Abstract
Acute intermittent hypoxia (AIH) induces sympathetic and phrenic long-term facilitation (LTF), defined as a sustained increase in nerve discharge. We investigated the effects of AIH and acute intermittent optogenetic (AIO) stimulation of neurons labeled with AAV-CaMKIIa, hChR2(H134R), and mCherry in the nucleus of the solitary tract (NTS) of anesthetized, vagotomized, and mechanically ventilated rats. We measured renal sympathetic nerve activity (RSNA), phrenic nerve activity (PNA), power spectral density, and coherence, and we made cross-correlation measurements to determine how AIO stimulation and AIH affected synchronization between PNA and RSNA. Sixty minutes after AIH produced by ventilation with 10% oxygen in balanced nitrogen, RSNA and PNA amplitude increased by 80% and by 130%, respectively (P < 0.01). Sixty minutes after AIO stimulation, RSNA and PNA amplitude increased by 60% and 100%, respectively, (P < 0.01). These results suggest that acute intermittent stimulation of NTS neurons can induce renal sympathetic and phrenic LTF in the absence of hypoxia or chemoreceptor afferent activation. We also found that while acute intermittent optogenetic and hypoxic stimulations increased respiration-related RSNA modulation (P < 0.01), they did not increase synchronization between central respiratory drive and RSNA. We conclude that mechanisms that induce LTF originate within the caudal NTS and extend to other interconnecting neuronal elements of the central nervous cardiorespiratory network.
Keywords: nucleus tractus solitarius, optogenetics, sympathetic long-term facilitation, synchronization
sleep apnea can lead to hypertension (25, 33), which is associated with elevated sympathetic nerve activity (SNA) (1, 37). Acute intermittent hypoxia (AIH) has been used as a model of arterial hypoxemia that occurs during sleep apnea (8, 22). Exposure to AIH results in sympathetic long-term facilitation (LTF), defined as a sustained increase in SNA in both humans (8, 41) and in rats (12, 42). However, the mechanisms that lead to AIH-induced sympathetic LTF are poorly understood.
AIH is also capable of inducing phrenic LTF (29), expressed as a progressive and sustained increase in the burst amplitude of phrenic nerve discharge (28). Phrenic LTF can be induced by carotid sinus nerve electrical stimulation (17), suggesting that hypoxia is not necessary to generate phrenic LTF. Whether or not hypoxia is required to generate sympathetic LTF has not been examined.
The nucleus of the solitary tract (NTS) is the central site of termination of various visceral and cardiorespiratory afferent fibers, including some from arterial chemoreceptors that synaptically activate NTS neurons (16), thereby augmenting discharge activities of phrenic and sympathetic neurons. Since during AIH, NTS neurons within the chemoreflex pathway are intermittently activated, they could contribute to generation of sympathetic and phrenic LTF. Therefore, one of the goals of the present study was to test whether intermittent hypoxic stimulation of NTS neurons induces sympathetic LTF.
The recent cloning of light-sensitive ion channels and their expression in mammalian cells has opened a new field in neuroscience: optogenetics (4, 10, 40). Channelrhodopsin-2 (ChR2) is a nonselective cation channel that opens during light stimulation, thereby depolarizing and activating neurons (44). Thus, the optogenetic technique is a useful tool to activate targeted NTS neurons. With this technique, we tested the effects of acute intermittent optogenetic (AIO) stimulation in the NTS on renal SNA (RSNA) and phrenic nerve activity (PNA) in adult rats in vivo, compared them with AIH, and found that AIO stimulation and AIH each increase discharge intensities in both phrenic and sympathetic neural motor pathways. Since caudal NTS neurons have powerful excitatory influences on cardiorespiratory responsiveness (39), we postulate that AIO activation of NTS neurons contributes significantly to the augmented discharge activity. We also measured power spectral density and coherence in PNA and RSNA and made autocorrelation and cross-correlation measurements to determine whether LTF induced by AIO stimulation and AIH affects synchronization between PNA and RSNA. Despite the presence of a strong respiratory rhythm in RSNA, cross-correlation measurements indicated that LTF does not enhance PNA-RSNA synchronization.
METHODS
The Institutional Animal Care and Use Committee of the University of North Texas Health Science Center approved all experimental procedures.
Surgical procedures.
Experiments were performed on 23 male adult Sprague-Dawley rats (350–500 g). All rats were given at least 1 wk to acclimate before inclusion in our protocols. They were assigned to either an AIO group (n = 9), a control group (n = 6), or an AIH group (n = 8). Animals were surgically prepared for acute experiments during anesthesia with isoflurane (2–3%). Adequacy of anesthesia was ensured by the absence of a nociceptive reflex response (to a hindpaw pinch) and corneal and pinna reflexes. The animals were intubated through a tracheotomy and mechanically ventilated with 100% oxygen during surgical preparation. Gallamine triethiodide (5 mg·kg−1·h−1) was infused intravenously to induce neuromuscular blockade. Following paralysis, depth of anesthesia was assessed by the stability of arterial pressure (AP), heart rate (HR), and the absence of large changes in AP during a hind paw pinch. Body temperature was maintained at ∼37°C with a heating pad. A catheter was inserted into the abdominal aorta via the femoral artery and was used for the measurement of AP. HR was calculated from the AP waveform. Arterial blood Pco2 and pH were within the physiological range (PaCO2 ∼34.5 torr, pH ∼7.5) when the rats were examined at the end of the surgical preparation and also at the end of the experiment.
