Abstract
An imbalance between production of reactive oxygen species (ROS) and its elimination by antioxidant defense system in the body has been implicated for causes of aging and neurodegenerative diseases. This study was design to assess the changes in activities of antioxidant enzymes (superoxide dismutase (SOD), glutathione-S-transferase (GST), catalase), lipid peroxidation and reduced glutathione (GSH) levels in the brain of 2, 10 and 20 month old rats, and to determine the effect of safranal on the status of selected oxidative stress indices in the 10 and 20 month old rats. The aged rats (10 and 20 months) were given intraperitoneal injections of safranal (0.5 mg/kg day) daily for one month. The results of this study demonstrated that aging caused significant increase in the level of lipid peroxidation as well decrease in the GSH level and activities of SOD and GST in the brain of aging rats. The results of this study showed that safranal ameliorated the increased lipid peroxidation level as well as decreased GSH content of the brain of 10 and 20 month old rats. In addition, safranal treatment to the 20 month old rats, which restored the SOD and GST activities. In conclusion, safranal can be effective to protect susceptible aged brain from oxidative damage by increasing antioxidant defenses.
Keywords: aged, brain, oxidative damage, safranal
Introduction
Oxidative stress is recognized as an important mechanism underlying aging and neurodegenerative diseases [3, 17]. Besides pathological hallmarks, brain failures exhibit clear evidence of oxidative damage. Today, data from studies using human and animal models support the concept that oxidative imbalance and subsequent oxidative stress are among the earliest events in the pathogenesis of neurodegenerative diseases [26]. Thus, an increase in lipid peroxidation, protein oxidation and DNA oxidation has been reported in nervous system diseases. Similarly, biochemical evidence of brain pathological conditions for these signatures of oxidative stress has been shown in animal models [25]. There is now strong confirmation to link an increase in oxidative damage to lipids (lipid peroxidation), proteins, and nucleic acids in the brain tissue with aging [38, 41], although some studies have failed to indorse these findings [5]. These conclusions have led to the notion that antioxidant defense mechanisms in the brain are not sufficient to prevent age-related increases in oxidative damage and that dietary intake of a variety of antioxidants might be beneficial for preserving brain function [4, 24, 27]. Malondialdehyde (MDA), the most abundant aldehyde resulting from lipid peroxidation, shows important changes in tissue with aging [1]. Enzymatic and non-enzymatic antioxidants during aging constitutes defense system to clear up reactive oxygen species (ROS) in brain tissue [19, 35]. However, a good diet strategy may be effective in preventing age-related disease. Several drugs and food supplements have been shown to retard or reverse the biological effects of aging in animal models [7, 12, 35]. Recently, natural antioxidants have received growing attention as a potential preventive agent by scavenging ROS and detoxifying potent genotoxic oxidants [10]. Saffron, the most expensive spice in the world is derived from Crocus sativus stigmas. This spice has increased in its human applications and commercial value. Italy accompanied with Iran, Spain, India, Greece, Azerbaijan and Morocco, are the ones of world saffron producer [9, 11, 22, 29]. Biomedical data has been demonstrated that saffron and its ingredients may be fruitful as a treatment for neurodegenerative disorders and the related memory impairments, ischemic retinopathy and/or age-related macular degeneration, coronary artery diseases, blood pressure abnormalities, acute and/or chronic inflammatory diseases, mild to moderate depression, seizure and Perkinsonism [10, 18, 19, 21, 32]. Furthermore, antioxidant, antimutagenic, antigenotoxic, tumoricidal and antioxidant activities of saffron and its ingredient have been found [10, 18, 19, 30, 33, 34]. Safranal is one of the major active constituent of saffron and responsible for the characteristic quality of saffron [10, 15, 17, 33, 34]. It has been found that safranal has several pharmacological effects including anti-platelets, anti-oxidant, anti-tumor, anti-arthritic and anti-inflammatory [7, 10, 17,18,19, 21, 32, 33]. However, there are few studies in the literature investigating the effects of safranal treatment on oxidative stress in aged animals. Therefore, this study was design to investigate the effect of safranal on pro-oxidant and antioxidant status in the aged rats. For this reason, MDA levels as well as a non-enzymatic (reduced glutathione – GSH) [28] and activity of enzymatic antioxidants (superoxide dismutase – SOD, glutathione transferase – GST) were determined in the brains of 10 and 20 month old rats compared with the respective 2 month old control rats.
