Abstract
A novel type of self-fluorescent unimolecular micelle nanoparticle (NP) formed by multi-arm star amphiphilic block copolymer, Boltron® H40 (H40, a 4th generation hyperbranched polymer)-biodegradable photo-luminescent polymer (BPLP)-poly(ethylene glycol) (PEG) conjugated with cRGD peptide (i.e., H40-BPLP-PEG-cRGD) was designed, synthesized, and characterized. The hydrophobic BPLP segment was self-fluorescent, thereby making the unimolecular micelle NP self-fluorescent. cRGD peptides, which can effectively target αvβ3 integrin-expressing tumor neovasculature and tumor cells, were selectively conjugated onto the surface of the micelles to offer active tumor-targeting ability. This unique self-fluorescent unimolecular micelle exhibited excellent photostability and low cytotoxicity, making it an attractive bioimaging probe for NP tracking for a variety of microscopy techniques including fluorescent microscopy, confocal laser scanning microscopy (CLSM), and two-photon microscopy. Moreover, this self-fluorescent unimolecular micelle NP also demonstrated excellent stability in aqueous solutions due to its covalent nature, high drug loading level, pH-controlled drug release, and passive and active tumor-targeting abilities, thereby making it a promising nanoplatform for targeted cancer theranostics.
Keywords: Self-fluorescence, unimolecular micelles, drug nanocarrier, drug delivery, cRGD peptide, bioimaging probe, cancer therapy, cancer theranostics
1. Introduction
Multifunctional nanoparticles (NPs) integrating therapeutic agents, imaging probes, and tissue/cell specific targeting ligands are desirable for a wide range of biomedical applications including targeted cancer theranostics[1–5]. Many different types of NPs have been reported as therapeutic and/or imaging agent nanocarriers, among which liposomes, polymer micelles, and vesicles have been the most widely studied[6–8]. Liposomes, polymer micelles, and vesicles are typically formed by a large number of linear amphiphilic molecules via a self-assembly process. Thus, their in vitro and in vivo stabilities are susceptible to a number of factors including the concentration of amphiphilic linear molecules, flow stress, and interactions with serum proteins, which often lead to insufficient in vivo stability[9–11]. Premature rupture of such self-assembled multi-molecular NPs during circulation can cause a burst release of high concentration anticancer drugs and/or imaging probes into the bloodstream, which can not only can lead to potential systemic toxicity, but can also undermine their tumor-targeting and imaging abilities[9–11]. To improve the in vitro and in vivo stability of drug/agent nanocarriers, we developed a series of unimolecular micelles made of judiciously engineered multi-arm star amphiphilic block copolymers[3, 4, 12–17]. Since each unimolecular micelle NP is formed by a single multi-arm star amphiphilic block copolymer molecule consisting only of covalent bonds, it possesses excellent stability. Unimolecular micelles also provide a high drug loading capacity, possess a narrow nanoparticle size distribution, and offer excellent chemical versatility that allows for further surface modification such as ligand conjugation[3, 4, 13–18].
Fluorescent drug nanocarriers are highly desirable for both in vitro and in vivo applications as the fluorescence property allows for easy tracking of the nanocarriers using a variety of microscopy imaging techniques[19–23]. For instance, cellular internalization and intracellular trafficking of fluorescent NPs, as well as in vivo biodistribution of fluorescent NPs, can be conveniently carried out using fluorescence microscopy[21–24]. Current strategies to create fluorescent NPs include conjugating or encapsulating organic dyes or utilizing inorganic fluorescent NPs such as quantum dots (QDs) or other metallic particles[25–27]. However, there are various limitations to these common approaches. For instance, the organic dyes conjugated onto or encapsulated into the NPs may dissociate from the NPs. Moreover, organic dyes often exhibit low photostability[24, 28]. Meanwhile, inorganic fluorescent NPs such as QDs may possess high cytotoxicity and can limit the design of drug nanocarriers that may also require complicated synthesis processes[29–31].
Recently, a family of biodegradable photo-luminescent polymers (BPLPs) has been reported by Yang et al.[21, 23, 32, 33]. The reactants used to synthesize BPLPs, including citric acid, amino acids, and aliphatic diols, are all compounds used in many Food and Drug Administration-regulated devices[21]. In contrast to organic dyes or QDs, BPLPs have demonstrated excellent photostability and biocompatibility[21, 33]. Due to their polymeric nature, BPLPs can be conveniently used to fabricate NPs or scaffolds[21, 23].
Here we report the first self-fluorescent unimolecular micelle NP that exhibits excellent aqueous stability and photostability, low cytotoxicity, and a pH-controlled drug release profile. Furthermore, this unique unimolecular micelle NP is conjugated with cRGD peptides that can effectively target αvβ3 integrin-expressing tumor neovasculature and/or cells[3, 34–36]. αvβ3 integrin plays an important role for both tumor development and tumor metastasis, and is over-expressed on both the tumor cells and the angiogenic endothelial cells of many types of solid tumors (e.g., glioblastoma, breast, prostate, ovarian cancer, and melanoma)[37, 38]. In addition, αvβ3 integrin is up-regulated in tumors following radiotherapy[34]. The self-fluorescent unimolecular micelle NP is formed by a multi-arm star amphiphilic block copolymer molecule, Boltron®H40 (H40, a 4th generation hyperbranched polymer)-biodegradable photo-luminescent polymer (BPLP)-poly(ethylene glycol) (PEG) conjugated with cRGD peptide (i.e., H40-BPLP-PEG-cRGD). This unique self-fluorescent unimolecular micelle NP exhibits excellent aqueous stability and photostability, low cytotoxicity, high drug loading level capacity, and pH-controlled drug release, making it an extremely promising nanoplatform for various biomedical applications including targeted cancer theranostics.
