Abstract
Haptoglobin (Hp), serum amyloid A (SAA), C-reactive protein (CRP), white blood cells (WBC), reactive oxygen metabolites (ROMs), the antioxidant barrier (Oxy-adsorbent) and thiol groups of plasma compounds (SHp) were measured in ten dogs that had been transported a distance of about 230 km within 2 h (experimental group) and in ten dogs that had not been subjected to road transportation (control group). Blood was collected via cephalic venipuncture before road transportation (T0), after road transportation (T1), and more than 6 (T6) and 24 (T24) hours after road transportation in the experimental group (Group A) and at the same time points in the control group (Group B). The GLM (general linear model) Repeated Measures procedure showed a significant difference between the two groups (P<0.0001) and a significant rise (P<0.0001) in the concentrations of Hp, SAA, CRP, WBC, ROMs, Oxy-adsorbent and SHp after road transportation in Group A, underlining that physiological and homeostatic mechanisms are modified differently at various sampling times.
Keywords: acute phase proteins, dog, oxidative parameters, transportation stress, white blood cells.
Introduction
Transportation, often considered one of the main causes of stress, represents a variety of physical and psychological stimuli that disrupt homeostasis and metabolism in animals [12, 30]. It has been shown to be stressful for many domestic animal species [6, 14, 16, 29] influencing physiological and hematological parameters [1, 3, 4], the mobilization of energy and protein metabolism [5], the activity of enzymes and hormones [1, 33] and the changes in the immune system [10]. Signals originating from stresses like transportation are transmitted to the hypothalamus in the brain, activating the hypothalamic-pituitary-adrenal and sympathoadrenal axes, which lead to release of glucocorticoids and catecholamines, respectively, that, through the induction of proinflammatory cytokines by macrophages and lymphocytes, promote the production of acute phase proteins (APPs) in hepatocytes, thereby augmenting peripheral APP levels in stressed animals [24]. Moreover, it is known that a stressful condition leads to the imbalance between oxidants and antioxidants in favor of oxidants at the cellular or individual level [19, 28]. The alteration of the oxidative balance, if not adequately restored by the antioxidant barrier, induces oxidative stress that causes cellular damage [28, 35], which makes the organism sensitive to serious degenerative diseases [23]. Although the linkage between transportation stress and variations in APP and oxidative balance is clear [25, 34], very little scientific research has been done on the influence of transportation in dogs [3, 27] in fact, canine health and welfare have been evaluated only through physiology and behavior [3, 36] and housing conditions [2], and there are no data available regarding the response of APPs and oxidative parameters. APPs are a group of negative and positive proteins whose serum concentrations decrease or increase, respectively, in response to challenge [11, 15]. In the dog, serum amyloid A (SAA) and C-reactive protein (CRP) are known to be major acute phase proteins that increase 10- to 100-fold respectively on stimulation, while haptoglobin (Hp) is considered a minor APP that increases by around 4 times [7, 9]. A stressful condition leads to excessive production of the radicals too, which results in oxidative stress [19]. Psychological stress due to road transportation elevates oxidative stress measured by serum total antioxidant capacity [31]. The plasma/serum ability to oppose the massive oxidative action of a hypochlorous acid solution is evaluated by means of the oxy-adsorbent test (Oxy-adsorbent), and a significant component of the plasma/serum barrier to oxidation is thiol. Its function is to oppose the propagation step of peroxidative processes by inactivating either alkoxyl or hydroxyl radicals (SHp test).
On the basis of the above, the purpose of this study was to investigate the influence of road transportation on the changes of Hp, SAA, CRP, white blood cells (WBC), reactive oxygen metabolites (ROMs), the antioxidant barrier (Oxy-adsorbent) and thiol groups of plasma compounds (SHp) in order to identify useful biomarkers to improve the transportation conditions of dogs.
Materials and Methods
The present study was carried out during the autumn season in Italy and involved a laboratory component and a veterinary clinic component at the Department of Veterinary Sciences, University of Messina. All treatments, housing and care of animals were reviewed and approved in accordance with the standards recommended by the US National Research Council’s Guide for the Care and Use of Laboratory Animals as established by the Italian Ministry of Health and European Council Directive 2010/63/EU.
