Abstract
Pathological fibrosis is driven by a feedback loop in which the fibrotic extracellular matrix is both a cause and consequence of fibroblast activation. However, the molecular mechanisms underlying this process remain poorly understood. Here we identify yes-associated protein (YAP) (homolog of drosophila Yki) and transcriptional coactivator with PDZ-binding motif (TAZ) (also known as Wwtr1), transcriptional effectors of the Hippo pathway, as key matrix stiffness-regulated coordinators of fibroblast activation and matrix synthesis. YAP and TAZ are prominently expressed in fibrotic but not healthy lung tissue, with particularly pronounced nuclear expression of TAZ in spindle-shaped fibroblastic cells. In culture, both YAP and TAZ accumulate in the nuclei of fibroblasts grown on pathologically stiff matrices but not physiologically compliant matrices. Knockdown of YAP and TAZ together in vitro attenuates key fibroblast functions, including matrix synthesis, contraction, and proliferation, and does so exclusively on pathologically stiff matrices. Profibrotic effects of YAP and TAZ operate, in part, through their transcriptional target plasminogen activator inhibitor-1, which is regulated by matrix stiffness independent of transforming growth factor-β signaling. Immortalized fibroblasts conditionally expressing active YAP or TAZ mutant proteins overcome soft matrix limitations on growth and promote fibrosis when adoptively transferred to the murine lung, demonstrating the ability of fibroblast YAP/TAZ activation to drive a profibrotic response in vivo. Together, these results identify YAP and TAZ as mechanoactivated coordinators of the matrix-driven feedback loop that amplifies and sustains fibrosis.
Keywords: Hippo, idiopathic pulmonary fibrosis, mechanotransduction, plasminogen activator inhibitor 1, extracellular matrix
fibrosis, defined as the excessive accumulation of extracellular matrix, is a common pathological process in soft tissues and organs, including the lung, liver, kidney, skin, and cardiovascular system (55). Despite the large clinical burden imposed by fibrotic pathologies, few therapeutic options exist, prompting intensive efforts to elucidate new strategies for treating fibrosis (14). Recent work has demonstrated that the fibrotic extracellular matrix itself plays an essential role in promoting fibrogenic cell activation and perpetuating fibrotic pathologies (6, 30, 45), prompting efforts to therapeutically target the matrix (4, 26) to interrupt or reorient pathological signaling from the fibrotic matrix to resident cells. Parallel efforts have been aimed at identifying cellular signaling mechanisms activated by the pathological matrix environment as potential therapeutic targets (24).
One feature of the matrix environment that is prominently changed across all organ and tissue manifestations of fibrosis is the elastic modulus, more commonly referred to as matrix stiffness, which dramatically increases with matrix deposition, cross linking, and remodeling (6, 15, 32). Changes in the matrix mechanical landscape exert profound influences on mesenchymal cell signaling and activation state (7, 17, 23, 25, 31, 32), with prominent contributions identified from mechanical activation of transforming growth factor (TGF)-β signaling (7, 53), Rho kinase activation (23, 32, 36), and myocardin-related transcription factor (MRTF)-dependent transcription (23). Interruption of mechanical signaling in an animal model of fibrosis has demonstrated the conceptual promise of targeting cellular activation by the matrix mechanical environment (64). However, our understanding of how matrix mechanical properties are transduced by the cell and translated into altered cellular activation states remains a major limitation in efforts to target this mechanobiological aspect of fibrosis.
Here, we investigate the mechanistic role that the transcription coactivators yes-associated protein (YAP) (homolog of drosophila Yki) and transcriptional coactivator with PDZ-binding motif (TAZ) (also known as Wwtr1) play in matrix stiffness-dependent fibroblast activation and pulmonary fibrosis. YAP (56) and TAZ (27) are central transcriptional coactivators in the Hippo pathway (60) and mediate their effects through interactions with a number of other pathways and transcriptional effectors (38). Recent studies have implicated YAP and TAZ in mechanical signaling (2, 9, 12, 52), with activation through a cytoskeletal pathway (2) independent of the Lats1/2 and Mst1/2 kinases of the canonical mammalian Hippo pathway (59). However, how YAP and TAZ mediate fibroblast mechanoactivation, and whether this pathway is relevant to fibrotic pathologies, remains less clear. In this study, we demonstrate enhanced expression and localization of YAP and TAZ in mechanically remodeled fibrotic human lung tissue. We then identify a central role for YAP and TAZ in mediating fibroblast activation and matrix synthesis in vitro and fibrogenesis driven by fibroblasts adoptively transferred to the lung in vivo. Matrix stiffness-dependent fibroblast activation is driven in part through TGF-β-independent expression of the YAP/TAZ target plasminogen activator inhibitor (PAI)-1, which attenuates pericellular plasmin activity and thereby promotes cell-matrix adhesion and continued YAP/TAZ nuclear localization. This feedback loop allows YAP/TAZ mechanoactivation to translate increasing matrix stiffness into persistent cellular activation and fibrogenesis.
MATERIALS AND METHODS
Immunohistochemistry.
Human lung tissue was obtained from explanted lungs of patients with idiopathic pulmonary fibrosis (IPF) who underwent lung transplantation at Brigham and Women's Hospital or from normal lung tissue obtained from organ donors not suitable for transplantation, under a protocol approved by the Partners Human Research Committee. Lung tissue was fixed, embedded in paraffin, sectioned to 5-μm slices, deparaffinized, and rehydrated through xylenes and graded alcohol series. After endogenous peroxidase activity was quenched in 0.3% H2O2, the sections were blocked with 10% normal goat serum for 30 min. The sections were stained with primary antibody (mouse anti-YAP 1:100, sc-101199, Santa Cruz Biotechnology; or rabbit anti-TAZ 1:100, HPA007415, Sigma), biotinylated secondary antibody and VECTASTAIN ABC Reagent (anti-Mouse IgG, PK-4002 or anti-Rabbit IgG, PK-6101, Vectorlabs), peroxidase substrate solution (ImmPACT DAB Kit, SK-4105, Vectorlabs), and then counterstained with VECTOR Hematoxylin (H-3401, Vectorlabs). Stained sections were cleared, mounted, and then imaged with an upright microscope.
Western blotting.
IPF and normal primary fibroblasts were cultured in 12-well tissue culture-treated plastic plates in DMEM containing 4.5 g/l glucose, L-glutamine, sodium pyruvate, and supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified incubator with 5% CO2 at 37°C. Cell lysates were collected with lysis buffer (Cell Signaling no. 9803), mixed with 2× Laemmli sample buffer and boiled, run on 10% Mini-Protean TGX Gel (Bio-Rad), and then subjected to immunoblot analysis using mouse anti-YAP (63.7) antibody (1:500, Santa Cruz Biotechnology) and rabbit anti-YAP/TAZ (D24E4) antibody (1:500, Cell Signaling).
Atomic force microscopy mechanical mapping.
Fresh tissue samples from IPF lungs obtained at the time of transplant at Brigham and Women's Hospital under a protocol approved by the Partners Human Research Committee were snap frozen in liquid nitrogen and stored at −80°C until use. For immunohistochemistry and atomic force microscopy (AFM) parallel imaging, snap-frozen lung tissue was embedded in optimal cutting temperature compound and cryo-sectioned into 5-μm slices for immunohistochemistry [rabbit anti-WWTR1 (TAZ) antibody, 1:100, Sigma Aldrich] and an adjacent parallel 30-μm slice for AFM stiffness measurement. Mechanical characterization was performed using an atomic force microscope (MFP-3D, Asylum Research) as previously described (32, 33) with minor modifications. Briefly, tissue stiffness was measured by performing AFM microindentation in PBS at room temperature using a sphere-tipped probe (Novascan) with a diameter of 5 μm and a nominal spring constant of ∼60 pN/nm. The cantilever spring constant was further confirmed by the thermal fluctuation method. The AFM system was calibrated following the manufacturer's instruction before each indentation measurement. High-resolution force-indentation profiles were acquired at an indentation rate of 20 μm/s separated by 1.25 μm in a 64-by-64 sample grid covering an 80-by-80-μm area. Elastic modulus (E) at each point on the grid was calculated from fitting force-indentation data using Hertz sphere model in MFP-3D software (Version 101010+2106, Asylum Research). For lung tissue, a Poisson's ratio of 0.4 (8) was used to convert elastic modulus to shear modulus (G), using the relationship E = 2 (1 + v)G, and resulting shear modulus data were plotted into contour maps using MATLAB.
Polyacrylamide hydrogels.
Polyacrylamide hydrogels were prepared as described previously (37, 40) with minor modifications. Briefly, 15-mm no. 1 glass coverslips (Fisher Scientific) were treated with a 0.4% solution of 3-methacryloxypropyltrimethoxysilane (Sigma Aldrich) in acetone for 20 min, rinsed once with fresh acetone, and air dried. Five prepolymerization solutions of variable ratios of acrylamide:bis-acrylamide (Bio-Rad) were prepared as described previously (40)[%acrylamide:%bisacrylamide (Shear Modulus, kPa), 3:0.05 (0.1), 3:0.11 (0.4), 7.5:0.05 (1.6), 7.5:0.24 (6.4), 12:0.24 (25.6)]. Thirty microliters of each prepolymerization mixture were delivered and sandwiched between the above silanized coverslip and a SurfaSil (Thermo Scientific)-treated, hydrophobic glass slide for 0.5–1.0 min. After polymerization, the silanized coverslips with hydrogel attached were carefully peeled off from the hydrophobic slide, covered with 2 ml water in a 12-well plate, and then sterilized by heating the plate in a microwave oven for 30–40 s, stopping immediately before the water reaches the boiling point. The hydrogels were incubated in 1 mg/ml sterile dopamine hydrochloride solution in 50 mM HEPES (pH 8.5) for 15 min to coat the gel surface with polymerized dopamine (28). The gels were rinsed three times with 50 mM HEPES (pH 8.5) to remove residual dopamine and functionalized by incubation for 30 min with 0.05 mg/ml sterile collagen I (PureCol, Advanced BioMatrix) in PBS.