2. Materials and Methods
2.1. Materials
Boltorn® H40 (a hyperbranched polyester with hydroxyl terminal groups) was provided by Perstorp Polyols Inc. The heterobifunctional poly(ethylene glycol) (PEG) derivatives, COOH-PEG-maleimide (Mw=5000 g/mol) and COOH-PEG-OCH3 (Mw=3500 g/mol), were acquired from JenKem Technology. Citric acid, 1,8-octanediol, and L-cysteine were purchased from Sigma-Aldrich. Succinic anhydrous, 4-dimethylamino pyridine (DMAP), and 1,3-dicyclohexylcarbodiimide (DCC) were purchased from ACROS and used without further purification. Triethylamine (TEA), anhydrous dimethyl sulfoxide (DMSO), anhydrous dimethylformamide (DMF), and tris(2-carboxyethyl)phosphine (TCEP) were purchased from Sigma-Aldrich. All other chemicals and reagents used were of analytical reagent grade. The anticancer drug, doxorubicin hydrochloride (DOX HC1) was purchased from Beijing Mesochem Technology Co., Ltd. Cyclo (Arg-Gly-Asp-D-Phe-Cys) (cRGD) peptide was purchased from Peptides International. Ultrapure deionized water (DI water, Milli-Q Water Systems) was used for all buffer solutions and experiments. Dulbecco's phosphate-buffered saline (DPBS, pH 7.4), Dulbecco's Modified Eagle Medium (DMEM, high glucose, pyruvate), Trypsin-EDTA (0.25%), and Fetal Bovine Serum (FBS) were purchased from Invitrogen, USA. The U87MG human glioblastoma cells (expressing high levels of intergrin αvβ3) were purchased from ATCC and cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.
2.2 Synthesis of BPLP Polymers
BPLP polymers were synthesized following a literature protocol with slight modifications[32]. Briefly, citric acid, 1, 8-octanediol, and L-cysteine with a molar ratio of 1:1.2:0.2 were added into a 100 mL two-neck flask and dried under vacuum for 2 h. After the mixture was melted at 160 °C in an oil bath under continuous stirring, the temperature was lowered to 120 °C for another 75 min. Next, the polymer was dissolved in 1,4-dioxane and the resulting polymer solution was added dropwise into DI water under constant stirring. The BPLP polymer was obtained after lyophilization.
2.3 Synthesis of COOH-PEG-cRGD
COOH-PEG-cRGD was prepared via a thiol-maleimide coupling reaction. Briefly, the COOH-PEG-Mal and cRGD-SH with a molar ratio of 1:1.2 were added in a 50 mL two-neck flask and dissolved in DMSO. TECP was added to avoid disulfide formation among cRGD peptides. The mixture was stirred under argon gas at room temperature for 24 h and followed by dialysis against DI water to remove impurities using a cellulose dialysis membrane (molecular weight cut-off, 2 kDa). After 48 h dialysis, the product was dried by lyophilization.
2.4 Synthesis of BPLP-PEG-cRGD and BPLP-PEG-OCH3
BPLP-PEG-cRGD was prepared via an esterification process in the presence of DCC and DMAP. Briefly, COOH-PEG-cRGD (5000 g/mol), BPLP, DCC, and DMAP with a molar ratio of 1.1:1:1.2:0.24 were dissolved in 10 mL of DMSO. The reaction was carried out at room temperature for 48 h. Next, the dicyclohexylurea (DCU) precipitated in the reaction solution was filtered out and the remaining solution was added dropwise into cold ethyl ether to obtain the crude polymers. The crude polymers were redissolved in DMSO and dialyzed against DI water for 48 h using a cellulose dialysis membrane (molecular weight cut-off, 5 kDa). The purified polymer was dried via lyophilization. BPLP-PEG-OCH3 was prepared following a similar procedure by using COOH-PEG-OCH3 (3500 g/mol) instead.
2.5 Synthesis of H40-COOH
To purify H40-OH, it was dissolved in acetone overnight and was then precipitated in cold ethyl ether. H40-COOH was prepared by converting the hydroxyl terminal groups into carboxyl terminal groups in the presence of succinic anhydrous. Briefly, H40-OH, succinic anhydrous, and DMAP with a molar ratio of 1:70:7 were dissolved into 10 mL of CH2Cl2. The reaction was carried out at room temperature for 48 h and the product was precipitated using diethyl ether and vacuum-dried.
2.6 Synthesis of H40-BPLP-PEG-OCH3/cRGD
H40-BPLP-PEG-OCH3/cRGD was synthesized by reacting H40-COOH with BPLP-PEG-cRGD and BPLP-PEG-OCH3 in 10 mL of DMF in the presence of DCC and DMAP. The molar ratio of reactants (H40-COOH:BPLP-PEG-cRGD:BPLP-PEG-OCH3) was 1:7:28. The reaction mixture was stirred at room temperature for 48 h and the by-product, DCU, was removed by filtration. The impurities were removed by dialysis against DMF for 12 h and against DI water for another 36 h using a cellulose dialysis membrane (molecular weight cut-off, 15 kDa). The resulting polymer H40-BPLP-PEG-OCH3/cRGD was obtained after lyophilization and used to prepare targeted unimolecular micelles. Multi-arm star amphiphilic block copolymer H40-BPLP-PEG-OCH3 (without cRGD conjugation) was also prepared following a similar procedure and was used to prepare non-targeted unimolecular micelles.
2.7 Preparation of DOX-Loaded Unimolecular Micelles
DOX·HCl (4 mg) was dissolved in 4 mL of anhydrous DMSO and treated with 2 moles excess of TEA for 2 h. Subsequently, the multi-arm star amphiphilic block copolymer H40-BPLP-PEG-cRGD or H40-BPLP-PEG-OCH3 (20 mg) was added to this solution. Thereafter, 12 ml of DI water was added dropwise into the solution under constant stirring. The resulting solution was stirred using a magnetic stirring bar for 4 h and then dialyzed against DI water using a cellulose dialysis membrane (molecular weight cut-off, 2 kDa) for 24 h followed by freeze-drying.