Materials and Methods
Chemicals
All purified enzymes, coenzymes, substrates, standards, buffers and kits were purchased from Sigma Chemicals Company, USA. Safranal and other chemicals were also supplied from Sigma-Aldrich Chemical (St. Louis, USA).
Study design
Ten male Wistar rats of different ages namely 2, 10, and 20 months were used for 2 different preparations (n=5), and (the number of samples in one group is 5). The average of life span of male Wistar rats is almost 25 months [2]. Accordingly, the percent lifespan of 2, 10, and 20 month old male Wistar rats are almost 8, 40 and 80% respectively.
Animals were obtained from the Center for Experimental Medical Research of Mashhad Medical University. The animals were kept at a constant temperature of 25°C, humidity of 55% at 8:00–20:00 h light, and 20:00–8:00 h dark cycle. The animals were fed standard chow (Javaneh Khorasan Ltd., Iran) until treatment or time of sacrifice. The animals were housed according to regulating the Walfare of experimented animals. The study was conducted in the Experimental Animal Research Laboratory of Mashhad Medical University. All the animal procedures were approved by the Institutional Animal Ethical Committee. The 10 and 20 month old rats were divided into two subgroups as untreated (vehicle) and safranal-treated old rats. The 10 and 20 month old rats were given intraperitoneal injection of safranal (0.5 mg/kg body weight) daily for one month. Control animals received an equal volume of vehicle (0.9% NaCl).
Biochemical analysis
Preparation of homogenates and subcellular fractions
After the 30 days of safranal treatment, the animals were sacrificed by cervical dislocation under the general anesthesia (pentobarbital (100 mg/kg) i.p.). The whole brains were dissected out, washed in 0.9% NaCl, weighed, and these samples were frozen in liquid nitrogen and kept at –80oC. The samples (whole brains) minced and homogenized in 9 times volume of cold isotonic sucrose buffer. The homogenizing buffer contained the following in the final concentration: 0.25 M sucrose and 0.02 M triethanolamine, pH 7.4, containing 0.12 mM dithiothreitol. The entire procedure was carried out at 0–4°C. The homogenate was centrifuged at 1,000 g for 10 min for removing the cell debris. The pellet was discarded and the supernatant was further centrifuged at 12,000 g for 20 min at 4°C in a refrigerated super-speed centrifuge to isolate the mitochondrial pellet. The obtained supernatant was centrifuged at 105,000 g for 65 min at 4°C in a ultracentrifuge to yield the cytosolic supernatant and microsomal pellet [31]. Whole homogenates were used for measurement of lipid peroxidation. Supernatant fraction that obtained by centrifuging at 1,000 g for 10 min was used for the estimation of GSH level and SOD activities and finally protein was estimated in the cytosolic and microsomal fractions.
Protein estimation
Protein concentration was estimated in the cytosolic and microsomal fractions by the method of Bradford using bovine serum albumin (BSA) as standard [8].
Measurements of enzymes
Measurement of lipid peroxidation and glutathione
The formation of lipid peroxides was measured in the homogenates of whole brain. The formation of MDA, an end product of fatty acid peroxidation was measured spectrophotometrically at 532 nm (Shimadzu UV-2550) by using a thiobarbituric acid reactive substance (TBARS) essentially by the method of Genet et al. [14]. Results are expressed as nmole of MDA formed/mg protein. Tissue total GSH levels were measured spectrophotometrically at 412 nm using 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) as the reagent [6, 37].
Assay of SOD
The activity of SOD was determined by the method of Marklund and Marklund [23], using inhibition of pyrogallol autoxidation at pH 8. The specific activity of SOD is expressed as units per mg protein per minute [23].
