Abstract
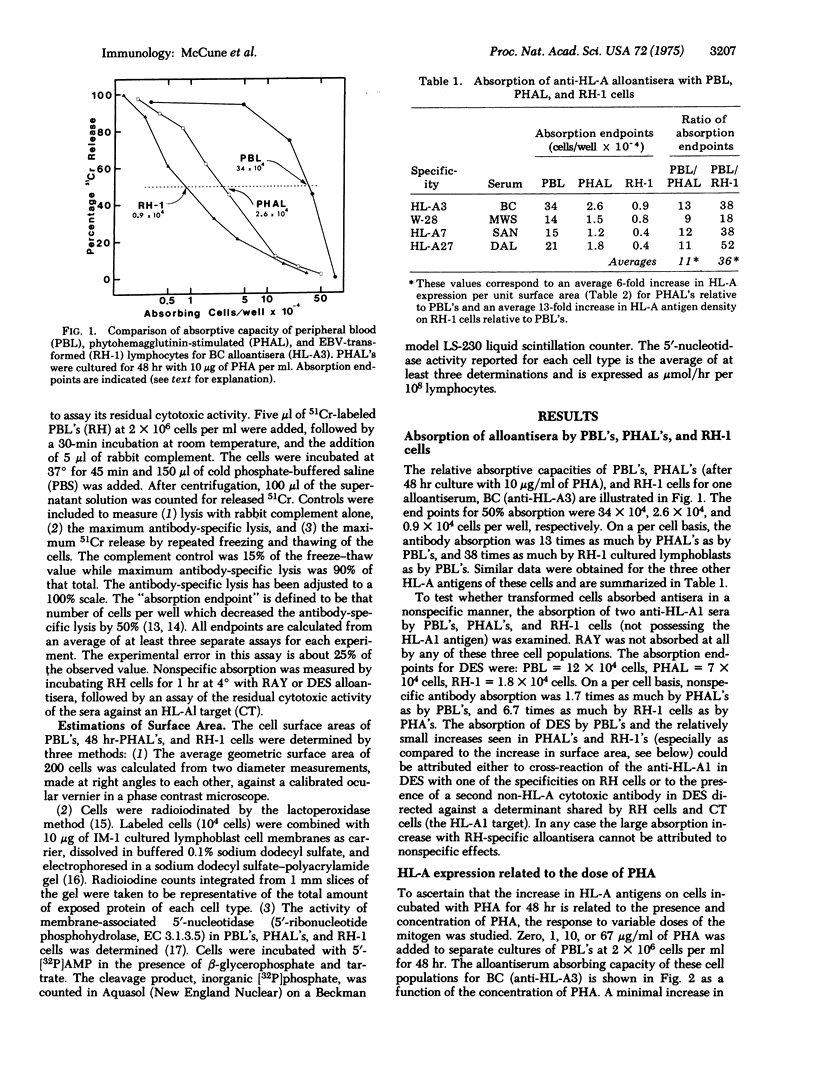
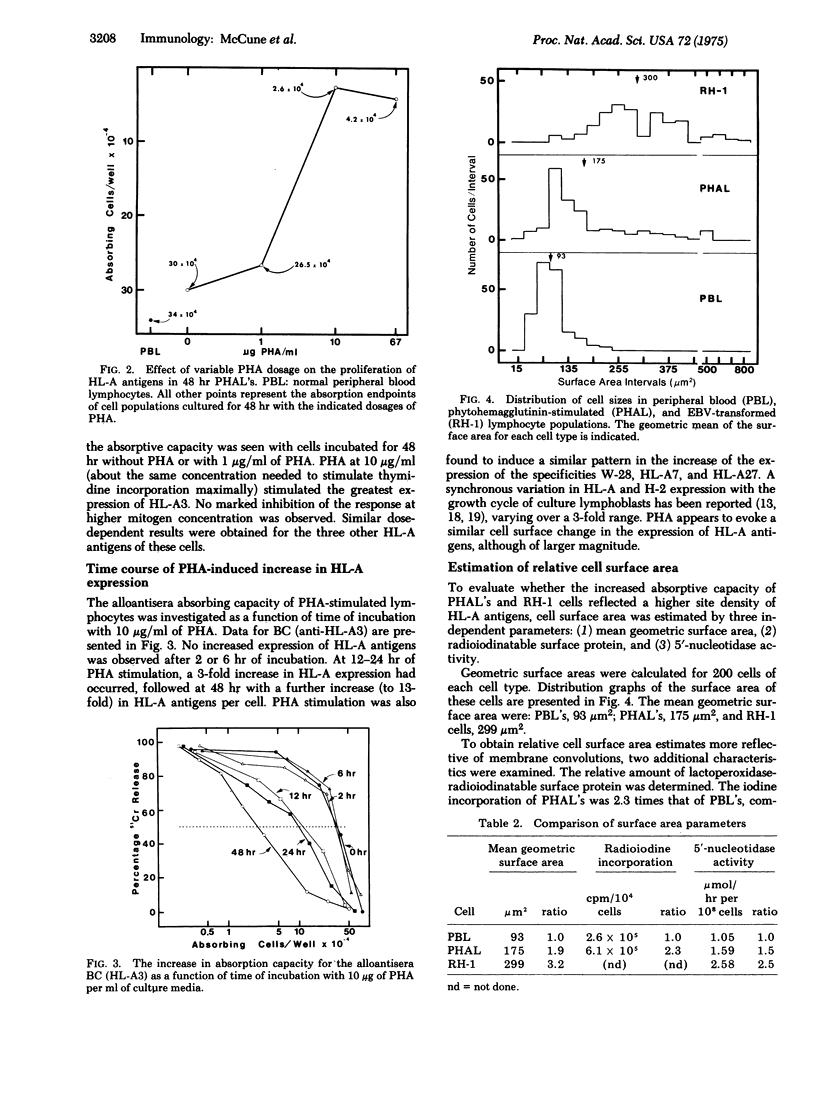
The amount of HL-A antigens present on the peripheral blood lymphocytes of a single human donor was increased about 11-fold after stimulation with phytohemagglutinin and 36-fold after transformation with Epstein-Barr virus. This increase applied to all four HL-A specificities of these cells. The response to phytohemagglutinin was dependent on dose and was first observed at 12 hr of incubation. Measurements of the amount of surface membranes by geometry, by radioiodinatable surface proteins, and by 5'-nucleotidase (5'-ribonucleotide phosphohydrolase, EC 3.1.3.5) assay all indicated that the enhanced representation of HL-A antigens after stimulation by phytohemagglutinin or transformation by Epstein-Barr virus must be due to an increase in the density of these antigens on the cell surface.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boldt D. H., MacDermott R. P., Jorolan E. P. Interaction of plant lectins with purified human lymphocyte populations: binding characteristics and kinetics of proliferation. J Immunol. 1975 May;114(5):1532–1536. [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr, Klein G. Progressive loss of H-2 antigens with concomitant increase of cell-surface antigen(s) determined by Moloney leukemia virus in cultured murine lymphomas. J Natl Cancer Inst. 1973 Feb;50(2):347–362. doi: 10.1093/jnci/50.2.347. [DOI] [PubMed] [Google Scholar]
- Cikes M., Klein G. Quantitative studies of antigen expression in cultured murine lymphoma cells. I. Cell-surface antigens in "Asynchronous" cultures. J Natl Cancer Inst. 1972 Dec;49(6):1599–1606. doi: 10.1093/jnci/49.6.1599. [DOI] [PubMed] [Google Scholar]
- Dick H. M., Steel C. M., Crichton W. B. HL-A typing of cultured peripheral lymphoblastoid cells. Tissue Antigens. 1972;2(2):85–93. doi: 10.1111/j.1399-0039.1972.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Response of human thymus-derived (T) and non-thymus-derived (B) lymphocytes to mitogenic stimulation in vitro. Eur J Immunol. 1974 Mar;4(3):193–199. doi: 10.1002/eji.1830040308. [DOI] [PubMed] [Google Scholar]