2.8 Characterization
1H NMR spectra of all intermediate and final polymer products were recorded on a Varian Mercury Plus 300 spectrometer using DMSO-d6 as a solvent at 25°C. Molecular weights (Mn and Mw) and polydispersity indices (PDI) of the polymers were determined by gel permeation chromatography (GPC) equipped with a refractive index detector, a viscometer detector, and a light scattering detector (Viscotek, USA). DMF with 0.1 mmol of LiBr was used as a mobile phase with a flow rate of 1 mL/min. Fluorescent spectra of the unimolecular micelle solutions were acquired on a Nanolog FL3-2iHR spectrofluorometer (HORIBA Jobin Yvon Inc., USA). The sizes and morphologies of the unimolecular micelles were determined by dynamic light scattering (DLS, ZetaSizer Nano ZS90, Malvern Instrument, USA) and transmission electron microscopy (TEM, FEI Tecnai G2 F30 TWIN 300 KV, E.A. Fischione Instruments, Inc. USA) at a polymer concentration of 0.05 mg/ml. The TEM sample was prepared by depositing a drop of the copolymer solution (0.05 mg/ml) containing 1 wt% of phosphotungstic acid onto a 200 mesh copper grid coated with carbon. The DOX loading level, defined as the weight percentage of DOX in the DOX-loaded unimolecular micelle NPs, was measured by a Cary 500 UV-Vis-NIR spectrophotometer based on a standard calibration curve of DOX at 485 nm.
2.9 In Vitro Drug Release Study
Drug release studies were carried out in a glass apparatus at 37 °C in either an acetate buffer (pH 5.3) or a phosphate buffer (pH 7.4) solution. Five mg of DOX-loaded non-targeted or targeted unimolecular micelles were dispersed uniformly in 5 ml of medium and then placed in a dialysis bag with a molecular weight cut-off of 2 kDa. The dialysis bag was immersed in 50 ml of the release medium and kept at 37 °C under a horizontal laboratory shaker at 100 rpm (Thermo Scientific MaxQ Shaker, USA). At specific time points, 3 ml of release media were collected and replaced by the same volume of fresh media. The amount of released DOX was analyzed by a UV-Vis-NIR spectrophotometer at 485 nm.
2.10 Cellular Uptake Study
The cellular internalization and intracellular distribution of the self-fluorescent unimolecular micelles in the U87MG cells were analyzed by fluorescence microscopy. U87MG cells (1.4×104 cells/cm2) were seeded in the 8-well chamber slide and cultured overnight. When about 80% confluence was reached, the cells were treated with H40-BPLP-PEG (non-targeted) and H40-BPLP-PEG-cRGD (targeted) unimolecular micelles at two different concentrations (i.e., 0.5 mg/ml or 1.0 mg/ml) for 6 and 20 h. A blocking experiment with 2 µM of free cRGD was also carried out for targeted micelles. After a certain period of treatment, cells were washed with DPBS and fixed by 4% polyformaldehyde (PFA). Cell nuclei were stained by propidium iodide (PI, Sigma, USA). Cells were first treated by RNaseA at 37 °C for 30 min to eliminate any interference from RNA, which PI can also bind to, followed by treatment with PI solution (1 µg/mL) at room temperature for 1 h. Thereafter, cells were imaged under a fluorescence microscope (Nikon, Japan) using two different filters. To image the self-fluorescent unimolecular micelle NPs, a UV-2E/C filter with an excitation wavelength of 325 to 375 nm and an emission wavelength of 435 to 485 nm was used. For cell nuclei imaging, a Texas-red filter with an excitation wavelength of 533 to 588 nm, and an emitter wavelength of 608 to 683 nm, was used. Digital monochromatic images were acquired using NIS-Element AR software.
Cell uptake studies of DOX-loaded unimolecular micelles (non-targeted and targeted NPs) were carried out using confocal laser scanning microscopy (CLSM), two-photon microscopy and flow cytometry. For CLSM and two-photon microscopy studies, after reaching 80% confluence, the cells (1.4×l04 cells/cm2) were treated with free DOX, DOX-loaded non-targeted, and DOX-loaded targeted micelles at a DOX concentration of 20 µg/ml. After 5 h incubation, cells were washed by DPBS and images were taken under a CLSM (Nikon Eclipse Ti inverted microscope equipped with Nikon AIR confocal diode lasers, Japan) or two-photon microscope (Bruker Ultima IV, USA) to observe the intracellular locations of both the self-fluorescent unimolecular micelle NPs and DOX. For the CLSM imaging, a UV-2E/C filter was used for unimolecular micelle imaging and a Texas-red filter was used for cell nuclei imaging. For two-photon microscope imaging, the excitation wavelength was set at 700 nm, and emissions at 445 nm (for unimolecular micelle imaging) and 620 nm (for DOX imaging) were collected.
For the flow cytometry study, U87MG cells (1.5×104 cells/cm2 ) were seeded in 24-well plates and cultured overnight. When about 80%, confluence was reached, cells were treated with free DOX, DOX-loaded non-targeted micelles, and DOX-loaded targeted micelles at a DOX concentration of 20 µg/ml for 5 h. A blocking experiment with 2 µM of free cRGD was also carried out for the DOX-loaded targeted micelles. After 5 h of treatment, DOX uptake was analyzed by an Accuri™C6 flow cytometer system (BD Bioscience, USA). A minimum of 2×104 cells for each sample were analyzed for DOX fluorescence intensity on a decade log scale.
2.11 Cytotoxicity Assay
The cytotoxicity of free DOX, DOX-loaded non-targeted micelles, and DOX-loaded targeted micelles for U87MG cells was analyzed by MTT assay. U87MG cells (3×104 cells/cm2 ) were seeded in a 96-well plate overnight. When about 80% confluence was reached, cells were treated with free DOX, DOX-loaded non-targeted micelles, DOX-loaded targeted micelles, and empty targeted and non-targeted micelles at two DOX concentrations (i.e., 2.5 and 5.0 µg/ml). After 48 h of treatment, the cells were incubated with media containing 500 µg/ml of MTT for another 4 h, followed by adding 100 µl of DMSO to dissolve the precipitates. An absorbance at 560 nm was measured by a GloMax®-Multi+ Detection System (Promega, USA) with an absorbance of 730 nm as the reference.
