Abstract
TNF-like weak inducer of apoptosis (TWEAK) is a growth factor for bipotent liver progenitors that express its receptor, fibroblast growth factor-inducible 14 (Fn14), a TNF receptor superfamily member. Accumulation of Fn14+ progenitors occurs in severe acute alcoholic steatohepatitis (ASH) and correlates with acute mortality. In patients with severe ASH, inhibition of TNF-α increases acute mortality. The aim of this study was to determine whether deletion of Fn14 improves the outcome of liver injury in alcohol-consuming mice. Wild-type (WT) and Fn14 knockout (KO) mice were fed control high-fat Lieber deCarli diet or high-fat Lieber deCarli diet with 2% alcohol (ETOH) and injected intraperitoneally with CCl4 for 2 wk to induce liver injury. Mice were euthanized 3 or 10 days after CCl4 treatment. Survival was assessed. Liver tissues were analyzed for cell death, inflammation, proliferation, progenitor accumulation, and fibrosis by quantitative RT-PCR, immunoblot, hydroxyproline content, and quantitative immunohistochemistry. During liver injury, Fn14 expression, apoptosis, inflammation, hepatocyte replication, progenitor and myofibroblast accumulation, and fibrosis increased in WT mice fed either diet. Mice fed either diet expressed similar TWEAK/Fn14 levels, but ETOH-fed mice had higher TNF-α expression. The ETOH-fed group developed more apoptosis, inflammation, fibrosis, and regenerative responses. Fn14 deletion did not reduce hepatic TNF-α expression but improved all injury parameters in mice fed the control diet. In ETOH-fed mice, Fn14 deletion inhibited TNF-α induction and increased acute mortality, despite improvement in liver injury. Fn14 mediates wound-healing responses that are necessary to survive acute liver injury during alcohol exposure.
Keywords: alcohol, liver injury, liver fibrosis, liver progenitors
fibroblast growth factor -inducible 14 (Fn14) is a tumor necrosis factor (TNF) superfamily receptor for TNF-like weak inducer of apoptosis (TWEAK) (12). TWEAK is expressed by macrophages (3) and acts as mitogen for Fn14+ liver progenitor cells (3, 12, 30). Although TWEAK-Fn14 interaction promotes expansion of progenitor populations that are involved in liver regeneration (13), it also induces fibrosis in many organs, including liver, kidney, and heart (15, 22, 31). Moreover, TWEAK-Fn14 pathway activation stimulates proinflammatory responses in many autoimmune and inflammatory diseases (6).
Fn14 is barely detectable in healthy adult livers, but induction of Fn14 expression has been reported in many types of liver injury. For example, partial hepatectomy triggers dramatically increased Fn14 mRNA and protein expression within a few hours (8, 13, 23). Hepatic Fn14 expression is also upregulated in other experimental liver injuries, such as that induced by CCl4, a 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet, acetaminophen, and choline-deficient, ethionine-supplemented diets (1, 30). Moreover, induction of Fn14 expression is observed in chronic human liver diseases, including nonalcoholic steatohepatitis, alcoholic liver disease, chronic hepatitis C (12), and hepatocellular carcinoma (8, 32).
In their recent transcriptome analysis, Affo et al. (1) showed that Fn14 is relatively overexpressed in patients with alcoholic steatohepatitis (ASH) compared with other liver diseases. In patients with ASH, Fn14 mRNA expression correlated with acute mortality, prompting Affo et al. to suggest that Fn14 might be a therapeutic target in severe ASH. More effective treatments for ASH are definitely needed, because the current 30-day mortality in patients who require hospitalization for severe ASH is ∼30% and more than half of the surviving patients die within 1 yr of discharge (17). Histologically, severe ASH is characterized by zone 3-predominant hepatocellular injury (ballooned hepatocytes), steatosis, and intrasinusoidal fibrosis (16). Although TNF-α produced by Kupffer cells is believed to play a critical role in the pathogenesis of ASH, the mechanisms underlying this disease are poorly understood (2). The purpose of this study is to determine if abrogation of Fn14 signaling improved the outcomes of severe acute ASH in a mouse model.
MATERIALS AND METHODS
Reagents.
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless stated otherwise.
Animal experiments.
Male wild-type (WT) C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). The Fn14 knockout (KO) mouse strain was provided by Biogen Idec. Animal surgery was performed and care was provided according to Duke University Institutional Animal Care and Use Committee-approved protocol (A007-13-01) as set forth in the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.”
Liver injury was established using a model of acute alcohol-related fibrosing steatohepatitis (ASH) reported by Nagy and colleagues (24). Male WT or Fn14 KO mice at 8- to 10 wk of age were fed control high-fat Lieber deCarli or 2% alcohol-supplemented high-fat Lieber deCarli (ETOH) diet and then injected with CCl4 (1 μl/g body wt ip, prediluted 1:3 in olive oil) or vehicle twice per week for 2 wk. Mice were euthanized 72 h after the last CCl4 injection to determine acute effects of Fn14 on liver injury. Seventeen additional WT and Fn14 KO mice were fed chow diets for 10 days after completion of 2 wk of ETOH + CCl4 treatment and then euthanized to assess how deletion of Fn14 impacted recovery from ASH. To evaluate the effects of treatment on proliferative activity, nine WT and nine Fn14 KO mice were routinely injected with bromodeoxyuridine (BrdU, 50 μg/g body wt ip) 2 h before they were euthanized. A more detailed list of the experimental groups is provided in Table 1.
Table 1.
Detailed usage of mice in experiments
| Before Treatment |
After Treatment (Analyzed) |
|||
|---|---|---|---|---|
| Treatment Group | WT | Fn14 KO | WT | Fn14 KO |
| C | 5 | 3 | 5 | 3 |
| ETOH | 4 | 3 | 4 | 3 |
| C + CCl4 | 4 | 3 | 4 | 3 |
| ETOH + CCl4* | 14 | 16 | 14 | 13 |
| ETOH + CCl4 + 10 days off | 10 | 7* | 10 | 4 |
| Total number | 37 | 32 | 37 | 26 |
WT, wild-type; Fn14 KO, fibroblast growth factor-inducible 14 knockout; C, control (high-fat Lieber DiCarli diet); ETOH, high-fat Lieber DiCarli diet with 2% alcohol. In the ETOH + CCl4 treatment group, 9 WT and 9 Fn14 KO mice received bromodeoxyuridine.