Twenty clinically healthy dogs (6–8 years old) of different breeds, including English setters (6), pointers (5), kurzhaars (5), and beagles (4), were used. All dogs were born and raised in Italy, where they were bred by private owners. Dogs were fed a high-quality commercial diet (Purina Pro Plan Adult, Nestle Italiana Spa, Milan, Italy) in accordance with their body condition score, as assessed by their owner. A full clinical history, including dietary regimens and average daily exercise, was obtained from each dog. All dogs underwent a full physical examination in order to exclude animals with injuries, swelling or any form of apparent diseases (Table 1).
Table 1. Mean ± SD values for rectal temperature (RT), respiratory rate (RR), heart rate (HR), red blood cells (RBC), white blood cells (WBC), hemoglobin (HGB), hematocrit (HCT), platelets (PLT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, creatinine and total proteins, expressed in their conventional units of measurement, obtained from the 20 healthy dogs used in present study (with reference ranges).
| Parameters | Mean ± SD | Range [18] |
|---|---|---|
| RT (°C) | 38.40 ± 0.14 | 38.3–38.7 |
| RR (breaths/min) | 22.00 ± 3.00 | 10–30 |
| HR (beats/min) | 80.00 ± 7.14 | 60–180 |
| RBC (M/µl) | 6.85 ± 0.80 | 5.5–7.9 |
| WBC(K/ µl) | 11.60 ± 1.35 | 6–16 |
| HGB (g/dl) | 14.44 ± 1.02 | 12–18 |
| HCT (%) | 42.11 ± 2.82 | 37–55 |
| PLT (K/µl) | 332.00 ± 43.04 | 240–400 |
| AST (U/l) | 37.00 ± 12.00 | 23–66 |
| ALT (U/l) | 82.00 ± 23.00 | 21–102 |
| Urea (mmol/l) | 2.64 ± 0.78 | 1.67–3.33 |
| Creatinine (μmol/l) | 83.35 ± 8.55 | 44.2–132.6 |
| Total proteins (g/dl) | 6.64 ± 0.35 | 5.4–7.1 |
Ten dogs were transported a distance of about 230 km within 3 h with an average speed of 80 km/h (experimental group) and ten dogs were not subjected to road transportation (control group). All dogs of the experimental group (Group A) had previous experiences of road transportation; however, one week prior to the start of the study, the dogs were loaded into a van and then unloaded to ensure that all would enter the van without hesitation. The road transportation started at 10:00 h and lasted for 3 h, and it involved a combination of road surfaces ranging from small country lanes (5 km) and secondary roads (50 km) to motorways (175 km). All dogs were transported into crates large enough to permit the animal to lie down.
Blood, collected from each dog via cephalic venepuncture by means of a 20 G needle connected to a 5 ml syringe, was transferred into two different types of venous blood collection tubes (Terumo Corporation, Tokyo, Japan): with no additive and containing ethylenediaminetetraacetic acid (EDTA). The serum samples obtained after centrifugation of tubes without additive were stored for 20 days at −80°C before all measurements. Dogs were restrained gently with a halter during blood collection. For all samples collected before road transportation at 09:30 (T0), after 3 h of road transportation (T1), and more than 6 (T6) and 24 (T24) hours after road transportation, Hp, SAA, CRP, WBC, ROMs, Oxy-adsorbent and SHp were assessed. Collection of samples in all subjects (transported and untransported animals) was performed at the same time. In fact, the sampling times were equal in order to exclude modifications not related to transport stress.
Serum concentrations of Hp were determined by use of the hemoglobin-binding method using a commercial kit (Tridelta Development Limited, Kildare, Ireland) and an automated biochemistry analyzer (Cobas Mira Plus multiparametric autoanalyzer, ABX Diagnostics, Montpellier, France). Concentrations of SAA were determined by use of a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Tridelta Development Limited, Kildare, Ireland). The ELISA was designed for use in determining concentrations of SAA in different animal species such as dogs. Final absorbance of samples was measured by use of a microtiter plate reader at 450 nm. Both methods were validated in the authors’ laboratory for canine serum [22]. A solid-phase sandwich immunoassay specific for canine CRP (Tridelta Development Limited, Kildare, Ireland) was used in accordance with directions provided by the manufacturer. Final absorbance of samples was measured by use of a microtiter plate reader (PowerWave XS, Bio Tek Instruments Inc., Winooski, VT, USA) at 450 nm. This method was validated in the authors’ laboratory for canine serum [22]. The WBC count was assessed in blood samples containing EDTA using a multiparametric automatic analyzer (HecoVet, SEAC, Florence, Italy).