Assay of GST
Tissue homogenates Assay of GST activity was determined in the post-mitochondrial fraction of tissues, which was separated by the sequential centrifugation. In brief, they were centrifuged at 600 g for 10 min at 4oC to remove crude fractions. Then, the supernatants were centrifuged at 10,000 g for 20 min to obtain the post-mitochondrial fraction. The GST activities were measured using cumene hydroperoxide and 1-chloro-2, 4-dinitrobenzene as substrates [36, 39].
Statistical analysis
Data were analyzed using one-way ANOVA by InStat 3.0 program followed by Tukey-Kramer post-hoc test for multiple comparisons. Kolmogorov Smirnov tests showed that the data was normally distributed. The evaluation was made by the comparison of groups. The results were presented as means ± SEM and P<0.05 was considered significant.
Results
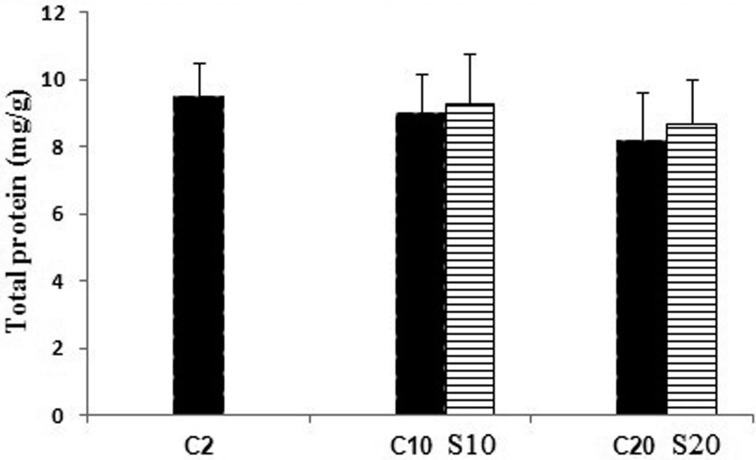
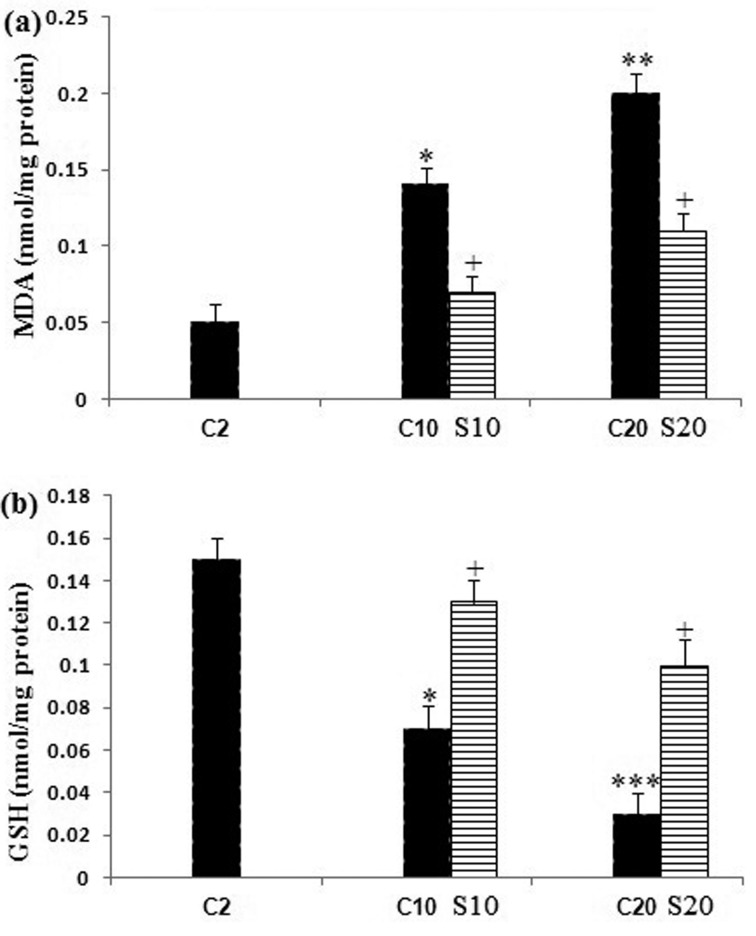
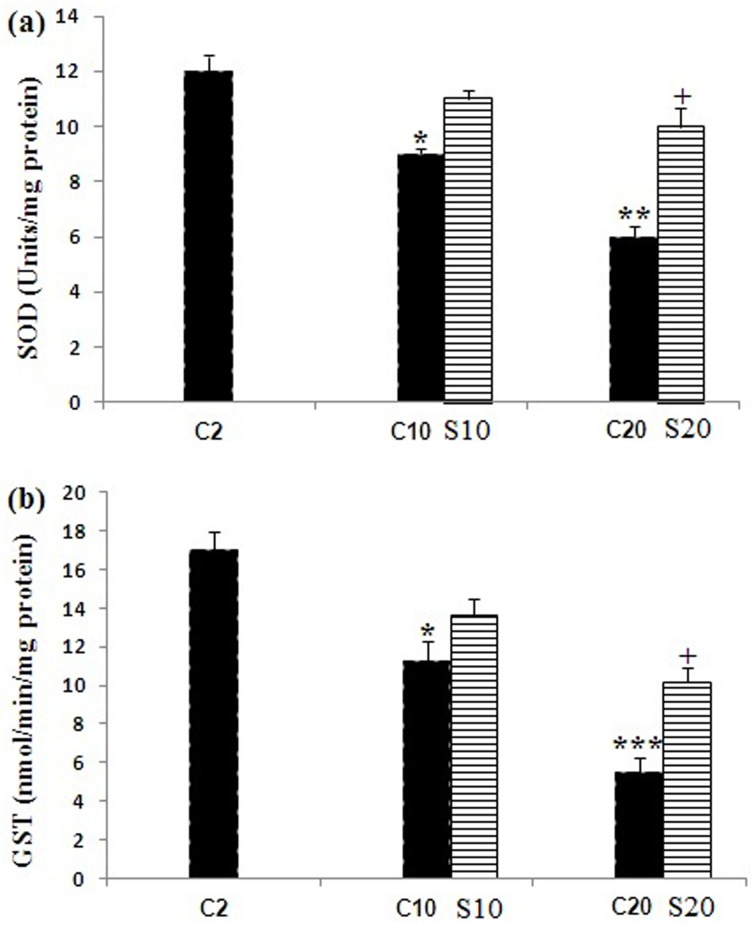
Changes in protein content are shown in (Fig. 1). The protein content in the pellet fractions did not statistically show significant changes with aging and safranal treatment when compared with the 2 month old rats. Lipid peroxidation was measured as the formation of MDA in the whole homogenates of aging rat brain from the untreated and safranal-treated aging animals. Our data