneutrality and have the opportunity to develop significant brown fat depots (40). By contrast, humans seek out thermoneutral environments and have a relatively small amount of BAT available for stress-induced thermogenesis.
Stress and Energy Balance in Humans
Because the objective of this review is to compare animal models of human stress and energy balance, it is appropriate to first describe the characteristic responses of humans and to evaluate why stress causes weight gain in some people but weight loss in others. The response to a particular stress is determined by the duration and nature of the stressor, whether it is physical or psychological, the sex of the individual experiencing the stress, and that individual's perception of the stress. This perception will be determined by familiarity with the stressful event, how much threat is considered to be associated with the stress, and availability of an appropriate support system. In the brain, multiple systems are activated and integrated to orchestrate the appropriate response to a specific stress. Although activation of the SNS and HPA optimize an immediate response to a short-lived, unpredictable environmental or physical challenge, chronic stress can lead to a variety of pathologies that eventually become life threatening (190). GC release during one stress has the potential to modify neuronal structure and influence physiological and behavioral responses to subsequent stressors (138); thus chronic or repetitive stress produces adaptive changes that allow the body to restabilize in a challenging environment. This adaptation is referred to as allostasis, and if it is either inadequate or inappropriate, then there will be a gradual development of chronic disease (137, 139). During the last decade there has been growing interest in the potential of chronic psychological stress as a causal factor in human obesity (201, 243) and metabolic syndrome (19) at the same time as clinicians address the maladaptive catabolic effects of sustained physiological stress to successfully treat critically ill patients (167).
Stress and Weight Gain in Humans
It has long been recognized that there is a direct association between stress responsiveness and eating disorders in both men and women (187, 247, 256), but more recently interest has focused on the association between everyday social stress and its impact on food choice and weight gain. An online survey of 1,848 adults conducted by the American Psychological Society in 2007 (3) found that one-third of the sample population was experiencing extreme stress caused by work responsibilities, financial issues, and children. Forty-three percent of the respondents reported that stress caused them to eat too much, whereas 36% reported that stress caused them to skip a meal. Similar results were obtained in a recent telephone poll of 2,505 adults by National Public Radio, the Robert Wood Johnson Foundation, and Harvard School of Public Health (154), which found that 49% of the sample had experienced a major stress in the past year. The most common causes of this stress were too many responsibilities, financial problems, work-related problems, and health issues. The participants reported many different ways of responding to stress, but 44% of those “experiencing a great deal of stress” said they ate less than usual, whereas 39% said they ate more. These surveys indicate that stress has diverse effects on food intake across the population and imply that chronic stressors contribute to overeating and weight gain in only a subset of the population. Even though many people consider their job to be stressful, the available literature fails to support an association between psychosocial pressure of work and body mass index (BMI) or body fat distribution for either men or women (161, 196). Job insecurity and high or low psychological demands at work have been shown to increase weight gain in obese individuals, but to cause weight loss in those with a low BMI (83). Even though the work environment is frequently perceived at stressful, a recent study with a relatively small number of participants found that cortisol was higher for both sexes when they were home than when they were at work (61).
Although cross-sectional data indicate that only some members of the population increase food intake in response to stress, because of the increasing incidence of obesity there is significant interest in the association between chronic stress, food choice, and weight gain and a general assumption that chronic stress is an independent risk factor for obesity. There have been repeated demonstrations that restrained eaters, especially women (79, 247), are more likely to overeat in response to stress than are unrestrained eaters (91, 263). Restrained eaters make a sustained conscious effort to restrict food intake and make food choices that allow control of body weight. Thus food intake is determined by a reliance on external cues related to specific foods rather than an internal state of hunger or satiety. Although restrained eaters may have energy intakes similar to those of unrestrained eaters (204, 205), because restraint requires extended periods of self-imposed restriction (235), it is associated with intermittent periods of disinhibition that result in overeating and consumption of “forbidden” foods (93). Restrained eaters that experience frequent bouts of disinhibition are those most likely to overeat in response to chronic stress; thus, in order for stress to increase food intake, an individual has to be both a restrained eater and someone who is easily disinhibited (248). A number of studies have clearly shown that stress has the potential to disinhibit restrained eaters, increasing energy intake by changing food choice to foods that have a higher carbohydrate and fat content (173, 176, 244, 247). Yeomans and Coughlan (259) confirmed that anxiety caused disinhibition only in women who were practicing ineffective restrained eating, whereas women who were restrained and resistant to disinhibition decreased their food intake in response to stress, but increased intake in relaxed conditions. These studies confirm that stress is an independent risk factor for obesity in only a select subset of the population.
Several studies have reported that restrained eaters have normal basal cortisol concentrations (66, 166) but have a hyperreactive HPA response to stress (153, 177). Women who showed an exaggerated cortisol response to a laboratory-based stressor also ate more on the day of stress (66) and reported consuming more snacks in response to daily “hassles” (153). A detailed evaluation of HPA activity in women demonstrated a positive association between restraint, basal cortisol release, and impaired negative feedback of the HPA axis (224).
Comfort Food Hypothesis
The “Comfort Food” hypothesis proposed by Dallman et al. (60) provides a mechanistic explanation for why consumption of high-fat, high-carbohydrate “preferred” foods helps animals and humans cope with chronic stress but also leads to weight gain. This hypothesis developed from a series of rodent studies examining the association between chronic stress, central GC levels, activation of the HPA, sucrose intake, and body composition (60). The first observation was that chronic elevation of GC caused by implanted CORT pellets (2) or repeated exposure to high-intensity stressors [e.g., 30 min footshock for 7 days, cold exposure for 5 days (261)] impaired the negative fast feedback activity of CORT such that there was a failure to suppress CRF expression in the PVH and an extended release of GC during exposure to a novel stress (218). In experimental conditions, chronically elevated GC in the amygdala increased CRF mRNA expression in the PVH (193) indicating that loss of sensitivity to negative feedback was rostral to the PVH and pituitary (22).
The second observation was that access to sucrose solution corrected ACTH and CORT secretion, hypophagia, hypoinsulinemia, inhibition of weight gain, and loss of body fat in ADX rats even though they drank only 50% as much sucrose as sham controls (16). Sucrose also modified the central effects of ADX, preventing the increase in PVH and amygdala CRF mRNA expression and suppressing activation of the locus coeruleus (125), an area of the brain implicated in stress-induced anxiety (33). Thus sucrose consumption mimics the negative feedback of the HPA axis that is normally performed by GC, and it has been suggested that downregulation of CRF in the amygdala provides the neuroanatomical basis for comfort food dampening the stress response (121). Saccharin does not correct ACTH, insulin, energy intake, or weight gain in ADX rats (21, 125), demonstrating that the negative effects of ADX are secondary to a reduced caloric deficit, and it has been proposed that the ratio of CORT to insulin determines energy balance (207).
Evidence from studies with ADX streptozotocin (STZ) diabetic rats support the notion that insulin is the metabolic signal that downregulates CRF system activation (207) and increases the salience of food reward (122) through a mechanism that is dependent on the hepatic vagus nerve (124). When ADX rats are replaced with chronically high levels of CORT they lose weight due to increased thermogenesis, but are hyperinsulinemic and have enlarged white fat pads with a slightly greater increase in intraperitoneal than subcutaneous fat (17), suggesting that ADX has little effect on peripheral metabolism unless circulating insulin levels are low (59).
Consistent with the comfort food hypothesis a 4-day treatment of young, lean volunteers with the synthetic GC methylprednisolone increased food intake and body weight. The increase in food intake was macronutrient specific and involved a fourfold increase in fat intake, a threefold increase in carbohydrate intake, but only a 70% increase in protein consumption (221). In a more recent study women with the greatest perceived stress were fatter, had a greater waist-to-hip ratio, reported more emotional eating, and had a smaller cortisol response but a greater psychological response to a laboratory-based social stress test than their controls (224). Animal studies designed to test the comfort food hypothesis have, however, provided mixed results. Rats with access to sucrose solution, lard, and chow have a greater energy intake both before and during 5 days of 3 h of restraint compared with chow-fed controls. Stress specifically inhibits chow intake, and during restraint the rats fed comfort food have an attenuated ACTH and CORT release. Comfort foods reduce hypothalamic CRF mRNA in control animals, however, there is no effect of diet in stressed animals (165). Additional studies have shown that access to comfort food prevents suppression of food intake, inhibits CORT release, and reduces anxiety behavior during 3 days of foot shock (159), and that access to a cafeteria diet prevents an increase in serum CORT of rats subjected to 3 wk of chronic unpredictable mild stress (CMS) but does not prevent anxiety-type behavior (158, 262). Thus these studies show that access to comfort foods prevents chronic stress from downregulating CORT negative feedback on the HPA axis, but the impact on various behaviors and the central mechanisms responsible for this are less well defined.
An alternative hypothesis is that consumption of palatable foods downregulates the stress response by activating reward pathways. Rats given access to 4 ml of sucrose solution on a daily basis have a reduced HPA response and fewer anxiety-type behaviors following 20 min of restraint stress than rats that do not have access to sucrose (230). The attenuation of CORT release during restraint also is present in rats given access to saccharin solution or males given the alternate pleasure of a brief daily exposure to a receptive female (230). The dampening of the stress response is independent of sucrose metabolism because inhibition of CORT release is absent in rats gavaged with sucrose but is exaggerated if sucrose is consumed in two daily exposures compared with one longer exposure that delivers the same amount of sucrose (232). Lesions of the basolateral amygdala (BLA), an area of the brain that integrates responses to reward in addition to stress, prevent the inhibition of CORT release by sucrose (230), and a microarray of the BLA indicates that brief access to sucrose changes the expression of a large number of genes including some involved in synaptic remodeling (230). Therefore, it is proposed that access to pleasurable activity, including consumption of preferred foods, downregulates the stress response through reward-based structural plasticity. This is supported by observations that the stress-dampening effect is sustained for at least 21 days after sucrose has been withdrawn (50). It should be noted that the reward and comfort food hypotheses are conceptually different in that the first suggests that pleasurable activity dampens the response to a novel acute stress (230), whereas the second proposes that consumption of palatable, energy-dense foods during chronic stress prevents an adaptation that makes the HPA axis hyperresponsive to subsequent stressors. Both of these paradigms appear at odds with evidence of increased anxiety in rats deprived of intermittent binging on sucrose solution (53). The large meals of sucrose in binging rats sensitize central reward systems and induce a state typical of addiction (18, 53, 54, 169). The increased anxiety in nonstressed bingeing rats that are deprived of sucrose is one manifestation of withdrawal. This is very different from protocols in which access to comfort foods or pleasurable experiences suppress stress responsiveness.