The left renal sympathetic and phrenic nerves were exposed and attached to a pair of Teflon-coated stainless-steel wires (AS632; Cooner Wire, Chatsworth, CA) to record RSNA and PNA. The nerves and electrodes were secured with silicone glue (Kwik-Sil; World Precision Instruments, Sarasota, FL). The nerve signals were amplified (×20,000–50,000), band-pass filtered (100–1,000 Hz), and full-wave rectified and integrated using 50-ms time constants to quantify RSNA and PNA in both time domain and frequency domain analyses. After surgical preparation, isoflurane was discontinued gradually as a mixture of α-chloralose (40 mg/kg), and urethane (800 mg/kg) was injected intravenously to maintain anesthesia. Adequacy of anesthesia was assessed as described above. Animals were mechanically ventilated with oxygen-enriched room air during baseline control conditions, with end-tidal (ET)CO2 maintained at 4 ± 0.4%. This basal level is consistent with control levels used in another study performed on chloralose–urethane-anesthetized rats (43).
Viral gene transfer of ChR2 to NTS neurons.
Young adult rats (∼200 g; n = 15) were anesthetized {ketamine, 75 mg/kg and Dormitor, [Medetomidine; (RS)-4-[1-(2,3-dimethylphenyl)ethyl]-3H-imidazole], 0.5 mg/kg ip} and placed in a stereotaxic frame. Breathing was spontaneous throughout gene transfer procedures. The dorsal surface of the brain stem was exposed, and adeno-associated virus (AAV2) containing CaMKIIa-hChR2 (H134R)-mCherry (titer, 1012 virus molecules per milliliter; purchased from the University of North Carolina vector core) was injected with a glass micropipette (25–30-μm tip diameter) into the caudal NTS. CaMKIIa is expressed in glutamatergic, and not GABAergic, neurons in the thalamus and cerebral cortex (21), and CaMKII mediates emergent respiratory rhythmic discharges in glutamatergic neurons of the medulla (27). NTS injection of 200 nl of the AAV-CaMKIIa-mCherry rhodopsin vector over a 5-min period was made using the calamus scriptorius as a landmark. The depth of injection was 0.6 mm below the dorsal surface. A second microinjection was made at a site 0.5 mm caudal to calamus scriptorius and 0.6 mm below the dorsal surface. After microinjections, the wound was sutured, and anesthesia was reversed with antisedan [Atipamezole; 4-(2-Ethyl-1,3-dihydroinden-2-yl)-3H-imidazole] at 1 mg/kg. Immediate postoperative care was given, and animals were left to recover for 3–4 wk before the experiments to ensure a high level of transgene expression.
Protocols.
All experimental protocols were initiated at least 1 h after completion of surgical procedures.
AIH.
After 5 min of baseline recording, AIH was produced by ventilating the rat with 10% oxygen in nitrogen for 1 min every 6 min for a total of 10 exposures to hypoxia in the AIH group. The parameters were recorded for 60 min after AIH (for a total of 120-min recording).
AIO stimulation.
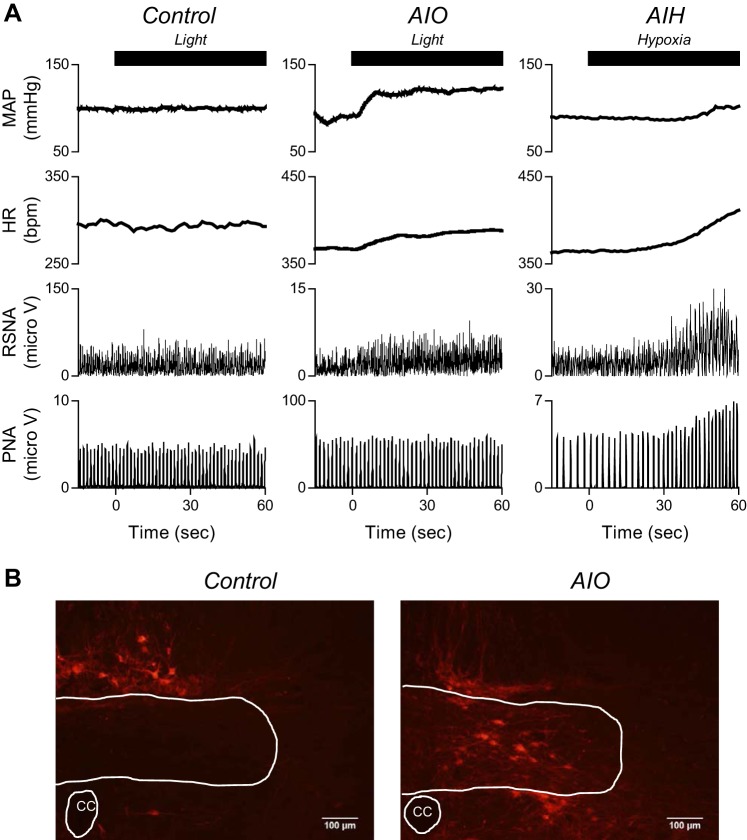
Stimulation of ChR2+ neurons was accomplished using a multimode patch cable (M54L01; Thorlabs, Newton, NJ) with a 400-μm core fiber (OGK4; Thorlabs) coupled to a 470-nm high-power LED (M470F1; Thorlabs) and a high-power LED driver (DC2100; Thorlabs). The fiber was placed against the calamus scriptorius on the dorsal surface. The same pattern of stimulation as in the AIH protocol was used for optogenetic stimulation of NTS neurons (a pulse of light of 10-ms duration delivered at 20 Hz and 1.65–2.23 mW for 1 min every 6 min for a total of 10 stimuli). Light intensity was measured using a PM100D power meter coupled to an S140C detector (Thorlabs). Intensity measurements through brain slices indicate that 500–700 μm below the slice surface intensity has fallen to 17–22% of that measured at the surface of the slice (2). The intensity of light stimulation was determined on the basis of preliminary experiments in which we examined AP response to optogenetic stimulation at 1, 5, 10, 20, and 40 Hz. The intensity at 20 Hz induced the largest increase in mean AP (MAP) of 19 ± 3 mmHg (n = 4). In six rats, mCherry-labeled neurons were near, but outside the boundaries of NTS (Fig. 1B, control), as confirmed by post-mortem histology. These animals served as controls in experiments that tested the effects of AIO stimulation on MAP, HR, RSNA, and PNA.