showed a significant increase (P<0.05, P<0.01) in the MDA levels in the 10 and 20 month old rats as compared with the 2 month old control rats; whereas safranal-treated aging in the 10 and 20 month old rats had a significantly (P<0.05) decreased in the brain MDA level as compared with the 2 and 10 month old rats. Significant decreases (P<0.05 and P<0.001) in the GSH content were detected in brain homogenates of aging 10 and 20 month old rats when compared with the 2 month old control rats. Safranal treatment increased the GSH content in supernatant of whole homogenates in the 10 and 20 month old rats compared with the untreated 10 and 20 month old rats (P<0.05) (Fig. 2b). Changes in the activities of SOD and GST in the brain of the untreated 2, 10, and 20 month old rats and safranal-treated the 10 and 20 month old rats are summarized in Fig. 3. In the untreated 10 and 20 month old rats a significant (P<0.05 and P<0.01, respectively) a decrease was seen in the SOD activity when compared with 2 month old control rats. Treatment of safranal to the aging animals increased the SOD activity in 20 month old rats, when compared with respective age-matched controls (P<0.05) (Fig. 3a).
Fig. 1.
Protein concentration of brain of the untreated 2, 10 and 20 month old rats (C) and safranal (S) treated 10 and 20 month old rats.
Fig. 2.