By contrast to the studies that suggest that consumption of “comfort foods” suppress the stress response there are others showing that a high-fat diet exaggerates the stress response. Rats fed a 40% kcal fat diet have elevated basal CORT levels with a greater effect of diet at 7 than at 21 days (220). CORT also takes longer to return to baseline after stress, and this is apparent for up to 12 wk of high-fat feeding (103, 220). The form in which dietary fat is consumed, rather than energy consumed as fat, determines the HPA response to stress. Rats offered a choice of chow and lard for 7 days have a dampened HPA response to restraint compared with controls or rats consuming a formulated high-fat diet (123). Exposing animals to a high-fat diet for extended periods of time attenuates stress-induced anxiety-type behavior (34) but exaggerates hypophagia and weight loss (70, 126), suggesting that responses mediated by CRFR2 are exaggerated, whereas those that are CRR1-dependent are downregulated. A limitation of these studies is that it is impossible to separate the effects of diet composition from that of obesity.
Stress and Weight Loss in Humans
Although it is well established that chronic physiological stress associated with critical illness can result in significant weight loss (167), there is only a small amount of literature on weight loss caused by psychological stress in humans. A review of 13 cross-sectional studies, including a total of 160,000 adults, found a U-shaped association between chronic work-related psychosocial stress and BMI in that stress was higher in underweight, overweight, and obese individuals compared with normal weight individuals. In a longitudinal analysis, an increase in job-related stress was associated with a similar odds ratio for development of obesity as for weight loss over 4 years (155). Block et al. (26) used self-report of BMI and stress in 1,552 adults to show that over a 9-year period psychosocial stress was associated with weight loss in those with a low BMI and weight gain in those with a high BMI. A similar relation has been reported for men in a 5-year follow-up of ∼8,000 British civil servants, but there was no association between job stress and weight change in women (109). In contrast to these studies, Stone and Brownell (206) examined 12 wk daily diaries of 158 subjects and found that both men and women were more likely to eat less than to eat more on stressful days. In addition to this information on chronic stress, there are some reports of weight loss in response to a traumatic event. A major life crisis in elderly adults, such as loss of a spouse, has been shown to cause a substantial inhibition of food intake and weight loss, both of which are reversed over time (250). The limited number of reports on weight loss caused by psychological stress in humans clearly indicates that this is not considered to be a significant health issue. By contrast, as discussed in detail below, weight loss is the predominant response in animal models of stress.
Animal Models of Stress-Induced Weight Loss
Research into stress-induced weight gain dominates the human literature, but weight loss is the most common outcome in experimental animal stress studies. This may be because a majority of laboratory-induced stressors are acute and nonpredictable and because experimental animals do not usually have access to a variety of palatable foods. Laboratory animals (most commonly rats and mice) are likely to have a more uniform genetic background than participants in a human study and the number of animals in a laboratory-based study is unlikely to be large enough to parse out a bimodal distribution. Even if there are individual differences in stress-responsiveness of these animals, weight loss predominates and only the most frequently used stress procedures will be considered here.
Restraint and immobilization stress.
Restraint stress is one of the most commonly used protocols for acute stress in laboratory rats and mice and has been the subject of a number of reviews (37, 72, 162). The procedure involves physically confining the animal to a holder that prevents locomotion, turning or crouching, but that is not restrictive enough to cause pressure on internal organs. Restraint has been achieved using Plexiglas tubes, decapicones, wire mesh holders, cut-off plastic bottles, or by wrapping animals in a towel (37). This confinement is a combined physical and psychological stress that may last for only minutes (105) or for hours (223) and there is no major energetic demand or physical injury. The stress is technically simple, uniform, and easily replicated. Immobilization stress is a more severe variant of restraint in which the animal is laid on either its front or its back and is immobilized by taping or tying down its limbs and sometimes head movement is limited by putting loops over the neck. This obviously produces more physical distress than restraint and is overall a more severe stress (7).
Prolonged (up to 18 h) exposure to restraint (223) or the combination of cold water immersion and restraint for 3.5 h (90) causes gastric ulcers, which would have a nonspecific inhibitory effect on food intake and general health of the animals. By contrast to these experiments, investigations of behavioral and neuroendocrine mechanisms use periods of restraint that last for minutes or several hours. In group-housed, 250-g rats brief, 20-min periods of restraint stimulate food intake during the hour immediately following restraint even though CORT does not change (13). Because weight gain also can be induced by brief handling of unstressed animals (13), it is possible that this results from a generalized arousal of the animals rather than a specific effect of restraint. By contrast, two bouts of 20 min of restraint 9 days apart each increase serum CORT and cause weight loss in single housed, 450-g, low-fat-fed rats, but weight gain in high-fat-fed rats susceptible to diet-induced obesity (144). The weight gain is independent of any change in PVH CRF mRNA, but is associated with a decrease in CRF mRNA in the central nucleus of the amygdala (144). Longer periods of restraint stress reliably induce a state of negative energy balance in addition to anxiety-type behaviors and learning deficits. In mice, 30 min of restraint significantly inhibits food intake for 4 h (212), whereas the more severe stress of 20 min immobilization inhibits food intake for up to 3 days (57). In rats, a single 3-h restraint elevates serum CORT and increases energy expenditure only during restraint (86), and stress-induced hyperthermia is corrected within an hour of the end of restraint (85), but food intake is inhibited for up to 9 h and body weight is reduced compared with controls for at least 10 days after the restraint (178). Weight loss is exaggerated if restraint is repeated each day, with a maximal effect after 3 days, but if these rats are exposed to a second bout of repeated restraint they experience additional weight loss (85). Food intake is inhibited on the days of restraint, but returns to control levels within a few days of the end of stress, with no attempt to overeat (87). The rats lose weight on the days of restraint but do not compensate for the weight loss and continue to weigh less than controls for at least 40 days after the end of stress (87). Initially, all of the difference in weight is accounted for by loss of lean body mass, but 5 days after the end of restraint the difference is a combination of lean and fat tissue (265) due to a temporary shift in adipocyte and liver metabolism (264, 265). This results in the rats being smaller, but having a proportionally similar body composition as controls.
The weight loss in restrained rats is not dependent on energy status during restraint (85) but can be prevented if rats are offered lard and 30% sucrose in addition to chow. The prevention of weight loss is associated with downregulation of HPA activation during restraint (165), and the importance of CORT in the initiation of long-term weight loss has been confirmed using ADX rats with and without an injection of CORT immediately before the start of restraint (188). The central mechanisms responsible for initiation of the long-term downregulation of body weight have not been identified, but receptors accessible to third ventricle infusion of antalarmin, a CRFR1-specific antagonist, are required for the sustained reduction in body weight (48). These receptors are not responsible for an inhibition of food intake or a reversible reduction in body weight that are apparent on the days of restraint (48). In addition to showing a sustained reduction in body weight, restrained rats remain hyperresponsive to novel acute stressors (47, 84). Exposure of previously stressed rats to a second stress leads to an exaggerated ACTH and CORT release (38, 134, 175) that is associated with a reduced number of hippocampal GCR (35, 134) and a reduction in CRFBP during the days immediately following stress (219). Third ventricle infusion of CRFR antagonists before restraint does not prevent this change in HPA sensitivity (49), suggesting that restraint produces long-lasting changes in the function of multiple control systems, consistent with observations that a single exposure to the stress of social defeat causes sustained changes in serotonergic and noradrenergic systems (20, 34).
The effects of restraint on energy balance are age dependent. Young, 3-mo-old rats are hyperthermic for 12 h after a 3-h restraint, whereas 21-mo-old rats are not (31). By contrast, young (5 wk old) rats rapidly recover weight loss after 3 days of 3 h of restraint, whereas older (300 g) rats maintain a reduced weight for at least 40 days (87). The age-related differences in response may be determined by testosterone, which can alter central regulation of HPA activity (74). Weight loss is not sex specific (Harris, RBS; unpublished observations), although a majority of studies are performed in males. Females show a greater CORT release during a 30-min restraint than males, and this is due to different levels of CRF expression in specific areas of the brain including the PVH, but is independent of the estrous cycle (11).
Social defeat.
Chronic social defeat is a combined physical and psychological stressor that has been used extensively as a mouse model of depression (117) and to investigate rewarding aspects of drug abuse (146). Male C57BL/6 mice exposed for 5 days to a resident-intruder paradigm that involves receiving 20 bites from an aggressive resident male lose weight, exhibit depression-like behavior (118), avoid social contact (10), and increase their voluntary alcohol intake (119). A single bout of defeat results in a threefold increase in serum CORT 30 min after the defeat but a 12-fold increase 24 h after the last of 12 defeats (107), indicative of the severity of the stress. A majority of this work involves male mice, because most females are not socially dominant. By contrast, California mice (Peromyscus californicus) are socially aggressive (197). The social defeat produces a greater increase in serum CORT and inhibition of social interaction in subordinate females than males (227, 228), but the effect of social defeat on body weight of the females has not been reported.
Anxiety and weight loss in male-defeated mice develop even when physical contact is minimized, but the intruder and resident are housed in constant sensory contact on opposite sides of a vented partition (106). Detailed analysis of behavior in individual C57BL/6 mice subjected to 10 days of 10 min of social defeat reveals different phenotypes in the defeated mice: “Susceptible” mice lose weight, reduce preference for sucrose solution, and reduce social interaction. By contrast, “unsusceptible” mice cannot be differentiated from controls based on body weight or behavior (116). The differences are independent of CORT release or anxiety-type behavior, but susceptibility is associated with increased activity of dopamine neurons in the ventral tegmental area (VTA), part of the limbic reward system. Interestingly, there are more changes in gene expression in the VTA from unsusceptible than susceptible mice, implying that resistance to social defeat is an active, rather than passive, response. The Chinese tree shrew (Tupaia belangeri chinensis) is a small mammal intermediate between insectivores and primates that is also used as a model for stress-induced depression. Subordinate males subjected to an hour a day of social defeat followed by sensory contact for the remainder of the day (55) respond in a similar manner to mice and display hypercortisolemia, anhedonia, and reduced activity. They also lose ∼6% of body weight, which is not readily recovered when stress ends (242).