3. Results and Discussion
3.1 Design of Multifunctional Self-Fluorescent Unimolecular Micelles
Figure 1 shows a schematic of a multifunctional self-fluorescent unimolecular micelle formed by multi-arm star amphiphilic block copolymer, H40-BPLP-PEG, conjugated with cRGD peptides. Our previous studies and others have demonstrated that properly engineered multi-arm star amphiphilic block copolymers can form stable unimolecular micelles in aqueous solutions that exhibit excellent aqueous stability due to their covalent nature[12–14]. Boltorn® H40 (H40), a 4th generation hyperbranched aliphatic polyester with hydroxyl terminal groups, serves as a desirable inner core of the multi-arm star amphiphilic block copolymer due to its globular structure as well as its good biodegradability and biocompatibility. The hydrophobic self-fluorescent BPLP segment plays two roles in this NP design. (1) Together with the H40 inner core, it forms a hydrophobic core to be used for hydrophobic drug encapsulation. (2) Since it is self-fluorescent, the resulting unimolecular micelle is also self-fluorescent. PEG, a hydrophilic and biocompatible polymer, was used to form a hydrophilic shell. PEGylation not only provides the unimolecular micelle excellent water solubility, but also reduce opsonization during circulation in the bloodstream, thereby increasing the circulation time of the NP[39, 40]. As discussed earlier, cRGD peptides, which can effectively target αvβ3 integrin-expressing tumor neovasculature and tumor cells, are selectively conjugated onto the surface of the micelles to give them their active tumor-targeting abilities.
Fig. 1.
A schematic illustration of a self-fluorescent unimolecular micelle NP made of multi-arm star amphiphilic block copolymer, H40-BPLP-PEG-cRGD, for tumor-targeted drug delivery and bioimaging.
3.2 Synthesis and Characterization of Self-Fluorescent Multi-Arm Star Amphiphilic Block Copolymer H40-BPLP-PEG-OCH3/cRGD
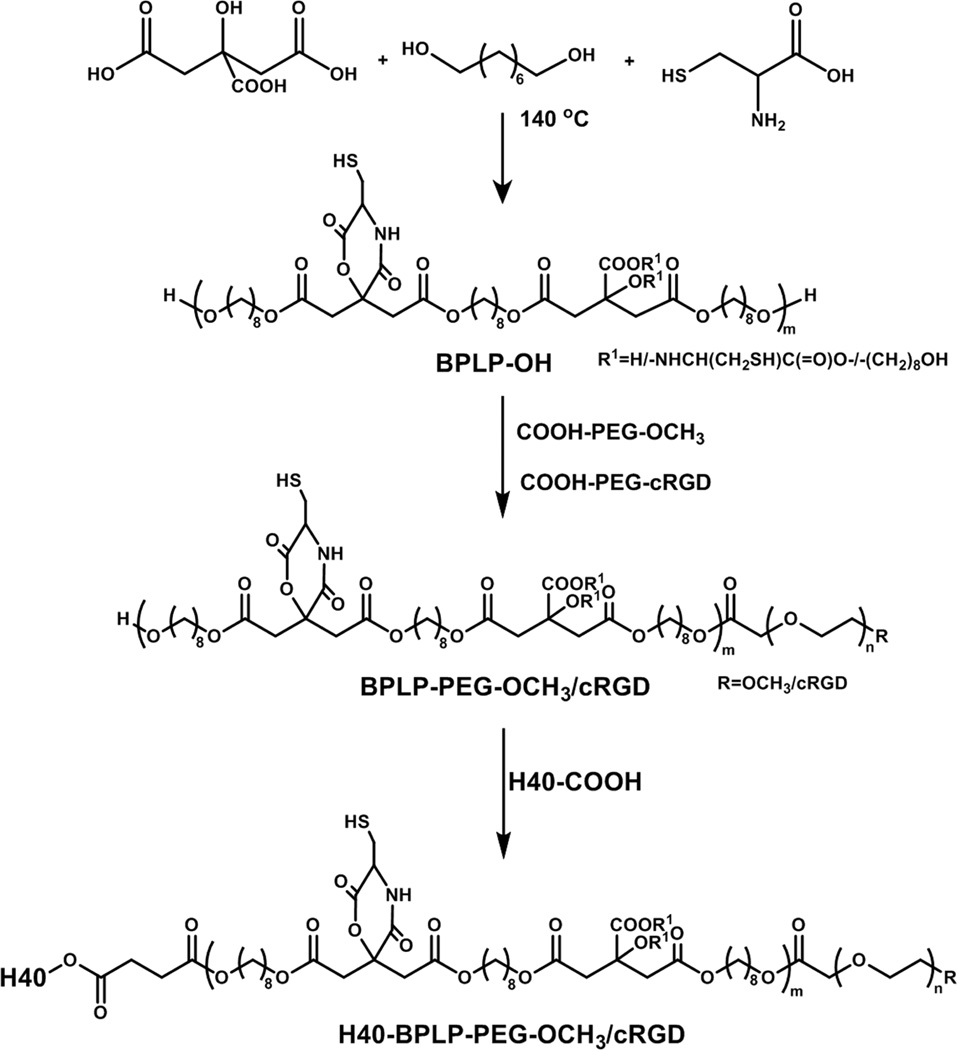
The self-fluorescent H40-BPLP-PEG-OCH3/cRGD copolymer was synthesized following Scheme 1. First, the hydrophobic self-fluorescent BPLP segment was synthesized, as reported, with some modification[32]. Citric acid was reacted with 1,8-octanediol to form a polyester backbone and then condensed with L-cysteine via the pendent carboxyl group and terminal hydroxyl group of the citrate unit to create a six-member ring. The fluorescence property of the BPLP polymer was attributed to its six-member ring[32]. The chemical structure was confirmed by 1H NMR as shown in Fig. 2(A). The presence of peaks at 1.02 ppm (e) and 5.60 ppm (d) were ascribed to the thiol (—SH) and amide groups (— NH—C(=O) —) of L-cysteine, respectively. The signals at 2.75 ppm (b) were assigned to the protons of citric acid (—CH2— from citric acid). The peaks at 1.2 ppm (h), 1.5 ppm (a), 3.2 ppm (f), and 4.0 (g) ppm corresponded to protons of the methylene groups in 1,8-octanediol (—CH2 — from 1,8-octanediol). The FT-IR spectrum (shown in Fig. 2(B)) further confirmed the presence of —C(=O) —NH— at 1,536 cm−1, –C(=O)—O — at 1,723 cm−1, —SH at 2,542 cm−1, —CH2— at 2912 cm−1, and −OH at 3259 cm−1. The combined 1H NMR and FT-IR analyses confirmed the structure of BPLP. The molecular weight of the BPLP polymer as measured by GPC was 1215 g/mol.