For evaluation of oxidative stress parameters, blood samples were centrifuged (ALC 4235 A Milan, Italy) at 3,000 g × 20 min. The obtained serum was immediately analyzed by means of an ultraviolet spectrophotometer (model Slim SEAC, Florence, Italy), and ROMs, Oxy-adsorbent and SHp were evaluated. These techniques are based on the “spin traps” system, in which molecules react with free radicals, creating complexes revealed by spectrophotometry. The dROMs test is a colorimetric test that assesses the levels of hydroperoxides (R-OOH), the “markers” and “amplifiers” of tissue damage generated by peroxidation of lipids, amino acids, proteins, and nucleic acids. In this test, these molecules, after reaction with a properly buffered chromogen, develop a colored derivative, which is photometrically detected. The concentration of ROMs, which directly parallels changes in color intensity, is expressed in Carratelli Units (1 CARR U=0.08 mg% hydrogen peroxide). Increased values directly correlate with increased levels of oxidative stress. The oxy-adsorbent test evaluates the ability of plasma to oppose the massive oxidant action of an excess of hypochlorous acid in water solution by assessing photometrically the residual unreacted radicals of the acid. Decreased values directly correlate with the injury severity of the “plasma barrier due to oxidation”. When the “excess” of radicals of hypochlorous acid after massive oxidation is high, the plasma barrier is reduced and vice versa. The SHp test is a colorimetric determination of the plasma/serum thiol antioxidant barrier, which opposes peroxidative processes inhibiting both alkoxyl and hydroxyl radicals. This test is based on the ability of thiol groups to develop a colored complex when reacted with DTNB (5,5-dithiobis-2-nitrobenzoic acid). The “titer” of thiols directly parallels color intensity. Decreased values directly correlate with a lower efficacy of the thiol antioxidant barrier.