Changes in (a) lipid peroxidation (MDA) and (b) reduced glutathione (GSH) levels in brain of the untreated 2, 10 and 20 month old rats (C) and safranal (S) treated 10 and 20 month old rats. Values are presented as mean ± SEM. The comparisons of experimental values are with the control values. Statistical significance + P<0.05, comparing age-matched controls versus safranal treatment and *P<0.05, **P<0.01 and ***P<0.001 versus the 2 month old control rats.
Fig. 3.
Changes in the activities of antioxidant enzymes (a) superoxide dismutase (SOD) and (b) glutathione S-transferase (GST) in brain of the untreated 2, 10, and 20 month old rats (C) and safranal (S) treated 10 and 20 month old rats. Values are presented as mean ± SEM.The comparisons of experimental values are with the control values. Statistical significance + P<0.05, comparing age-matched controls versus safranal treatment and *P<0.05, **P<0.01 and ***P<0.001 versus the 2 month old rats.
In the untreated 10 and 20 month old rats there was a significant (P<0.05 and P<0.001) decrease in the GST activity when compared with the 2 month old rats. When compared with the respective age control group, an increased of the GST activity at 20 month safranal-treated animals was seen (P<0.05) (Fig. 3b).
Discussion
In the current study, association between aging and increasing level of oxidation was evaluated by measuring the endogenous MDA and GSH levels as well as enzymatic antioxidants in brain homogenate of aging rats (10 and 20 month old rats). Our data showed that the increased endogenous MDA and decreased SOD, GST and GSH level ameliorate after the treatment of safranal in the 10 and 20 month old rats. Therefore, in this study, we investigated the anti-aging and protective potential of safranal treatment on the activities of antioxidant enzymes (SOD, GST), lipid peroxidation and GSH levels in the brain of aging rats. Our study confirmed that the GSH content, the SOD, and GST activities in brain were significantly higher in the 2 month old rats than the 10 and 20 month old rats. Furthermore, current results showed that lipid peroxidation level was lower in the 2 month old rats than the 10 and 20 month old rats. Previous studies performed in the rat brain have shown an elevation in the level of lipid peroxidation with a reduction in the GSH content and the activity of antioxidant enzyme during aging [35, 42]. These results are fully in agreement with those obtained in the present work. These studies suggest that the age-related degeneration of brain tissue in the rat may be due to a rise in free radical production in the mitochondria [35], and difference in results of studies might be related to variations in species, strain, sex, and experimental design [43]. In this study, it was determined that the MDA level was significantly lower in the safranal treated rats compared to the untreated 10 and 20 month old rats. In addition, these results indicated that the GSH content was higher in the safranal treated rats compared with the untreated 10 and 20 month old rats. The brain is rich in lipids and especially in polyunsaturated fatty acids, which is very sensitive to peroxidation [24, 25]. The MDA content is formed through the peroxidation of unsaturated fatty acids and widely used as an index of biogenic macromolecules, particularly lipid peroxidation [43]. In mammals GSH activity as mainly endogen antioxidant controls the production of ROS [13]. An increase in GSH level signifies that ROS and oxidative stress are decreasing what probably protects the tissues and organs against oxidative stress [25]. In this study, it was determined that the brain SOD and GST levels were significantly higher in the 2 month old control rats when compared with the untreated 10 and 20 month old rats. The brain SOD and GST levels were significantly increased in the safranal treated 20 month old rats when compared with the 2 month old control rats. It has been found that the SOD activity decreased in rat liver, brain, heart, kidney, and uterus [40] during aging and this component is an important defense system to clear up ROS in vivo [16, 20]. GST belongs to a group of multigene and multifunctional detoxification enzymes, which defend cells against a wide variety of toxic insults from chemicals, metabolites, and oxidative stress [39, 44]. The lower activities of SOD and GST in the untreated 10 and 20 month old rats may be a consequence of inhibitory effects due to down-regulation phenomenon or excess ROS generation. Safranal may also inhibit lipid peroxidation by inducing GST and SOD.