Scheme 1.
Synthesis scheme of the self-fluorescent multi-arm star amphiphilic block copolymer H40-BPLP-PEG-OCH3/cRGD.
Fig. 2.
(A) 1H NMR and (B) FT-IR spectra of the BPLP polymers.
Next, the hydrophilic PEG chain was conjugated to BPLP to form an amphiphilic linear block copolymer BPLP-PEG via an ester bond. PEGs with two different molecular weights (3.5 and 5.0 kDa) were used in this step. PEG-cRGD (5.0 KDa) was prepared via a thiol-maleimide coupling reaction and was used to synthesize BPLP-PEG-cRGD. The disappearance of the maleimide proton (6.67 ppm) demonstrated the successful conjugation of cRGD on PEG-maleimide. BPLP-PEG-OCH3 was synthesized through a reaction between BPLP and PEG (3.5 KDa) and was used to control the molar ratio of cRGD on the surface of the unimolecular micelle. One potential advantage of using longer PEGs to conjugate cRGD targeting ligands is to reduce the potential steric hindrance of the surrounding PEG-OCH3 arms, thus potentially allowing for better targeting abilities. 1H NMR confirmed the formation of BPLP-PEG polymers (Fig. 3(A)). In addition to the peaks of the BPLP segment, the peaks at 3.67 ppm (c) were observed due to the methylene protons of PEG (— O —CH2—CH2— O —). The signals at 7.95 to 8.15 ppm were ascribed to the cRGD targeting ligands. The molecular weights measured by GPC (shown in Table 1) were 6, 341 and 4, 623 Da for BPLP-PEG-cRGD and BPLP-PEG-OCH3, respectively.
Fig. 3.
1H NMR spectra of (A) BPLP-PEG-cRGD and (B) H40-BPLP-PEG-OCH3/cRGD polymers.
Table 1.
Molecular weights of all polymers
| Polymers | Mn (g/mol) |
Mw (g/mol) |
Mw/Mn |
|---|---|---|---|
| H40-OH | 2833 | 5100 | 1.80 |
| H40-COOH | 7658 | 13021 | 1.70 |
| BPLP | 1215 | 2214 | 1.82 |
| BPLP-PEG-OCH3 | 4623 | 6686 | 1.45 |
| BPLP-PEG-cRGD | 6341 | 9862 | 1.41 |
| H40-BPLP-PEG- OCH3/cRGD | 115364 | 164472 | 1.43 |
In the next step, BPLP-PEG-cRGD and BPLP-PEG-OCH3 block copolymers were conjugated onto the H40 core through esterification to form the multi-arm star amphiphilic block copolymer H40-BPLP-PEG-OCH3/cRGD. The feed molar ratio of BPLP-PEG-cRGD and BPLP-PEG-OCH3 was set at 1:4 (corresponding to 20% of cRGD terminal groups and 80% of methoxy terminal groups). The 1H NMR spectrum of H40-BPLP-PEG-OCH3/cRGD is shown in Fig. 3(B). While the peaks of the BPLP and PEG polymer segments were observed, the characteristic peaks of H40 (1.2 ppm) overlapped with those of 1,8-octanediol. Hence, GPC analysis was employed to confirm the successful formation of the multi-arm star amphiphilic block copolymer H40-BPLP-PEG-OCH3/cRGD. As shown in Table 1, the molecular weight of H40-BPLP-PEG-OCH3/cRGD was measured to be 115,364 Da, which was significantly larger than that of the liner BPLP-PEG polymers, indicating the formation of the multi-arm star amphiphilic block polymer H40-BPLP-PEG-OCH3/cRGD as designed. The average number of arms (#arms) of H40-BPLP-PEG-OCH3/cRGD was calculated to be 22 using Eq. 1, based on the molecular weights measured by GPC. This result was consistent with previous reports on the number of arms of H40-based multi-arm star amphiphilic block copolymers[12, 13].
| Eq. 1 |
3.3 The Properties of Self-Fluorescent Unimolecular Micelles
The self-fluorescent multi-arm star amphiphilic block copolymer H40-BPLP-PEG-OCH3/cRGD can form stable unimolecular micelles in an aqueous solution due to its globular structure as well as a large number of amphiphilic arms with a proper hydrophobic-to-hydrophilic ratio. The morphologies and sizes of the unimolecular micelles were studied by DLS and TEM. As shown in Fig. 4(A), the hydrodynamic diameter of the unimolecular micelles ranged from 30 to 100 nm, with an average diameter of 52 nm (PDI = 0.140). The TEM images of the unimolecular micelles (Fig. 4(B)) showed a spherical morphology with an average diameter of around 33 ± 4 nm, which was smaller than that measured by DLS. This was because DLS measured the hydrodynamic diameter of the micelles with the hydrophilic PEG arms extending into the aqueous solution, while TEM measured the diameter of dried unimolecular micelles.
Fig. 4.
(A) DLS histogram and (B) TEM images of the unimolecular micelles.