Social defeat produces similar physiological and behavioral responses in rats as it does in mice, and these were reviewed by Bulwalda et al. (36). Subordinate rats exposed to an aggressive dominant male for a few minutes each day for several weeks gain weight more slowly than controls even though they do not remain in sensory contact with the dominant male (179). A single 1-h exposure to an aggressive resident rat can cause a sustained hyperthermia, reduced activity, and inhibition of food intake and weight gain for 8 days (140). The severity of response is inversely related to the ability of the subordinate to fight back rather than the number of attacks made by the resident (141). Housing conditions also influence the rate of recovery of the subordinate as single-housed rats fail to gain weight, show anxiety-type behavior, and are hyperresponsive to acute stressors for several weeks after a defeat, whereas group-housed subordinates recover within days of the defeat (175, 240). These data suggest that the long-lasting response to a single stress results from modification of control systems during the poststress period and that this is prevented by social interaction with nonaggressive cage mates.
Chronic mild stress.
Another common rat model of combined physical and psychological stress is the CMS protocol in which animals are exposed to a different stressor each day for a number of weeks (88, 252) and has been reviewed in detail by Willner (251). This is considered a reliable model of depression because the rats show anxiety-type behavior and anhedonia (253). Food intake is inhibited during CMS (88), but these measures are not entirely reliable because some of the mild stressors interfere with normal feeding and others make it difficult to accurately measure intake. There is a steady inhibition of growth in both male and female CMS rats (67, 170) and in mice offered high-fat diet (143). The severity of each stress in this protocol is less than the resident-intruder protocol, but the unpredictability of the procedure increases the degree of psychological stress imposed. Because each laboratory uses a different number and type of stressor, there is some variability in study outcomes and there is no information on whether the weight loss is reversible once the stress protocol ends.
Glucocorticoid infusion.
As noted above loss of adrenal hormones in ADX rats causes a significant reduction in food intake that can be restored by replacement of aldosterone and CORT. The aldosterone stimulates fluid intake, but CORT stimulates food intake and increases insulin release (183). CORT lowers PVH CRF expression (189), and acute injections of CRF facilitate the stimulation of carbohydrate intake by NPY and norepinephrine in ADX rats (222). By contrast, sustained supraphysiological replacement doses of CORT suppress food intake. The high levels of CORT stimulate insulin release which, in turn, downregulates NPY expression to suppress food intake. Insulin also promotes fat storage in the periphery at the same time as CORT has a catabolic effect on lean tissue (183). Rats infused with high-dose CORT are in a catabolic state, but have relatively large intraabdominal fat depots (17) due to a simultaneous, site-specific stimulation of both lipolysis and adipocyte proliferation (39). Unlike rats and mice, hamster species secrete both cortisol and CORT and each of which is regulated independently (160). Although both CORT and cortisol are increased by acute stress, only cortisol shows a sustained elevation in response to chronic stress (160). Daily injections of either GC have no effect on body weight, whereas chronic infusion of cortisol, but not CORT, causes hyperinsulinemia, weight loss, and reduced body fat without changing food intake (200). Thus chronic elevation of GC alone does not replicate the obese phenotype typical of Cushing's syndrome even though it does cause a redistribution of adipose tissue to the viscera.
Animal Models of Stress-Induced Weight Gain
As discussed above, inhibition of food intake and weight loss are typical physiological responses to stress, but the increasing incidence of obesity has led to development of experimental animal models that can be used to investigate the association between chronic stress and weight gain in humans (see Table 1). Different methods of inducing stress in rodents and other animal models produce divergent behavioral and physiological responses (25), but it has been suggested that chronic social stress is more typical of stressful events in an animal's natural habitat in addition to representing the etiology of stress-related disorders in humans (25, 112).
Table 1.
Comparison of animal models of stress-induced weight gain to characteristics of humans susceptible to stress-induced weight gain
| Humans | Monkeys | Hamsters | Socially Defeated mice | GC-Infused Mice | |
|---|---|---|---|---|---|
| Nature of chronic stress | Constant psychological pressure at work or home | Constant presence of socially dominant monkey | Intermittent physical contact with dominant male/foot shock | Constant presence of dominant mouse with limited physical contact | Continuous corticosterone administration |
| Sex of subject | Usually female | Only females tested | Only males tested | Only males tested | |
| Daily Food Intake | Restrained | Increased | Increased | Increased/not different | Increased |
| Food intake after acute stress | Increased | ||||
| Body weight/adiposity | Increased | Increased | Increased | Increased/no change/reduced | Increased |
| Body weight after stress ends | Maintained | Normalized in young, gain maintained in old | Normalized | ||
| Baseline GC level | Normal | Suppressed | Normal | Same as individually housed controls | Atrophied adrenals |
| GC response to acute stress | Increased | Increased | |||
| HPA reactivity | Impaired DST | Hypersensitive to ACTH, impaired DST | Impaired DST | ||
| References | 66, 79, 91, 153, 166, 177, 224, 247, 248, 259, 263 | 6, 184, 195, 254 | 71, 199 | 14, 15, 77, 148, 163, 182 | 41, 104 |
Summary of phenotype of humans who are susceptible to stress-induced weight gain and animal models of stress-induced weight gain. Empty boxes indicate that the parameter has not been reported.
GC, glucocorticoid; HPA, hypothalamic pituitary adrenal axis; DST, dexamethasone suppression test; ACTH, adrenocorticotropic hormone.
Tail pinch.
There is little literature associating acute stress with overeating or weight gain; however, Rowland and Antelman (174) found that tail-pinch caused rats to immediately start eating if food was present. The oral responses to tail-pinch include gnawing, licking, and eating (4) and are dependent on activation of the striatal dopaminergic system. Weight gain is achieved if the procedure is performed repeatedly each day when rats have access to sweetened milk, but cannot be replicated in chow-fed rats (73, 127). Tail-pinch-induced eating can be blocked by opiate receptor antagonists (150), by CRFR1 antagonists (181), and by a variety of hormones and neuropeptides known to influence feeding behavior (73, 127) even though behavioral analysis of young animals indicates a difference between stress-induced feeding and hunger (210). The feeding response is likely a nonspecific behavior because tail-pinch also initiates maternal behaviors in dams with pups (211) and sexual behavior in males (129). Consistent with this, it has been proposed that mastication is a coping response that downregulates the HPA axis (92), the stress-induced increase in striatal dopamine (75), and the stress-induced suppression of spatial memory (147). More recently bingeing on palatable food has been reported in a rat model that combines repeated food restriction and stress (27, 82). The bingeing is driven by sensitization of opioid receptors (28, 81) and does not result in weight gain. It has been proposed that the history of food restriction, maintenance of normal body weight, and bingeing on palatable food represents a model of bulimia nervosa (52, 82).
Social conflict.
By direct contrast to studies described above in which defeated mice lose weight, there are some recent studies in which subchronic and mild social defeat result in reversible weight gain of the defeated animal. Moles et al. (148) reported that subordinate male NMRI mice housed in continuous visual, olfactory, and auditory contact with a dominant male and intermittently exposed to short periods of physical contact with the same dominant male each day for 8 days gain more weight than either individually housed, nonstressed controls or dominant male mice. Both subordinate and dominant mice eat more than controls, but only the subordinate is heavier because of increased nonactivity energy expenditure in the dominant mouse. In other studies, however, Swiss CD1 mice subjected to the same protocol gain less weight and have smaller epididymal fat pads than their controls (15), or eat more, but gain the same amount of weight and are equally fat as CD-1 controls (182). When the mice are offered a high-fat diet subordinate mice gain more weight than group-housed controls and are fatter than dominant mice, but are no fatter than nonstressed mice (14, 182). In a similar protocol offering mice continuous access to chow, but also 4 h access to high-fat diet each day, subordinates eat more than nonstressed controls and gain weight during stress (164). The increase in energy intake is associated with a threefold increase in chow intake but a 20% reduction in consumption of high-fat diet (164). Once the stress ends young (8 wk old) subordinate mice normalize their food intake and start to lose weight, whereas 6-mo-old stressed mice are significantly fatter than their controls and hypothalamic expression of the orexigenic peptides NPY and agouti-related protein (AgRP) are elevated even 2 wk after stress has ended (164).
Stress-induced weight gain is associated with a significant increase in circulating ghrelin that is reversed during recovery (164). Ghrelin is a hormone that is increased in states of hunger (226), stimulates food intake (257), and increases adiposity with repeated administration (229). The increased weight gain of defeated mice is associated with increased food intake due to larger meal size, which correlates with elevated ghrelin concentrations before the start of feeding (51, 120). Consistent with the potential for ghrelin to promote food intake during stress, wild-type mice receiving central infusions of ghrelin receptor antagonist or ghrelin knock-out mice do not gain weight during chronic stress (164).
Mice also gain weight on a modified social stress protocol in which the subordinate is subjected to progressively shorter durations of exposure to different dominant resident mice each day, starting with 10 min on day 1 and ending with only 0.5 min on day 10 (77). The subordinate C57BL/6 mouse gains more weight than controls during stress and maintains this weight difference throughout a 30-day poststress period. Water intake of the mice is substantially increased on the days of stress, but food intake is increased only during the recovery period. Even though the defeated mice weigh more than controls, this is due to an increase in body water content, and at the end of the 30-day recovery period there are no differences in fat pad size, which tend to be smaller in the defeated mice (P < 0.1). Overall, weight gain in socially defeated mice is inconsistent and composition of the gain may be determined by the specifics of experimental protocol that is used.
Weight gain is more consistent in chronically stressed Syrian hamsters. In 1988 Borer et al. (29) reported that group-housed female golden Syrian hamsters grew faster and gained 50% more body fat than single-housed controls. The increased weight gain was attributed to a reduction in heat loss due to huddling by the group-housed animals. Subsequently, Meisel et al. (142) repeated the experiment and noted that not only were growth and adiposity increased, but that adrenals were enlarged in group-housed animals and concluded that group housing animals that were normally individually housed represented a model of chronic social stress. This work was followed up in studies in which male Syrian hamsters were subjected to daily 7-min sessions of social defeat in a resident intruder test (71, 199). Weight gain was apparent in submissive intruders within 5 days and did not differ between animals that were subjected to social defeat on 4 consecutive days or on 4 days distributed across the first 10 days of the study. Weight gain was associated with a small, but significant, increase in cumulative food intake and an increase in feed efficiency (71). Importantly, neither weight gain nor cumulative energy intake of subordinates returned to control levels once the social stress ended. In a second study mesenteric fat was the only fat depot that significantly increased in the subordinate animals (199), suggesting a link between stress, abdominal fat deposition, and increased risk for chronic disease, but this was not the case for the first experiment in which all of the measured fat depots were significantly larger in subordinate than control animals (71). The dominant animals also gained more weight than controls, but the gain was not as great as that in the subordinates and was due to increased lean tissue, not body fat (199). Foot-shock induces identical increases in body fat and feed efficiency in hamsters (199), whereas repeated restraint stress decreases food intake and causes weight loss (108) suggesting that the weight gain could be generalized to some, but not all types of chronic stress.