Cell culture.
IMR-90 lung fibroblasts were obtained from ATCC. Fibroblasts isolated from seven subjects diagnosed with IPF or seven normal subjects were cultured from the explanted lungs of patients who underwent lung transplantation or whose organs were rejected for transplantation at the University of Pittsburgh Medical Center, under a protocol approved by the University of Pittsburgh Institutional Review Board. Approximately 2-cm3 pieces of peripheral lung from which the pleural margin was removed were minced, and fibroblasts were cultured as previously described (47). Both IMR-90 and primary lung fibroblasts were cultured in DMEM containing 4.5 g/l glucose, L-glutamine, and sodium pyruvate, and supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin (all from Mediatech) in a humidified incubator with an atmosphere containing 5% CO2 at 37°C. For all experiments, cells were used at passages 4–8.
RNA interference.
IMR-90 cells were transfected using Lipofectamine RNAiMAX (Life Technologies) with an siRNA ON-TARGETplus SMARTpool (Thermo Scientific) containing four siRNAs targeting human YAP (L-012200-00-0005) and TAZ (WWTR1, L-016083-00-0005) or a nontargeting SMARTpool (D-001810-10-05). Cells were seeded onto 0.4-kPa or 25.0-kPa polyacrylamide hydrogels in 24-well plates using DMEM medium with 1% FBS and without antibiotics overnight and then exposed to 25 nM of each targeting siRNAs or equivalent amounts of nontargeting siRNA. Cells were cultured for 3 days. Knockdown of each siRNA target was confirmed by RT-PCR following 72 h of transfection. For TGF-β1 treatment, 2 ng/ml human TGF-β1 (100-B-001, R&D Systems) was added 72 h after transfection, and cells were incubated for another 24 h before RT-PCR analysis.
Immunofluorescence.
Cells were seeded at 50 cells per mm2 and transfected as described above. The cells were fixed in formalin, permeabilized with 0.5% Triton X-100, and blocked with 10% normal goat serum in PBS for 1 h. The cells were incubated overnight with a mouse monoclonal antibody against YAP (H-9, sc-271134, Santa Cruz Biotechnologies), a rabbit polyclonal antibody against TAZ (H-70, sc-48805, Santa Cruz Biotechnologies), a mouse monoclonal antibody against vinculin (hVIN-1, V9131, Sigma Aldrich), a mouse monoclonal antibody against procollagen I (PCIDG10, MAB1913, Millipore), all diluted at 1:500 in PBS with 1% BSA. They were then washed three times and incubated for 1 h with a secondary goat anti-mouse (or rabbit) Alexa Fluor 488- or 546-conjugated antibody (Life Technologies) diluted 1:500 in PBS with 1% BSA, and washed three times before fluorescence imaging.
For measurements of cell area, F-actin and nuclei were stained with Alexa Fluor 546-Phalloidin and Hoechst 33342 (Life Technologies), respectively. Fluorescence images were obtained with a Nikon TE300 fluorescent microscope and analyzed with MetaMorph 6.1 (Universal Imaging) to calculate 2D projected cell area based on F-actin staining.
Focal adhesion morphology was analyzed using software MetaMorph 6.1 and based on established methods (11). The length of stained focal adhesions was defined by their longest axis in the direction of lamellar spreading and was measured within the lamellar zone of cell edge. F-actin staining was quantified by measuring the average F-actin fluorescence intensity (normalized to stained areas) within each image with MetaMorph 6.1. Procollagen I staining was quantified by counting the procollagen I staining-positive cells and then normalizing to the total cell number in each image.
Soluble collagen assay.
Cells were seeded and transfected as described above on 0.4-kPa or 25.0-kPa gels in DMEM medium with 1% FBS and without antibiotics. After 72 h after transfection, cell culture media were collected and concentrated, and collagen concentration was measured using Sircol Soluble Collagen Assay (S1000, Biocolor, UK), following manufacturer's instructions. Cell number was determined using the CyQUANT NF Cell Proliferation Assay (Life Technologies, C35007). Collagen concentrations were normalized to the corresponding cell numbers as measured with CyQuant Assay.
Apoptosis.
Apoptosis was assessed using a fluorescence-based, ApoONE Caspase 3/7 Activity Assay (G7790, Promega). Apoptosis ratio was calculated by dividing ApoONE signal with CyQuant signal from parallel control wells.
PAI-1 ELISA.
Cells were seeded on discrete stiffness gels in F12K DMEM with 10% FBS. Cell culture media were collected and analyzed for PAI-1 (Human PAI-1 Quantikine ELISA Kit, DSE100, R&D Systems) concentrations by ELISA. Concentration results were normalized to the corresponding cell numbers.
Plasmin activity.
Plasmin activity in cell culture media was determined using the SPECTROZYME PL chromogenic substrate (no. 251, American Diagnostica) according to manufacturer's instructions. Briefly, 50 μl of the cell culture media was transferred to a single well of a 96-well plate containing 50 μl of SPECTROZYME PL chromogenic substrate and 50 μl of buffer solution. Absorbance was measured at 405 nm using a Bio-Rad 680 microplate reader (20).
Traction forces.
Contractile forces exerted by cells on different stiffness matrices were assessed by traction force microscopy as described previously (37). Briefly, polyacrylamide substrates with shear moduli of 0.4 and 6.4 kPa were prepared, and fluorescent sulfate-modified latex microspheres (0.2 μm, 505/515 ex/em, FluoSpheres, Life Technologies) were conjugated to the gel surfaces after treatment with 1 mg/ml of 3-hydroxytyramine hydrochloride (Sigma Aldrich) in 50 mM HEPES solution (pH 8.5) for 5 min. For this, 6.4 kPa was chosen, as this intermediate modulus hydrogel allows substantial displacements and robust quantification of tractions relative to stiffer 25-kPa gels (37). Cells were plated on the gels overnight and then transfected for YAPi/TAZi and incubated for 72 h as described above before traction force measurements. Images of gel surface-conjugated fluorescent beads were acquired for each cell before and after trypsinization using a Pathway HT fluorescence imaging system (BD Biosciences) and a ×20 magnification objective. Cell area was visualized using CellLight Plasma Membrane-RFP (Life Technologies) and obtaining a paired fluorescent image of the cell membrane. Tractions exerted by IMR-90 were estimated by measuring bead displacement fields, computing corresponding traction fields using Fourier Transform Traction Microscopy, and calculating root-mean-square traction with MATLAB (MathWorks).
Quantitative PCR.
Cells cultured on eight replicate stiffness wells in 24-well plate were lysed sequentially with 350 μl of RNA lysis buffer (RLT buffer, QIAGEN), and RNA was isolated (RNeasy mini kit) and quantified with RiboGreen reagent. Each sample (50 ng) was primed with oligo (dT) and reverse transcribed into cDNA using TaqMan reverse transcription reagents (Applied Biosystems) at 25°C for 10 min, 37°C for 60 min, and 95°C for 5 min. Equivalent amounts of each cDNA sample were added to Power SYBR green PCR master mix (Applied Biosystems) containing 1 μM each of forward and reverse primer for mRNA amplification. Primers were designed with qPrimerDepot (http://primerdepot.nci.nih.gov) and are intron overlapping. qPCR was performed by incubating at 95°C for 10 min to activate AmpliTaq Gold DNA polymerase (Applied Biosystems) and then cycling 40 times at 95°C for 15 s and 60°C for 1 min. Ct values within each experiment were normalized against GAPDH.
Primers for qPCR.
Primers are as follows: YAP1: (FW) ACGTTCATCTGGGACAGCAT, (RV) GTTGGGAGATGGCAAAGACA; TAZ (WWTR1): (FW) TTCCTAGGGTCTTGCCATGT, (RV) AGTCCTACGACGTGACCGAC; SERPINE1 (PAI-1): (FW) TGGTGCTGATCTCATCCTTG, (RV) AGAAACCCAGCAGCAGATTC; COL1A1: (FW) CACACGTCTCGGTCATGGTA, (RV) AAGAGGAAGGCCAAGTCGAG; GAPDH: (FW) AATGAAGGGGTCATTGATGG, (RV) AAGGTGAAGGTCGGAGTCAA; FN1: (FW) ACCTCGGTGTTGTAAGGTGG, (RV) CCATAAAGGGCAACCAAGAG.
Doxycycline-inducible Tet-On NIH3T3 cells.
The Lenti-X HTX packaging system (Clontech) along with the pLVX-Tet-On Advanced plasmid (no. 632162, Clontech) were used to generate lentivirus that was then used to transduce the tetracycline-controlled transactivator, rtTA-Advanced, into NIH3T3 cells. Stable pools of Tet-On-NIH3T3 were selected with 1 μg/ml G-418 sulfate, and these cells were then infected with lentivirus transducing the expression 3xFLAG-tagged human TAZ-4SA [S66A, S89A, S117A, and S311A (29)] or YAP-5SA [5SA: S61A, S109A, S127A, S164A, S397A (61)] from the pLVX-Tight-Puro plasmid (no. 632162, Clontech). Stable dox-inducible TAZ4SA- or YAP5SA-expressing cells were grown in DMEM plus 10% FBS, 10,000 U/ml of penicillin, 10,000 μg/ml of streptomycin, and 25 μg/ml of amphotericin B, along with continuous exposure to selection reagents 1 μg/ml G-418 sulfate and 2 μg/ml puromycin.