Fig. 1.
A: typical recordings of mean arterial pressure (MAP), heart rate (HR), renal sympathetic nerve activity (RSNA), and phrenic nerve activity (PNA) in response to a single optogenetic stimulation for 1 min in control rat (left) and in acute intermittent optogenetic (AIO) stimulation rat (middle), and in response to a single hypoxic stimulation for 1 min in acute intermittent hypoxia (AIH) rat (right). Optogenetic stimulation of neurons expressing Channelrhodopsin2 (ChR2) in the nucleus of the solitary tract (NTS) was sufficient to trigger the response of MAP, HR, and RSNA. B: typical mCherry expression near and in caudal NTS in control and AIO rats. CC, central canal.
Data analysis.
AP, HR, RSNA, PNA, and ETCO2 were recorded at a sampling rate of 2,000 Hz. Because the absolute magnitude of RSNA and PNA depended on recording conditions, integrated RSNA and PNA were normalized as a percentage (%). In time domain analysis, an intravenous bolus injection of the ganglionic blocker hexamethonium bromide (60 mg/kg) was used to determine 0% RSNA. The 0% level was subtracted from baseline RNSA, and this value was considered to be 100% RSNA. The amplitude of PNA at baseline was considered to be 100%. MAP, HR, RSNA, frequency, and amplitude of PNA and ETCO2 for 4 min were obtained before treatments (baseline), at 5 min after and at 60 min after AIH or AIO stimulation.
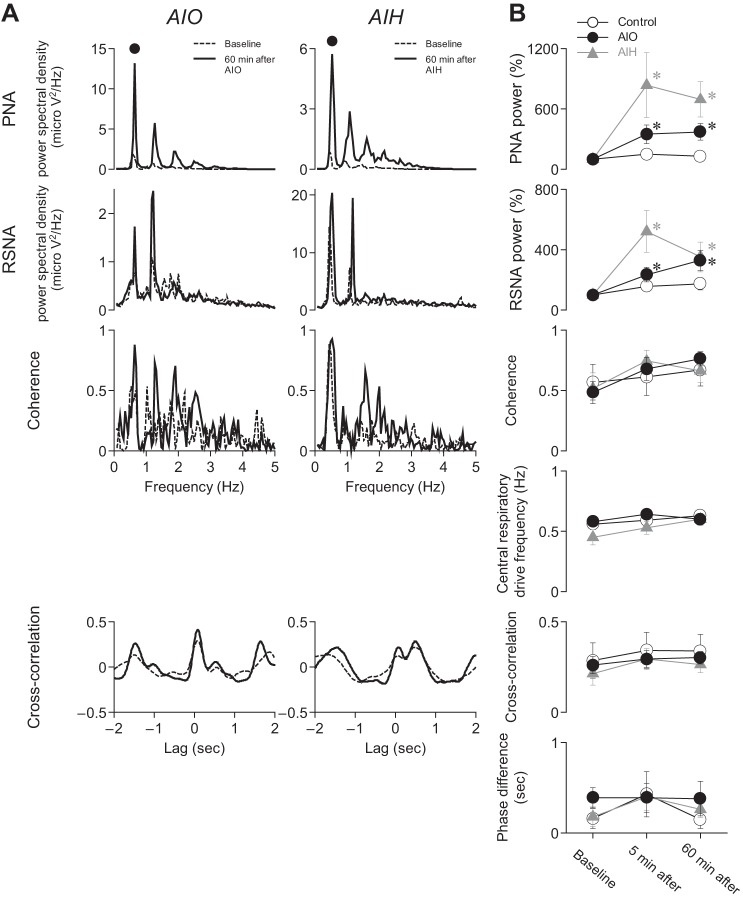
To examine synchronization between PNA and RSNA, power spectral density, coherence, and cross correlation between PNA and RSNA were measured using MatLab (v8.2.0.701; MathWorks, Natick, MA) (36). To estimate coherence, a 240-s data set of PNA and RSNA was sampled at 100 Hz and segmented into 50%-overlapping bins of 2,048 points each. For each segment, a linear trend was subtracted, and a Hamming window was applied. A fast Fourier transform was performed to obtain the frequency spectrum of PNA and RSNA signals. The ensemble averages of PNA power spectral density, RSNA power spectral density, and cross-spectral density between the PNA and RSNA were obtained over 22 segments. The squared coherence function was estimated by normalizing the cross-spectral density. The coherence value ranges from 0 to 1 (unity). Unity coherence indicates perfect linear dependence between the two signals, whereas zero coherence indicates total independence between the two signals. The values of PNA and RSNA powers and the coherence were obtained at the frequency of the central respiratory drive (defined as frequency of the main peak in PNA spectrum; Fig. 3A). The PNA and RSNA powers at baseline were considered to be 100% in frequency domain analysis. Using the same 240-s data set (bin width, 0.01 s) of PNA and RSNA, a normalized cross-correlation function was estimated. Records in Fig. 3A, taken from a single experiment, are representative of all experiments. The normalized cross-correlation value ranges from −1 (negative perfect correlation) to 1 (positive perfect correlation). The cross-correlation value and phase difference were obtained at peak cross correlation between PNA and RSNA.
Fig. 3.
Acute intermittent optogenetic and hypoxic stimulation increased respiration-related RSNA modulation, but they did not increase synchronization between central respiratory drive and RSNA. A: examples of power and coherence spectral plots and cross-correlation function at baseline and 60 min after AIO stimulation and AIH. Solid circle indicates central respiratory drive frequency at 60 min after AIO stimulation and AIH. B: AIO stimulation and AIH increased PNA and RSNA powers at central respiratory drive frequency. However, AIO stimulation and AIH did not increase the coherence and cross-correlation values between PNA and RSNA. *P < 0.05 compared with baseline within the same group.