As noted above, an attempt to replicate stress-induced weight gain with chronic infusions of GC into adrenal-intact animals was unsuccessful (200). One reason may be because endogenous GC are released in hourly bursts which are necessary for maintenance of normal HPA responsiveness, and it is likely that a stable high level of cortisol activates different GR and MR populations from those that respond to the acute stimulation of GC release during a social defeat encounter. Increased growth and accumulation of fat mass in social-defeated hamsters remains uncorrected once stress ends, but it is not known whether this also is the case for humans subjected to chronic stress. Therefore, further work is required to elucidate the mechanistic basis of weight gain and a more in-depth evaluation of the human phenotype is required to determine whether hamsters represent a good model for chronic psychological stress in humans.
The reason for the dramatic difference between animals that lose weight during social stress compared with those that gain weight has not been explained. Because different strains of mice respond differently to the same stressor (191, 192), it is possible that mouse strain impacts the response of the submissive mouse or even the degree of aggression displayed by the resident mouse. C57BL/6 mice, however, have been used in studies that show either weight gain (77) or weight loss (118). One consistent difference is that the degree of stress is greater in experiments where subordinate mice lose weight (77, 148). Consistent with this the aggressive behavior of Syrian hamsters is ritualized such that the physical damage to the subordinate is limited compared with that sustained by subordinate mice (95). Despite the reduced level of physical stress in animals that gain weight, there remains a significant level of psychological stress because chronic social stress induces social avoidance behavior irrespective of whether mice gain (77) or lose (70) weight.
Chronic social stress.
A unique social stressor in laboratory rats is the visible burrow system (VBS) (24). The burrow consists of a large chamber divided into an open surface area that is lit 12 h/day and several smaller dark chambers connected to the surface area by tunnels. Food and water are available in both the open area and small chambers (216). Mixed gender groups of rats are housed together in the burrow which results in one of the males becoming dominant and the remaining males being subordinate. The subordinate rats have elevated CORT, low testosterone, are hypophagic, and lose weight. They have enlarged adrenals, small thymus glands, a greatly blunted CORT response to novel stress, and changes in brain neurotransmitters typical of depression (24, 214, 215). The dominant rats also have a slightly reduced body weight, enlarged adrenals, and small thymus glands, but maintain normal testosterone levels and respond normally to an acute novel stress (215). Once the rats are separated and stress ends the subordinate rats overeat until body weight has returned to control levels, but fat depots are larger than controls with a greater proportion of fat deposited in intraperitoneal than subcutaneous depots (216). In this model of continuous stress, consumption of a high-fat diet does not protect the animals against weight loss during stress, but does exaggerate fat gain during recovery (214). The mechanism for poststress weight gain has not been determined, but subordinate animals that lose weight have an elevated HPA response to acute stress (180), and it is possible that HPA reactivity during the early stages of recovery facilitate overeating and fat gain.
Housing of nonhuman primates in social groups has allowed investigation of behavior in an environment that is comparable to the VBS in rats because the captive monkeys develop a social hierarchy that induces a state of chronic stress in subordinates (184). Subordinate members of groups of female cymologous monkeys housed in experimental conditions tend to be socially isolated, fearful, dexamethasone insensitive, and hypersensitive to ACTH (195). The recent development of an electronic chip detection system has allowed precise monitoring of food intake of individual members of colonies of female macaques (254). Subordinate monkeys consistently eat more than the dominant females regardless of dietary composition or choice (254), consuming large meals and increasing night-time eating (149). Consistent with the comfort food hypothesis, subordinate monkeys have an exaggerated preference for high-fat diet, which increases their weight gain and reduces anxiety-type behaviors (6). The increased intake of subordinates has been attributed to a greater sensitivity to postprandial ghrelin (145) that increases intake by selectively stimulating intake of the low-fat diet. By contrast, ghrelin suppresses food intake of dominant animals by selectively inhibiting intake of low-fat diet (145). Astressin B, a nonspecific CRFR antagonist that does not cross the blood-brain barrier, significantly inhibits intake of both low- and high-fat diet in dominant animals but selectively increases intake of the high-fat diet without changing intake of the low-fat diet in subordinates, despite decreasing serum cortisol (145). By contrast, antalarmin, a CRFR1 antagonist that can cross the blood-brain barrier, suppresses intake of subordinate monkeys to the same levels as in dominant animals (255). These results imply that although the subordinates have a preference for palatable high-fat diet, consumption is not directly driven by cortisol, but does result from activation of the central CRF system.
The reason for the difference in response between subordinate rats in the VBS compared with subordinates in a monkey colony has not been explored. Both animals are subjected to continuous social stress, but an obvious difference is that the rats in the VBS are male, whereas the monkeys are females, and it is possible that there a sex effect on response to social stress, similar to that in humans. Another potential physiological factor is the role of emotional hyperthermia. The monkeys are housed in outside enclosures in the southeast United States where ambient temperature is close to thermoneutrality [25–28°C for a monkey (245)], whereas the rats are in an environmental-controlled facility where ambient temperature is likely to be 8 or 9°C below neutral temperature [∼30°C for a rodent (76)]. It is possible that environmental temperature inhibits stimulation of thermogenesis in monkeys to prevent an excessive elevation of body temperature, but irrespective of differences in heat loss, stress has opposite effects on food intake in the rats versus the monkeys, presumably due to differences in central neuroendocrine responses to the chronic stress.
Glucocorticoid infusion.
Unlike Syrian hamsters (200) and rats, ADX has little sustained effect on food intake or body weight of wild-type mice (43, 198), but GC replacement at supraphysiological levels stimulates food intake even in CRF knockout mice (99). These data indicate a direct effect of GC, rather than a downregulation of central CRF by GC, which stimulates food intake. In a study with intact mice (104) water was replaced with solution of 25 or 100 ug/ml CORT for 4 wk. Mice offered the high-dose CORT, ate more, were less active, and gained twice as much body weight and fat as the control and low-dose animals. Serum CORT was increased threefold only during the dark phase in mice drinking low-dose CORT, but was increased 10-fold during both the light and dark phase in mice offered high-dose CORT. Adrenal weights were significantly reduced by both levels of CORT indicative of effective negative feedback control. The weight gain in these mice, compared with weight loss in cortisol-treated Syrian hamsters (200) or CORT-treated rats, reflect a clear species effect on the relation between HPA activity and energy balance; however, the benefit of adding CORT to drinking water means that CORT intake will be determined by food intake, which is greater at night than during the day in mice. Thus diurnal periodicity is layered on top of an elevated basal CORT level, which is similar to that found in Cushing's syndrome, but different from the majority of obese individuals who maintain normal circadian cortical release (258). Weight gain and changes in tissue morphology are fully reversible once the CORT is withdrawn (41).
Perspectives and Significance
Activation of CRF and SNS systems during stress can have potent effects on food intake. In humans an acute trauma (250) or chronic physiological stress (167) will suppress appetite and cause wasting. This catabolic effect is reflected uniformly in laboratory animal models of acute mixed physical and psychological stress (32), is exaggerated if the acute stress is repeated on a daily basis (85), and is not reversed once stress ends (87). A reduced body weight is not the only prolonged response to acute stress because rats and mice show an exaggerated HPA response to acute novel stress and anxiety-type behaviors in the poststress period (140, 141). By contrast, chronic psychological stress may cause either weight loss or weight gain in humans (155). The current high incidence of obesity has led to a significant interest in stress-induced weight gain, even though it is apparent for only a subset of the general population and it is not clear which aspects of daily life are perceived as stressful (61). Several animal models of chronic stress have been developed to explore the association between psychological stress and obesity (see Table 1). Mouse models of social defeat, which have the potential to be the most useful due to availability of transgenics, are not entirely reliable (70, 148). Syrian hamsters may provide a reproducible model of stress-induced weight gain, but this needs to be confirmed as currently there are only two papers reporting on this model (71, 199). Energy balance in the subordinate member of monkey colonies may represent another good model of human social stress (255); however, there are few investigators who have access to these animals and the opportunity for invasive end points is more limited than with rodent studies.
Few of the experiments that include measures of food intake and body weight are designed to investigate energy balance because a majority of stress research is focused on anxiety and depression. To understand the relation between acute or chronic stress and energy balance in humans, it will be necessary not only to establish and fully characterize reliable experimental animal models, but also to gather more information on the phenotype of human subjects both during and after exposure to stress. Even though current interest is focused on stress and weight gain, insight into mechanisms of sustained weight loss in animal models of acute or chronic stress has the potential to provide a better understanding of central and peripheral mechanisms that control body weight and may even provide new opportunities to develop interventions for inducing weight loss in nonstressed individuals who are overweight.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK 053903 awarded to R. B. S. Harris.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.B.H. prepared figures; R.B.H. drafted manuscript; R.B.H. edited and revised manuscript; R.B.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Dr. Timothy Bartness, Georgia State University and Bhavna Desai, Georgia Regents University for helpful comments and discussion during the preparation of this paper.