All mice died within 2 wk. No mice died during the 10-day recovery period.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded liver tissues were cut into 5-μm-thick sections and mounted on glass slides. Sections were deparaffinized with xylene, dehydrated with ethanol, and then incubated for 10 min in 3% hydrogen peroxide to block endogenous peroxidase. For antigen retrieval, the sections were heated in 10 mM sodium citrate buffer (pH 6.0) for 10 min or incubated with pepsin (catalog no. 00-3009, Invitrogen) for 5 min. Nonspecific binding was inhibited by 15 min of incubation in protein block (catalog no. X9090, Dako). Sections were then incubated at 4°C overnight with primary antibodies: Fn14 (catalog no. 314102, Biolegend), desmin (catalog no. ab6322, Abcam), α-smooth muscle actin (αSMA; catalog no. ab32575, Abcam), α-fetoprotein (αFP; catalog no. A0008, Dako), pan-cytokeratin (pan-CK; catalog no. 180132, Zymed), LGR5 (catalog no. TA301323, Origene), Sox9 (catalog no. 5535, Millipore), Ki67 (catalog no. M7249, Dako), proliferating cell nuclear antigen (PCNA; catalog no. sc7907, Santa Cruz Biotechnology), F4/80 (catalog no. MCA497GA, AbD Serotec), and horseradish peroxidase-conjugated anti-rabbit (catalog no. K4003, Dako). Secondary antibodies were used to visualize target proteins. 3,3′-Diaminobenzidine reagent (catalog no. K3466, Dako) was applied in the detection procedure. Terminal deoxynucleotidyl transferase dUTP nick end label (TUNEL) staining was performed following the manufacturer's instructions (Roche). The proportions of tissue stained with Sirius red, αSMA, desmin, pan-CK, LGR5, αFP, and F4/80 were assessed by morphometric analysis using MetaView software (Universal Imaging, Downington, PA) in 10 randomly chosen ×20 fields per section per mouse, as described elsewhere (19). TUNEL, Ki67, PCNA, BrdU, and Sox9 staining was quantified by counting the numbers of hepatocytes with stained nuclei in 10 randomly chosen ×10 fields per section per mouse.
mRNA quantification by real-time RT-PCR.
Total RNA was isolated using commercial reagents (TRIzol, Invitrogen). RNA concentration and purity were determined with a spectrophotometer (model ND-1000, NanoDrop Technologies, Palo Alto, CA), and samples with a 260 nm-to-280 nm absorbance ratio >1.8 were used in subsequent analyses. RNA was quantified by RT-PCR as described elsewhere (34). All samples were analyzed in duplicate. Gene expression levels were normalized to the reference gene S9, and fold change was calculated by the comparative threshold (2−ΔΔCt) method. Sequences of primers are listed in Table 2.
Table 2.
Sequence of mouse primers
| Sequence |
||
|---|---|---|
| Product | Forward | Reverse |
| Collagen 1α1 | GAGCGGAGAGTACTGGATCG | GCTTCTTTTCCTTGGGGTTC |
| Desmin | TACACCTGCGAGATTGATGC | ACATCCAAGGCCATCTTCA |
| Fn14 | GTGTTGGGATTCGGCTTGGT | CCAGGCAGAAGTCGCTGTG |
| IL-6 | AAAGCCAGAGTCCTTCAGAGAGATACAG | ATGAATTGGATGGTCTTGGTCCTTAG |
| S9 | GACTCCGGAACAAACGTGAGGT | CTTCATCTTGCCCTCGTCCA |
| αSMA | GATGAAGCCCAGAGCAAGAG | CTTTTCCATGTCGTCCCAGT |
| TNF-α | TCGTAGCAAACCACCAAGTG | AGATAGCAAATCGGCTGACG |
| LGR5 | GGATTCCACAGCAACAACATCAG | CGAGGCACCATTCAAAGTCAGTG |
αSMA, α-smooth muscle actin.
Western blot analysis.
Protein extracts were prepared by homogenization of liver tissue in RIPA buffer (catalog no. R0278, Sigma) and quantified (Pierce). Proteins were visualized by Western blot analysis using the primary antibodies Fn14 (Abcam) and β-actin (Santa Cruz Biotechnology).
Hydroxyproline assay.
Liver hydroxyproline content was quantified in flash-frozen liver samples, as described elsewhere (27). Concentrations were calculated from a standard curve prepared with high-purity hydroxyproline (Sigma-Aldrich) and expressed as milligrams of hydroxyproline per gram of liver.
Assessment of hepatic injury.
Serum alanine aminotransferase levels were measured using commercially available kits (Biotron Diagnostics) according to the manufacturer's instructions.
Statistical analysis.
Values are means ± SE from at least three animals per group (see Table 1 for details). Student's t-test was used for comparisons between two groups, and one- or two-way ANOVA followed by Tukey's multiple-comparison post hoc test was used for comparisons among more than two groups. Survival was calculated using log-rank analysis. P < 0.05 was considered to be statistically significant.
RESULTS
Subacute liver injury triggers hepatic accumulation of Fn14+ cells.
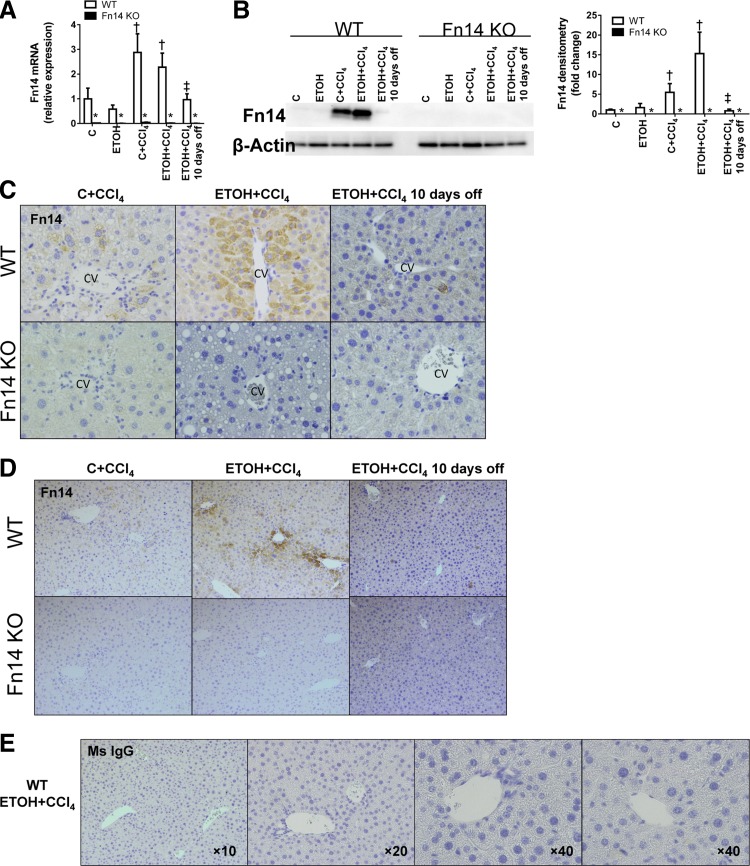
To determine if alcohol ingestion influenced changes in Fn14 expression during liver injury, we performed quantitative RT-PCR, Western blot analysis, and immunohistochemistry on livers from mice that were treated with CCl4 while being fed the control or the ETOH diet. Results were compared with those from mice fed the same diets but treated with vehicle. Expression of Fn14 mRNA and protein was barely detectable in vehicle-treated mice fed either diet. Regardless of alcohol consumption, CCl4 strongly induced Fn14 expression. Moreover, when CCl4 was withdrawn and chow diets were resumed, Fn14 expression fell back to baseline levels (Fig. 1, A, B, and D). Thus, subacute liver injury provided a strong stimulus for Fn14 induction, and, at these levels of ingestion, alcohol did not appreciably alter injury-related increases in hepatic Fn14 expression.