Adoptive transfer.
Immunocompromised NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) male mice were obtained from Jackson Laboratories, housed in a specific pathogen-free animal facility at Massachusetts General Hospital, and entered into experiments at 8–12 wk old. All mice were maintained in microisolator cages, fed autoclaved food and water, and treated according to Institutional Animal Care and Research Committee-approved protocols. Groups of five NSG mice were injected with 1 × 106 YAP5SA- or TAZ4SA-expressing fibroblasts resuspended in 300 μl sterile PBS via tail vein in an air-filtered laminar flow hood. Other groups of five NSG mice were injected with control fibroblasts and PBS as control groups. All mice were given either water or water containing doxycycline at 1 mg/ml from day 0. Mice were then euthanized by exsanguination under anesthesia after 14 days, and whole-lung tissue was harvested for histological and biochemical analysis. The total collagen content of the left lung was measured by hydroxyproline assay as previously described (49). Right lungs were fixed for 72 h in 4% paraformaldehyde, processed, and embedded in paraffin. Sections were stained with hematoxylin and eosin for histopathological analysis or with Masson's trichrome for the evaluation of collagen accumulation and distribution.
Cell-derived matrix experiments.
Preparation of cell-derived matrices was performed using methods adapted from published protocols (5). Control or TAZ4SA-transduced NIH3T3 fibroblasts were plated at 35,000 cells per well onto 15-mm coverslips coated with 2% gelatin in 24-well plates. Cells were seeded and fed every 48 h with doxycycline at 0, 1, or 100 ng/ml. Matrix decellularization was performed at day 7 by exposure to 1 ml cell extraction buffer composed of PBS plus 2% NH4OH and 0.5% Triton X-100 at room temperature followed by washing in PBS. To solubilize cell-derived extracellular matrix proteins, dishes were placed on ice and extracted with 5 M guanidine HCl and 0.1 M dithiothreitol in deionized H2O followed by scraping. Extracellular matrix protein content was determined by BCA protein assay kit using a 1:25 dilution. In parallel experiments, intact cells were removed from dishes using trypsin, and cell number was measured by CyQuant assay. Cell-derived matrix protein quantity was normalized to total cell number per well. Naïve cell adhesion to cell-derived matrices were performed by first extracting cells using the matrix decellularization protocol described above, followed by seeding of control NIH3T3 cells on cell-derived matrices for 4 h. Plates were agitated gently, and nonadherent cells were removed by aspiration, followed by fixation in 4% paraformaldehyde and counting of adherent cells in three high-power fields per well, four replicates per condition.
RESULTS
YAP and TAZ are mechanoactivated by pathological matrix stiffness in lung fibroblasts.
YAP and TAZ have recently been implicated in mechanosignaling in multiple cell types and contexts (2, 9, 12). To investigate their potential role in pathological fibroblast-matrix stiffness responses, we first analyzed a microarray dataset (GEO Accession: GSE22011) documenting transcripts whose expression in human lung fibroblasts changed across substrate elastic moduli spanning the range observed in normal and fibrotic lungs (32). We focused on the 86 transcripts whose expression robustly increased across matrix stiffness in cells from three independent donors and compared them to a list of transcripts differentially regulated in NIH 3T3 fibroblasts by YAP overexpression (61, 62). Remarkably, of the 86 stiffness-enhanced transcripts, 45 were previously found to be increased in 3T3 cells overexpressing YAP (Table 1), yielding a Fisher exact P value of 7.528E-32. In contrast, the matrix stiffness-induced transcripts were previously shown to have little overlap with a TGF-β-responsive transcript dataset (32). These findings suggest substantial contributions of YAP and TAZ to matrix stiffness-dependent transcript expression in human lung fibroblasts in vitro.
Table 1.
Microarray analysis implicates YAP/TAZ in fibroblast stiffness response
| Genes Increased by Matrix Stiffness | |||||
|---|---|---|---|---|---|
| ABCC9 | C6orf173 | DIAPH3 | KIF11 | NUSAP1 | SULF1 |
| ABI3BP | CASC5 | DLGAP5 | KIF14 | OLFML1 | SYTL2 |
| ACACA | CCNB1 | ENPP1 | KIF15 | OXTR | TAGLN |
| ADAMTSL1 | CCNB2 | EPGN | KIF18A | PCDCILG2 | TK1 |
| ANLN | CDC2 | ESM1 | KIF20A | PDGFRL | TNFSF4 |
| ANXA3 | CDC20 | FABP3 | KIF20B | PRC1 | TOP2A |
| AOX1 | CDCA2 | FANCB | KIF2C | PRR11 | TPX2 |
| AP1S3 | CDKN3 | FGF1 | KIF4A | PTX3 | TRIM55 |
| APOBEC3B | CENPE | HMMR | KRTAP2-4 | PUS7L | TTK |
| ARHGAP29 | CENPF | HSD17B7 | METTL7A | RELN | UBE2C |
| ASPM | CEP55 | IFIT1 | MKI67 | SCD | VEPH1 |
| BUB1 | CIT | IL7R | NCAPG | SCVEP1 | |
| BUB1B | CKAP2L | INSIG1 | NDC80 | SERPINB7 | |
| C13ORF3 | CPA4 | IQGAP3 | NLRP10 | SLC16A4 | |
| C2orf44 | DHCR24 | KIAA1632 | NUF2 | SLIT2 | |
Transcripts for the 86 genes shown in this table were previously identified as significantly increased by matrix stiffness across a panel of three independent primary lung fibroblast lines using microarray analysis (32). The 45 genes in boldface font denote the overlap of this list with transcripts whose expression was found to be significantly enhanced by yes-associated protein (YAP) overexpression in NIH 3T3 cells (61, 62). TAZ, transcriptional coactivator with PDZ-binding motif.
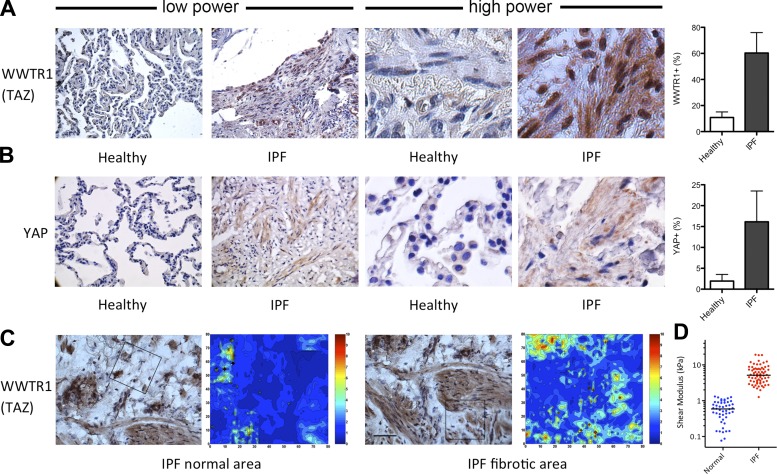
Before embarking on studies of YAP and TAZ roles in fibroblast activation and fibrogenesis, we sought to evaluate their expression and localization in diseased lung cells and tissues to evaluate their potential roles in human fibrosis. We first analyzed fixed tissue obtained from the lungs of individuals with IPF (n = 4) and normal lung tissue (n = 4). Normal lung tissue was largely devoid of cells positive for immunohistochemical staining with antibodies against YAP and TAZ, with infrequent staining restricted to round/cuboidal cells in the parenchyma (Fig. 1). Notably, normal lung tissue exhibits a relative paucity of readily identifiable interstitial fibroblasts compared with the fibrotic lung (44). In contrast to normal lung, IPF lung tissue displayed prominent staining with antibodies against both YAP and TAZ, with pronounced expression in spindle-shaped cells populating areas of the lung marked by architectural distortion and cellular hyperplasia characteristic of IPF. High-magnification observation of these regions demonstrated nuclear localization of TAZ, consistent with its role as a transcriptional cofactor, and more diffuse staining for YAP. Analysis of tissue stiffness by AFM and TAZ immunostaining in adjacent serial sections demonstrated colocalization of TAZ-immunopositive cell nuclei and areas of elevated matrix stiffness, consistent with a role for the local mechanical environment in TAZ localization in situ. We also confirmed previous measures of tissue stiffness in fibrotic lungs (6, 7, 32), documenting median shear moduli in normal lungs of 0.59 kPa (range 0.07–1.32 kPa) and in fibrotic regions of IPF lungs of 5.16 kPa (range ∼1.27–19.33 kPa), providing a pathophysiological range of matrix mechanical properties over which to study fibroblast function in vitro. For purposes of comparison, we assumed a Poisson's ratio (ν) of lung tissue of 0.4; thus the conversion from shear modulus (G) to Young's modulus (E) requires multiplication of our observed values by 2.8 [E = 2 × (1 + ν) × G], resulting in median values of 1.65 and 14.45 kPa in normal and fibrotic lung tissue.
Fig. 1.