Responses to brief systemic hypoxia.
To determine whether chemoreflex responses to brief exposures to systemic hypoxia are altered after AIO stimulation or AIH, systemic hypoxia was induced by ventilation with 10% oxygen for 1 min in AIO (n = 6), control (n = 5), and AIH (n = 6) groups. The RSNA, PNA amplitude and frequency, MAP, and HR responses to hypoxia were measured before and 60 min after the end of AIO stimulation or AIH. In the AIH group, the first 1 min of hypoxic exposure during the AIH protocol was used as the control hypoxic stimulation.
Statistical analysis.
Statistical analysis was performed using SigmaPlot 12. The PNA power and RSNA power in the spectral analyses were transformed into their natural logarithms for statistical analysis. The logarithmic transformations produced a normal distribution verified by a Shapiro-Wilk test. Effects of AIH and AIO stimulation on PNA, RSNA, MAP, and HR were tested by two-way ANOVA with repeated measurements. In the case of a significant F value in the interaction between group and time, a post hoc test with the Bonferroni method identified significant differences between mean values. Univariate regression and correlation analyses were used to analyze the relationship between change in PNA amplitude and RSNA at 60 min after AIO stimulation or AIH from baseline. Differences were considered significant when P < 0.05.
RESULTS
Comparative effects of AIH and AIO stimulation on PNA, RSNA, MAP, and HR.
Here, we present comparative effects of global hypoxia and optogenetic stimulation in the caudal NTS and the progression of effects that occur over an hour after cessation of stimulation. We first show that responses to repetitive, AIO stimulation and AIH challenges differ remarkably from effects of a single optogenetic or hypoxic presentation. We then present the time-dependent changes in PNA, RSNA, MAP, and HR that form the basis for long-term facilitation (LTF), and we illustrate sites in caudal NTS responsible for responses to AIO stimulation.
Figure 1A illustrates typical recordings of MAP, HR, RSNA, and PNA in response to a single optogenetic stimulus lasting 1 min in a control rat and an AIO rat, and a single hypoxic stimulation of 1 min in an AIH rat. Across experiments, the responses to 1 min of optogenetic stimulation were variable. A single optogenetic stimulation for 1 min increased MAP and HR significantly (P < 0.05) in all rats, whereas RSNA either increased (n = 5), decreased (n = 2), or did not change (n = 2). PNA amplitude and frequency were increased in four of the nine rats. In the AIH group, single hypoxic stimulation increased HR, RSNA, and PNA amplitude and frequency.
In six control rats, in which injection sites outside the boundaries of caudal NTS were targeted, 1 min of optogenetic stimulation had no discernible effects on any of the measured parameters. Figure 1B left panel shows typical mCherry expression outside the boundaries of NTS in the control rat, and the right panel shows typical mCherry expression inside the boundaries of NTS in the AIO rat.
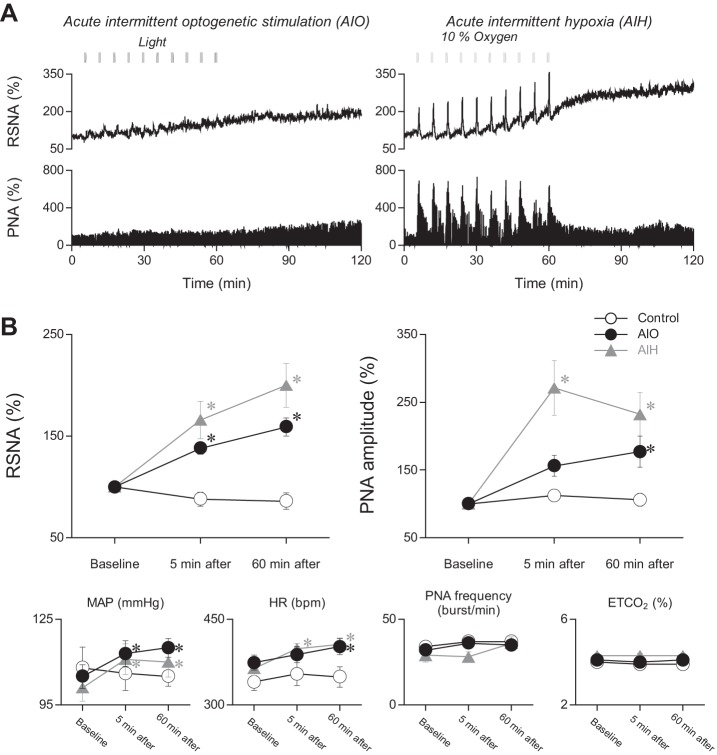
Figure 2 shows the effect of AIO stimulation (10 bursts of optogenetic stimulation) and AIH (10 bursts of hypoxic stimulation) on RSNA, PNA amplitude and frequency, MAP, and HR. Figure 2A illustrates RSNA and PNA responses recorded for 120 min during and after AIO stimulation or AIH in two experiments. AIO stimulation and AIH each produced LTF, characterized by progressive increases in RSNA and PNA, with each stimulus presentation greater during AIH than AIO stimulation, which continues to increase gradually after stimulus cessation. End-tidal CO2 was carefully monitored and kept within the control range (see methods) to ensure that no change occurred that might have altered respiratory and sympathetic nerve discharge. Figure 2B graphically summarizes results from all experiments. Measurements represent RSNA (left panel) and PNA (right panel) before (baseline) and at 5-min and 60-min intervals after AIH and AIO stimulation. Ordinate values are normalized as percentages. RSNA was significantly increased relative to baseline (P < 0.05) at 5 and 60 min after AIO stimulation and AIH. PNA was increased significantly (P < 0.05) at 5 and 60 min after AIH and 60 min after AIO stimulation. PNA was also increased 5 min after AIO stimulation, but the increase was not statistically significant (P = 0.052). After 60 min, RSNA was greater in the AIH group than in the AIO group (P < 0.05). Percent differences between the AIH and AIO groups in PNA, MAP, and HR at 60 min were not significant. There was no relationship between the percent change in PNA amplitude vs. the percent change in RSNA measured 60 min after AIH (r = 0.35; P = 0.39) or AIO stimulation (r = 0.09; P = 0.81).