Fig. 1.
Subacute liver injury triggers hepatic accumulation of fibroblast growth factor-inducible 14 (Fn14)-positive (Fn14+) cells. Healthy adult wild-type (WT) mice fed control, high-fat Lieber deCarli diet (C) or high-fat Lieber deCarli diet with 2% alcohol (ETOH) were treated with CCl4 for 2 wk and euthanized at the time points described in materials and methods. Expression of hepatic Fn14 was evaluated by quantitative RT-PCR analysis (normalized to the housekeeping gene S9; A), representative Western blot analysis for Fn14 (normalized to β-actin loading control; B, left), and quantification of Western blot analysis by densitometry (B, right) and representative immunohistochemistry (C; magnification ×40). To ensure reagent specificity, similar analysis was performed in mice that were genetically deficient in Fn14 [Fn14 knockout (KO)]. CV, central vein; PV, portal vein. D: representative immunohistochemistry of Fn14 in liver sections from WT and Fn14 KO mice at lower magnification (original magnification ×10). E: representative images from immunohistochemistry of a mouse IgG (Ms IgG) control on liver sections from WT mice fed ETOH diet and treated with CCl4. *P < 0.05 vs. WT. †P < 0.05 vs. C. ‡P < 0.05 vs. ETOH + CCl4.
Immunostaining with anti-Fn14 antibody or control IgG demonstrated that a subpopulation of nonnecrotic hepatocytes in CCl4-treated WT mice specifically expressed Fn14 (Fig. 1, C–E, see Figs. 7A and 8B), whereas no Fn14 mRNA or protein was detected in Fn14-deficient (Fn14 KO) CCl4-treated mice. Since ETOH feeding alone was insufficient to induce significant Fn14 expression, we chose to study the effects of Fn14 deletion on the liver injury induced by exposure to the control diet + CCl4 or the ETOH diet + CCl4.
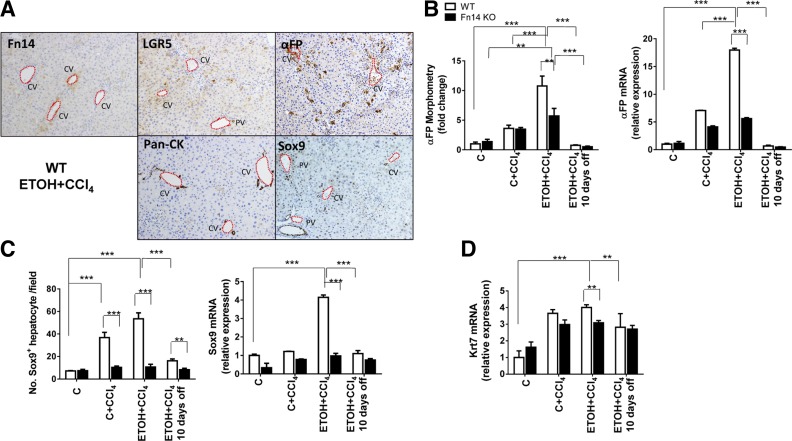
Fig. 7.
Deletion of Fn14 impairs liver progenitor expansion. A: representative immunohistochemistry of Fn14, LGR5, α-fetoprotein (αFP), pan-CK, and Sox9 in liver sections from WT mice fed ETOH diet and treated with CCl4 for 2 wk. Original magnification ×10. CV, central vein; PV, portal vein. B–D: morphometry data and mRNA expression for αFP (B) and Sox9 (C) and mRNA expression for keratin 7 (Krt7; D) for livers from WT and Fn14 KO mice. Results are expressed as fold change compared with control. Results were normalized to the housekeeping gene S9. All comparisons were calculated by 2-way ANOVA followed by Tukey's multiple-comparison post hoc test: **P < 0.01, ***P < 0.001.
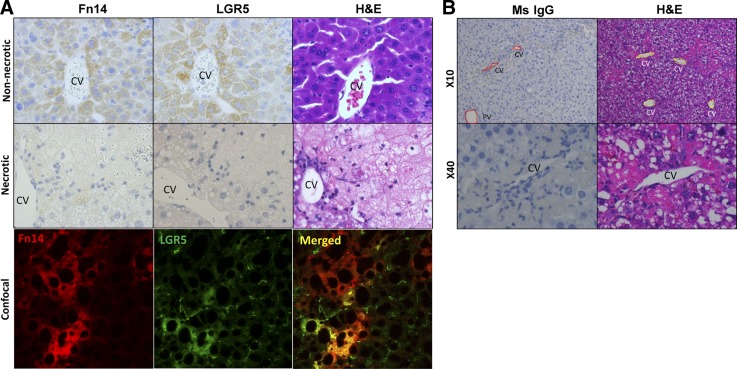
Fig. 8.
Liver Fn14+ cells coexpress LGR5. A: serial section staining for Fn14, LGR5, and hematoxylin-eosin (H&E) from nonnecrotic and necrotic regions in WT mice after injury induced by CCl4 and ETOH (top and middle) and confocal image of Fn14 and LGR5 in injured liver (bottom). Original magnification, ×40. B: representative images from immunohistochemistry of a mouse IgG (Ms IgG) control for Fn14 and hematoxylin-eosin on necrotic area in liver sections from WT mice fed ETOH diet and treated with CCl4. CV, central vein; PV, portal vein.