All samples were analyzed in duplicate by the same operator. The samples exhibited parallel 98 displacement to the standard curve. The overall intra-assay coefficient of variation was<5%.
All results were expressed as the mean ± standard deviation (SD). Data were normally distributed (P<0.05, Kolmogorov-Smirnov test). The GLM (general linear model) Repeated Measures procedure was used to determine the differences between the experimental and control groups, and the statistically significant effect of sampling time (before transport, after transport, and after recovery period). Values of P<0.05 were considered statistically significant. Duncan’s multiple range test was applied for post hoc comparisons.
Results
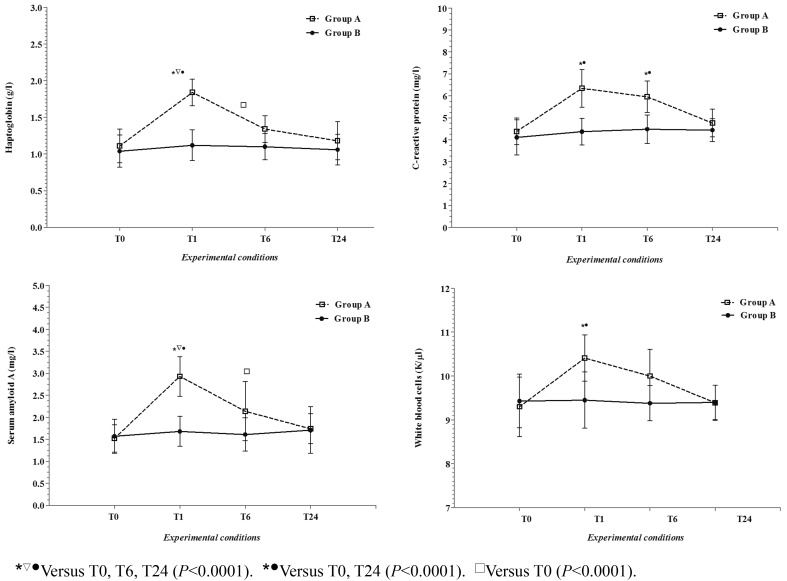
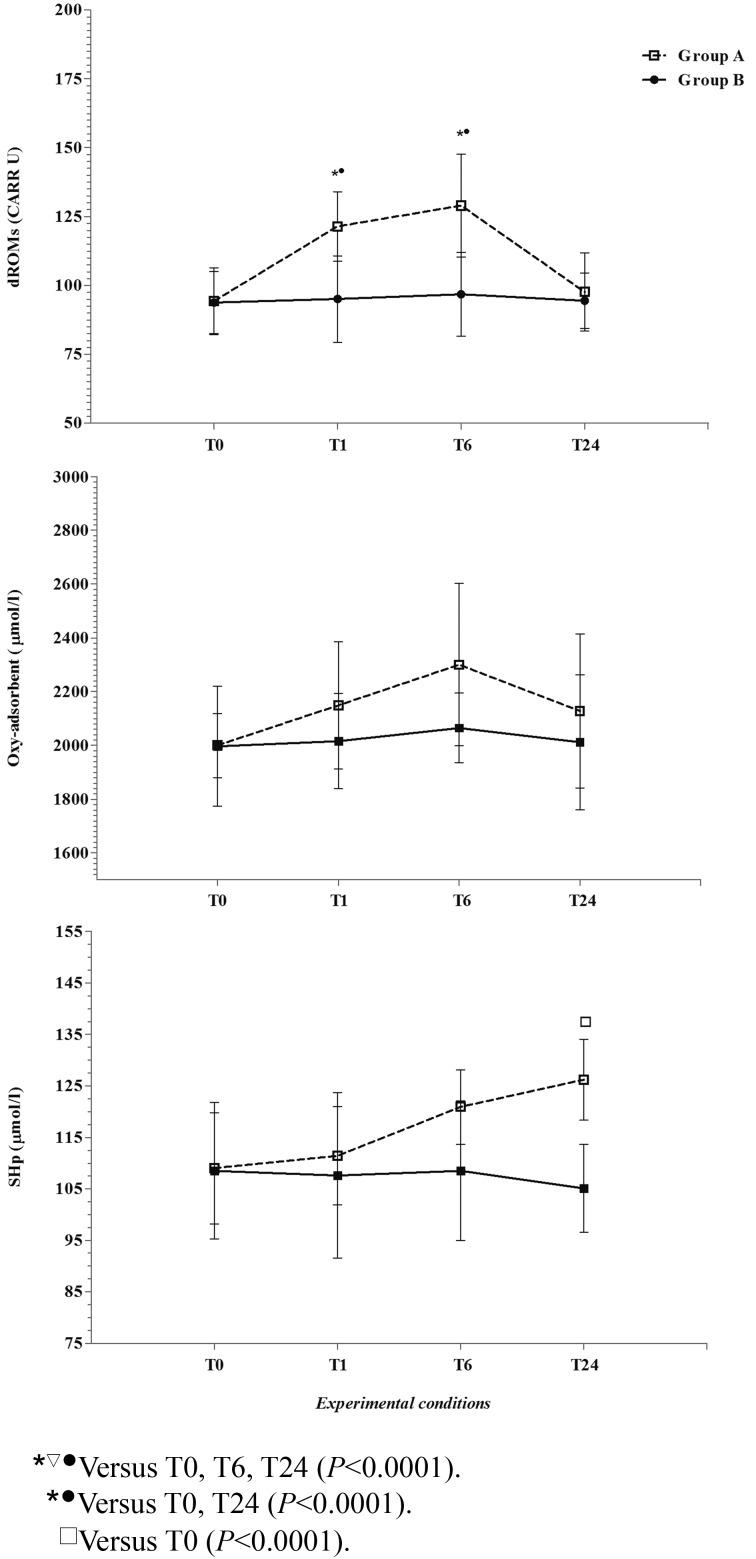
Application of the GLM revealed a significant differences between Groups A and B (P<0.0001) on Hp, SAA, CRP, WBC, ROMs, Oxy-adsorbent and SHp and for the influence of sampling time (P<0.0001) on Hp, SAA, CRP, WBC, ROMs and Oxy-adsorbent in Group A. Particularly, the Hp and SAA values showed a statistically significant increase at T1 with respect to T0, T6 and T24, whereas the CRP and WBC values showed a statistically significant increase at T1 and T6 with respect to T0 and T24. Also, the ROMs values were statistically increased at T1 and T6 with respect to T0 and T24, and SHp increased gradually, with significant values at T24 with respect to T0.
Figures 1 and 2 show average values for all studied parameters, expressed in conventional units of measurement with SD, measured during the experimental period in the dogs.
Fig. 1.