REFERENCES
- 1.Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev 31: 41–59, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Akana SF, Dallman MF. Chronic cold in adrenalectomized, corticosterone (B)-treated rats: facilitated corticotropin responses to acute restraint emerge as B increases. Endocrinology 138: 3249–3258, 1997. [DOI] [PubMed] [Google Scholar]
- 3.American Psychological Society. Stress in America. Am Psychological Soc, 2007, http://www.apa.org/news/press/releases/2007/10/stress.aspx. [Google Scholar]
- 4.Antelman SM, Szechtman H, Chin P, Fisher AE. Tail pinch-induced eating, gnawing and licking behavior in rats: dependence on the nigrostriatal dopamine system. Brain Res 99: 319–337, 1975. [DOI] [PubMed] [Google Scholar]
- 5.Arase K, York DA, Shimizu H, Shargill N, Bray GA. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am J Physiol Endocrinol Metab 255: E255–E259, 1988. [DOI] [PubMed] [Google Scholar]
- 6.Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol Behav 101: 446–455, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armario A, Jolin T. Influence of intensity and duration of exposure to various stressors on serum TSH and GH levels in adult male rats. Life Sci 44: 215–221, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Armario A, Marti O, Molina T, de Pablo J, Valdes M. Acute stress markers in humans: response of plasma glucose, cortisol and prolactin to two examinations differing in the anxiety they provoke. Psychoneuroendocrinology 21: 17–24, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M. Diagnosis and complications of Cushing's Syndrome: a consensus statement. J Clin Endocrinol Metab 88: 5593–5602, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. A model of anxious depression: persistence of behavioral pathology. Neurosci Behav Physiol 35: 917–924, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience 234: 40–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backstrom T, Winberg S. Central corticotropin releasing factor and social stress. Front Neurosci 7: 117, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badiani A, Jakob A, Rodaros D, Stewart J. Sensitization of stress-induced feeding in rats repeatedly exposed to brief restraint: the role of corticosterone. Brain Res 710: 35–44, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell'Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLos One 4: e4331, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology 29: 899–910, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF, Dallman MF. Sucrose intake and corticosterone interact with cold to modulate ingestive behaviour, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. J Neuroendocrinol 14: 330–342, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF. Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol 12: 461–470, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport 13: 1575–1578, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergmann N, Gyntelberg F, Faber J. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect 3: R55–R80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience 92: 327–341, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF. Corticosterone facilitates saccharin intake in adrenalectomized rats: does corticosterone increase stimulus salience? J Neuroendocrinol 12: 453–460, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84: 1025–1039, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci 20: 1142–1156, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20: 117–134, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav 73: 261–271, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among us adults. Am J Epidemiol 170: 181–192, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boggiano MM, Chandler PC. Binge eating in rats produced by combining dieting with stress. Curr Protoc Neurosci, Chapter 9: Unit9 23A, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci 119: 1207–1214, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Borer KT, Pryor A, Conn CA, Bonna R, Kielb M. Group housing accelerates growth and induces obesity in adult hamsters. Am J Physiol Regul Integr Comp Physiol 255: R128–R133, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Brown MR, Fisher LA. Glucocorticoid suppression of the sympathetic nervous system and adrenal medulla. Life Sci 39: 1003–1012, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Buechel HM, Popovic J, Staggs K, Anderson KL, Thibault O, Blalock EM. Aged rats are hypo-responsive to acute restraint: implications for psychosocial stress in aging. Front Aging Neurosci 6: 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulwalda B, de Boer SF, Van Kalkeren AA, Koolhaas JM. Physiological and behavioral effects of chronic intracerebroventricular infusion of corticotropin-releasing factor in the rat. Physychoneuroendocrinology 22: 297–309, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci 10: 176–183, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buwalda B, Blom WA, Koolhaas JM, van Dijk G. Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav 73: 371–377, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, Tilders FJ, Bohus B, Koolhaas JM. Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral crf challenge in socially defeated rats. J Neuroendocrinol 11: 513–520, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev 29: 83–97, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev 33: 1089–1098, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Caggiula AR, Antelman SM, Aul E, Knopf S, Edwards DJ. Prior stress attenuates the analgesic response but sensitizes the corticosterone and cortical dopamine responses to stress 10 days later. Psychopharmacology (Berl) 99: 233–237, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Campbell JE, Peckett AJ, D'Souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol 300: C198–C209, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214: 242–253, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Cassano AE, White JR, Penraat KA, Wilson CD, Rasmussen S, Karatsoreos IN. Anatomic, hematologic, and biochemical features of C57Bl/6NCRL mice maintained on chronic oral corticosterone. Comp Med 62: 348–360, 2012. [PMC free article] [PubMed] [Google Scholar]
- 42.Castonguay TW. Glucocorticoids as modulators in the control of feeding. Brain Res Bull 27: 423–428, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Castonguay TW, Beaulieu S, Eskay RL, Barden N, Kamara K, Khozin S, Lustberg L, Brown L. The effects of adrenalectomy and aldosterone replacement in transgenic mice expressing antisense RNA to the Type 2 glucocorticoid receptor. Physiol Behav 77: 417–423, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Castonguay TW, Dallman MF, Stern JS. Some metabolic and behavioral effects of adrenalectomy on obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 251: R923–R933, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Cerri M, Morrison SF. Corticotropin releasing factor increases in brown adipose tissue thermogenesis and heart rate through dorsomedial hypothalamus and medullary raphe pallidus. Neuroscience 140: 711–721, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Chanson P, Salenave S. Metabolic syndrome in Cushing's Syndrome. Neuroendocrinology 92, Suppl 1: 96–101, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Chotiwat C, Harris RB. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm Behav 50: 489–495, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Chotiwat C, Harris RB. Antagonism of specific corticotropin-releasing factor receptor subtypes selectively modifies weight loss in restrained rats. Am J Physiol Regul Integr Comp Physiol 295: R1762–R1773, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chotiwat C, Kelso EW, Harris RB. The effects of repeated restraint stress on energy balance and behavior of mice with selective deletion of CRF receptors. Stress 13: 203–213, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Christiansen AM, Dekloet AD, Ulrich-Lai YM, Herman JP. “Snacking” Causes long term attenuation of hpa axis stress responses and enhancement of brain Fosb/deltaFosb expression in rats. Physiol Behav 103: 111–116, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest 121: 2684–2692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cifani C, Micioni Di BM, Vitale G, Ruggieri V, Ciccocioppo R, Massi M. Effect of salidroside, active principle of rhodiola rosea extract, on binge eating. Physiol Behav 101: 555–562, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res 10: 478–488, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport 12: 3549–3552, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 98: 12796–12801, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D'Allaire S, DeRoth L. Physiological responses to treadmill exercise and ambient temperature in normal and malignant hyperthermia susceptible pigs. Can J Vet Res 50: 78–83, 1986. [PMC free article] [PubMed] [Google Scholar]
- 57.Dal-Zotto S, Marti O, Delgado R, Armario A. Potentiation of glucocorticoid release does not modify the long-term effects of a single exposure to immobilization stress. Psychopharmacology (Berl) 177: 230–237, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Dallman MF, Akana SF, Bhatnagar S, Bell ME, Strack AM. Bottomed out: Metabolic significance of the circadian trough in glucocorticoid concentrations. Int J Obes Relat Metab Disord 24: S40–S46, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview. Glucocorticoids–food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology 145: 2633–2638, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA 100: 11696–11701, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Damaske S, Smyth JM, Zawadzki MJ. Has work replaced home as a haven? Re-examining arlie hochschild's time bind proposition with objective stress data. Soc Sci Med 115: 130–138, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Groote L, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor Type 1 and Type 2 agonists. J Neurochem 94: 45–56, 2005. [DOI] [PubMed] [Google Scholar]
- 63.De Souza EB, Van Loon GR. Differential plasma beta-endorphin, beta-lipotropin, and adrenocorticotropin responses to stress in rats. Endocrinology 116: 1577–1586, 1985. [DOI] [PubMed] [Google Scholar]
- 64.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23: 4850–4857, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Endo Y. Do long-term glucocorticoid treatments induce behavioral rhythm disturbances in rats? Physiol Behav 66: 751–755, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26: 37–49, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Farhan M, Ikram H, Kanwal S, Haleem DJ. Unpredictable chronic mild stress induced behavioral deficits: a comparative study in male and female rats. Pak J Pharm Sci 27: 879–884, 2014. [PubMed] [Google Scholar]
- 68.Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective Type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology 32: 1052–1068, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fekete EM, Zhao Y, Szucs A, Sabino V, Cottone P, Rivier J, Vale WW, Koob GF, Zorrilla EP. Systemic urocortin 2, but not urocortin 1 or stressin 1-a, suppresses feeding via CRF2 receptors without malaise and stress. Br J Pharmacol 164: 1959–1975, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience 192: 351–360, 2011. [DOI] [PubMed] [Google Scholar]
- 71.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 290: R1284–R1293, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev 18: 223–249, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Goebel-Stengel M, Stengel A, Wang L, Tache Y. Orexigenic response to tail pinch: role of brain NPY(1) and corticotropin releasing factor receptors. Am J Physiol Regul Integr Comp Physiol 306: R164–R174, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez F, Manalo S, Dallman MF. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology 145: 59–70, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Gomez FM, Giralt MT, Sainz B, Arrue A, Prieto M, Garcia-Vallejo P. A possible attenuation of stress-induced increases in striatal dopamine metabolism by the expression of non-functional masticatory activity in the rat. Eur J Oral Sci 107: 461–467, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Gordon CJ. Relationship between preferred ambient temperature and autonomic thermoregulatory function in rat. Am J Physiol Regul Integr Comp Physiol 252: R1130–R1137, 1987. [DOI] [PubMed] [Google Scholar]