Deletion of Fn14 increases mortality, despite improving liver injury.
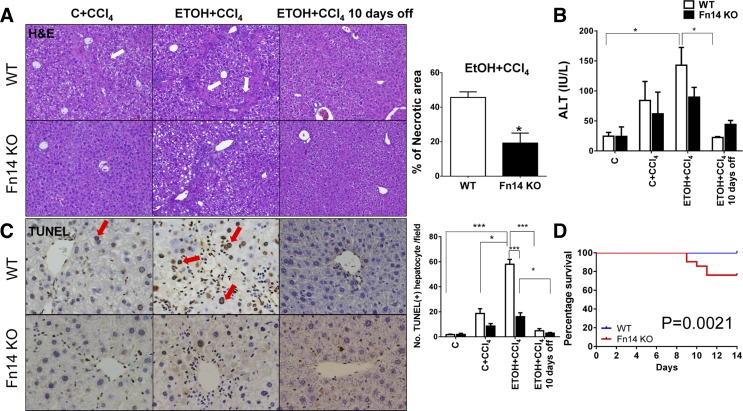
In WT mice, CCl4 induced necrosis in the liver. Consistent with findings reported by others (24), a greater degree of acute injury was noted when alcohol was present in the diet of animals treated with CCl4 (Fig. 2A). The histochemical findings demonstrating more extensive hepatocellular injury in the ETOH + CCl4 group correlated with elevations in serum alanine aminotransferase (ALT; Fig. 2B). Similarly treated Fn14 KO mice developed less histological necrosis and inflammation than WT mice (Fig. 2A). Consistent with decreased injury on hematoxylin-eosin staining, TUNEL staining demonstrated that ETOH + CCl4 induced greater hepatocyte apoptosis than CCl4 alone, while Fn14 deletion significantly reduced the number of apoptotic hepatocytes relative to WT animals (Fig. 2C). Despite the decrease in overall injury, approximately one-quarter of the Fn14 KO mice that were treated with ETOH + CCl4 died during the treatment period, while all the other Fn14 KO mice survived, as did all the WT mice, including the ETOH + CCl4 group (Fig. 2D). Thus, Fn14 deletion selectively increased liver injury-related mortality in ETOH-consuming mice. However, Fn14 KO and WT mice that survived acute liver injury and were examined 10 days after discontinuation of ETOH + CCl4 were similar with regard to hepatic hematoxylin-eosin staining, serum ALT, and hepatocyte apoptosis markers (Fig. 2, A–C). Furthermore, no surviving mice in either group died during the recovery period.
Fig. 2.
Deletion of Fn14 increases mortality, despite improving liver injury. A–D: liver histology (A), serum alanine aminotransferase (ALT) levels (B), terminal deoxynucleotidyl transferase dUTP nick end label (TUNEL) staining [red arrows indicate TUNEL+ cells; C], and survival (D) in WT and Fn14 KO mice fed control or ETOH diet and treated with intraperitoneal injections of CCl4 for 2 wk. A: hematoxylin-eosin stained sections of livers from WT mice fed ETOH diet and treated with CCl4 and similarly treated Fn14 KO mice. Percent necrosis was quantified from 50 randomly chosen fields per section in ETOH + CCl4 groups. Comparisons for ALT and TUNEL+ hepatocytes per field were caculated by 2-way ANOVA followed by Tukey's multiple-comparison post hoc test: *P < 0.05, ***P < 0.001. Significance for survival curve was calculated using log-rank test.
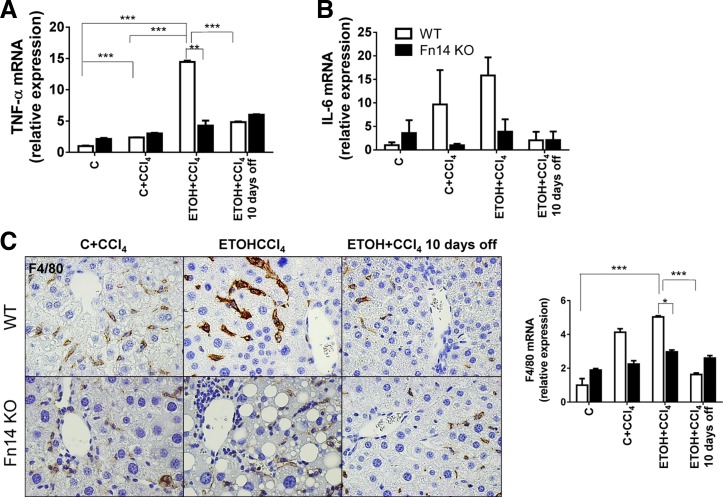
Deletion of Fn14 reduces proinflammatory cytokine expression.
Severe ASH in humans is associated with dramatic induction of proinflammatory cytokines, such as TNF-α (10). TNF-α plays a key role in the pathogenesis of ETOH-mediated liver injury in rodent models (35). However, neutralization of TNF-α increased acute mortality in patients with severe acute ASH (21). To determine if Fn14 influenced inflammatory responses to liver injury in our models, we evaluated whole liver expression of two key inflammatory cytokines, TNF-α and interleukin-6 (IL-6), and one macrophage-specific marker, F4/80.