Our observations confirmed that safranal may be effective to control of age related tissue damage by decrease in free radicals generation, increase in antioxidant defenses and restore SOD and GST activities in brain. The results of our previous study also demonstrated that safranal could be a candidate to suppress the development of age-induced liver damage by protecting against oxidative stress and increasing antioxidant defenses. Farahmand and co-workers showed that safranal inhibited lipid peroxidation by the elevation GST and SOD activities [10]. Safranal is the major active constituent of Crocus sativus, owing to its strong antioxidant, could prevent tissue damages in animal modeling. It was reported that it could be a valuable molecule in alleviating myocardial ischemia-reperfusion (IR) injury by normalizing myocardial antioxidant [7]. Hosseinzadeh and co-workers demonstrated that safranal ameliorate the ischemia-reperfusion injury (IRI)-induced oxidative damage in rat hippocampus by elevating total sulfhydryl contents, antioxidant capacity and decreasing malondialdehyde [19]. They also reported that safranal had protective effects against the skeletal muscle injury during ischemia-reperfusion by elevating total sulfhydryl contents, antioxidant capacity and decreasing malondialdehyde [18]. Another study indicated that safranal may prevent the gastric mucosa damage due to their antioxidant properties by increasing the gluthatione levels and diminishing the lipid peroxidation in the rat gastric mucosa [21].
In summery, aging generated higher oxidative stress in the rat brain, by decreasing GSH level and suppressing the SOD and GST activities, while increasing the lipid peroxidation. Safranal found effective in enhancing the levels of GSH, SOD and GST accompanied with decreasing lipid peroxidation. Thus, safranal can be effective to protect susceptible brain aging from oxidative damage by increasing antioxidant defenses. There is another confirmation to use of antioxidant as a health beneficial food component during aging.
Conflict of Interest
The authors report no declarations of interest.
Acknowledgments
The authors would like to thank Research Affairs of Neyshabur University of Medical Sciences for financially supporting this work.
References
- 1.Akbulut H., Akbulut K.G., Gonul B. 1997. Age-related changes in malondialdehyde and glutathione levels of gastric mocosa of the rats and effects of exogenous melatonin. Dig. Diss. Sci. 42: 1381–1382. [DOI] [PubMed] [Google Scholar]
- 2.Altun M., Bergman E., Edström E., Johnson H., Ulfhake B.2007. Behavioral impairments of the aging rat. Physiol. Behav. 92: 911–923. doi: 10.1016/j.physbeh.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 3.Arivazhagan P., Thilakavathy T., Ramanathan K., Kumaran S., Panneerselvam C.2002. Effect of DL-α-lipoic acid on the status of lipid peroxidation and protein oxidation in various brain regions of aged rats. J. Nutr. Biochem. 13: 619–624. doi: 10.1016/S0955-2863(02)00217-6 [DOI] [PubMed] [Google Scholar]
- 4.Baquer N.Z., Taha A., Kumar P., McLean P., Cowsik S.M., Kale R.K., Singh R., Sharma D.2009. A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology 10: 377–413. doi: 10.1007/s10522-009-9226-2 [DOI] [PubMed] [Google Scholar]
- 5.Barja de Quiroga G., Pérez-Campo R., López Torres M.1990. Anti-oxidant defences and peroxidation in liver and brain of aged rats. Biochem. J. 272: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler E., Duron O., Kelly B.M.1963. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61: 882–888. [PubMed] [Google Scholar]
- 7.Bharti S., Golechha M., Kumari S., Siddiqui K.M., Arya D.S.2012. Akt/GSK-3β/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia-reperfusion injury in rats. Eur. J. Nutr. 51: 719–727. doi: 10.1007/s00394-011-0251-y [DOI] [PubMed] [Google Scholar]