Yes-associated protein (YAP) (homolog of drosophila Yki) and transcriptional coactivator with PDZ-binding motif (TAZ) (also known as Wwtr1) expression and nuclear localization are enhanced in remodeled fibrotic lungs. A: immunohistochemical staining of TAZ (WWTR1) in representative low-power and high-power fields from normal (healthy) and idiopathic pulmonary fibrosis (IPF) lungs and quantification of nuclei staining positive for TAZ (WWTR1+/total nuclei) from 4 donors per group, 4 low-power images per donor, and 2–3 high-power sampling areas per image. B: immunohistochemical staining of YAP and quantification of cells (nuclei) staining positive for YAP from 4 donors per group, 4 low-power images per donor, and 2–3 high-power sampling areas per image. C: immunohistochemical staining of TAZ in IPF lung tissue and comparison with corresponding high-resolution stiffness maps measured in adjacent serial sections by atomic force microscopy (AFM). Scale bar in immunohistochemistry (IHC), 50 mm. Box in each IHC image indicates the approximate stiffness mapping area (80 × 80 μm). Color scale in stiffness maps indicates shear modulus in kPa. D: tissue stiffness (shear modulus) measured in several regions from snap-frozen unfixed normal and IPF lungs by AFM (n = 6 each of IPF and normal lungs).
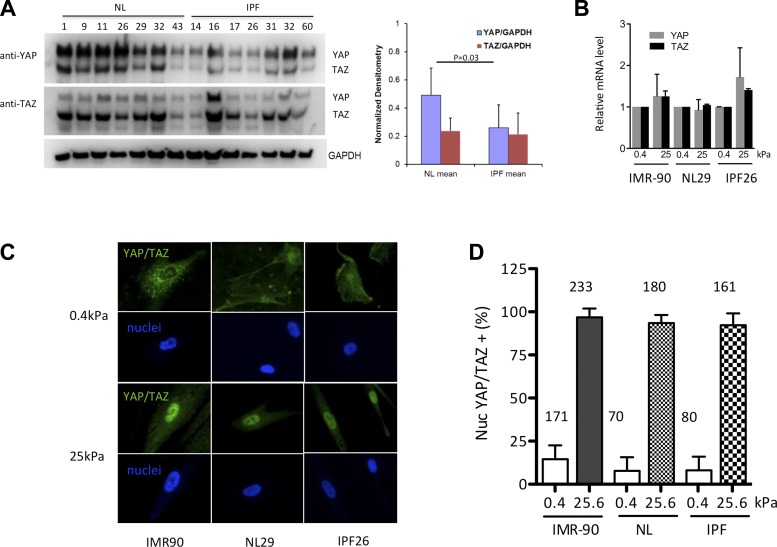
We next examined YAP and TAZ protein levels in isolated normal fibroblasts and those derived from patients with IPF to determine whether these proteins were aberrantly expressed in disease-derived cells. IPF fibroblasts have been found previously to maintain selected phenotypic differences in culture relative to cells derived from normal lung tissue (18, 21, 30, 36). However, when we examined YAP and TAZ protein levels, we noted little difference in their expression between cultured normal and IPF-derived cells (Fig. 2A), with a modest trend toward lower YAP expression in IPF-derived cells. This suggested that the increased staining for YAP and TAZ observed in fibrotic lung tissue results from a change in the relative abundance of fibroblasts in the tissue, a known feature of fibrotic pathologies (44), and/or a response of fibroblasts to the specific local fibrotic environment present in situ. Indeed, multiple signals have been implicated in regulation of YAP and TAZ expression and localization (38, 58, 59), several of which may be altered in the context of fibrosis, including soluble signaling molecules and matrix mechanical properties (12).
Fig. 2.
YAP and TAZ respond to pathophysiological increases in matrix stiffness by relocalizing to fibroblast nuclei. A: despite immunohistochemical evidence for enhanced YAP/TAZ expression in IPF, IPF-derived fibroblasts do not overexpress YAP/TAZ in a cell-autonomous fashion. Cells from 7 different normal and IPF lungs were grown on tissue culture plastic, and YAP/TAZ protein levels were analyzed by Western blotting with mouse anti-YAP (63.7) antibody (Santa Cruz Biotechnology) and rabbit anti-YAP/TAZ (D24E4) antibody (Cell Signaling). Both antibodies cross react with YAP and TAZ, which appear at molecular weights of ∼70 kDa and ∼55 kDa, respectively. GAPDH was used as a loading control. Densitometry normalized to GAPDH demonstrates a modest decrease in YAP protein levels in IPF fibroblasts relative to normal lung fibroblasts. NL, normal cells. B: YAP and TAZ transcript levels vary little across matrix stiffness conditions spanning the pathophysiological range in IPF-derived, normal, and IMR-90 fibroblasts (n = 3 independent experiments). C: in contrast, YAP and TAZ nuclear localization is dramatically altered by matrix stiffness. Immunostaining with mouse anti-YAP (63.7) antibody (Santa Cruz Biotechnology, detects both YAP and TAZ) and Hoechst 33342 after culturing 48 h on 0.4- and 25.0-kPa shear moduli polyacrylamide hydrogels demonstrates pronounced nuclear localization on stiff matrices. D: cells with predominantly nuclear YAP/TAZ localization were counted in a blinded fashion on 0.4- and 25.0-kPa gels and expressed as a fraction of total cell number. N = total number of cells analyzed from 2 independent experiments from 3 normal (NL29, NL43, and NL45) and 3 IPF (IPF14, IPF26, and IPF32) lines.
To test the role the mechanical environment plays in YAP and TAZ expression and localization, we cultured normal and IPF-derived fibroblasts as well as IMR-90 lung-derived fibroblasts on collagen I-coated polyacrylamide gels of elastic moduli spanning the range observed in normal and fibrotic lung tissue (Fig. 1) (6). We observed a modest (20–60%) increase in YAP and TAZ transcripts across matrix stiffness in IPF cells and little difference in YAP and TAZ message levels in normal and IMR-90 fibroblasts across this matrix stiffness range (Fig. 2B). In contrast to the modest effects on overall transcript levels, there was a dramatic change in YAP/TAZ protein localization between soft and stiff matrices (Fig. 2, C and D), with almost all cells exhibiting predominant nuclear localization of YAP/TAZ on stiff matrix and far fewer cells exhibiting distinct nuclear localization on soft matrix, in agreement with recent observations (2, 9, 12). Strikingly, our observation of matrix stiffness-dependent nuclear localization was consistent across normal, IMR-90, and IPF-derived fibroblasts, demonstrating that IPF-derived cells remained highly responsive to the mechanical environment. Taken together, these results demonstrate that TAZ and, to a lesser extent, YAP are prominently expressed in IPF lung tissue (Fig. 1), and, whereas YAP and TAZ overall expression levels are relatively unresponsive to matrix stiffness, their nuclear localization is highly dependent on a pathologically stiffened extracellular matrix (Figs. 1 and 2).
YAP and TAZ control fibroblast function.
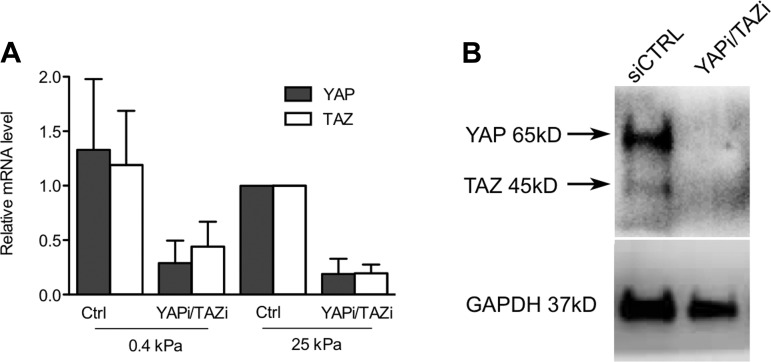
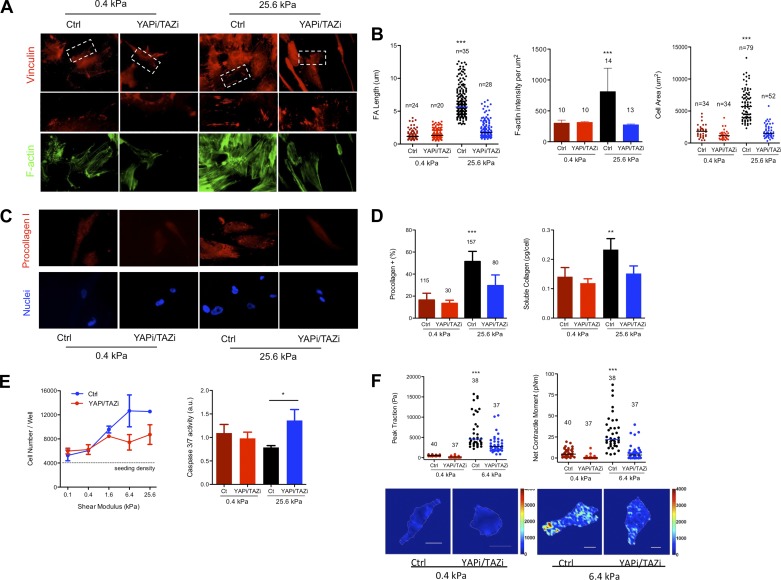
To test the functional importance of YAP and TAZ in lung fibroblast function in vitro, we began by studying IMR-90 lung fibroblasts, as these cells provide a stable and abundant cell source that exhibits matrix stiffness-dependent YAP and TAZ relocalization indistinguishable from primary normal and IPF-derived fibroblasts (Fig. 2). Moreover, we have previously shown that both primary and IMR-90 fibroblasts respond to increases in matrix stiffness with morphological and cytoskeletal changes, increased proliferation, suppressed apoptosis, and increased collagen synthesis and matrix contractile force generation (32, 37). To test YAP and TAZ roles in these functions, we knocked down expression of YAP and TAZ simultaneously using siRNA (Fig. 3). Remarkably, simultaneous YAP and TAZ knockdown reversed or attenuated all matrix stiffness-dependent changes in cell morphology and function (Fig. 4). Whereas matrix stiffness resulted in increased cell spreading, actin polymerization, and elongation of focal adhesions, YAP and TAZ knockdown reversed these outcomes to levels observed on soft matrices. Strikingly, YAP and TAZ knockdown exerted no distinguishable effect on cells cultured on soft matrices, indicating a specific effect restricted to a stiffened extracellular matrix. In a similar fashion, increasing matrix stiffness enhanced procollagen I expression and collagen synthesis, proliferation, and contractile force generation, while attenuating caspase 3/7 activity, as previously observed (32, 37). Again, YAP and TAZ knockdown reversed these matrix stiffness-dependent functional changes and did so in a fashion largely restricted to cells grown on stiff matrices (Fig. 4).