Fig. 2.
Acute intermittent optogenetic stimulation of NTS neurons as well as acute intermittent hypoxia induced sympathetic and phrenic long-term facilitation. A: typical recordings of RSNA and PNA for 120 min in response to AIO stimulation and AIH. B: AIO stimulation and AIH increased RSNA, PNA amplitude, MAP, and HR measured at 60 min after the stimulations. *P < 0.05 compared with baseline within the same group.
To briefly highlight and recapitulate the effects of AIH and AIO tests in all experiments, 1) dramatic and sustained increases in RSNA and PNA amplitude, along with elevated MAP and HR, occurred after AIO and AIH; 2) AIH was more effective than AIO in elevating RSNA; 3) no correlations were found between the percentage increases in RSNA and PNA after AIO stimulation or AIH; 4) RSNA LTF was consistently observed after AIH and AIO stimulation, whereas phrenic LTF, defined as an increase of more than 20% (42), was observed in seven of nine AIO experiments and in seven of eight AIH experiments.
AIO stimulation and AIH effects on PNA and RSNA power spectral density, coherence, and synchronization between central respiratory drive and RSNA.
A prominent rhythm associated with central respiratory drive is observed in most sympathetic neural discharges even under basal conditions (9, 19). In addition, during chemoreflex activation, phrenic and sympathetic discharge coupling is attenuated by glutamatergic antagonism in the NTS (7). These studies, as well as others, not cited here indicate that the NTS plays an important, if not essential, role in coordinating sympathetic and respiratory motor outputs. Hence, in the present study, 1) we looked for augmented respiratory rhythm in RSNA and PNA by measuring power spectral densities and coherence, under basal conditions, as well as during global AIH and during AIO stimulation in the NTS; and 2) we made cross-correlation measurements to determine whether LTF following global AIH and AIO stimulation in the NTS increased PNA-RSNA synchronization.
Figure 3 shows the effects of AIO stimulation and AIH on PNA and RSNA power spectral densities, coherence between PNA and RSNA in the frequency domain, and cross correlations in the time domain. Figure 3A shows results from two experiments. AIO stimulation or AIH increased power spectral density in both PNA and RSNA at the primary harmonic frequency (large dot over the PNA power spectral density plots) of 36 epochs per minute, corresponding to the burst discharge frequency recorded from the phrenic nerve. By contrast, AIH and AIO stimulation had only modest effects on coherence between PNA and RSNA and on PNA-RSNA cross correlation.
Results from all experiments are summarized in Fig. 3B. Across experiments, PNA and RSNA power spectral densities increased significantly (P < 0.05), reflecting intensification of respiratory rhythmic components in PNA and RSNA. The effects were generally well developed at 5 min after AIO stimulation and AIH and remained so until the last measurements at 60 min. On the other hand, there were no significant changes (P > 0.05) in coherence and in strength of cross correlation between PNA and RSNA.
Thus, although augmented respiratory drive was evident from the persistent boost in PNA and RSNA power spectral density after AIO stimulation and AIH, there was no evidence from cross correlation that LTF increased synchronization between respiratory and sympathetic motor outputs. This dichotomy caused us to consider whether PNA synchronization may not have been transmitted to the renal sympathetic motor network. To test this possibility, we made PNA autocorrelation measurements (not illustrated) and compared them with PNA-RSNA cross correlations. After AIH, PNA synchronization increased in three of eight experiments (38%) and was unchanged in the remainder. After AIO stimulation, PNA synchronization increased in four of nine experiments (44%) and was unchanged in the others. When we compared each of the PNA autocorrelation responses with PNA-RSNA cross correlations, we found no obvious relationship.
In summary, 1) AIO stimulation and AIH increased RSNA modulation at the central respiratory drive frequency; 2) LTF appeared to have inconsistent and insignificant effects on synchronization within the phrenic motor pool; and 3) for instances that PNA synchronization increased, it was not effectively transferred into the renal sympathetic motor pathway.
Effects of LTF conditioning on RSNA, PNA, MAP, and HR responsiveness to a single hypoxic episode.