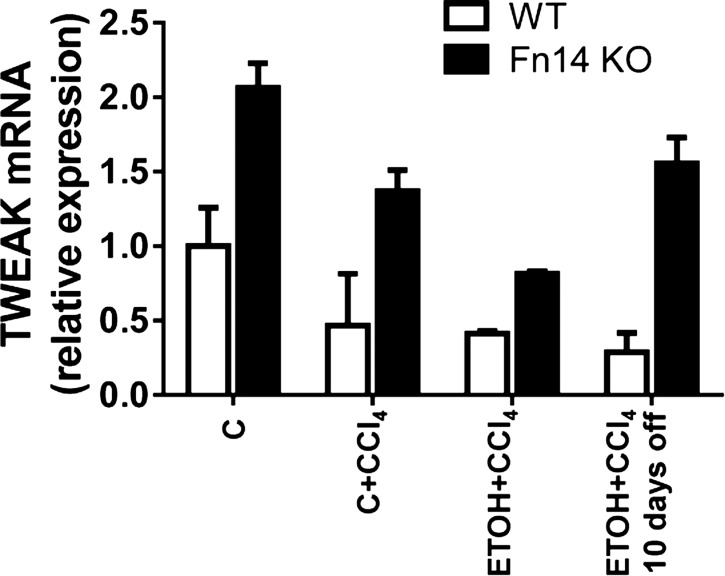
CCl4 treatment increased mRNA levels of both cytokines in WT mice. ETOH-consuming mice demonstrated severalfold greater induction of TNF-α and IL-6 than mice fed the control diet. Fn14 deletion did not prevent TNF-α mRNA upregulation in mice fed the control diet and treated with CCl4 but abrogated the additive effects of ETOH on TNF-α induction during liver injury (Fig. 3, A and B). Fn14 is a member of the TNF receptor superfamily, and its natural ligand is TWEAK. Unlike TNF-α, hepatic expression of TWEAK did not increase during CCl4 treatment in any of the WT mice. Indeed, Fn14 deletion increased (rather than decreased) TWEAK mRNA levels in all groups (Fig. 4). Fn14 deletion inhibited induction of IL-6 in mice fed the ETOH or the control diet, further suggesting that the inhibitory effects of Fn14 deletion on TNF-α expression in ETOH-fed mice were specific (Fig. 3, A and B). Also, although ETOH diet-fed mice tended to accumulate more F4/80+ cells than control diet-fed mice during liver injury, Fn14 deletion inhibited CCl4-related accumulation of macrophages similarly in both groups of mice (Fig. 3C). The aggregate data suggest that induction of TNF-α during ETOH-associated liver injury occurs via Fn14-dependent and -independent mechanisms and that the former are particularly important for increasing hepatic TNF-α production. Withdrawal of the injury-inducing factors resulted in a decline of TNF-α and IL-6 in WT mice, leading to similar expression of these cytokines in WT and Fn14 KO livers at the end of the 10-day recovery period.
Fig. 3.
Deletion of Fn14 reduces proinflammatory cytokine expression. A and B: whole liver mRNA expression of the inflammatory cytokines TNF-α and IL-6 in WT and Fn14 KO mice. Results were normalized to the housekeeping gene S9. C: representative immunohistochemistry (original magnification ×40) and mRNA expression for the macrophage marker F4/80. Results are expressed as fold change compared with control. Comparisons for TNF-α and F4/80 were calculated by 2-way ANOVA followed by Tukey's multiple-comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA showed that IL-6 levels differ significantly across exposure in WT (P = 0.04), but not Fn14 KO, mice.
Fig. 4.
ETOH feeding + CCl4 injection does not induce hepatic expression of the Fn14 ligand TNF-like weak inducer of apoptosis (TWEAK). Expression of hepatic TWEAK was evaluated by quantitative RT-PCR analysis in livers from healthy adult WT mice fed control or ETOH diet, treated with CCl4 for 2 wk, and euthanized at the time points described in materials and methods. Results were normalized to expression of the housekeeping gene S9. One-way ANOVA showed that TWEAK levels did not differ significantly across exposure in WT or Fn14 KO mice.
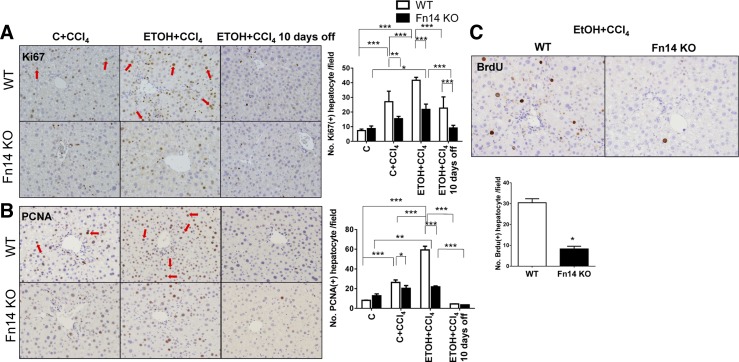
Deletion of Fn14 inhibits liver progenitor accumulation and impairs hepatocyte proliferation.
Fn14 is expressed by progenitor cells, which can differentiate into hepatocytes (1, 12, 13). The effects of subacute injury on expression of other progenitor markers and hepatocyte proliferation and the impact of Fn14 deletion on these parameters were evaluated by quantitative immunohistochemistry and quantitative RT-PCR analysis (Figs. 5–7). Rates of hepatocyte turnover are known to be low in healthy adult mice. Consistent with this, very few hepatocytes in the healthy livers of control diet-fed WT mice were labeled with the proliferation markers Ki67 and PCNA (25, 37; data not shown). These mice also demonstrated very few cells that were positive for pan-CK, a liver progenitor marker that is also expressed by Fn14+ cells (30; data not shown). Staining for LGR5 [an endodermal progenitor marker (11)], αFP [a marker of hepatocytic progenitors (29)], and Sox9 [a bipotent liver progenitor marker (9)] was observed rarely and localized primarily around periportal areas (data not shown).
Fig. 5.
Deletion of Fn14 impairs hepatocyte proliferation. Liver sections from WT and Fn14 KO mice were stained for Ki67 (A), proliferating cell nuclear antigen (PCNA, B), and bromodeoxyuridine (BrdU; C). Original magnification ×10. Red arrows indicate Ki67+ or PCNA+ hepatocytes. Positive hepatocytes were quantified in 10 randomly selected fields per section per mouse. Comparisons for Ki67+ and PCNA+ hepatocytes per field were calculated by 2-way ANOVA followed by Tukey's multiple-comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
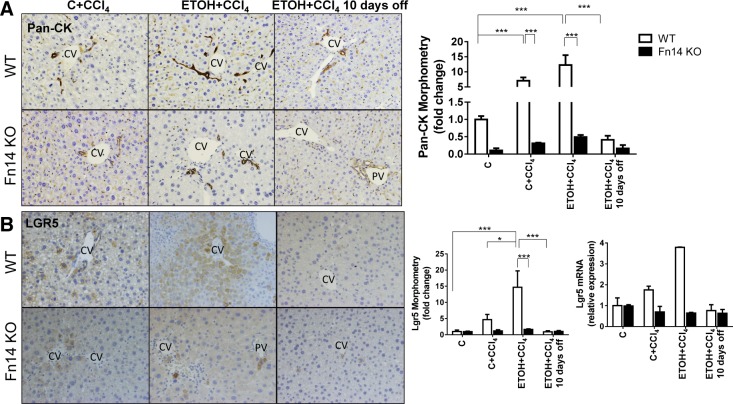
CCl4 injections increased numbers of Ki67- and PCNA-labeled hepatocytes in WT mice (Fig. 5, A and B). Injury-related accumulation of proliferating hepatocytes was greater in ETOH diet-fed mice (Fig. 5, A and B), which also demonstrated more liver injury than control diet-fed controls (Fig. 2). CCl4 injections also increased progenitors in both groups of WT mice and promoted greater expansion of progenitor cells in ETOH-fed mice (Figs. 6 and 7). This was accompanied by increased whole liver mRNA expression of LGR5, αFP, and Sox9 in the ETOH + CCl4 group (Fig. 6B and Fig. 7, B and C). Furthermore, careful examination of serially stained sections demonstrated that Fn14+ cells were closely localized with LGR5+ cells and αFP+ cells but distinct from cells that expressed pan-CK or Sox9 (Figs. 7A and 8A), consistent with our previous findings after partial hepatectomy (13).