Fig. 3.
YAP/TAZ siRNA (YAPi/TAZi) attenuates YAP/TAZ message (relative mRNA) and protein levels (Western blot) in IMR-90 fibroblasts. A: YAP and TAZ transcript levels measured by qPCR, normalized to GAPDH, and to 25-kPa control samples. B: Western blotting with mouse anti-YAP (63.7) antibody (Santa Cruz Biotechnology) detects YAP (65 kDa) and TAZ (45 kDa) bands in siCTRL but not YAPi/TAZi samples.
Fig. 4.
Profibrotic responses to matrix stiffness are dramatically reduced by YAP/TAZ knockdown. A: IMR-90 fibroblasts were transfected with YAP/TAZ siRNA on 0.4- and 25.0-kPa shear moduli polyacrylamide hydrogels functionalized with collagen I for 72 h, fixed, and stained with mouse anti-vinculin antibody to visualize focal adhesions and phalloidin to visualize F-actin. Regions of interest indicated by white dashed boxes are shown at higher magnification in second row of images, with contrasting large and small vinculin-positive adhesions indicated by white arrows. B: increasing matrix stiffness enhances and YAP/TAZ knockdown attenuates focal adhesion (FA) length, F-actin assembly, and cell spreading. ***P < 0.0001 by 1-way ANOVA; FA length, n = number of cells analyzed from 2 independent experiments; F-actin, n = number of images analyzed from 3 independent experiments; cell area, n = number of cells analyzed from 3 independent experiments. C: immunostaining with mouse anti-procollagen I antibody and Hoechst 33342 to visualize cell nuclei. D: increased matrix stiffness enhances and YAP/TAZ knockdown attenuates procollagen I-positive cells (***P < 0.0001 by 1-way ANOVA, n = number of cells analyzed from 3 independent experiments) and soluble collagen production in cell culture medium, as measured with SirCol assay (**P < 0.05, by 1-way ANOVA from 2 independent experiments). E: increasing matrix stiffness promotes and YAP/TAZ knockdown attenuates cell accumulation over 72 h, with the converse trend observed for caspase 3/7 activity (*P < 0.05 by t-test from 2 independent experiments). F: increasing matrix stiffness enhances contractility, as measured by net contractile moment and peak tractions in traction force microscopy. YAP/TAZ siRNA knockdown selectively attenuates tractions on stiff matrices (***P < 0.0001 by 1-way ANOVA, n = number of cells analyzed from 2 independent experiments). Representative traction maps are shown from 0.4- and 6.4-kPa matrices, where color scale indicates traction magnitudes (kPa) and scale bar indicates 20 μm.
To extend these findings implicating YAP/TAZ in matrix stiffness-dependent fibroblast function to primary disease-derived cells, we studied IPF fibroblasts in a similar fashion (Fig. 5), focusing on a single IPF line that exhibited YAP/TAZ expression levels typical of both normal and IPF-derived cells (IPF 26). We first demonstrated that we could achieve robust and equivalent knockdown of YAP and TAZ transcripts on soft and stiff matrices (Fig. 5A). We then measured cellular force generation using traction microscopy and confirmed that matrix stiffness-induced increases in primary IPF fibroblast tractions were significantly attenuated by simultaneous YAP/TAZ knockdown (Fig. 5B). Similarly, we observed that simultaneous siRNA knockdown of YAP and TAZ attenuated matrix stiffness-induced proliferation of IPF fibroblasts (Fig. 5C). Finally, as indicators of fibrotic matrix synthesis, we measured transcript levels for COL1A1 and protein levels for fibronectin (FN) (39) and found that simultaneous siRNA knockdown of YAP and TAZ reversed matrix stiffness-induced increases in expression of each (Fig. 5D) and consistently suppressed FN protein levels across multiple IPF-derived lines (Fig. 5E). As in IMR-90 fibroblasts, the effects of YAP and TAZ knockdown were largely limited to cells resident on pathologically stiffened matrices, further implicating YAP/TAZ function in matrix stiffness-dependent activation of disease-derived IPF fibroblasts.
Fig. 5.
IPF fibroblast responses to matrix stiffness are ablated by YAP/TAZ knockdown. IPF fibroblasts were transfected with YAP/TAZ siRNA for 72 h on 0.4- and 25.0-kPa shear moduli polyacrylamide hydrogels functionalized with collagen I. A: equivalent knockdown of YAP and TAZ on soft and stiff matrices was observed (n = 3 independent experiments). B: increasing matrix stiffness enhances IPF fibroblast contractility, as measured by net contractile moment and peak tractions in traction force microscopy. YAP/TAZ siRNA knockdown selectively attenuates tractions on stiff matrices. ***P < 0.0001 by 1-way ANOVA, n = number of cells analyzed from 2 independent experiments. C: increasing matrix stiffness promotes cell accumulation over 72 h. YAP/TAZ siRNA knockdown selectively reduces cell accumulation on stiff matrix (n = 2 independent experiments). D: increasing matrix stiffness promotes IPF fibroblast gene expression of extracellular matrix transcripts COL1A1 and FN1. YAP/TAZ siRNA knockdown selectively attenuates matrix stiffness-promoted gene expression on stiff matrices (n = 2 independent experiments). Similarly, increasing matrix stiffness enhances expression of EDA fibronectin (FN) protein, as measured by Western blot from cell lysates, and YAP/TAZ siRNA knockdown reduces EDA FN expression on pathologically stiff but not physiologically compliant matrix (n = 3 independent experiments). E: YAP/TAZ siRNA knockdown evokes consistent effects on EDA FN protein expression across 3 independent IPF fibroblast lines. cFN, cellular fibronectin.
PAI-1 is a pivotal profibrotic YAP/TAZ target.
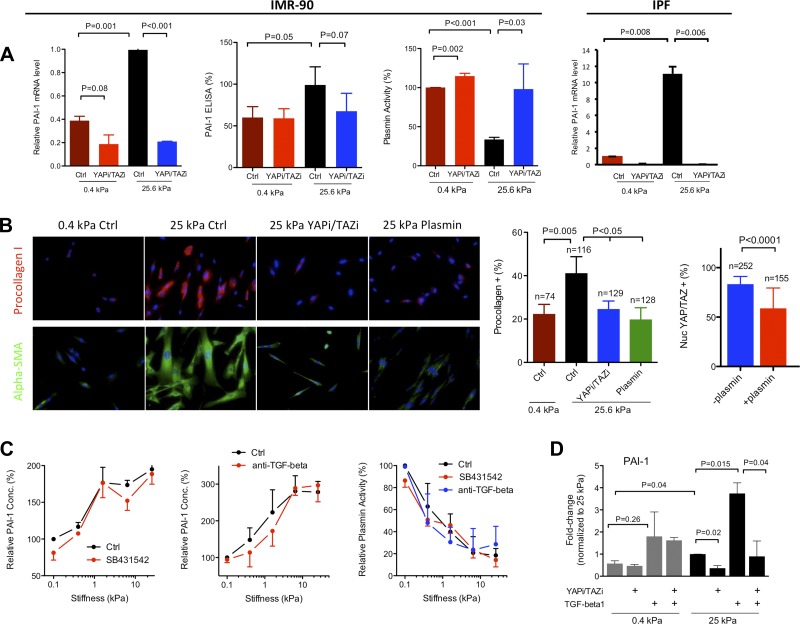
Taken together, the above results indicate broad functional effects of YAP/TAZ knockdown on matrix stiffness-dependent fibroblast activation that are preserved in IPF fibroblasts. How such effects are coordinated through transcriptional effects of YAP and TAZ is not well understood. Although multiple matrix stiffness-dependent, putative YAP/TAZ target genes were identified in prior work (9, 32), none were obvious candidates to serve as central nodes in profibrotic function of fibroblasts. Interestingly, SERPINE1, which encodes PAI-1 and is a target of YAP/TAZ (61, 62), narrowly missed inclusion in our previous study of matrix stiffness-responsive transcripts (32), as it failed to reach the minimum fold change cutoff in cells from one of three independent fibroblast lines. Importantly, PAI-1 has been definitively linked to both profibrotic functions of cultured fibroblasts (20) and fibrosis in animal models and human disease (10, 16, 19, 22). We confirmed that matrix stiffness promotes an increase in SERPINE1 transcripts and that YAP/TAZ knockdown attenuates this response in both IMR-90 and IPF primary fibroblasts (Fig. 6A). Intriguingly, IPF fibroblast SERPINE1 expression levels displayed a greater sensitivity to matrix stiffness. Soluble PAI-1 levels in cell culture supernatants were also elevated with matrix stiffness and reversed by YAP/TAZ knockdown although this response failed to reach statistical significance (Fig. 6A). Although the magnitude of changes in PAI-1 levels were relatively modest, the resulting effect on endogenous plasmin activity in cell culture supernatants was highly significant, demonstrating that increasing matrix stiffness suppresses plasmin activity, whereas YAP/TAZ knockdown returns plasmin activity to levels found on soft matrices.