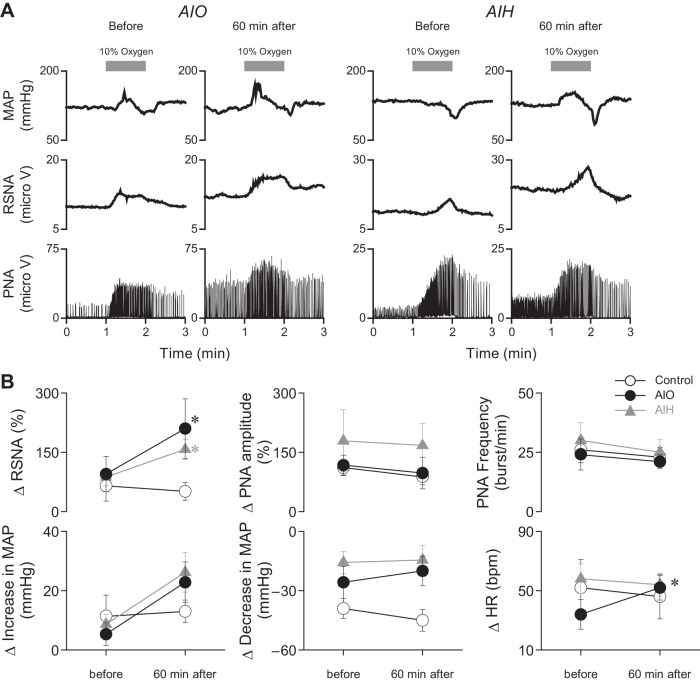
LTF was shown in a recent study to sensitize cardiovascular responsiveness and lumbar splanchnic sympathetic nerve discharges in response to a single hypoxic episode given long after AIH (42). Therefore, we tested whether AIH and AIO stimulation would produce late facilitation of RSNA and PNA responses to a single episode of hypoxia. Figure 4 shows the effects of 1 min of exposure to hypoxia on MAP, RSNA, PNA amplitude, PNA frequency, and HR. Records taken before (control) and at 60 min after the AIO stimulation or AIH are illustrated. Results illustrated in Fig. 4A are from two experiments, and Fig. 4B summarizes results from all experiments (n = 17). These tests show that RSNA responses to 1 min of hypoxia increased after AIO stimulation and AIH, whereas PNA amplitude and frequency were not altered. MAP responses to hypoxia were variable, with 16 of 17 rats showing an initial fall in MAP. In 12 of 17 rats, the fall in MAP was followed by an increase. Thus, increases and decreases in MAP are plotted separately.
Fig. 4.
Acute intermittent optogenetic and hypoxic stimulation enhance RSNA response to hypoxic stimulation. A: typical recordings of MAP, RSNA, and PNA in response to hypoxic stimulation (10% oxygen for 1 min) before and at 60 min after AIO stimulation and AIH. B: response of RSNA was increased after AIO stimulation or AIH. PNA amplitude and frequency response did not alter before and after AIO stimulation or AIH. *P < 0.05 compared with before AIO stimulation or AIH within the same group.
In summary, 1) RSNA responses to a single episode of hypoxia were increased by AIO stimulation and AIH; 2) HR responses to 1 min of hypoxia increased after AIO stimulation but not after AIH; and 3) neither AIH nor AIO stimulation affected the PNA response to a single hypoxic challenge.
Effects of angiotensin receptor blockade on RSNA and PNA long-term facilitation and cardiovascular responsiveness.
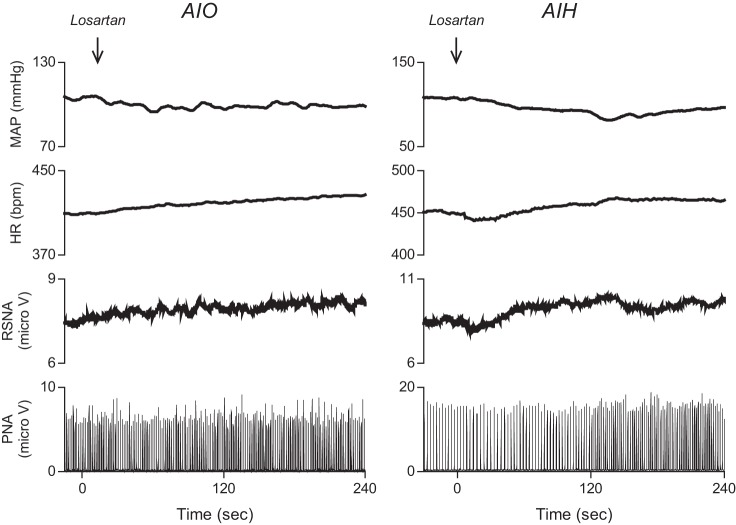
Intermittent hypoxia increases sympathetic activity in humans (8, 41), perhaps as a result of LTF, and ANG II contributes to the increase in MAP induced by chronic exposures of rats to IH (13, 14, 20). Hence, we tested whether ANG II receptor effects might contribute to renal sympathetic LTF and the cardiovascular responses that accompany it. Figure 5 illustrates the effects in two experiments of losartan, an ANG II AT1A receptor antagonist (1 mg/kg iv) on MAP, HR, RSNA, and PNA responsiveness to AIO stimulation and AIH. Losartan injection slightly decreased MAP responses to AIH (n = 2) and AIO stimulation (n = 3). HR and RSNA increased, presumably by baroreflex activation. PNA responses to AIO stimulation and AIH were unchanged. While these results do not reflect the sympathetic effects of ANG II release in humans, they indicate that global blockade of ANG II receptors does not appreciably alter PNA, RSNA, and cardiovascular responsiveness to AIO stimulation and AIH.
Fig. 5.
Typical recordings of MAP, HR, RSNA, and PNA in response to losartan, an ANG II AT1A receptor antagonist (1 mg/kg iv) injected 60 min after AIO stimulation and AIH.
DISCUSSION
It is well known from many studies over the years that the nucleus of the solitary tract integrates and modulates respiratory-sympathetic interactions under basal conditions and during various stress situations. One stress condition, acute intermittent hypoxia (AIH), produces a persistent increase in both phrenic and splanchnic sympathetic nerve activities (LTF) in the rat (12, 42), but the central nervous system sites responsible for the augmentation were not investigated. So one of the goals in our study was to determine whether local activation of neurons in the NTS by acute intermittent optogenetic activation could produce LTF in phrenic and renal sympathetic motor outputs. Having found LTF in both outputs after local AIO stimulation, we compared it with LTF produced by global intermittent acute hypoxia. A third objective was to determine whether LTF in phrenic and renal sympathetic pathways evoked by AIO in the NTS and by global AIH was synchronized.