Fig. 6.
Deletion of Fn14 inhibits liver progenitor accumulation. Liver sections from WT and Fn14 KO mice were stained for pan-cytokeratin (pan-CK; A) and LGR5 (B). Original magnification ×20. CV, central vein; PV, portal vein. Morphometric data and LGR5 mRNA expression in the respective groups are shown. Results are expressed as fold change compared with control. Results were normalized to the housekeeping gene S9. All comparisons were calculated by 2-way ANOVA followed by Tukey's multiple-comparison post hoc test: *P < 0.05, ***P < 0.001.
Baseline levels of hepatocyte proliferation and progenitor accumulation were similar in Fn14 KO and WT mice fed control diets. After CCl4 treatment, however, Fn14 KO mice accumulated fewer pan-CK-, LGR5-, αFP-, and Sox9-expressing cells, expressed less keratin 7 [a biliary/progenitor cell marker (7)] mRNA, and showed decreased induction of Ki67 and PCNA labeling in hepatocytes. The reduced proliferative and progenitor responses occurred in control- and ETOH diet-fed Fn14 KO mice (Fig. 5, A and B, Fig. 6, and Fig. 7, B–D). BrdU incorporation confirmed significantly decreased hepatocyte proliferation during liver injury in Fn14 KO mice (Fig. 5C). Hence, Fn14 promotes liver progenitor accumulation and hepatocyte proliferative activity during subacute liver injury. After discontinuation of ETOH and CCl4, hepatic pan-CK, LGR5, αFP, and Sox9 protein and/or mRNA expression fell to similar levels in surviving WT and Fn14 KO mice, while the number of Ki67- and PCNA-labeled hepatocytes declined by ≥50% in both groups (Fig. 5, A and B). Declines in regenerative responses (Figs. 5–7) paralleled the resolution of liver injury (Fig. 2) and accompanying fall in Fn14 expression (Fig. 1).
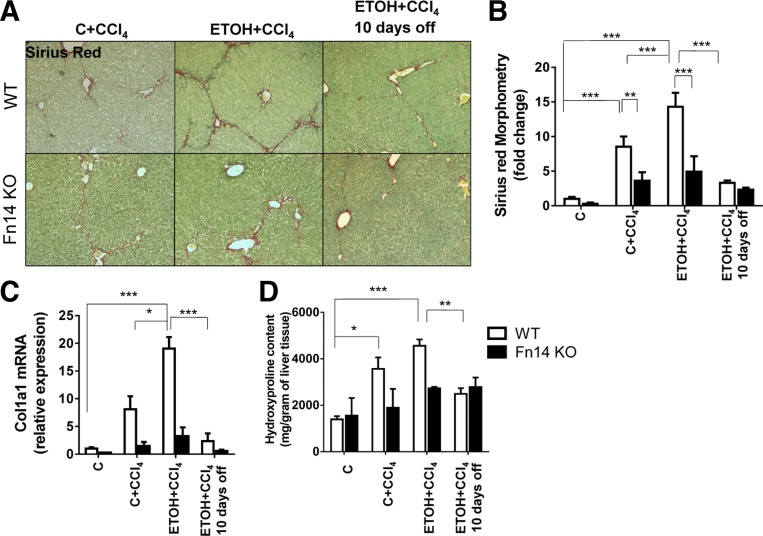
Deletion of Fn14 reduces liver fibrosis.
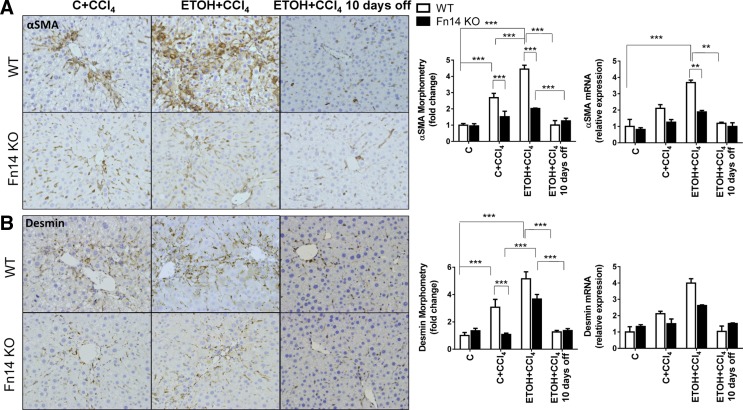
Fibrosis occurs during severe ASH (10). CCl4 injections increased hepatic fibrosis in WT mice. As with liver injury (Fig. 2) and injury-related regenerative responses (Figs. 5–7), consumption of ETOH diets exacerbated liver fibrosis, as evidenced by increased Sirius red staining (Fig. 9, A and B), collagen 1α1 mRNA (Fig. 9C), and liver hydroxyproline content (Fig. 9D). Because myofibroblasts derived from activated hepatic stellate cells (HSC) are major sources of collagen-producing cells in injured livers (14), we evaluated several HSC activation markers, including αSMA and desmin, by quantitative immunohistochemistry and quantitative RT-PCR (Fig. 10). Both markers increased at the protein and mRNA levels during injury and were greater in ETOH- than control diet-fed mice during CCl4 treatment.
Fig. 9.
Deletion of Fn14 reduces liver fibrosis. Liver sections from WT and Fn14 KO mice were stained with Sirius red to demonstrate fibrosis (A; original magnification ×10), with morphometry displayed as means ± SE (B). C: quantitative RT-PCR analysis of collagen 1α1 (Col 1α1) mRNA levels, with results normalized to the housekeeping gene S9. D: hepatic hydroxyproline content in the respective groups. Results are expressed as fold change compared with control. All comparisons were calculated by 2-way ANOVA followed by Tukey's multiple-comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 10.