- Götze D., Pellegrino M. A., Ferrone S., Reisfeld R. A. Expression of H-2 antigens during growth of cultured tumor cells. Immunol Commun. 1972;1(6):533–544. doi: 10.3109/08820137209022962. [DOI] [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Mackintosh P., Wallin J., Hardy D. A., Ling N. R., Steel C. M. The interaction of normal lymphocytes and cells from lymphoid cell lines. IV. HL-A typing of the cell line cells. Immunology. 1973 Feb;24(2):315–331. [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeim F., Stoddard A., Fritze D., Herrmann C., Zeller E., Walford R. L. HL-A antigens on hyperplastic and neoplastic thymic tissue. Cancer Res. 1974 Mar;34(3):654–656. [PubMed] [Google Scholar]
- Pegrum G. D., Balfour I. C., Evans C. A., Middleton V. L. HL-A typing of "leukaemic" cells. Lancet. 1971 Apr 24;1(7704):852–853. doi: 10.1016/s0140-6736(71)91511-x. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Natali P. G., Pellegrino A., Reisfeld R. A. Expression of HL-A antigens in synchronized cultures of human lymphocytes. J Immunol. 1972 Feb;108(2):573–576. [PubMed] [Google Scholar]
- Pious D., Hawley P., Forrest G. Isolation and characterization of HL-A variants in cultured human lymphoid cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1397–1400. doi: 10.1073/pnas.70.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliata F., Faig D., Conklyn M., Silber R. Studies on the lymphocyte 5'-nucleotidase in chronic lymphocytic leukemia, infectious mononucleosis, normal subpopulations, and phytohemagglutinin-stimulated cells. Cancer Res. 1974 Dec;34(12):3197–3202. [PubMed] [Google Scholar]
- Rogentine G. N., Jr, Gerber P. HL-A antigens of human lymphoid cells in long-term tissue culture. Transplantation. 1969 Jul;8(1):28–37. doi: 10.1097/00007890-196907000-00004. [DOI] [PubMed] [Google Scholar]
- Seigler H. F., Kremer W. B., Metzgar R. S., Ward F. E., Haung A. T., Amos D. B. HL-A antigenic loss in malignant transformation. J Natl Cancer Inst. 1971 Mar;46(3):577–584. [PubMed] [Google Scholar]
- Strominger J. L., Cresswell P., Grey H., Humphreys R. E., Mann D., McCuneJ, Parham P., Robb R., Sanderson A. R., Springer T. A. The immunoglobulin-like structure of human histocompatibility antigens. Transplant Rev. 1974;21(0):126–143. doi: 10.1111/j.1600-065x.1974.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Miyajima T., Sengar D. P., Stiehm E. R. Extraneous lymphocytic HL-A antigens in severe combined immunodeficiency disease. Transplantation. 1972 Mar;13(3):250–255. doi: 10.1097/00007890-197203000-00009. [DOI] [PubMed] [Google Scholar]