- 77.Goto T, Kubota Y, Tanaka Y, Iio W, Moriya N, Toyoda A. Subchronic and mild social defeat stress accelerates food intake and body weight gain with polydipsia-like features in mice. Behav Brain Res 270: 339–348, 2014. [DOI] [PubMed] [Google Scholar]
- 78.Grace CR, Perrin MH, Cantle JP, Vale WW, Rivier JE, Riek R. Common and divergent structural features of a series of corticotropin releasing factor-related peptides. J Am Chem Soc 129: 16102–16114, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Greeno CG, Wing RR. Stress-induced eating. Psychol Bull 115: 444–464, 1994. [DOI] [PubMed] [Google Scholar]
- 80.Grill HJ, Markison S, Ginsberg A, Kaplan JM. Long-term effects on feeding and body weight after stimulation of forebrain or hindbrain CRH receptors with urocortin. Brain Res 867: 19–28, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Hagan MM, Moss DE. An animal model of bulimia nervosa: opioid sensitivity to fasting episodes. Pharmacol Biochem Behav 39: 421–422, 1991. [DOI] [PubMed] [Google Scholar]
- 82.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav 77: 45–54, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Hannerz H, Albertsen K, Nielsen ML, Tuchsen F, Burr H. Occupational factors and 5-year weight change among men in a Danish national cohort. Health Psychol 23: 283–288, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Harris RB, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav 81: 557–568, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Harris RB, Mitchell TD, Simpson J, Redmann SM Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol 282: R77–R88, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav 49: 615–625, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol Regul Integr Comp Physiol 275: R1928–R1938, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Harris RB, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav 63: 91–100, 1997. [DOI] [PubMed] [Google Scholar]
- 89.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer's and Parkinson's disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging 18: 285–289, 1997. [DOI] [PubMed] [Google Scholar]
- 90.Hayase M, Takeuchi K. Gastric acid secretion and lesion formation in rats under water-immersion stress. Dig Dis Sci 31: 166–171, 1986. [DOI] [PubMed] [Google Scholar]
- 91.Heatherton TF, Herman CP, Polivy J. Effects of physical threat and ego threat on eating behavior. J Pers Soc Psychol 60: 138–143, 1991. [DOI] [PubMed] [Google Scholar]
- 92.Hennessy MB, Foy T. Nonedible material elicits chewing and reduces the plasma corticosterone response during novelty exposure in mice. Behav Neurosci 101: 237–245, 1987. [DOI] [PubMed] [Google Scholar]
- 93.Herman CP, Polivy J. A boundary model for the regulation of eating. Res Publ Assoc Res Nerv Ment Dis 62: 141–156, 1984. [PubMed] [Google Scholar]
- 94.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 27: 260–286, 2006. [DOI] [PubMed] [Google Scholar]
- 95.Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav 50: 640–646, 2006. [DOI] [PubMed] [Google Scholar]
- 96.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci 11: 585–599, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest 96: 231–238, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the Type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther 305: 385–393, 2003. [DOI] [PubMed] [Google Scholar]
- 99.Jacobson L. Glucocorticoid replacement, but not corticotropin-releasing hormone deficiency, prevents adrenalectomy-induced anorexia in mice. Endocrinology 140: 310–317, 1999. [DOI] [PubMed] [Google Scholar]
- 100.Jahn O, Eckart K, Sydow S, Hofmann BA, Spiess J. Pharmacological characterization of recombinant rat corticotropin releasing factor binding protein using different sauvagine analogs. Peptides 22: 47–56, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in bac transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511: 479–496, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci 16: 5555–5565, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamara K, Eskay R, Castonguay T. High-fat diets and stress responsivity. Physiol Behav 64: 1–6, 1998. [DOI] [PubMed] [Google Scholar]
- 104.Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151: 2117–2127, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kearns RR, Spencer RL. An unexpected increase in restraint duration alters the expression of stress response habituation. Physiol Behav 122: 193–200, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keeney AJ, Hogg S. Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol 10: 753–764, 1999. [DOI] [PubMed] [Google Scholar]
- 107.Keeney AJ, Hogg S, Marsden CA. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol Behav 74: 177–184, 2001. [DOI] [PubMed] [Google Scholar]
- 108.King-Herbert AP, Hesterburg TW, Thevenaz PP, Hamm TE Jr,. Moss OR, Janszen DB, and Everitt JI. Effects of immobilization restraint on Syrian Golden hamsters. Lab Anim Sci 47: 362–366, 1997. [PubMed] [Google Scholar]
- 109.Kivimaki M, Head J, Ferrie JE, Shipley MJ, Brunner E, Vahtera J, Marmot MG. Work stress, weight gain and weight loss: Evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. Int J Obes (Lond) 30: 982–987, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Kluger MJ, O'Reilly B, Shope TR, Vander AJ. Further evidence that stress hyperthermia is a fever. Physiol Behav 39: 763–766, 1987. [DOI] [PubMed] [Google Scholar]
- 111.Knol BW. Stress and the endocrine hypothalamus-pituitary-testis system: a review. Vet Q 1991; 13: 104–114. [DOI] [PubMed] [Google Scholar]
- 112.Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev 21: 775–782, 1997. [DOI] [PubMed] [Google Scholar]
- 113.Kovacs KJ. Crh: The link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat 54: 25–33, 2013. [DOI] [PubMed] [Google Scholar]
- 114.Krahn DD, Gosnell BA, Levine AS, Morley JE. Behavioral effects of corticotropin-releasing factor: localization and characterization of central effects. Brain Res 443: 63–69, 1988. [DOI] [PubMed] [Google Scholar]
- 115.Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of crh and restraint stress decrease with repeated exposures. Biol Psychiatry 27: 1094–1102, 1999. [DOI] [PubMed] [Google Scholar]
- 116.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404, 2007. [DOI] [PubMed] [Google Scholar]
- 117.Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7: 121–147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57Bl/6J strain. Pharmacol Biochem Behav 38: 315–320, 1991. [DOI] [PubMed] [Google Scholar]
- 119.Kudryavtseva NN, Madorskaya IA, Bakshtanovskaya IV. Social success and voluntary ethanol consumption in mice of C57Bl/6J and CBA/Lac strains. Physiol Behav 50: 143–146, 1991. [DOI] [PubMed] [Google Scholar]
- 120.Kumar J, Chuang JC, Na ES, Kuperman A, Gillman AG, Mukherjee S, Zigman JM, McClung CA, Lutter M. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite 64: 81–88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.la Fleur SE. The effects of glucocorticoids on feeding behavior in rats. Physiol Behav 89: 110–114, 2006. [DOI] [PubMed] [Google Scholar]
- 122.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology 145: 2174–2185, 2004. [DOI] [PubMed] [Google Scholar]
- 123.la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology 146: 2193–2199, 2005. [DOI] [PubMed] [Google Scholar]
- 124.la Fleur SE, Manalo SL, Roy M, Houshyar H, Dallman MF. Hepatic vagotomy alters limbic and hypothalamic neuropeptide responses to insulin-dependent diabetes and voluntary lard ingestion. Eur J Neurosci 21: 2733–2742, 2005. [DOI] [PubMed] [Google Scholar]
- 125.Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology 142: 2796–2804, 2001. [DOI] [PubMed] [Google Scholar]
- 126.Legendre A, Papakonstantinou E, Roy MC, Richard D, Harris RB. Differences in response to corticotropin-releasing factor after short- and long-term consumption of a high-fat diet. Am J Physiol Regul Integr Comp Physiol 293: R1076–R1085, 2007. [DOI] [PubMed] [Google Scholar]
- 127.Levine AS, Morley JE. Stress-induced eating in rats. Am J Physiol Regul Integr Comp Physiol 241: R72–R76, 1981. [DOI] [PubMed] [Google Scholar]
- 128.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leyton M, Stewart J. Acute and repeated activation of male sexual behavior by tail pinch: opioid and dopaminergic mechanisms. Physiol Behav 60: 77–85, 1996. [DOI] [PubMed] [Google Scholar]
- 130.Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci 34: 1442–1452, 2011. [DOI] [PubMed] [Google Scholar]
- 131.Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology 143: 3905–3915, 2002. [DOI] [PubMed] [Google Scholar]
- 132.Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology 63: 97–110, 2012. [DOI] [PubMed] [Google Scholar]
- 133.Marazziti D, Di Muro A, Castrogiovanni P. Psychological stress and body temperature changes in humans. Physiol Behav 52: 393–395, 1992. [DOI] [PubMed] [Google Scholar]
- 134.Marti O, Gavalda A, Gomez F, Armario A. Direct evidence for chronic stress-induced facilitation of the adrenocorticotropin response to a novel acute stressor. Neuroendocrinology 60: 1–7, 1994. [DOI] [PubMed] [Google Scholar]
- 135.Marti O, Gavalda A, Jolin T, Armario A. Acute stress attenuates but does not abolish circadian rhythmicity of serum thyrotrophin and growth hormone in the rat. Eur J Endocrinol 135: 703–708, 1996. [DOI] [PubMed] [Google Scholar]
- 136.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol 556: 221–234, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McEwen BS. Stress adaptation, disease. Allostasis and allostatic load. Ann NY Acad Sci 840: 33–44, 1998. [DOI] [PubMed] [Google Scholar]
- 138.McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol 72: 878–890, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15, 2003. [DOI] [PubMed] [Google Scholar]
- 140.Meerlo P, Overkamp GJ, Koolhaas JM. Behavioural and physiological consequences of a single social defeat in roman high- and low-avoidance rats. Psychoneuroendocrinology 22: 155–168, 1997. [DOI] [PubMed] [Google Scholar]
- 141.Meerlo P, Sgoifo A, De Boer SF, Koolhaas JM. Long-lasting consequences of a social conflict in rats: Behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci 113: 1283–1290, 1999. [DOI] [PubMed] [Google Scholar]
- 142.Meisel RL, Hays TC, Del Paine SN, Luttrell VR. Induction of obesity by group housing in female Syrian hamsters. Physiol Behav 47: 815–817, 1990. [DOI] [PubMed] [Google Scholar]
- 143.Michel C, Duclos M, Cabanac M, Richard D. Chronic stress reduces body fat content in both obesity-prone and obesity-resistant strains of mice. Horm Behav 48: 172–179, 2005. [DOI] [PubMed] [Google Scholar]
- 144.Michel C, Levin BE, Dunn-Meynell AA. Stress facilitates body weight gain in genetically predisposed rats on medium-fat diet. Am J Physiol Regul Integr Comp Physiol 285: R791–R799, 2003. [DOI] [PubMed] [Google Scholar]
- 145.Michopoulos V, Loucks T, Berga SL, Rivier J, Wilson ME. Increased ghrelin sensitivity and calorie consumption in subordinate monkeys is affected by short-term astressin B administration. Endocrine 38: 227–234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miczek KA, Yap JJ, Covington HE 3rd. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120: 102–128, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miyake S, Yoshikawa G, Yamada K, Sasaguri K, Yamamoto T, Onozuka M, Sato S. Chewing ameliorates stress-induced suppression of spatial memory by increasing glucocorticoid receptor expression in the hippocampus. Brain Res 1446: 34–39, 2012. [DOI] [PubMed] [Google Scholar]
- 148.Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, Rizzi R, Ciani B, D'Amato FR. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology 31: 623–633, 2006. [DOI] [PubMed] [Google Scholar]
- 149.Moore CJ, Lowe J, Michopoulos V, Ulam P, Toufexis D, Wilson ME, Johnson Z. Small changes in meal patterns lead to significant changes in total caloric intake. Effects of diet and social status on food intake in female rhesus monkeys. Appetite 62: 60–69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morley JE, Levine AS. Stress-induced eating is mediated through endogenous opiates. Science 209: 1259–1261, 1980. [DOI] [PubMed] [Google Scholar]
- 151.Murazumi K, Yahata T, Kuroshima A. Effects of cold and immobilization stress on noradrenaline turnover in brown adipose tissue of rat. Jpn J Physiol 37: 601–607, 1987. [DOI] [PubMed] [Google Scholar]
- 152.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24: 5370–5380, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology 32: 125–132, 2007. [DOI] [PubMed] [Google Scholar]
- 154.NPR/Robert Wood Johnson Foundation/Harvard School of Public Health. The Burden of Stress in America. http://media.npr.org/documents/2014/july/npr_rwfj_harvard_stress_poll.pdf.