Deletion of Fn14 reduces myofibroblast accumulation. A and B: representative immunohistochemistry (original magnification × 20), morphometry, and mRNA expression for αSMA and desmin. Results are expressed as fold change compared with control. All comparisons were calculated by 2-way ANOVA followed by Tukey's multiple-comparison post hoc test: **P < 0.01, ***P < 0.001.
Fn14 KO mice demonstrated significantly less liver fibrosis than WT mice after CCl4 injections, regardless of diet. Fibrosis (as assessed by morphometry) and collagen gene expression (as assessed by quantitative RT-PCR) in Fn14 KO mice were about half the levels observed in WT livers (Fig. 9, B and C). The hepatic hydroxyproline content was also lower in CCl4-treated Fn14 KO than CCl4-treated WT mice (Fig. 9C). Consistent with the decrease in liver fibrosis, Fn14 KO livers also accumulated only about half as many myofibroblastic cells as WT livers (Fig. 10). Deletion of Fn14 did not retard regression of fibrosis when ETOH and CCl4 were withdrawn in surviving animals (Figs. 9 and 10).
DISCUSSION
In this study we examined, for the first time, the effect of Fn14 deletion in an animal model that mimics features of human ASH. Similar to patients with severe ASH, ETOH-consuming mice with subacute liver injury demonstrated striking increases in Fn14 mRNA and protein expression without a concomitant increase in TWEAK expression (Fig. 4) (1). In mice, as in humans (10, 20), severe ASH is also associated with increased production of inflammatory cytokines and macrophage accumulation, liver cell death, a progenitor response, and fibrosis. In the current animal model, the intensity of each of these processes generally paralleled the level of Fn14 expression, being significantly weaker in Fn14 KO mice than WT controls. These data suggest that targeting Fn14 might improve the outcome of ASH. Therefore, it is particularly noteworthy that we observed the opposite effect when Fn14 was deleted. Namely, acute mortality was higher in animals deficient in Fn14. Interestingly, all deaths occurred during the 2nd wk of ETOH feeding and were restricted to the subgroup of Fn14 KO mice that received CCl4 injections to induce subacute liver injury. When ETOH and CCl4 were withdrawn, no further mortality occurred.
The reduced short-term survival in the Fn14 KO group is not easily explained by differences in liver injury, because serum aminotransferases and hepatocyte apoptosis were similar in WT and Fn14 KO mice that survived ETOH + CCl4 treatment. However, surviving ETOH diet-fed Fn14-deficient mice demonstrated significantly decreased progenitor accumulation and fibrosis compared with ETOH diet-fed WT mice immediately upon termination of treatment. The latter findings raise the possibility that the increase in ETOH-related mortality might have resulted from defective liver wound healing during subacute liver injury, a concept supported by recent evidence for impaired liver regeneration and increased mortality in Fn14 KO mice after partial hepatectomy (13). In this regard, it is noteworthy that hepatic expression of TNF-α was significantly higher in ETOH- than control diet-fed mice during liver injury and that Fn14 deletion selectively suppressed the ETOH-related “super”-induction of TNF-α. TNF-α has important proregenerative actions (5). In humans with severe ASH, treatments that inhibited TNF-α increased short-term mortality (21), and this negative outcome was attributed to loss of the proregenerative effects of TNF-α (18). Thus it is possible that inhibition of TNF-α induction by Fn14 deletion is responsible for the high mortality in Fn14 KO mice. Definitive proof would require supplementation of TNF-α in the Fn14 KO mice either pharmacologically or genetically. Since the Fn14 KO was global, it is also conceivable that the increased mortality might have resulted from loss of Fn14 function in extrahepatic tissue(s). Further research is needed to clarify this, because necropsies were not performed in the current study. While it will be interesting to determine whether liver-specific deletion of Fn14 impacts subacute liver injury-related mortality, the clinical relevance of such studies will be doubtful, because it is not feasible to achieve liver-specific inhibition of Fn14 in patients with ASH by a genetic or a pharmacological approach. Moreover, the present work in WT mice demonstrates that hepatic Fn14 expression declined rapidly once ASH-inducing insults were stopped. Indeed, levels of Fn14 mRNA and protein were found to be similar in WT and Fn14 KO mice within 10 days. At that time point, both groups also had comparable levels of liver function, progenitors, fibrosis, and inflammatory cytokine levels. Similar findings were observed when these studies were repeated in mice that are genetically deficient in TWEAK (data not shown), although the present studies and at least one other study (1) demonstrated that TWEAK itself was not elevated when ETOH injury was augmented with injections of CCl4. These findings might be consistent with those seen in human ASH and support evidence that Fn14 is able to signal in a ligand-independent manner (33). Lack of TWEAK mRNA induction also does not preclude the possibility that TWEAK mRNA induction might have occurred at some other time point(s), that local levels of TWEAK protein increased in our system, or that the injured livers might have been exposed to increased levels of soluble TWEAK that were generated by some extrahepatic source. The aggregate data, nevertheless, indicate that inhibition of Fn14 activity does not facilitate the resolution of ASH-related injury, casting doubt about the utility of treating ASH patients with Fn14 or TWEAK inhibitors. It is possible that pharmacological targeting of Fn14 or TWEAK may not have the same effect as genetic deletion of Fn14. Although we did not examine the effects of anti-TWEAK antibodies in the current studies, we recently published a very comprehensive comparison of Fn14 deletion, TWEAK deletion, and anti-TWEAK antibody treatment in several hundred mice at various time points after partial hepatectomy. In those experiments, all the approaches to abrogate TWEAK-Fn14 signaling had the same effect: each profoundly inhibited liver regeneration (both accumulation of liver progenitors and replication of mature-appearing liver cells) (13). Thus it is unlikely that dramatic differences will occur between pharmacological inhibition of Fn14 or TWEAK and genetic deletion of Fn14 in the current model.