Fig. 6.
Matrix stiffness promotes profibrotic functions through the YAP/TAZ target plasminogen activator inhibitor (PAI)-1. A: increasing matrix stiffness promotes IMR-90 fibroblast PAI-1 (SERPINE1) gene expression and PAI-1 protein levels in conditioned media while decreasing plasmin activity (n = 3 independent experiments). YAP/TAZ siRNA knockdown selectively attenuates matrix stiffness-promoted PAI-1 expression on stiff matrices and correspondingly increases plasmin activity on stiff matrices. IPF fibroblasts exhibit similar stiffness- and YAP/TAZ-dependent PAI-1 (SERPINE1) expression (n = 2 independent experiments). B: exogenous plasmin (0.1 U/ml) attenuates matrix stiffness-promoted procollagen I and α-smooth muscle actin (SMA) expression on stiff matrices in a fashion similar to YAP/TAZ siRNA knockdown. Plasmin also significantly reduces the proportion of fibroblasts with predominant nuclear localization of YAP/TAZ on stiff matrices; n = number of cells analyzed per condition. C: inhibition of transforming growth factor (TGF)-β receptor with ALK5 inhibitor SB431542 (1 μM) or a pan anti-TGF-β antibody (10 μg/ml) has no effect on matrix stiffness-induced PAI-1 protein production or plasmin activity decrease in IMR-90-conditioned media (n = 2 independent experiments). D: exogenous TGF-β (2 μg/ml) potentiates matrix stiffness-promoted PAI-1 gene expression. YAP/TAZ siRNA double knockdown (YAPi/TAZi) ablates TGF-β effect on stiff matrices but has no effect on soft matrices (n = 2 independent experiments).
To evaluate the functional consequences of increased plasmin levels, we added exogenous plasmin to fibroblast cultures on stiff matrices. Both procollagen I and α-smooth muscle actin (SMA) staining were dramatically reduced to levels observed on soft matrices (Fig. 6B) when exposed to exogenous plasmin. These results confirm a role for pericellular plasmin activity in suppressing profibrotic collagen and α-SMA expression and reveal one mechanism by which matrix stiffness-dependent YAP/TAZ nuclear localization produces far-reaching effects on fibroblast function through a specific transcriptional target. Treatment with exogenous plasmin also altered cell shape and reduced cell spreading (Fig. 6B), consistent with prior evidence that plasmin engages proteolytic degradation of extracellular matrix proteins and thereby alters cell-matrix adhesions (20). Plasmin treatment also partially reversed YAP/TAZ nuclear localization on stiff matrices (Fig. 6B), implicating YAP/TAZ-dependent PAI-1 expression and subsequent inhibition of plasmin in a positive feedback loop that promotes and sustains YAP/TAZ nuclear localization on stiff matrices.
SERPINE1 and its protein product PAI-1 are well-known targets of TGF-β signaling (1), which itself has previously been linked to fibroblast mechanoactivation through tension-mediated liberation of active TGF-β on stiff matrices (54). Thus we tested whether matrix stiffness effects on PAI-1/plasmin levels required TGF-β signaling. To interrupt TGF-β signaling, we used SB431542, a small-molecule inhibitor of the TGF-β type I receptor activin receptor-like kinase ALK5, or a pan-TGF-β-blocking antibody. Matrix stiffness effects on PAI-1 and plasmin levels were unchanged by ALK5 inhibition or anti-TGF-β treatment, demonstrating that the YAP/TAZ-dependent, matrix stiffness-induced changes in PAI-1/plasmin levels were independent of TGF-β receptor signaling (Fig. 6C).
When we added exogenous TGF-β to stimulate SERPINE1 expression on stiff matrices, we were able to enhance transcript levels, but this effect was also attenuated by YAP/TAZ knockdown, suggesting a supportive role for YAP/TAZ in TGF-β-induced transcriptional effects (Fig. 6D), consistent with prior evidence of functional interactions between YAP/TAZ and SMAD signals downstream of TGF-β (51). Interestingly, and unique to this study, we observed that YAP/TAZ knockdown was ineffective at suppressing TGF-β responses on soft matrices (Fig. 6D), demonstrating that the influence of YAP/TAZ on TGF-β-induced gene expression is highly dependent on mechanical context. Together, these results demonstrate that matrix stiffness and biochemical activation can act independently on PAI-1 expression. Moreover, these findings suggest that YAP/TAZ nuclear localization may be necessary for the full effects of profibrotic biochemical signaling to be seen, but only on pathologically stiff matrices. Hence, targeting YAP/TAZ function may limit, not only mechanical, but also biochemical activation of fibroblasts in a mechanical context-dependent fashion.
Active YAP and TAZ are fibrogenic in the lung.
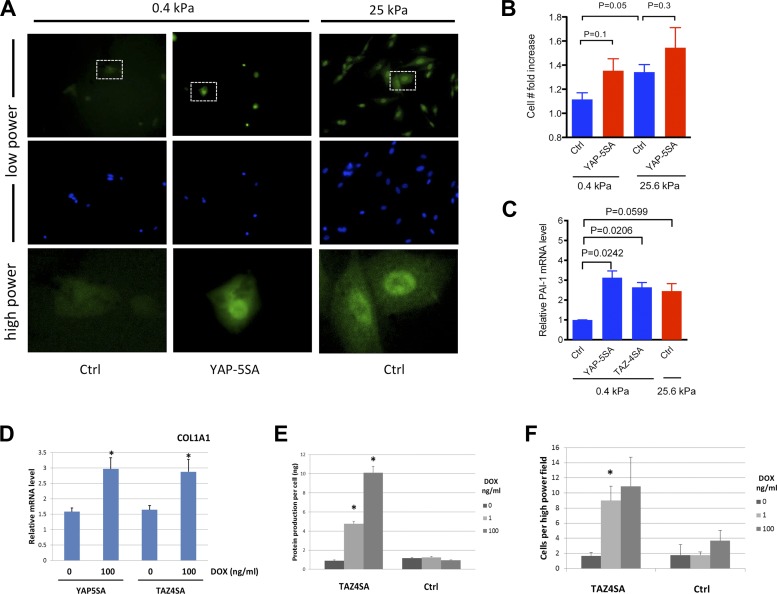
Genetic deletion of Yap in mice results in early embryonic lethality (42), whereas deletion of Taz is associated with greatly reduced survival and several developmental defects, including an emphysematous phenotype in the lungs (35). Interestingly, Taz heterozygous mice, with global inactivation of one copy of Taz, are protected from bleomycin-induced lung fibrosis (41). However, it is unclear whether this protection is attributable to abnormalities resulting from reduced TAZ function during development or to reduced TAZ function at the time of bleomycin challenge and, if the latter, whether this protection is due to reduced Taz function in fibroblasts or in other lung cell types (34, 63). Therefore, we focused our efforts on determining whether the induction of YAP or TAZ activation in fibroblasts alone is sufficient to drive the development of fibrosis in the lung. To address this question, we first generated NIH3T3 fibroblasts expressing doxycycline-inducible mutant YAP or TAZ constructs in which the serine residues targeted by LATS1/2 and necessary for cytoplasmic retention and destabilization are substituted with alanine (hereafter referred to as YAP5SA or TAZ4SA cells) (51, 61). Notably, culture of these cells on soft matrices demonstrated that the mutant proteins accumulated in the nucleus and promoted increased, although not statistically significant, proliferation along with statistically significant increases in PAI-1 (SERPINE1) transcript levels equivalent to that seen in control cells on pathologically stiff matrices (Fig. 7, A–C). These data indicate that constitutive nuclear localization of YAP or TAZ is sufficient to overcome matrix compliance limitations on fibroblast activation.
Fig. 7.
Overexpression of YAP5SA or TAZ4SA promotes fibroblast proliferation and matrix synthesis. YAP5SA-expressing NIH3T3 fibroblasts exhibit nuclear YAP localization on soft (0.4 kPa) matrices comparable to control NIH3T3 cells grown on stiff (25 kPa) matrices. In contrast, control NIH3T3 cells exhibit little nuclear localization on soft matrices. Immunostaining performed with mouse anti-YAP (63.7) antibody (Santa Cruz Biotechnology, detects both YAP and TAZ) and Hoechst 33342. All cells exposed to 100 ng/ml doxycycline (DOX) for 48 h. Dashed boxes in top row indicate areas detailed under higher power in bottom row. B: YAP5SA-expressing NIH3T3 fibroblasts exhibit a trend toward enhanced proliferative capacity on soft matrix (0.4 kPa) comparable to that of control cells on stiff matrix (25 kPa). Data shown are fold increases in cell number relative to seeding density, n = 2 independent experiments. C: YAP5SA- and TAZ4SA-expressing NIH3T3 fibroblasts exhibit increased PAI-1 (SERPINE1) transcript levels on soft matrix (0.4 kPa) comparable to control cells on stiff matrix (25 kPa), as measured by qPCR normalized to GAPDH and 0.4 kPa control (Ctrl) samples, n = 2 independent experiments. D: doxycycline-induced YAP5SA or TAZ4SA expression augments expression of COL1A1 transcripts in NIH3T3 cells cultured 48 h on 25-kPa hydrogels. *P < 0.05 compared with 0 ng/ml doxycycline, unpaired t-test. E: doxycycline-induced TAZ4SA expression increases extracellular matrix protein deposition on a per-cell basis by NIH3T3 cells on gelatin-coated coverslips. *P < 0.05 compared with NIH3T3 control cells (Ctrl) treated with same dose doxycycline. F: naïve NIH3T3 adhesion is enhanced on matrices derived from doxycycline-treated TAZ4SA cells relative to control cells (Ctrl). *P < 0.05 compared with NIH3T3 control cells treated with the same dose of doxycycline.