The H40-BPLP-PEG-OCH3/cRGD unimolecular micelle is a unique theranostic nano-platform due to its unique fluorescence property inherited from its hydrophobic BPLP segments. The fluorescence property of the unimolecular micelle was investigated using a series of excitation wavelengths ranging from 320 to 390 nm. As shown in Fig. 5(A), the unimolecular micelles showed a bright fluorescent emission peak at 420 nm (blue) within the range of excitation studied. The strongest emission intensity was observed at 360 nm excitation, which is in agreement with previous reports[21, 32]. The bright fluorescence was also found under a UV-lamp as shown in Fig. 5(B). The photostability of the unimolecular micelles was also investigated. The fluorescent intensity of the unimolecular micelle solution was reduced by less than 2% of the initial intensity after 3 h of UV exposure (365 nm excitation; 6 W) (Fig 5(C)), suggesting excellent photostability. In contrast, under the same conditions, DAPI lost more than 50% of its initial fluorescent intensity within 3 h.
Fig. 5.
Fluorescence properties of the unimolecular micelles. (A) Fluorescent spectra of the unimolecular micelles in water at various excitations. (B) Digital photograph of the unimolecular micelles (in water) taken under a UV lamp. (C) Photostability evaluation of the unimolecular micelles.
3.4 Self-Fluorescent Unimolecular Micelles as a Bioimaging Probe
Encouraged by the fluorescence property of the unimolecular micelle solution, we investigated the potential of a self-fluorescent unimolecular micelle NP as a tumor-targeting bioimaging probe. cRGD peptide, a small peptide that has been demonstrated to effectively target αvβ3 integrin-expressing tumor neovasculature and/or cells, was conjugated onto the unimolecular micelles. U87MG, a human glioblastoma cell line with a high expression of integrin αvβ3, was used in this study. U87MG cells were incubated with either non-targeted or targeted micelles for 6 and 20 h. Cells without any treatment were used as a negative control. The cells were imaged under a fluorescence microscope with the unimolecular micelle NP shown in blue and cell nuclei shown in red. Fig. 6(A) shows the fluorescent images of the U87MG cells incubated with cRGD-conjugated (targeted, T) and non-targeted (NT) unimolecular micelles at 0.5 mg/mL for 6 h. Based on the blue fluorescent color generated by the self-fluorescent unimolecular micelle NP, the cellular uptake of the cRGD-conjugated NPs was significantly higher than that of the non-targeted NPs, demonstrating that the cRGD peptide can effectively target the αvβ3 integrin through receptor-mediated endocytosis. At a higher micelle solution (1 vs. 0.5 mg/mL or, correspondingly, Fig. 6(B) vs. Fig. 6(A)), or a longer incubation time (20 vs. 6 h, or, correspondingly, Fig. 7 vs. Fig. 6), stronger blue signals were detected, thus indicating higher cellular uptake of the NPs. Figure 7 also shows the blocking experiment using free cRGD peptides. Incubating U87MG cells with both free cRGD peptides and cRGD-conjugated unimolecular micelles significantly reduced the blue fluorescence intensity of the targeted micelles to a degree that was comparable to that of the non-targeted micelles due to the competitive binding between free cRGD and cRGD-conjugated NPs[3]. Taken together, these results demonstrated that the self-fluorescent unimolecular micelles can be an excellent bioimaging probe, and that cRGD conjugation can markedly increase the cellular uptake of the unimolecular micelles in an integrin αvβ3 specific manner.
Fig. 6.
Fluorescence microscopy images of U87MG cells incubated with H40-BPLP-PEG non-targeted micelles (NT) and H40-BPLP-PEG-cRGD targeted unimolecular micelles (T) at 37 °C for 6 h at (A) 0.5 mg/ml and (B) 1.0 mg/ml, respectively. PI was used to stain the cell nucleus (red). The blue color was ascribed to the fluorescent property of the unimolecular micelles.
Fig. 7.
Fluorescence microscopy images of U87MG cells incubated with H40-BPLP-PEG non-targeted micelles (NT) and H40-BPLP-PEG-cRGD targeted micelles (T), or targeted micelles with a blocking dose of free cRGD, at 37 °C for 20 h at (A) 0.5 mg/ml and (B) 1.0 mg/ml, respectively. PI was used to stain the cell nucleus (red). The blue fluorescence was attributed to the self-fluorescent unimolecular micelles.
3.5 Self-Fluorescent Unimolecular Micelles as Tumor-Targeting Drug Nanocarriers
The utility of the self-fluorescent unimolecular micelles as tumor-targeted drug nanocarriers and bioimaging agents was further investigated. DOX, a model fluorescent hydrophobic anticancer drug, was encapsulated within the H40-BPLP hydrophobic inner core. The self-fluorescent unimolecular micelles had a very high DOX loading level (i.e., 15.7 wt%). To study the effects of pH-dependent drug release properties of the DOX-loaded unimolecular micelles, in vitro drug release studies were performed under simulated physiological (pH 7.4) and cellular (pH 5.3) conditions at 37 °C. As shown in Fig. 8, the pH value of the medium had a strong effect on the release rate of DOX from the unimolecular micelles. The release rate of DOX was much higher at a pH of 5.3 than at a pH of 7.4. At a pH of 7.4, the amount of DOX released after 78 h was only 25%. However, at a pH value of 5.3, 43% of the DOX was released within the first 10 h, and nearly 73% of the DOX was released after 78 h. DOX-loaded unimolecular micelles with pH-sensitive release profiles can reduce the amount of the drug lost during circulation in the blood stream (pH 7.4), and once the micelles are internalized into the acid endocytic compartments (e.g., endosomes and lysosomes), a faster release of DOX would occur. This pH-dependent drug release behavior may be attributed to the protonation of the amino group of DOX and the faster degradation of the micelle core at a lower pH[3, 4, 14]. This pH-dependent drug release behavior is desirable for targeted cancer therapy since it can enhance therapeutic efficacy while minimizing non-specific systemic toxicity.
Fig. 8.
In vitro drug release profiles of DOX-loaded unimolecular micelles at a pH of 7.4 and 5.3.