- 8.Bradford M.M.1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 9.D’Archivio A.A., Giannitto A., Incani A., Nisi S.2014. Analysis of the mineral composition of Italian saffron by ICP-MS and classification of geographical origin. Food Chem. 157: 485–489. doi: 10.1016/j.foodchem.2014.02.068 [DOI] [PubMed] [Google Scholar]
- 10.Farahmand S.K., Samini F., Samini M., Samarghandian S.2013. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology 14: 63–71. doi: 10.1007/s10522-012-9409-0 [DOI] [PubMed] [Google Scholar]
- 11.Festuccia C., Mancini A., Gravina G.L., Scarsella L., Llorens S., Alonso G.L., Tatone C., Di Cesare E., Jannini E.A., Lenzi A., D’Alessandro A.M., Carmona M.2014. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. Biomed. Res. Int. 2014: 135048. doi: 10.1155/2014/135048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusco D., Colloca G., Lo Monaco M.R., Cesari M.2007. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2: 377–387. [PMC free article] [PubMed] [Google Scholar]
- 13.Gałecka E., Mrowicka M., Malinowska K., Gałecki P.2008. [Chosen non-enzymatic substances that participate in a protection against overproduction of free radicals]. Pol. Merkuriusz Lek. 25: 269–272 (in Polish). [PubMed] [Google Scholar]
- 14.Genet S., Kale R.K., Baquer N.Z.2002. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek (Trigonellafoenum graecum). Mol. Cell. Biochem. 236: 7–12. doi: 10.1023/A:1016103131408 [DOI] [PubMed] [Google Scholar]
- 15.Geromichalos G.D., Lamari F.N., Papandreou M.A., Trafalis D.T., Margarity M., Papageorgiou A., Sinakos Z.2012. Saffron as a source of novel acetylcholinesterase inhibitors: molecular docking and in vitro enzymatic studies. J. Agric. Food Chem. 60: 6131–6138. doi: 10.1021/jf300589c [DOI] [PubMed] [Google Scholar]
- 16.Haddadi M., Jahromi S.R., Sagar B.K., Patil R.K., Shivanandappa T., Ramesh S.R.2014. Brain aging, memory impairment and oxidative stress: a study in Drosophila melanogaster. Behav. Brain Res. 259: 60–69. doi: 10.1016/j.bbr.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 17.Harman D.1981. The ageing process: major risk factor for disease and death. Proc. Natl. Acad. Sci. USA 78: 7124–7128. doi: 10.1073/pnas.78.11.7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H., Modaghegh M.H., Saffari Z.2009. Crocus sativus L. (Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid. Based Complement. Alternat. Med. 6: 343–350. doi: 10.1093/ecam/nem125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseinzadeh H., Sadeghnia H.R.2005. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 8: 394–399. [PubMed] [Google Scholar]
- 20.Keaney M., Matthijssens F., Sharpe M., Vanfleteren J., Gems D.2004. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 37: 239–250. doi: 10.1016/j.freeradbiomed.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 21.Kianbakht S., Mozaffari K.2009. Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. J. Med. Plants 8: 30–38. [Google Scholar]
- 22.Makri O.E., Ferlemi A.V., Lamari F.N., Georgakopoulos C.D.2013. Saffron administration prevents selenite-induced cataractogenesis. Mol. Vis. 19: 1188–1197. [PMC free article] [PubMed] [Google Scholar]
- 23.Marklund S., Marklund G.1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47: 469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Lara E., Siles E., Hernández R., Cañuelo A.R., Luisa del Moral M., Jiménez A., Blanco S., López-Ramos J.C., Esteban F.J., Pedrosa J.A., Peinado M.A.2003. Glutathione S-transferase isoenzymatic response to aging in rat cerebral cortex and cerebellum. Neurobiol. Aging 24: 501–509. doi: 10.1016/S0197-4580(02)00139-2 [DOI] [PubMed] [Google Scholar]
- 25.Mecocci P.2004. Oxidative stress in mild cognitive impairment and Alzheimer disease: a continuum. J. Alzheimers Dis. 6: 159–163. [DOI] [PubMed] [Google Scholar]
- 26.Praticò D., Delanty N.2000. Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am. J. Med. 109: 577–585. doi: 10.1016/S0002-9343(00)00547-7 [DOI] [PubMed] [Google Scholar]