The patterns of the mean values (±SD) of haptoglobin (Hp), serum amyloid A (SAA), C-reactive protein (CRP), and white blood cells (WBC) together with the statistical significances obtained in Group A (dogs subjected to transportation) and in Group B (dogs not subjected to transportation) during the experimental period (T0, before the transportation; T1, immediately after 3 hours of transportation; T6, more than 6 hours after the end of transportation; T24, more than 24 hours after the end of transportation).
Fig. 2.
The patterns of the mean values (±SD) reactive oxygen metabolites (ROMs), antioxidant barrier (Oxy-adsorbent), and thiol groups of plasma compounds (SHp) together with the statistical significances obtained in Group A (dogs subjected to transportation) and in Group B (dogs not subjected to transportation) during the experimental period (T0, before the transportation; T1, immediately after 3 hours of transportation; T6, more than 6 hours after the end of transportation; T24, more than 24 hours after the end of transportation).
Discussion
The analysis of the results obtained in the present study indicated that there was an influence of road transportation on the studied APPs and on antioxidant mechanisms in the dogs.
As shown in Fig. 1, the Hp, SAA and CRP concentrations showed a high increase after road transportation, together WBC values, with the increase maintained after 6 h and a subsequent decline during the recovery period. Although SAA and CRP are known to be major APPs in the dog and Hp is considered a minor APP, all parameters showed this increase. Our results are in agreement with those of some researchers who emphasized the role of APPs not only in inflammation but also under some conditions such as road transportation, which can be highly stressful and compromise the animal’s welfare [6, 14, 29]. So, the significant increase of Hp, SAA and CRP during the experimental period might not be associated with pathological conditions but could be due to a physical “stress” resulting in numerous cellular changes that occur after transportation.
Regarding the WBCs values, since APPs have bacteriostatic and immunomodulatory effects [8], the changes in WBCs could represent an initial immune system’s response linked to the increase in the studied APPs. The simultaneous changes in APPs and WBCs could just be the result of cortisol release, as steroids are known to affect leukocytes and have a permissive effect on hepatic APP synthesis [8]. As previously demonstrated, the WBC number changes in response to transport stress [32], and the changes are associated with an increase in neutrophils and a decrease in lymphocytes [13, 18].
Moreover, the pattern of oxidative parameters, characterized by a significant increase after transportation, is in agreement with the findings of other researchers who reported that external stress factors can lead to increased generation of free radicals and other reactive oxygen [18]. In fact, as previously demonstrated in dromedaries, higher concentrations of oxidative status biomarkers after transportation may be explained by higher levels of glucocorticoids and adrenaline-induced pathways of aerobic energy production associated with stress, which generate reactive oxygen metabolites [26]. In fact, when animals are subjected to stressful conditions, such as road transportation, the hypothalamic-pituitary-adrenal axis is active, and glucocorticoids are released from the adrenal cortex [21]. Moreover, transportation induced changes in the oxidant/antioxidant equilibrium that could be essentially due to increased mitochondrial electron transport within muscle cells. Endogenous and exogenous antioxidants counterbalance the oxidative processes and so maintain the oxidant/antioxidant equilibrium [20].
In conclusion, the obtained results showed that road transportation has an important impact on the influence of the oxidant-antioxidant balance and APPs in the dog. The responses of APPs and changes in oxidative parameters represent a reaction in which the physiological and homeostatic mechanisms are modified differently at various sampling times confirming the complexity of variations due to road transportation. So, these findings concerning the studied parameters should be of concern for the health and welfare of dogs.