To evaluate the effect of YAP5SA or TAZ4SA on fibrogenic outcomes, we first measured COL1A1 transcript levels 48 h after seeding on 25-kPa hydrogels. Compared with cells cultured without doxycycline, and therefore without expression of mutant YAP or TAZ, cells expressing YAP5SA or TAZ4SA in the presence of 100 ng/ml doxycycline exhibited a nearly twofold elevation in COL1A1 transcripts (Fig. 7D), consistent with augmented matrix synthesis under the control of nuclear YAP or TAZ. As a complement to this approach, we also seeded control NIH3T3- and TAZ4SA-expressing cells on gelatin-coated coverslips, cultured them for 7 days in the absence or presence of doxycycline, and then quantified both cell number and the amount of cell-derived matrix protein deposited on the coverslips. Doxycycline induction of TAZ4SA resulted in robust, dose-dependent increases in extracellular matrix deposition on a per-cell basis but had no effect in control cells (Fig. 7E). To further evaluate the functional capacity of these cell-derived matrices, we repeated the above experimental protocol and reseeded the cell-derived matrices with naïve NIH3T3 cells. Reseeded cells were allowed to adhere for 4 h and then counted to assess their adhesion and survival. Extracellular matrices deposited by doxycycline-treated TAZ4SA cells supported significantly increased adhesion of naïve fibroblasts relative to matrices deposited by either control cells or TAZ4SA cells in the absence of doxycycline (Fig. 7F). Taken together, these findings support the concept that fibroblasts, acting under the control of nuclear TAZ, deposit both a more abundant and highly functional extracellular matrix that promotes fibroblast adhesion and survival.
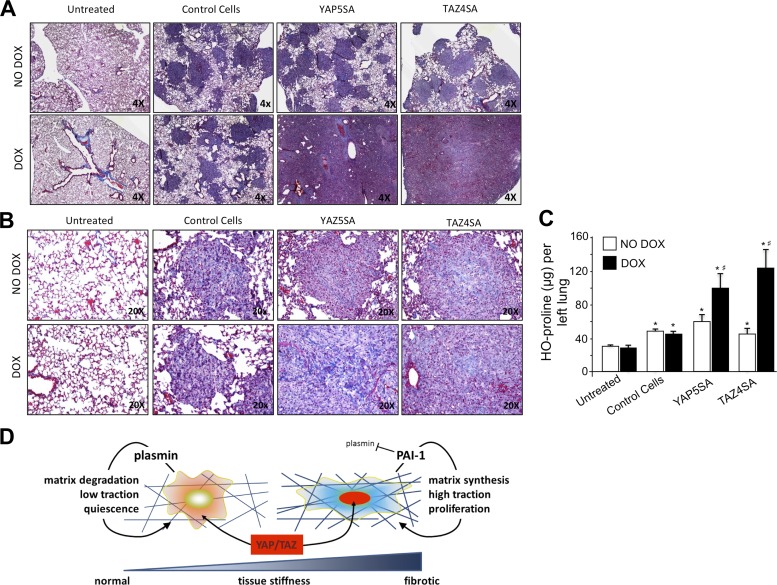
To evaluate the function of these cells in the lung, we employed an adoptive cell transfer model that has previously been shown to distinguish the fibrogenic potential of fibroblasts in the murine lung (43, 46, 50). We injected YAP5SA, TAZ4SA, or control NIH3T3 cells into the tail vein of 6–10-wk-old immunocompromised mice, followed by feeding with or without doxycycline at 1 mg/ml in the drinking water. Both control NIH3T3 cells, which have undergone spontaneous immortalization, and YAP5SA- and TAZ4SA-transduced NIH3T3 cells were able to survive in the lung and form cellular nodules even in the absence of doxycycline-induced transgene expression (Fig. 8, A and B), likely reflecting the robust survival capacity of these immortalized cells and the receptive host environment provided in these severely immunocompromised mice. However, in the absence of doxycycline, we observed little fibrogenic response, as measured by hydroxyproline content of the left lung (Fig. 8C). In contrast, doxycycline-induced expression of YAP5SA or TAZ4SA resulted in profound increases in lung cellularity and abundant matrix deposition evident in tissues stained with Masson's trichrome (Fig. 8B) and confirmed by significant increases in lung hydroxyproline (Fig. 8C). These results demonstrate that overexpression of mutant, nuclear-localizing YAP or TAZ is sufficient to confer a profound increase in fibrogenic potential to cells. In combination with the evidence provided here that YAP and TAZ are abundantly expressed in remodeled regions of the fibrotic lung, and that YAP/TAZ control mechanical stiffness-induced fibroblast activation, these results support a model in which YAP and TAZ activation plays a pivotal role in the mechanical feedback loop that amplifies and sustains fibrosis (Fig. 8D).
Fig. 8.
Conditional overexpression of YAP5SA or TAZ4SA confers fibrogenic potential to fibroblasts adoptively transferred to mouse lung. A: adoptive transfer of control 3T3 fibroblasts or 3T3 fibroblasts expressing doxycycline-inducible YAP5SA or TAZ4SA to immunocompromised mice results in cellular nodule formation in the lungs evident under low magnification in Masson's trichrome-stained tissue sections. Feeding with doxycycline dramatically increases nodular lung fraction in mice with YAP5SA- or TAZ4SA-expressing cells. B: higher magnification images from Masson's trichrome-stained sections demonstrate collagen deposition in nodules from doxycycline-fed mice with YAP5SA- or TAZ4SA-expressing cells. C: robust increases in lung hydroxyproline were observed in doxycycline-fed mice with YAP5SA- or TAZ4SA-expressing cells relative to no doxycycline feeding and relative to doxycycline-fed mice with control 3T3 cells, demonstrating the fibrogenic capacity of adoptively transferred cells expressing YAP5SA or TAZ4SA. *P < 0.05 relative to Untreated, #P < 0.05 between No Dox and Dox within same group. D: schematic illustrates key findings of this work: increasing matrix stiffness promotes nuclear YAP/TAZ localization, shifting fibroblasts from a relatively quiescent state to a more activated fibrogenic and matrix-remodeling state, supported in part by upregulation of PAI-1 and attenuation of pericellular plasmin activity.
DISCUSSION
Recent evidence indicates that the pathological matrix is central to the persistent fibroblast activation that drives progressive fibrosis (6, 45). Identifying mechanisms that coordinate fibroblast responses to the matrix is thus a high priority. We present several lines of evidence implicating YAP and TAZ as central regulators of pathological fibroblast activation in lung fibrosis. In human IPF lung tissue, YAP and TAZ are abundantly expressed in spindle-shaped, fibroblastic cells, with particularly prominent nuclear localization of TAZ (Fig. 1). These areas of nuclear TAZ localization are associated with a stiffened extracellular matrix, and increasing matrix stiffness drives nuclear localization of YAP and TAZ in fibroblasts in culture (Fig. 2). On matrices of defined stiffness spanning the normal and pathological range, YAP and TAZ siRNA knockdown dramatically attenuates profibrotic matrix synthesis, contraction, and proliferation and does so preferentially on pathologically stiffened matrices (Figs. 4 and 5). Such results are consistent with matrix stiffness-dependent transcriptional profiles in human fibroblasts, which exhibit highly significant overrepresentation of YAP/TAZ target genes (Table 1). Finally, fibroblasts transduced with constitutively active YAP or TAZ mutant constructs overcome soft matrix limitations on growth and display augmented matrix synthesis (Fig. 7); adoptive transfer of these fibroblasts in mice results in profound remodeling and fibrosis in the lungs (Fig. 8), demonstrating that nuclear YAP/TAZ localization is sufficient to confer potent fibrogenic potential to fibroblasts. Several of these findings are entirely novel, including, most notably, our demonstration that YAP and TAZ loss of function through siRNA knockdown attenuates key fibrogenic activities in IPF fibroblasts and that YAP or TAZ gain of function is sufficient to promote fibrogenesis both in vitro and in vivo.
Our results both echo and contrast with recent observations of YAP-mediated mechanoregulation of cancer-associated fibroblasts (CAFs) (9). In agreement with our findings, CAFs exhibited matrix stiffness-enhanced matrix remodeling and contractile functions; however, CAFs did so predominantly under the influence of YAP rather than TAZ nuclear localization. In contrast, our results demonstrate prominent TAZ nuclear localization in lung fibroblast activation and sufficiency of either YAP or TAZ to drive a fibrogenic response, suggesting overlapping or redundant function of YAP and TAZ in lung fibroblasts in the development of pulmonary fibrosis. Moreover, our study demonstrates that disease-derived IPF fibroblasts in culture are indistinguishable from normal lung-derived fibroblasts in their levels of YAP/TAZ expression and remain fully able to reverse YAP and TAZ localization and function when cultured on physiologically soft matrices. These findings are distinct from observations in CAFs, in which persistent upregulation and constitutive activation of YAP are seen in a disease progression-associated manner (9). Taken together, our findings and those in CAFs broadly implicate YAP/TAZ mechanoregulation in fibroblast activation and matrix remodeling and suggest that further dissection of the similarities and differences in CAFs and IPF fibroblasts may help to elucidate overlapping and distinct mechanisms controlling activation of these cell types. One key takeaway from our studies is the demonstration that either normalizing matrix mechanical properties or interrupting pathological matrix signaling through YAP/TAZ appears sufficient to reverse key aspects of fibroblast activation and pathological cell function. One caveat of our work is that all fibroblasts were cultured and serially passaged on rigid matrices before transfer to hydrogels for experiments; we are thus unable to assess the role mechanical memory may have played in influencing our results (3, 57). Nevertheless, the robust reversal of YAP and TAZ nuclear localization by relatively short-term culture on physiologically soft matrices suggests that lung fibroblasts, even those derived from pathologically stiffened lungs, retain the capacity to respond appropriately to changes in their mechanical environment.