- 155.Nyberg ST, Heikkila K, Fransson EI, Alfredsson L, De Bacquer D, Bjorner JB, Bonenfant S, Borritz M, Burr H, Casini A, Clays E, Dragano N, Erbel R, Geuskens GA, Goldberg M, Hooftman WE, Houtman IL, Jockel KH, Kittel F, Knutsson A, Koskenvuo M, Leineweber C, Lunau T, Madsen IE, Hanson LL, Marmot MG, Nielsen ML, Nordin M, Oksanen T, Pentti J, Rugulies R, Siegrist J, Suominen S, Vahtera J, Virtanen M, Westerholm P, Westerlund H, Zins M, Ferrie JE, Theorell T, Steptoe A, Hamer M, Singh-Manoux A, Batty GD, Kivimaki M. Job strain in relation to body mass index: pooled analysis of 160,000 adults from 13 cohort studies. J Intern Med 272: 65–73, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, Oosting R, Bouwknecht A, Veening J, van der Gugten J, Groenink L. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol 463: 117–132, 2003. [DOI] [PubMed] [Google Scholar]
- 157.Ootsuka Y, Blessing WW, Nalivaiko E. Selective blockade of 5-HT2a receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress 11: 125–133, 2008. [DOI] [PubMed] [Google Scholar]
- 158.Ortolani D, Garcia MC, Melo-Thomas L, Spadari-Bratfisch RC. Stress-induced endocrine response and anxiety: the effects of comfort food in rats. Stress 17: 211–218, 2014. [DOI] [PubMed] [Google Scholar]
- 159.Ortolani D, Oyama LM, Ferrari EM, Melo LL, Spadari-Bratfisch RC. Effects of comfort food on food intake, anxiety-like behavior and the stress response in rats. Physiol Behav 103: 487–492, 2011. [DOI] [PubMed] [Google Scholar]
- 160.Ottenweller JE, Tapp WN, Burke JM, Natelson BH. Plasma cortisol and corticosterone concentrations in the Golden hamster (Mesocricetus auratus). Life Sci 37: 1551–1558, 1985. [DOI] [PubMed] [Google Scholar]
- 161.Overgaard D, Gyntelberg F, Heitmann BL. Psychological workload and body weight: is there an association? A review of the literature. Occup Med 54: 35–41, 2004. [DOI] [PubMed] [Google Scholar]
- 162.Pare WP, Glavin GB. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev 10: 339–370, 1986. [DOI] [PubMed] [Google Scholar]
- 163.Patterson ZR, Abizaid A. Stress induced obesity: lessons from rodent models of stress. Front Neurosci 7: 130, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patterson ZR, Khazall R, Mackay H, Anisman H, Abizaid A. Central ghrelin signaling mediates the metabolic response of C57Bl/6 male mice to chronic social defeat stress. Endocrinology 154: 1080–1091, 2013. [DOI] [PubMed] [Google Scholar]
- 165.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology 145: 3754–3762, 2004. [DOI] [PubMed] [Google Scholar]
- 166.Pirke KM, Tuschl RJ, Spyra B, Laessle RG, Schweiger U, Broocks A, Sambauer S, Zitzelsberger G. Endocrine findings in restrained eaters. Physiol Behav 47: 903–906, 1990. [DOI] [PubMed] [Google Scholar]
- 167.Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response to the stress of critical illness. Br J Anaesth 113: 945–954, 2014. [DOI] [PubMed] [Google Scholar]
- 168.Raber J. Detrimental effects of chronic hypothalamic-pituitary-adrenal axis activation. From obesity to memory deficits. Molec Neurobiol 18: 1–22, 1998. [DOI] [PubMed] [Google Scholar]
- 169.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 134: 737–744, 2005. [DOI] [PubMed] [Google Scholar]
- 170.Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res 203: 264–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 98: 2843–2848, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol 440: 189–197, 2002. [DOI] [PubMed] [Google Scholar]
- 173.Roberts CJ, Campbell IC, Troop N. Increases in weight during chronic stress are partially associated with a switch in food choice towards increased carbohydrate and saturated fat intake. Eur Eat Disord Rev 22: 77–82, 2014. [DOI] [PubMed] [Google Scholar]
- 174.Rowland NE, Antelman SM. Stress-induced hyperphagia and obesity in rats: a possible model for understanding human obesity. Science 191: 310–312, 1976. [DOI] [PubMed] [Google Scholar]
- 175.Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 24: 285–300, 1999. [DOI] [PubMed] [Google Scholar]
- 176.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring) 17: 72–77, 1999. [DOI] [PubMed] [Google Scholar]
- 177.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Hyperactivity of the HPA axis is related to dietary restraint in normal weight women. Physiol Behav 96: 315–319, 2009. [DOI] [PubMed] [Google Scholar]
- 178.Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol Regul Integr Comp Physiol 273: R1612–R1622, 1997. [DOI] [PubMed] [Google Scholar]
- 179.Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res 162: 127–134, 2005. [DOI] [PubMed] [Google Scholar]
- 180.Sakai RR, Tamashiro KL, Sottas CM, Ma LY, Hardy MP, Lucas LR, Celen Z, Fujikawa T, Blanchard RJ, McEwan BS. Endocrine changes following long-term psychosocial stress and recovery. In: Society for Neuroscience. Orlando, FL: 2002. [Google Scholar]
- 181.Samarghandian S, Ohata H, Yamauchi N, Shibasaki T. Corticotropin-releasing factor as well as opioid and dopamine are involved in tail-pinch-induced food intake of rats. Neuroscience 116: 519–524, 2003. [DOI] [PubMed] [Google Scholar]
- 182.Sanghez V, Razzoli M, Carobbio S, Campbell M, McCallum J, Cero C, Ceresini G, Cabassi A, Govoni P, Franceschini P, de Santis V, Gurney A, Ninkovic I, Parmigiani S, Palanza P, Vidal-Puig A, Bartolomucci A. Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the metabolic syndrome. Psychoneuroendocrinology 38: 2933–2942, 2013. [DOI] [PubMed] [Google Scholar]
- 183.Santana P, Akana SF, Hanson ES, Strack AM, Sebastian RJ, Dallman MF. Aldosterone and dexamethasone both stimulate energy acquisition whereas only the glucocorticoid alters energy storage. Endocrinology 136: 2214–2222, 1995. [DOI] [PubMed] [Google Scholar]
- 184.Sapolsky RM. Hypercortisolism among socially subordinate wild baboons originates at the CNS level. Arch Gen Psychiatry 46: 1047–1051,1989. [DOI] [PubMed] [Google Scholar]
- 185.Sapolsky RM, Armanini MP, Packan DR, Sutton SW, Plotsky PM. Glucocorticoid feedback inhibition of adrenocorticotropic hormone secretagogue release. Relationship to corticosteroid receptor occupancy in various limbic sites. Neuroendocrinology 51: 328–336, 1990. [DOI] [PubMed] [Google Scholar]
- 186.Sawchenko PE, Arias C. Evidence for short-loop feedback effects of ACTH on CRF and vasopressin expression in parvocellular neurosecretory neurons. J Neuroendocrinol 7: 721–731, 1995. [DOI] [PubMed] [Google Scholar]
- 187.Schaumberg K, Earleywine M. Evaluating the acquired preparedness model for bulimic symptoms and problem drinking in male and female college students. Eat Behav 14: 47–52, 2013. [DOI] [PubMed] [Google Scholar]
- 188.Scherer IJ, Holmes PV, Harris RB. The importance of corticosterone in mediating restraint-induced weight loss in rats. Physiol Behav 102: 225–233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schwartz MW, Strack AM, Dallman MF. Evidence that elevated plasma corticosterone levels are the cause of reduced hypothalamic corticotrophin-releasing hormone gene expression in diabetes. Regul Pept 72: 105–112, 1997. [DOI] [PubMed] [Google Scholar]
- 190.Seyle H. The Stress of Life. McGraw-Hill, 1976. [Google Scholar]
- 191.Shanks N, Anisman H. Stressor-provoked behavioral changes in six strains of mice. Behav Neurosci 102: 894–905, 1988. [DOI] [PubMed] [Google Scholar]
- 192.Shanks N, Griffiths J, Zalcman S, Zacharko RM, Anisman H. Mouse strain differences in plasma corticosterone following uncontrollable footshock. Pharmacol Biochem Behav 36: 515–519, 1990. [DOI] [PubMed] [Google Scholar]
- 193.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 861: 288–295, 2000. [DOI] [PubMed] [Google Scholar]
- 194.Shibata H, Nagasaka T. Role of sympathetic nervous system in immobilization- and cold-induced brown adipose tissue thermogenesis in rats. Jpn J Physiol 34: 103–111, 1984. [DOI] [PubMed] [Google Scholar]
- 195.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry 41: 871–882, 1997. [DOI] [PubMed] [Google Scholar]
- 196.Siegrist J, Rodel A. Work stress and health risk behavior. Scand J Work Environ Health 32: 473–481, 2006. [DOI] [PubMed] [Google Scholar]
- 197.Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res 208: 528–534, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Smith CK, Romsos DR. Effects of adrenalectomy on energy balance of obese mice are diet dependent. Am J Physiol Regul Integr Comp Physiol 249: R13–R22, 1985. [DOI] [PubMed] [Google Scholar]
- 199.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 292: R283–R290, 2007. [DOI] [PubMed] [Google Scholar]
- 200.Solomon MB, Sakai RR, Woods SC, Foster MT. Differential effects of glucocorticoids on energy homeostasis in Syrian hamsters. Am J Physiol Endocrinol Metab 301: E307–E316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Spencer SJ, Tilbrook A. The glucocorticoid contribution to obesity. Stress 14: 233–246, 2011. [DOI] [PubMed] [Google Scholar]
- 202.Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science 273: 1561–1564, 1996. [DOI] [PubMed] [Google Scholar]
- 203.Stengel A, Tache Y. Crf and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front Neurosci 8: 52, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychol Assess 16: 51–59, 2004. [DOI] [PubMed] [Google Scholar]
- 205.Stice E, Sysko R, Roberto CA, Allison S. Are dietary restraint scales valid measures of dietary restriction? Additional objective behavioral and biological data suggest not. Appetite 54: 331–339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Stone AA, Brownell K. The stress-eating paradox: mulitple daily measuremetns in adult males and females. Psychology Health 9: 425–436, 1994. [Google Scholar]