The key new findings of the present study are as follows. First, AIO increased PNA and RSNA measured out to 60 min after AIO localized to the NTS. This finding supports our hypothesis that acute intermittent stimulation of NTS neurons is sufficient to induce sympathetic and phrenic LTF. Second, AIO stimulation in the NTS and global AIH did not increase synchronization between renal sympathetic and phrenic motor nerve outputs. Third, both sympathetic and phrenic LTF can be generated without the necessity of systemic hypoxia and without activation of afferent input from arterial chemoreceptors. Fourth, each hypoxic stimulation in the AIH protocol induced robust responses in RSNA and PNA in all rats. In the AIO protocol, optogenetic stimulation did not induce robust RSNA responses in all rats (Fig. 2A). Nonetheless, AIO consistently generated sympathetic LTF, suggesting that the generation of sympathetic LTF does not require intermittent robust increases in RSNA or PNA.
At a minimum, AIH brings into play three elements to generate sympathetic and phrenic LTF: 1) systemic tissue hypoxia, 2) activation of afferent inputs from arterial chemoreceptors, and 3) activation of neurons in the brain stem. AIO activation of NTS neurons alone seems to be sufficient to generate sympathetic and phrenic LTF, although it is less intense than AIH (Fig. 2B), probably because peripheral chemoreceptor sites and several chemosensitive regions of the brain stem that act in concert with commissural NTS neurons during AIH are not affected. The data suggest that the LTF produced by repeated activation of the carotid bodies may be at least partially caused by some plasticity change of the downstream network activated by the carotid bodies. This change could occur within the NTS or at any stage further down the network that causes RSNA to increase.
The mechanisms by which AIH leads to sympathetic and phrenic LTF are still not clearly resolved. Dick et al. (12) found that AIH induces both sympathetic and phrenic LTF and concluded that sympathetic LTF was a consequence of phrenic LTF. In contrast, Xing and Pilowsky (42) found that sympathetic LTF could be induced by AIH in the absence of a discernible change in phrenic nerve discharge. Although our study does not resolve the debate regarding where AIH mediates its effects on phrenic-renal sympathetic synchronization, it does provide some insight and a direction for future investigations. Power spectral density and coherence analysis (Fig. 3) revealed peaks that correspond with a respiratory rhythm in RSNA, as others have shown in other types of sympathetic postganglionic neurons (36). AIO and AIH each produced a remarkable increase of power spectral density in both PNA and RSNA, which reflects an increase in the magnitude of central respiratory drive. According to respiratory network theory, increased drive originates from a tonic excitatory source that elevates recurrent excitation of bulbospinal inspiratory neurons (34). On the other hand, we found no evidence of changes in spectral frequency distributions, indicating that there is no change in respiratory drive frequency (Fig. 3B). The absence of a change in the frequency spectrum suggests that bulbar respiratory neurons involved in phase switching between inspiration and expiration are unaffected, whereas neurons that increase phrenic motor discharge intensity are likely targets.
Taken a step further, our data indicate that LTF does not have a dominant effect on phrenic-renal sympathetic rhythm synchronization. This might be expected if phrenic LTF is primarily organized within the phrenic motor pool, where it is upregulated and downregulated by serotonin receptor subtypes and their associated protein kinases (28). On the other hand, there is also evidence that AIH increases discharge synchronization between respiratory rhythmic neurons of the rostroventrolateral respiratory column and phrenic motoneurons, at least in the cat (30). Potential sites where LTF synchronizes phrenic and sympathetic discharges have been discussed by others. For example, an earlier theory was that phrenic-sympathetic synchronization is set up within the rostroventrolateral medulla (RVLM) by connections between the respiratory pattern generator and barosensitive sympathetic neurons (16). More recent data suggest that barosensitive GABAergic neurons in the caudal ventrolateral medulla may also indirectly contribute to central respiratory drive-related modulation of presympathetic RVLM neurons and sympathetic nerve activity (24). Another site involved in respiratory modulation of sympathetic nerve activity is the dorsolateral pons (11). In addition, a tonic increase of sympathetic tone is also present that is independent of phrenic LTF (42). Whether in the phrenic motor pool or the brain stem respiratory network, our study indicates that the coupling between phrenic and renal sympathetic motor pools must be variable and conditional. We found that increased phrenic synchronization was inconsistent and not significant across all experiments, as was synchronization between phrenic and renal sympathetic neural circuits. We conclude from our analysis that sympathetic LTF is influenced by, rather than directly coupled to, central respiratory drive.
At 60 min after AIO or AIH, renal sympathetic nerve LTF was not greater in magnitude than phrenic nerve LTF. On the other hand, other studies (12, 42) showed that AIH produced an increase in sympathetic nerve activity that was greater than the increase in PNA. The three studies, ours and the other two, are similar in their use of adult rats, although anesthetic combinations differed and RSNA was recorded in our study, whereas splanchnic nerve discharges were recorded in the others. The latter difference may have some bearing on the relative effects of AIH on phrenic and sympathetic nerve discharges. Although many different types of sympathetic nerve activity, including lumbar splanchnic and renal sympathetic nerve discharges, exhibit robust respiratory-rhythmic discharges entrained to PNA (32), enhancement of PNA-gated discharge activity in renal sympathetic nerves may be more constrained by baroreceptor activation, at least during severe acute hypoxia (23).
Our results indicate that neurons in the commissural NTS play an important role in the induction of sympathetic and phrenic LTF. We assume that glutamatergic transmission plays a role since chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats (6). However, although CaMKIIa is exclusively expressed in glutamatergic neurons of the thalamus and cerebral cortex and mediates emergent respiratory rhythmic discharges in glutamatergic neurons in the pre-Bötzinger complex of the medulla, there are a variety of monoamines and peptides, as well as acetylcholine, normally present in the NTS that might contribute to LTF. For example, ∼80% of catecholaminergic A2 neurons in the brain stem are also glutamatergic (38), and studies from our laboratory show that A2 neurons in the NTS contribute to the increased blood pressure induced by exposures to chronic intermittent hypoxia (3).