Since both the unimolecular micelle NPs and DOX are fluorescent, CLSM analyses on U87MG cells were performed to study the subcellular localizations of DOX and unimolecular micelles simultaneously. Cells were treated with free DOX, DOX-loaded non-targeted micelles (DOX-NT), and DOX-loaded targeted micelles (DOX-T). As shown in Fig. 9, for cells treated with free DOX, the red fluorescence of the DOX was located in the cell nuclei where the anti-cancer effect was executed[41]. For cells treated with DOX-NT and DOX-T, the blue fluorescence arising from the unimolecular micelle NPs was clearly visible in the cytoplasm, but not in the nuclei. This indicates that the unimolecular micelles did not translocate into the cell nuclei. Meanwhile, the red DOX fluorescence was localized in both the cell nuclei and the cytoplasm. Furthermore, in the cytoplasm, the blue fluorescence from the unimolecular micelles and the red DOX fluorescence largely overlapped each other to generate a purple color, suggesting that DOX was encapsulated inside of the unimolecular micelles and that DOX-loaded unimolecular micelle NPs were taken up by cells via endocytosis. Once the DOX-loaded unimolecular micelles entered the acid endocytic pathways (e.g., endosomes or lysosomes), DOX was released from the DOX-loaded unimolecular micelles and was then translocated into the cell nucleus where it interacted with the DNA by intercalation and inhibition of macromolecular biosynthesis[42]. Clearly, the DOX fluorescence intensity was much lower in cells treated with DOX-loaded non-targeted micelles than those treated with targeted micelles due to the receptor (i.e., αvβ3 integrin) mediated endocytosis discussed earlier. The DOX fluorescence intensity in cells treated with DOX-loaded targeted micelles was similar to those treated with free DOX because free DOX can quickly diffuse into the cellular cytoplasm, while NPs must use the endocytotic pathway to gain access to the cells[3, 13, 36]. Finally, it is worth mentioning that the utility of these self-fluorescent unimolecular micelles as bioimaging agents for two-photon excited fluorescence microscopy was also demonstrated (Fig. S1). The excitation wavelength used for two-photon imaging was set at 700 nm, which is suitable for in vivo applications. The maximum emission wavelength of these self-fluorescent unimolecular micelles is 425 nm, which is a relatively short wavelength. However, as previously reported, fluorescence around this region is still detectable in vivo [21]. Furthermore, the fluorescence wavelengths of the self-fluorescent unimolecular micelles can be tuned by changing the amino acids used in the self-fluorescent hydrophobic polymer segments [21, 32].
Fig. 9.
CLSM images of U87MG cells incubated with free DOX, DOX-loaded targeted micelles (DOX-T), and DOX-loaded non-targeted micelles (DOX-NT) at 37 °C for 5 h with a DOX concentration of 20 µg/ml. The red fluorescence is attributed to DOX, while the blue fluorescence is attributed to the unimolecular micelles.
To further verify the cellular uptake behavior of DOX-loaded targeted and non-targeted unimolecular micelles and free DOX, quantitative flow cytometry analyses were conducted (Fig. 10). Cells without any treatment were used as a negative control, which showed only a negligible level of autofluorescence. Consistent with the findings observed by CLSM analyses, cells treated with targeted micelles or free DOX exhibited a significantly higher level of cellular uptake than cells treated with non-targeted micelles. The presence of free cRGD peptides significantly reduced the DOX fluorescence intensity in cells treated with DOX-loaded unimolecular micelles due to competitive binding.
Fig. 10.
Flow cytometry analysis of U87MG cells incubated with free DOX (green line), DOX-T (blue line), DOX-NT micelles (red line), or DOX-T with a blocking dose of free cRGD (purple line) at 37 °C for 5 h with a DOX concentration of 20 µg/ml.
To determine the cytotoxicity of DOX-loaded unimolecular micelles, MTT-based cell viability assays were performed using U87MG cells. The cells were treated with the DOX-loaded targeted and non-targeted unimolecular micelles at two DOX concentrations (i.e., 2.5 and 5.0 mg/ml). Cells treated with media, free DOX, empty targeted (T) and non-targeted (NT) unimolecular micelles were used as controls. As shown in Fig. 11, the cell viability of cells treated with DOX-loaded targeted micelles and free DOX were similar, but both were significantly lower than the cell viability of cells treated with DOX-loaded non-targeted micelles. However, free DOX could lead to severe side effects in vivo, such as systemic toxicity, due to the lack of tumor-targeting ability. The increased cytotoxicity of DOX-loaded targeted micelles over the non-targeted ones is attributable to the enhanced cellular uptake of the targeted micelles via receptor-mediated endocytosis as discussed earlier. Finally, empty unimolecular micelles did not exhibit any apparent cytotoxicity.
Fig. 11.
Cytotoxicity of free DOX, DOX-loaded targeted micelles (DOX-T), DOX loaded non-targeted micelles (DOX-NT), empty (DOX-free) non-targeted micelles (NT), or empty targeted micelles (T) against U87MG cells at two different DOX concentrations. *p<0.05; **p<0.01.
4. Conclusion
A unique self-fluorescent unimolecular micelle nanoplatform formed by cRGD-conjugated multi-arm star amphiphilic block copolymer H40-BPLP-PEG-cRGD was synthesized and characterized as a tumor-targeting drug nanocarrier and bioimaging probe. The average hydrodynamic diameter of the spherical micelles was around 52 nm. The resulting unimolecular micelles were self-fluorescent since they contained a number of self-fluorescent BPLP segments. These self-fluorescent, cRGD-conjugated unimolecular micelles possessed excellent photo-stability and aqueous stability, low cytotoxicity, high drug loading level (15.7 wt.% DOX), pH-controlled drug release, and tumor-targeting abilities, thereby making them a promising candidate for many potential biomedical applications including tumor-targeted drug delivery and bioimaging.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the financial support from the National Institutes of Health (1K25CA166178) and the National Science Foundation (DMR 1032187) for this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
Supporting Information is available online or from the author.