- 207.Strack AM, Horsley CJ, Sebastian RJ, Akana SF, Dallman MF. Glucocorticoids and insulin: complex interaction on brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 268: R1209–R1216, 1995. [DOI] [PubMed] [Google Scholar]
- 208.Stratakis CA, Gold PW, Chrousos GP. Neuroendocrinology of stress: implications for growth and development. Horm Res 43: 162–167, 1995. [DOI] [PubMed] [Google Scholar]
- 209.Strijbos PJ, Hardwick AJ, Relton JK, Carey F, Rothwell NJ. Inhibition of central actions of cytokines on fever and thermogenesis by lipocortin-1 involves CRF. Am J Physiol Endocrinol Metab 263: E632–E636, 1992. [DOI] [PubMed] [Google Scholar]
- 210.Szechtman H, Hall WG. Ontogeny of oral behavior induced by tail pinch and electrical stimulation of the tail in rats. J Comp Physiol Psychol 94: 436–445, 1980. [DOI] [PubMed] [Google Scholar]
- 211.Szechtman H, Siegel HI, Rosemblatt JS, Komisaruk BR. Tail-pinch facilitates onset of maternal behavior in rats. Physiol Behav 19: 807–809, 1977. [DOI] [PubMed] [Google Scholar]
- 212.Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD, Datta R, Drago F, Vale WW, Koob GF, Zorrilla EP, Contarino A. Role of the corticotropin-releasing factor receptor Type 2 in the control of food intake in mice: A meal pattern analysis. Eur J Neurosci 26: 2303–2314, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Takahashi K, Totsune K, Sone M, Murakami O, Satoh F, Arihara Z, Sasano H, Iino K, Mouri T. Regional distribution of urocortin-like immunoreactivity and expression of urocortin mRNA in the human brain. Peptides 19: 643–647, 1998. [DOI] [PubMed] [Google Scholar]
- 214.Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav 89: 536–542, 2006. [DOI] [PubMed] [Google Scholar]
- 215.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav 80: 683–693, 2004. [DOI] [PubMed] [Google Scholar]
- 216.Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol 293: R1864–R1874, 2007. [DOI] [PubMed] [Google Scholar]
- 217.Tanaka C, Asakawa A, Ushikai M, Sakoguchi T, Amitani H, Terashi M, Cheng K, Chaolu H, Nakamura N, Inui A. Comparison of the anorexigenic activity of CRF family peptides. Biochem Biophys Res Commun 390: 887–891, 2009. [DOI] [PubMed] [Google Scholar]
- 218.Tanimura SM, Watts AG. Corticosterone modulation of ACTH secretogogue gene expression in the paraventricular nucleus. Peptides 22: 775–783, 2001. [DOI] [PubMed] [Google Scholar]
- 219.Tannenbaum B, Rowe W, Sharma S, Diorio J, Steverman A, Walker M, Meaney MJ. Dynamic variations in plasma corticosteroid-binding globulin and basal HPA activity following acute stress in adult rats. J Neuroendocrinol 9: 163–168, 1997. [DOI] [PubMed] [Google Scholar]
- 220.Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol Endocrinol Metab 273: E1168–E1177, 1997. [DOI] [PubMed] [Google Scholar]
- 221.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol Endocrinol Metab 271: E317–E325, 1996. [DOI] [PubMed] [Google Scholar]
- 222.Tempel DL, Leibowitz SF. Glucocorticoid receptors in PVN: interactions with NE, NPY, and Gal in relation to feeding. Am J Physiol Endocrinol Metab 265: E794–E800, 1993. [DOI] [PubMed] [Google Scholar]
- 223.Thayer WR, Toffler AH, Chapo G, Spiro HM. Inhibition of restraint ulcers in the rat by pyridoxine deficiency. Yale J Biol Med 38: 257–264, 1965. [PMC free article] [PubMed] [Google Scholar]
- 224.Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 36: 1513–1519, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacology (Berl) 116: 346–356, 1994. [DOI] [PubMed] [Google Scholar]
- 226.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281: 1220–1225, 2001. [DOI] [PubMed] [Google Scholar]
- 227.Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ:. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav 63: 543–550, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Trainor BC, Takahashi EY, Silva AL, Crean KK, Hostetler C. Sex differences in hormonal responses to social conflict in the monogamous California mouse. Horm Behav 58: 506–512, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. [DOI] [PubMed] [Google Scholar]
- 230.Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci USA 107: 20529–20534, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. Caloric consumption. Physiol Behav 103: 104–110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res 39: 245–270, 1983. [DOI] [PubMed] [Google Scholar]
- 234.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000. [DOI] [PubMed] [Google Scholar]
- 235.van Strien T, Engels RC, van Staveren W, Herman CP. The validity of dietary restraint scales: comment on Stice et al. (2004). Psychol Assess 18: 89–94; discussion 95-89, 2006. [DOI] [PubMed] [Google Scholar]
- 236.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378: 287–292, 1995. [DOI] [PubMed] [Google Scholar]
- 237.Vidal C, Suaudeau C, Jacob J. Hyper- and hypothermia induced by non-noxious stress: Effects of naloxone, diazepam and gamma-acetylenic GABA. Life Sci 33, Suppl 1: 587–590, 1993. [DOI] [PubMed] [Google Scholar]
- 238.Vinkers CH, Groenink L, van Bogaert MJ, Westphal KG, Kalkman CJ, van Oorschot R, Oosting RS, Olivier B, Korte SM. Stress-induced hyperthermia and infection-induced fever: two of a kind? Physiol Behav 98: 37–43, 2009. [DOI] [PubMed] [Google Scholar]
- 239.Vinkers CH, Penning R, Hellhammer J, Verster JC, Klaessens JH, Olivier B, Kalkman CJ. The effect of stress on core and peripheral body temperature in humans. Stress 16: 520–530, 2013. [DOI] [PubMed] [Google Scholar]
- 240.Von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res 117: 137–146, 2000. [DOI] [PubMed] [Google Scholar]
- 241.Wallace WH, Crowne EC, Shalet SM, Moore C, Gibson S, Littley MD, White A. Episodic ACTH and cortisol secretion in normal children. Clin Endocrinol (Oxf) 34: 215–221, 1991. [DOI] [PubMed] [Google Scholar]
- 242.Wang J, Chai A, Zhou Q, Lv L, Wang L, Yang Y, Xu L. Chronic clomipramine treatment reverses core symptom of depression in subordinate tree shrews. PLos One 8: e80980, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: a meta-analysis of longitudinal studies. Obesity (Silver Spring) 19: 771–778, 2011. [DOI] [PubMed] [Google Scholar]
- 244.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res 48: 195–202, 2000. [DOI] [PubMed] [Google Scholar]
- 245.Wark JD, Kuhar CW, Lukas KE. Behavioral thermoregulation in a group of zoo-housed colobus monkeys (Colobus guereza). Zoo Biol 33: 257–266, 2014. [DOI] [PubMed] [Google Scholar]
- 246.Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol 26: 109–130, 2005. [DOI] [PubMed] [Google Scholar]
- 247.Weinstein SE, Shide DJ, Rolls BJ. Changes in food intake in response to stress in men and women: psychological factors. Appetite 28: 7–18, 1997. [DOI] [PubMed] [Google Scholar]
- 248.Westenhoefer J, Broeckmann P, Munch AK, Pudel V. Cognitive control of eating behaviour and the disinhibition effect. Appetite 23: 27–41, 1994. [DOI] [PubMed] [Google Scholar]
- 249.Widmaier EP, Dallman MF. The effects of corticotropin-releasing factor on adrenocorticotropin secretion from perifused pituitaries in vitro: rapid inhibition by glucocorticoids. Endocrinology 115: 2368–2374, 1984. [DOI] [PubMed] [Google Scholar]
- 250.Willis L, Thomas P, Garry PJ, Goodwin JS. A prospective study of response to stressful life events in initially healthy elders. J Gerontol 42: 627–630, 1987. [DOI] [PubMed] [Google Scholar]
- 251.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52: 90–110, 2005. [DOI] [PubMed] [Google Scholar]
- 252.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364, 1987. [DOI] [PubMed] [Google Scholar]
- 253.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93: 358–364, 1987. [DOI] [PubMed] [Google Scholar]
- 254.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiol Behav 94: 586–594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wilson ME, Moore CJ, Ethun KF, Johnson ZP. Understanding the control of ingestive behavior in primates. Horm Behav 66: 86–94, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wolf EM, Crowther JH. Personality and eating habit variables as predictors of severity of binge eating and weight. Addict Behav 8: 335–344, 1983. [DOI] [PubMed] [Google Scholar]
- 257.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141: 4325–4328, 2000. [DOI] [PubMed] [Google Scholar]
- 258.Yaneva M, Mosnier-Pudar H, Dugue MA, Grabar S, Fulla Y, Bertagna X:. Midnight salivary cortisol for the initial diagnosis of Cushing's syndrome of various causes. J Clin Endocrinol Metab 89: 3345–3351, 2004. [DOI] [PubMed] [Google Scholar]
- 259.Yeomans MR, Coughlan E:. Mood-induced eating. Interactive effects of restraint and tendency to overeat. Appetite 52: 290–298, 2009. [DOI] [PubMed] [Google Scholar]
- 260.Yokoi Y. Effect of ambient temperature upon emotional hyperthermia, and hypothermia in rabbits. J Appl Physiol 21: 1795–1798, 1966. [DOI] [PubMed] [Google Scholar]
- 261.Young EA, Akana S, Dallman MF:. Decreased sensitivity to glucocorticoid fast feedback in chronically stressed rats. Neuroendocrinology 51: 536–542, 1990. [DOI] [PubMed] [Google Scholar]
- 262.Zeeni N, Daher C, Fromentin G, Tome D, Darcel N, Chaumontet C. A cafeteria diet modifies the response to chronic variable stress in rats. Stress 16: 211–219, 2013. [DOI] [PubMed] [Google Scholar]