- 27.Praticò D., Clark C.M., Liun F., Rokach J., Lee V.Y., Trojanowski J.Q.2002. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch. Neurol. 59: 972–976. doi: 10.1001/archneur.59.6.972 [DOI] [PubMed] [Google Scholar]
- 28.Richie J.P., Jr1992. The role of glutathione in aging and cancer. Exp. Gerontol. 27: 615–626. doi: 10.1016/0531-5565(92)90015-R [DOI] [PubMed] [Google Scholar]
- 29.Samarghandian S., Tavakkol Afshari J., Davoodi S.2011. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl. Biochem. Biotechnol. 164: 238–247. doi: 10.1007/s12010-010-9130-x [DOI] [PubMed] [Google Scholar]
- 30.Samarghandian S., Boskabady M.H., Davoodi S.2010. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn. Mag. 6: 309–314. doi: 10.4103/0973-1296.71799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samarghandian S., Borji A.2014. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 6: 99–107. doi: 10.4103/0974-8490.128963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samarghandian S., Borji A., Delkhosh M.B., Samini F.2013. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J. Pharm. Pharm. Sci. 16: 352–362. [DOI] [PubMed] [Google Scholar]
- 33.Samarghandian S., Shabestari M.M.2013. DNA fragmentation and apoptosis induced by safranal in human prostate cancer cell line. Indian J. Urol. 29: 177–183. doi: 10.4103/0970-1591.117278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samarghandian S., Borji A., Farahmand S.K., Afshari R., Davoodi S.2013. Crocus sativus L. (saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. Biomed. Res. Int. 2013: 417928. doi: 10.1155/2013/417928 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Sawada M., Carlson J.C.1987. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech. Ageing Dev. 41: 125–137. doi: 10.1016/0047-6374(87)90057-1 [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui M.R., Taha A., Moorthy K., Hussain M.E., Basir S.F., Baquer N.Z.2005. Amelioration of altered antioxidant status and membrane linked functions by vanadium and Trigonella in alloxan diabetic rat brains. J. Biosci. 30: 483–490. doi: 10.1007/BF02703722 [DOI] [PubMed] [Google Scholar]
- 37.Singh S.P., Janecki A.J., Srivastava S.K., Awasthi S., Awasthi Y.C., Xia S.J., Zimniak P.2002. Membrane association of glutathione S-transferase mGSTA4-4, an enzyme that metabolizes lipid peroxidation products. J. Biol. Chem. 277: 4232–4239. doi: 10.1074/jbc.M109678200 [DOI] [PubMed] [Google Scholar]
- 38.Siqueira I.R., Fochesatto C., de Andrade A., Santos M., Hagen M., Bello-Klein A., Netto C.A.2005. Total antioxidant capacity is impaired in different structures from aged rat brain. Int. J. Dev. Neurosci. 23: 663–671. doi: 10.1016/j.ijdevneu.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 39.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C.1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. doi: 10.1016/0003-2697(85)90442-7 [DOI] [PubMed] [Google Scholar]
- 40.Sudheesh N.P., Ajith T.A., Ramnath V., Janardhanan K.K.2010. Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin. Nutr. 29: 406–412. doi: 10.1016/j.clnu.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 41.Tian L., Cai Q., Wei H.1998. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med. 24: 1477–1484. doi: 10.1016/S0891-5849(98)00025-2 [DOI] [PubMed] [Google Scholar]
- 42.Vanella A., Geremia E., D’Urso G., Tiriolo P., Di Silvestro I., Grimaldi R., Pinturo R.1982. Superoxide dismutase activities in aging rat brain. Gerontology 28: 108–113. doi: 10.1159/000212519 [DOI] [PubMed] [Google Scholar]
- 43.Vendemiale G., Grattagliano I., Altomare E.1999. An update on the role of free radicals and antioxidant defense in human disease. Int. J. Clin. Lab. Res. 29: 49–55. doi: 10.1007/s005990050063 [DOI] [PubMed] [Google Scholar]
- 44.Vyskocilova C., Szotakova B., Skalova L., Bartikováa H., Hlavacova J., Bousova I.2013. Age-Related Changes in Hepatic Activity and Expression of Detoxification Enzymes in Male Rats. BioMed Res. Int. 2013: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]