References
- 1.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Xiao Y, Hong H, Javadi A, Engle JW, Xu W, Yang Y, et al. Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging. Biomaterials. 2012;33:3071–3082. doi: 10.1016/j.biomaterials.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J, Hong H, Chen G, Shi S, Zheng Q, Zhang Y, et al. Image-guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles. Biomaterials. 2013;34:8323–8332. doi: 10.1016/j.biomaterials.2013.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasongkla N, Shuai X, Ai H, Weinberg BD, Pink J, Boothman DA, et al. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew Chem Int Ed. 2004;43:6323–6327. doi: 10.1002/anie.200460800. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 7.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 8.Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed-Nanotechnol. 2010;6:714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Heise A, Hedrick JL, Frank CW, Miller RD. Starlike block copolymers with amphiphilic arms as models for unimolecular micelles. JACS. 1999;121:8647–8648. [Google Scholar]
- 10.Lawrence MJ. Surfactant systems: their use in drug delivery. Chem Soc Rev. 1994;23:417–424. [Google Scholar]
- 11.Kim S, Shi Y, Kim J, Park K, Cheng J. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv. 2010;7:49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 12.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(L-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery. Biomaterials. 2009;30:3009–3019. doi: 10.1016/j.biomaterials.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials. 2009;30:5757–5766. doi: 10.1016/j.biomaterials.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H, et al. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials. 2013;34:5244–5253. doi: 10.1016/j.biomaterials.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Burke JF, Pilla S, Chen H, Jaskula-Sztul R, Gong S. Octreotide-functionalized and resveratrol-loaded unimolecular micelles for targeted neuroendocrine cancer therapy. Nanoscale. 2013;5:9924–9933. doi: 10.1039/c3nr03102k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Grailer JJ, Pilla S, Steeber DA, Gong S. Tumor-Targeting, pH-Responsive, and Stable Unimolecular Micelles as Drug Nanocarriers for Targeted Cancer Therapy. Bioconjugate Chem. 2010;21:496–504. doi: 10.1021/bc900422j. [DOI] [PubMed] [Google Scholar]
- 17.Guo J, Hong H, Chen G, Shi S, Nayak TR, Theuer CP, et al. Theranostic Unimolecular Micelles Based on Brush-Shaped Amphiphilic Block Copolymers for Tumor-Targeted Drug Delivery and Positron Emission Tomography Imaging. ACS Appl Mater Interfaces. 2014 doi: 10.1021/am5002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Qian Y, Liu T, Hu X, Zhang G, You Y, et al. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials. 2011;32:6595–6605. doi: 10.1016/j.biomaterials.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 19.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27:2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JH, von Maltzahn G, Ong LL, Centrone A, Hatton TA, Ruoslahti E, et al. Cooperative nanoparticles for tumor detection and photothermally triggered drug delivery. Adv Mater. 2010;22:880–885. doi: 10.1002/adma.200902895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyawali D, Zhou S, Tran RT, Zhang Y, Liu C, Bai X, et al. Fluorescence imaging enabled biodegradable photostable polymeric micelles. Adv Healthc Mater. 2014;3:182–186. doi: 10.1002/adhm.201300145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, et al. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 23.Wadajkar AS, Kadapure T, Zhang Y, Cui W, Nguyen KT, Yang J. Dual-imaging enabled cancer-targeting nanoparticles. Adv Healthc Mater. 2012;1:450–456. doi: 10.1002/adhm.201100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Wu DQ, Lu B, Chang C, Chen CS, Wang T, Zhang YY, et al. Galactosylated fluorescent labeled micelles as a liver targeting drug carrier. Biomaterials. 2009;30:1363–1371. doi: 10.1016/j.biomaterials.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Huang CK, Lo CL, Chen HH, Hsiue GH. Multifunctional Micelles for Cancer Cell Targeting, Distribution Imaging, and Anticancer Drug Delivery. Adv Funct Mater. 2007;17:2291–2297. [Google Scholar]
- 27.Lee YK, Hong SM, Kim JS, Im JH, Min HS, Subramanyam E, et al. Encapsulation of CdSe/ZnS quantum dots in poly(ethylene glycol)-Poly(D,L-lactide) micelle for biomedical imaging and detection. Macromolecular Research. 2007;15:330–336. [Google Scholar]
- 28.Eggeling C, Widengren J, Rigler R, Seidel CAM. Photobleaching of fluorescent dyes under conditions used for single-molecule detection: Evidence of two-step photolysis. Anal Chem. 1998;70:2651–2659. doi: 10.1021/ac980027p. [DOI] [PubMed] [Google Scholar]
- 29.Chen N, He Y, Su Y, Li X, Huang Q, Wang H, et al. The cytotoxicity of cadmium-based quantum dots. Biomaterials. 2012;33:1238–1244. doi: 10.1016/j.biomaterials.2011.10.070. [DOI] [PubMed] [Google Scholar]
- 30.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otsuka H, Akiyama Y, Nagasaki Y, Kataoka K. Quantitative and reversible lectin-induced association of gold nanoparticles modified with alpha-lactosyl-omega-mercapto-poly(ethylene glycol) JACS. 2001;123:8226–8230. doi: 10.1021/ja010437m. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, et al. Development of aliphatic biodegradable photoluminescent polymers. Proc Natl Acad Sci USA. 2009;106:10086–10091. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Z, Zhang Y, Liu L, Weng H, Mason RP, Tang L, et al. Development of intrinsically photoluminescent and photostable polylactones. Adv Mater. 2014;26:4491–4496. doi: 10.1002/adma.201306070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, et al. Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11:6270–6279. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- 35.Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer Res. 2006;66:9673–9681. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, et al. cRGD-functionalized, DOX-conjugated, and (6)(4)Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials. 2011;32:4151–4160. doi: 10.1016/j.biomaterials.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai W, Niu G, Chen X. Imaging of integrins as biomakers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 38.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 39.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue X, Zhao Y, Dai L, Zhang X, Hao X, Zhang C, et al. Spatiotemporal drug release visualized through a drug delivery system with tunable aggregation-induced emission. Adv Mater. 2014;26:712–717. doi: 10.1002/adma.201302365. [DOI] [PubMed] [Google Scholar]
- 42.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.