Many mechanisms operating at cellular, membrane channel, and intracellular levels are likely to contribute to the induction of LTF. We have mentioned the involvement of serotonin receptors and associated protein kinases in the phrenic motor pool in response to AIH. Chronic exposure to intermittent hypoxia reduces the expression and function of KATP channels in NTS neurons receiving arterial chemoreceptor inputs (46). In the hippocampus, acute exposure to hypoxia induces reductions in KATP channel function, which are dependent upon release of adenosine (18). A rapid reduction in KATP channel function in NTS neurons during AIO and AIH protocols could increase neuronal discharge in response to an excitatory input and induce LTF. Another potential mechanism involves glial release of glutamate during activation of NTS neurons (5, 35). Alternatively, direct vagal afferent inputs to NTS astrocytes have been shown to induce glutamate release (26); perhaps glia receive similar afferent innervation from arterial chemoreceptor afferent inputs. Finally, severe AIH can induce phrenic LTF via an adenosine A2A receptor mechanism in the spinal cord (31).
Perspectives and Significance
Obstructive sleep apnea is known to be one of the causal factors in hypertension because it increases sympathetic tone (25, 33, 37). AIH induces sympathetic LTF that might be associated with the hypertension linked to obstructive sleep apnea in humans (8, 41). Our hope is that findings from the present study, added to the previous experimental database, will lead to a better understanding of sympathetic LTF and its relationship to hypertension. The optogenetic technique using halorhodopsin (light-driven chloride pump) can inhibit neuronal activity (15, 45). It would be interesting to determine whether optogenetic inhibition of NTS neurons prevents generation of the sympathetic LTF generated by AIH. It would also be of interest to identify the phenotype of the NTS neurons mediating sympathetic LTF.
GRANTS
This work was supported by National Institutes of Health Grant HL-088052.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.Y. and S.W.M. performed experiments; K.Y., P.M.L., and S.W.M. analyzed data; K.Y., P.M.L., and S.W.M. interpreted results of experiments; K.Y. and P.M.L. prepared figures; K.Y. drafted manuscript; K.Y., P.M.L., and S.W.M. edited and revised manuscript; K.Y., P.M.L., and S.W.M. approved final version of manuscript; S.W.M. conception and design of research.
ACKNOWLEDGMENTS
The authors acknowledge the expert technical assistance of Anuradha Rajulapati. The authors also thank Drs. Glenn Toney and Daniel Lodge, University of Texas Health Science Center at San Antonio, Texas, for assistance in the intensity measurements.
REFERENCES
- 1.Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest 124: 1454–1457, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bathina CS, Rajulapati A, Franzke M, Yamamoto K, Cunningham JT, Mifflin S. Knockdown of tyrosine hydroxylase in the nucleus of the solitary tract reduces elevated blood pressure during chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1031–R1039, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron 76: 70–81, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa-Silva JH, Zoccal DB, Machado BH. Chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol 302: R785–R793, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Costa-Silva JH, Zoccal DB, Machado BH. Glutamatergic antagonism in the NTS decreases post-inspiratory drive and changes phrenic and sympathetic coupling during chemoreflex activation. J Neurophysiol 103: 2095–2106, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 96: 754–761, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Czyzyk-Krzeska MF, Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol 426: 355–368, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisseroth K. Optogenetics. Nat Methods 8: 26–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dick TE, Baekey DM, Paton JF, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol 168: 76–85, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34: 309–314, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol (1985) 92: 627–633, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141: 154–165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol 121: 147–162, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron 38: 417–432, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Huang WX, Yu Q, Cohen MI. Fast (3 Hz and 10 Hz) and slow (respiratory) rhythms in cervical sympathetic nerve and unit discharges of the cat. J Physiol 523: 459–477, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight WD, Saxena A, Shell B, Nedungadi TP, Mifflin SW, Cunningham JT. Central losartan attenuates increases in arterial pressure and expression of FosB/DeltaFosB along the autonomic axis associated with chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1051–R1058, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XB, Jones EG. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Natl Acad Sci USA 93: 7332–7336, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Malpas SC, Ninomiya I. Effect of chemoreceptor stimulation on the periodicity of renal sympathetic nerve activity in anesthetized cats. J Auton Nerv Syst 37: 19–28, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 572: 881–896, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 307: 2169–2176, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci 31: 14,037–14,045, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mironov SL. Calmodulin and calmodulin kinase II mediate emergent bursting activity in the brainstem respiratory network (pre-Botzinger complex). J Physiol 591: 1613–1630, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Morris KF, Arata A, Shannon R, Lindsey BG. Long-term facilitation of phrenic nerve activity in cats: responses and short time scale correlations of medullary neurones. J Physiol 490: 463–480, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985) 112: 1678–1688, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: the regional differences. Neurosci Lett 81: 279–284, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Richter DW. Neural regulation of respiration: rhythmogenesis, and afferent control. In: Comprehensive Human Physiology, edited by Gregor R and Windhorst U, 1996, p. 2079–2095. [Google Scholar]
- 35.Seagard JL, Dean C, Patel S, Rademacher DJ, Hopp FA, Schmeling WT, Hillard CJ. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am J Physiol Heart Circ Physiol 286: H992–H1000, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Smith JE, Gilbey MP. Coherent rhythmic discharges in sympathetic nerves supplying thermoregulatory circulations in the rat. J Physiol 523: 449–457, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol 444: 191–206, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Talman WT. Glutamatergic transmission in the nucleus tractus solitarii: from server to peripherals in the cardiovascular information superhighway. Braz J Med Biol Res 30: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 13: 251–266, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol (1985) 89: 1333–1339, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol 588: 3075–3088, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu F, Zhang Z, Frazier DT. Microinjection of acetazolamide into the fastigial nucleus augments respiratory output in the rat. J Appl Physiol (1985) 91: 2342–2350, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron 71: 9–34, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature 446: 633–639, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1555–R1562, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]