References
- 1.Adenkola A.Y., Ayo J.O.2010. Physiological and behavioural response of livestock to road transportation stress: A review. Afr. J. Biotechnol. 9: 4845–4856. [Google Scholar]
- 2.Beerda B., Schilder M.B., Bernadina W., van Hooff J.A., de Vries H.W., Mol J.A.1999. Chronic stress in dogs subjected to social and spatial restriction. II. Hormonal and immunological responses. Physiol. Behav. 66: 243–254. doi: 10.1016/S0031-9384(98)00290-X [DOI] [PubMed] [Google Scholar]
- 3.Bergeron R., Scott S.L., Èmond J.P., Mercier F., Cook N.J., Schaefer A.L.2002. Physiology and behavior of dogs during air transport. Can. J. Vet. Res. 66: 211–216. [PMC free article] [PubMed] [Google Scholar]
- 4.Broom D.M.2003. Transport stress in cattle and sheep with details of physiological, ethological and other indicators. Dtsch. Tierarztl. Wochenschr. 110: 83–89. [PubMed] [Google Scholar]
- 5.Broom D.M.2008. The welfare of livestock during road transport. pp. 157–181. In: Long distance transport and the welfare of farm animals, Ed., Cabi, Wallingford. [Google Scholar]
- 6.Casella S., Fazio F., Giannetto C., Giudice E., Piccione G.2012. Influence of transportation on serum concentrations of acute phase proteins in horse. Res. Vet. Sci. 93: 914–917. doi: 10.1016/j.rvsc.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 7.Cerón J.J., Eckersall P.D., Martýnez-Subiela S.2005. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet. Clin. Pathol. 34: 85–99. doi: 10.1111/j.1939-165X.2005.tb00019.x [DOI] [PubMed] [Google Scholar]
- 8.Cray C., Zaias J., Altman N.H.2009. Acute phase response in animals: a review. Comp. Med. 59: 517–526. [PMC free article] [PubMed] [Google Scholar]
- 9.Cray C.2012. Acute phase proteins in animals. Prog. Mol. Biol. Transl. Sci. 105: 113–150. doi: 10.1016/B978-0-12-394596-9.00005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early B., O’riordan E.G.2006. Effects on transporting bulls at different space allowance on physiological, haematological and immunological responses to 12-h journey by road. Ir. J. Agric. Food Res. 45: 39–50. [Google Scholar]
- 11.Eckersall P.D., Bell R.2010. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet. J. 185: 23–27. doi: 10.1016/j.tvjl.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 12.Friend T.H.2011. A review of recent research on the transportation of horses. J. Anim. Sci. 79: E32–E40. [Google Scholar]
- 13.Galipalli S., Gadiyaram K.M., Kouakou B., Terrill T.H., Kannan G.2004. Physiological responses to preslaughter transportation stress in Tasco-supplemented Boer goats. S. Afr. J. Anim. Sci. 34: 198–200. [Google Scholar]
- 14.Giannetto C., Fazio F., Casella S., Marafioti S., Giudice E., Piccione G.2011. Acute phase protein response during road transportation and lairage at a slaughterhouse in feedlot beef cattle. J. Vet. Med. Sci. 73: 1531–1534. doi: 10.1292/jvms.11-0157 [DOI] [PubMed] [Google Scholar]
- 15.González F.H.D., Tecles F., Martínez-Subiela S., Tvarijonaviciute A., Soler L., Cerón J.J.2008. Acute phase protein response in goats. J. Vet. Diagn. Invest. 20: 580–584. doi: 10.1177/104063870802000507 [DOI] [PubMed] [Google Scholar]
- 16.Goumon S., Brown J.A., Faucitano L., Bergeron R., Widowski T.M., Crowe T., Connor M.L., Gonyou H.W.2013. Effects of transport duration on maintenance behavior, heart rate and gastrointestinal tract temperature of market-weight pigs in 2 seasons. J. Anim. Sci. 91: 4925–4935. doi: 10.2527/jas.2012-6081 [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B.1996. Vitamin C: antioxidant or pro-oxidant in vivo? Free Radic. Res. 25: 439–454. doi: 10.3109/10715769609149066 [DOI] [PubMed] [Google Scholar]
- 18.Idrus Z., Bahyuddin N., Wai C.Y., Farjam A.S., Sazili A.Q., Rajion M.A., Meng G.Y.2010. Physiological responses in goats subjected to road transportation under the hot, humid tropical conditions. Int. J. Agr. Biol. 12: 840–844. [Google Scholar]
- 19.Khadija A., Ati A., Mohammed A., Saad A.M., Mohamed H.E.2009. Response of broiler chicks to dietary monosodiumglutamate. Pak.Vet. J. 29: 165–168. [Google Scholar]