Our findings do, however, support the concept that recovery from ASH (and other injuries that kill hepatocytes) requires hepatocyte regeneration. The present study is important, because it provides novel evidence that Fn14 regulates regenerative responses to ASH, a clinically important form of human liver injury. Because Fn14 promotes progenitor-mediated regeneration (12, 30) and both progenitor accumulation and hepatocyte proliferative activity were impaired when Fn14 was absent, our findings suggest that progenitors mediate the outcomes of ASH. Although the role of liver progenitor cells during liver injury is still poorly defined (28), we and others have reported that expansion of progenitor cells occurs during many types of hepatic injury in humans and mice and proposed that progenitor compartments contribute considerably to the proper restoration of functional hepatic parenchyma (4, 11, 26, 36). Thus, inhibition of Fn14 might negatively affect liver regeneration by disrupting processes that normally modulate progenitor fate during and after ASH-related liver injury. Somewhat surprisingly, we also observed that Fn14 influences liver repair during ASH by supporting HSC differentiation into myofibroblasts. In the present study, stellate cell transformation into myofibroblasts was disrupted during ASH in mice lacking Fn14, and this accompanied reduced accumulation of liver progenitors and impaired wound-healing responses during acute ASH, including decreased hepatocyte proliferative activity and decreased survival. On the basis of these results (worse acute mortality during ASH and no obvious long-term benefits after ASH), we conclude that it would be premature to treat patients with severe acute ASH with Fn14/TWEAK signaling inhibitors.
GRANTS
This work was supported in part by National Institutes of Health Grants R37 AA-010154 and T32 DK-007568 (A. M. Diehl).
DISCLOSURES
L. C. Burkly is an employee and stockholder in Biogen Idec.
AUTHOR CONTRIBUTIONS
G.K., G.X., and A.M.D. developed the concept and designed the research; G.K., G.X., M.S.-S., C.D.G., L.K., M.V.M., S.S.C., and G.A.M. performed the experiments; G.K., G.X., and C.M. analyzed the data; G.K., G.X., and A.M.D. interpreted the results of the experiments; G.K. and G.X. prepared the figures; G.K., G.X., L.C.B., and A.M.D. approved the final version of the manuscript; G.X. drafted the manuscript; L.C.B. and A.M.D. edited and revised the manuscript.
REFERENCES
- 1.Affo S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millan C, Loaeza-del-Castillo A, Altamirano J, Garcia-Pagan JC, Arroyo V, Gines P, Caballeria J, Schwabe RF, Bataller R. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 62: 452–460, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol 86: 1337–1348, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, Taube J, Thomas JA, Wojtacha D, Gambardella A, Sansom OJ, Iredale JP, Forbes SJ. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA 110: 6542–6547, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18: 572–579, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diehl AM, Yin M, Fleckenstein J, Yang SQ, Lin HZ, Brenner DA, Westwick J, Bagby G, Nelson S. Tumor necrosis factor-α induces c-jun during the regenerative response to liver injury. Am J Physiol Gastrointest Liver Physiol 267: G552–G561, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Dohi T, Burkly LC. The TWEAK/Fn14 pathway as an aggravating and perpetuating factor in inflammatory diseases: focus on inflammatory bowel diseases. J Leukoc Biol 92: 265–279, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 49: 138–151, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Feng SL, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, Peifley KA, Winkles JA. The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol 156: 1253–1261, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43: 34–41, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494: 247–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baetscher M, Wang B, Bissell DM, Burkly LC. TWEAK induces liver progenitor cell proliferation. J Clin Invest 115: 2330–2340, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaca G, Swiderska-Syn M, Xie G, Syn WK, Kruger L, Machado MV, Garman K, Choi SS, Michelotti GA, Burkly LC, Ochoa B, Diehl AM. TWEAK/Fn14 signaling is required for liver regeneration after partial hepatectomy in mice. PLos One 9: e83987, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocabayoglu P, Friedman SL. Cellular basis of hepatic fibrosis and its role in inflammation and cancer. Front Biosci 5: 217–230, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, Hanto DW, Otterbein LE, Popov Y. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 183: 182–194, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 360: 2758–2769, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallee JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 365: 1790–1800, 2011. [DOI] [PubMed] [Google Scholar]
- 18.McClain CJ, Hill DB, Barve SS. Infliximab and prednisolone: too much of a good thing? Hepatology 39: 1488–1490, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, Premont R, Yang L, Syn WK, Metzger D, Diehl AM. Smoothened is a master regulator of adult liver repair. J Clin Invest 123: 2380–2394, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci 32: 453–468, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broet P, Emilie D, Foie-Alcool Group of the Association Francaise pour l'Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 39: 1390–1397, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Novoyatleva T, Schymura Y, Janssen W, Strobl F, Swiercz JM, Patra C, Posern G, Wietelmann A, Zheng TS, Schermuly RT, Engel FB. Deletion of Fn14 receptor protects from right heart fibrosis and dysfunction. Basic Res Cardiol 108: 325, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M, Choi SS, Diehl AM. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology 51: 1712–1723, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roychowdhury S, Chiang DJ, Mandal P, McMullen MR, Liu X, Cohen JI, Pollard J, Feldstein AE, Nagy LE. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4-induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res 36: 1139–1147, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Swiderska-Syn M, Syn WK, Xie G, Kruger L, Machado MV, Karaca G, Michelotti GA, Choi SS, Premont RT, Diehl AM. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut 63: 1333–1344, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, Teaberry V, Choi SS, Conde-Vancells J, Karaca GF, Diehl AM. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology 137: 1478–1488, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theise ND, Dolle L, Kuwahara R. Low hepatocyte repopulation from stem cells: a matter of hepatobiliary linkage not massive production. Gastroenterology 145: 253–254, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology 30: 1425–1433, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Tirnitz-Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, Knight B. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology 52: 291–302, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Ucero AC, Benito-Martin A, Fuentes-Calvo I, Santamaria B, Blanco J, Lopez-Novoa JM, Ruiz-Ortega M, Egido J, Burkly LC, Martinez-Salgado C, Ortiz A. TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta 1832: 1744–1755, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Jiang W, Chen X, Zhang C, Li H, Hou W, Liu Z, McNutt MA, Lu F, Li G. α-Fetoprotein acts as a novel signal molecule and mediates transcription of Fn14 in human hepatocellular carcinoma. J Hepatol 57: 322–329, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discovery 7: 411–425, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, Chan IS, Chen Y, Diehl AM. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut 62: 299–309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor-α in alcohol-induced liver injury in mice. Gastroenterology 117: 942–952, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Yoon SM, Gerasimidou D, Kuwahara R, Hytiroglou P, Yoo JE, Park YN, Theise ND. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 53: 964–973, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Yu CC, Woods AL, Levison DA. The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem J 24: 121–131, 1992. [DOI] [PubMed] [Google Scholar]