YAP and TAZ contribute to expression of a wide array of transcripts, including a coherent panel of genes associated with the contractile actomyosin cytoskeleton (9, 62). Our results extend these findings by demonstrating a key role for the YAP/TAZ target gene SERPINE1 (encoding PAI-1), through its regulation of plasmin, in controlling matrix stiffness-dependent fibroblast activation (Fig. 6). PAI-1 has numerous profibrotic functions (16) and has previously been shown to regulate FN degradation and matrix adhesion of lung fibroblasts through its effect on pericellular plasmin activity (20). Our results confirm that PAI-1-dependent plasmin inhibition on stiff matrices is necessary for expression of α-SMA and procollagen I, two hallmarks of activation of these cells, and that addition of exogenous plasmin reverses these changes while also partially reversing YAP and TAZ nuclear localization back to the cytoplasm. Thus our findings are consistent with opposing self-sustaining feedback loops in which soft matrices promote pericellular matrix proteolysis and cytoplasmic YAP/TAZ, while stiff matrices promote YAP/TAZ nuclear localization and PAI-1 expression to inhibit plasmin-dependent proteolysis (Fig. 8D). Interestingly, matrix stiffness effects on PAI-1 levels were independent of TGF-β signaling, demonstrating one way in which matrix stiffness can sustain a profibrotic state independent of this key fibrogenic cytokine. It has been shown previously that matrix stiffness can also, over extended periods, promote fibroblast activation of TGF-β (53), potentially amplifying the PAI-1 response noted here and further modulating matrix stiffness-dependent patterns of fibroblast gene expression (32). Interestingly, we observed that knockdown of YAP and TAZ expression was sufficient to disrupt TGF-β-augmented expression of PAI-1 and did so selectively on a pathologically stiff matrix. Hence, YAP/TAZ targeting in vivo may provide pleiotropic benefits by inhibiting matrix stiffness-dependent activation of fibroblasts while also blocking profibrotic biochemical signaling in areas of remodeled and stiffened matrix.
Taken together, our results identify YAP and TAZ as key regulators of fibroblast mechanoactivation and fibrogenic function. The localization of these factors in the lungs of patients with IPF and the profound fibrogenic potential of fibroblasts expressing active YAP and TAZ in the adoptive transfer model support a central role for these factors in driving fibrogenesis in vivo. Our findings are consistent with and build on earlier work demonstrating that mice haploinsufficient for Taz expression are protected from bleomycin-induced pulmonary fibrosis (41). In that work, global Taz haploinsufficiency prevented ascribing attenuated fibrosis to fibroblast function alone; here, we show for the first time that fibroblast-expressing active YAP or TAZ are sufficient to drive a fibrogenic program in the lungs. Finally, the conservation of YAP/TAZ responsiveness in IPF fibroblasts confirms the attractiveness of these transcription coactivators as potential targets for therapy, as RNAi knockdown of YAP and TAZ expression was sufficient to significantly attenuate key functions including proliferation, traction forces, and profibrotic gene and protein expression. An important goal for future work will be to evaluate the relative roles of YAP and TAZ in fibrosis and compare their potency relative to other mechanoactivated pathways, including Rho, serum response factor, and MRTF (23, 48), which share multiple downstream transcriptional targets with YAP and TAZ (13). Further elucidation of upstream and downstream components of the YAP and TAZ mechanoactivation pathway and development of strategies for targeting YAP and TAZ function in fibroblasts in vivo may provide attractive new strategies for intervening in the progression of fibrotic pathologies.
GRANTS
This work was supported by NIH HL092961 and HL113796 (D. Tschumperlin), NIH HL095732 and HL108975 (A. Tager), support from the Concern Cancer Foundation and NIH DK046200 (X. Varelas), NIH HL105489 (J. Horowitz), NIH HL078871 (T. Sisson), and NIH HL115106 and HL114839 (L. Fredenburgh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.L., T.H.S., J.C.H., I.O.R., L.E.F., C.F.-B., X.V., A.M.T., and D.J.T. conception and design of research; F.L., D.L., K.M.C., L.S., A.M., V.V., C.K.P., and S.E.H. performed experiments; F.L., D.L., K.M.C., L.S., A.M., X.V., A.M.T., and D.J.T. analyzed data; F.L., D.L., K.M.C., L.S., A.M., T.H.S., J.C.H., L.E.F., C.F.-B., X.V., A.M.T., and D.J.T. interpreted results of experiments; F.L., D.L., K.M.C., L.S., A.M., and D.J.T. prepared figures; F.L. and D.J.T. drafted manuscript; F.L., D.L., L.S., T.H.S., J.C.H., X.V., A.M.T., and D.J.T. edited and revised manuscript; F.L., D.L., K.M.C., L.S., A.M., V.V., C.K.P., S.E.H., T.H.S., J.C.H., I.O.R., L.E.F., C.F.-B., X.V., A.M.T., and D.J.T. approved final version of manuscript.
REFERENCES
- 1.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154: 1047–1059, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 4: 410–421, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16: 1009–1017, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol 10: 19, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186: 866–876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AC, Fiore VF, Sulchek TA, Barker TH. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J Pathol 229: 25–35, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler JP, Nakamura M, Sasaki H, Sasaki T, Takishima T. Poissons' ratio of lung parenchyma and parenchymal interaction with bronchi. Jpn J Physiol 36: 91–106, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15: 637–646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang-Tsai S, Sisson TH, Hattori N, Tsai CG, Subbotina NM, Hanson KE, Simon RH. Reduction in fibrotic tissue formation in mice genetically deficient in plasminogen activator inhibitor-1. Am J Pathol 163: 445–452, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugina V, Fontao L, Chaponnier C, Vasiliev J, Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci 114: 3285–3296, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Esnault C, Stewart A, Gualdrini F, East P, Horswell S, Matthews N, Treisman R. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev 28: 943–958, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5: 167sr161, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 293: G1147–G1154, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol 227: 493–507, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172: 259–268, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 175: 3–16, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattori N, Mizuno S, Yoshida Y, Chin K, Mishima M, Sisson TH, Simon RH, Nakamura T, Miyake M. The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism. Am J Pathol 164: 1091–1098, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol 38: 78–87, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SK, Fisher AS, Scruggs AM, White ES, Hogaboam CM, Richardson BC, Peters-Golden M. Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol 177: 2245–2255, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang WT, Vayalil PK, Miyata T, Hagood J, Liu RM. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am J Respir Cell Mol Biol 46: 87–95, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janmey PA, Wells RG, Assoian RK, McCulloch CA. From tissue mechanics to transcription factors. Differentiation 86: 112–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson LA, Rodansky ES, Sauder KL, Horowitz JC, Mih JD, Tschumperlin DJ, Higgins PD. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis 19: 891–903, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 10: 945–963, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J 19: 6778–6791, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science 318: 426–430, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 28: 2426–2436, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208: 1459–1471, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 46: 1246–1256, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp 28: 2911, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell 30: 137–150, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol 294: F542–F553, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Marinkovic A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 48: 422–430, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinkovic A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol 303: L169–L180, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauviel A, Nallet-Staub F, Varelas X. Integrating developmental signals: a Hippo in the (path)way. Oncogene 31: 1743–1756, 2012. [DOI] [PubMed] [Google Scholar]
- 39.McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition: Fab' to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol 92: 485–492, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mih JD, Sharif AS, Liu F, Marinkovic A, Symer MM, Tschumperlin DJ. A multiwell platform for studying stiffness-dependent cell biology. PLoS One 6: e19929, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitani A, Nagase T, Fukuchi K, Aburatani H, Makita R, Kurihara H. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am J Respir Crit Care Med 180: 326–338, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol 26: 77–87, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, Flaherty KR, Lee J, Bell M, Knight DA, Martinez FJ, Sleeman MA, Herzog EL, Hogaboam CM. Targeting IL-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID IPF model. Am J Respir Cell Mol Biol 50: 985–994, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 122: 2756–2762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Flaherty KR, Martinez FJ, Hogaboam CM. Therapeutic targeting of CC ligand 21 or CC chemokine receptor 7 abrogates pulmonary fibrosis induced by the adoptive transfer of human pulmonary fibroblasts to immunodeficient mice. Am J Pathol 170: 1152–1164, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol 166: 399–407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandbo N, Kregel S, Taurin S, Bhorade S, Dulin NO. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-beta. Am J Respir Cell Mol Biol 41: 332–338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14: 45–54, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Trujillo G, Meneghin A, Flaherty KR, Sholl LM, Myers JL, Kazerooni EA, Gross BH, Oak SR, Coelho AL, Evanoff H, Day E, Toews GB, Joshi AD, Schaller MA, Waters B, Jarai G, Westwick J, Kunkel SL, Martinez FJ, Hogaboam CM. TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci Transl Med 2: 57ra82, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10: 837–848, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development 138: 3907–3914, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J 18: 2551–2562, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater 13: 645–652, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150: 780–791, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao B, Li L, Guan KL. Hippo signaling at a glance. J Cell Sci 123: 4001–4006, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 24: 862–874, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J. YAP tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell 30: 151–165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123: 1096–1108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]