- 20.Kirschvink N., de Moffarts B., Lekeux P.2008. The oxidant/antioxidant equilibrium in horses. Vet. J. 177: 178–191. doi: 10.1016/j.tvjl.2007.07.033 [DOI] [PubMed] [Google Scholar]
- 21.Liu H.W., Zhong R.Z., Zhou D.W., Sun H.X., Zhao C.S.2012. Effects of lairage time after road transport on some blood indicators of welfare and meat quality traits in sheep. J. Anim. Physiol. Anim. Nutr. (Berl.) 96: 1127–1135. doi: 10.1111/j.1439-0396.2011.01230.x [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Subiela S., Cerón J.J.2005. Validation of commercial assays for the determination of haptoglobin, C-reactive protein and serum amyloid A in dogs. Arch. Med. Vet. 37: 61–66. [Google Scholar]
- 23.McCord J.M.2000. The evolution of free radicals and oxidative stress. Am. J. Med. 108: 652–659. doi: 10.1016/S0002-9343(00)00412-5 [DOI] [PubMed] [Google Scholar]
- 24.Murata H., Shimada N., Yoshioka M.2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet. J. 168: 28–40. doi: 10.1016/S1090-0233(03)00119-9 [DOI] [PubMed] [Google Scholar]
- 25.Murata H.2007. Stress and acute phase protein response: an inconspicuous but essential linkage. Vet. J. 173: 473–474. doi: 10.1016/j.tvjl.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 26.Nazifi S., Saeb M., Baghshani H., Saeb S.2009. Influence of road transportation during hot summer conditions on oxidative status biomarkers in Iranian dromedary camels (Camelus dromedaries). Afr. J. Biochem. Res. 3: 282–287. [Google Scholar]
- 27.Ochi T., Nishiura I., Tatsumi M., Hirano Y., Yahagi K., Sakurai Y., Sudo Y., Koyama H., Hagita Y., Fujimoto Y., Kitamura S., Hashimoto H., Nakamura T., Yamada A., Tanimoto M., Nishina N.2013. Effects of transport stress on serum alkaline phosphatase activity in beagle dogs. Exp. Anim. 62: 329–332. doi: 10.1538/expanim.62.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paltrinieri S.2013. Oxidative stress and canine leishmaniasis: more than a simple consequence of host-parasite interaction. Vet. J. 198: 547–548. doi: 10.1016/j.tvjl.2013.09.071 [DOI] [PubMed] [Google Scholar]
- 29.Piccione G., Casella S., Giannetto C., Giudice E., Fazio F.2012. Utility of acute phase proteins as biomarkers of transport stress in ewes. Small Rumin. Res. 107: 167–171. doi: 10.1016/j.smallrumres.2012.05.008 [DOI] [Google Scholar]
- 30.Piccione G., Casella S., Bazzano M., Giudice E., Fazio F.2013. Oxidative stress associated with road transportation in ewes. Small Rumin. Res. 112: 235–238. doi: 10.1016/j.smallrumres.2012.11.001 [DOI] [Google Scholar]
- 31.Pregel P., Bollo E., Cannizzo F.T., Biolatti B., Contato E., Biolatti P.G.2005. Antioxidant capacity as a reliable marker of stress in dairy calves transported by road. Vet. Rec. 156: 53–54. [DOI] [PubMed] [Google Scholar]
- 32.Buckham Sporer K.R., Weber P.S.D., Burton J.L., Earley B., Crowe M.A.2008. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress. J. Anim. Sci. 86: 1325–1334. doi: 10.2527/jas.2007-0762 [DOI] [PubMed] [Google Scholar]
- 33.Stull C.L., Rodiek A.V.2000. Physiological responses of horses to 24 hours of transportation using a commercial van during summer conditions. J. Anim. Sci. 78: 1458–1466. [DOI] [PubMed] [Google Scholar]
- 34.Todd S.E., Mellor D.J., Stafford K.J., Gregory N.G., Bruce R.A., Ward R.N.2000. Effects of food withdrawal and transport on 5- to 10-day-old calves. Res. Vet. Sci. 68: 125–134. doi: 10.1053/rvsc.1999.0345 [DOI] [PubMed] [Google Scholar]
- 35.Trevisan M., Browne R., Ram M., Muti P., Freudenheim J., Carosella A.M., Armstrong D.2001. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 154: 348–356. doi: 10.1093/aje/154.4.348 [DOI] [PubMed] [Google Scholar]
- 36.Tvarijonaviciute A., Martinez S., Gutierrez A., Ceron J.J., Tecles F.2011. Serum acute phase proteins concentrations in dogs during experimentally short-term induced overweight. A preliminary study. Res. Vet. Sci. 90: 31–34. doi: 10.1016/j.rvsc.2010.05.008 [DOI] [PubMed] [Google Scholar]