Abstract
In-feed Medication has been used for a long time to prevent coccidiosis, a worldwide protozoal disease in rabbits. Florfenicol (FFC) has been widely used in veterinary clinics for bacterial diseases treatment. Therefore, the use of combinations of coccidiostats with FFC in rabbits is common. In the present study, we aimed to evaluate the effect of three coccidiostats, sulfaquinoxaline (SUL), robenidine (ROB), and toltrazuril (TOL), as feed additives on the pharmacokinetic profile of FFC in rabbits. The disposition kinetics of FFC in rabbits were investigated after a single intravenous injection (25 mg/kg) in rabbits fed anticoccidial-free diets or feeds containing SUL (250 ppm), ROB (66 ppm), or TOL (2 ppm), respectively, for 20 days. Plasma FFC concentrations were determined by the high performance liquid chromatography (HPLC) method. The pharmacokinetic parameters of FFC were analyzed using a non-compartmental analysis based on the statistical moment theory. The results demonstrated that ROB feeding resulted in an obvious decrease in plasma FFC level as compared with anticoccidial-free feeding. The terminal elimination half-life (t1/2z), area under the concentration–time curve (AUC), area under the first moment curve (AUMC), and mean residence time (MRT) significantly decreased, whereas the elimination rate constant (λz) and total body clearance (CLz) obviously increased in rabbits pretreated with ROB. However, we did not find that SUL or TOL feeding had any effect on the pharmacokinetic profile of FFC. Our findings suggested that more attention should be paid to the use of FFC in rabbits supplemented with ROB.
Keywords: coccidiostats, drug-drug interactions, florfenicol, pharmacokinetics
Introduction
In poultry farms, two or more drugs, such as antimicrobial, antifungal, anticoccidial drugs, and growth promoters, were usually added to feeds to prevent and cure diseases [17]. The simultaneous use of multiple drugs may give rise to pharmcokinetic drug-drug interactions (DDIs). It is well-known that DDIs can cause changes in drug concentrations in the body, which may impair or exaggerate the proposed efficacy of the antibacterial drugs used for treatment of bacterial diseases. For example, it has been reported that continuous supplementation of the daily diet with small amounts of the anticoccidial-diclazuril or halofuginone in broiler chickens can affect the pharmcokinetic profile, tissue distribution, and efficacy of doxycycline, a common antibiotic used in poultry farms [10]. Coccidiosis is a common and worldwide protozoal disease in rabbits that can lead to large economic losses in the rabbit farming industry. Supplementation of feed with coccidiostats for prevention of coccidiosis in rabbits is very common [9]. In addition, antibiotic drugs are often added to the feed or administered directly to cure or prevent bacterial infection, a common disease in rabbits [8]. Therefore, combination of coccidiostats with other antimicrobials in rabbits is inevitable.
Florfenicol (FFC), a synthetic broad-spectrum antibiotic derived from chloramphenicol, has been widely used in veterinary clinics for treatment of bacterial diseases [2, 6, 16]. Previous researches have demonstrated that FFC has better antibacterial activity than chloramphenicol and thiamphenicol [7, 14] and has few adverse effects [22]. FFC also shows activity against many chloramphenicol-resistant bacterial strains involved in common infections in most animals [4, 7]. Recent studies have indicated that FFC can effectively inhibit the growth of Streptococcus agalactiae, an important pathogen resulting in a severe infectious disease in domestic rabbits that is resistant to penicillin, amoxicillin, and tetracycline [27]. The pharmacokinetics of FFC have been extensively studied in many species of animals [25], especially in rabbits [1, 18, 23]. The pharmacokinetic DDIs of FFC and other drugs such as anthelmintics (ivermectin, albendazole, and rafoxanide) in goats [3] or polyether ionophore antibiotics (salinomycin, monensin, and maduramycin) in broiler chickens [32] have also been investigated.
However, little is known about the DDIs of the pharmacokinetic profile between FFC and anticoccidial drugs in rabbits. At present, three coccidiostats, sulfaquinoxaline (SUL), robenidine (ROB), and toltrazuril (TOL), are routinely used as in-feed medications in rabbit cultivation [9]. In order to avoid the adverse effects of DDIs and provide guidance concerning rational use of drug combinations, the present study aimed to evaluate the effects of three coccidiostats (SUL, ROB, and TOL) as feed additives on the pharmacokinetic profile of FFC in rabbits during concomitant administration.
Material and Methods
Chemicals and reagents
SUL, ROB, TOL, and FFC were provided by Zhejiang Kangmu Pharmceutical Co., Ltd. (Shengzhou, Zhejiang, PR China). FFC was dissolved in polyethylene glycol-300 (TCI (Shanghai) Chemical Industry Co., Ltd., Shanghai, PR China) to a concentration of 50 mg/ml for intravenous administration. FFC analytical standards were purchased from Jiangsu Institute of Veterinary Drug Control (Nanjing, Jiangsu, PR China). Chloramphenicol (internal standard) analytical standards and high performance liquid chromatography (HPLC) grade acetonitrile and methanol were both obtained from ANPEL Scientific Instrument Co., Ltd. (Shanghai, PR China). All other reagents were of analytical grade or better.
Rabbits
Thirty-two healthy New Zealand white male rabbits (weight range 2–2.5 kg) were provided by Qing Long Shan Laboratory Animal Center (Nanjing, Jiangsu, PR China). Rabbits were maintained in the laboratory environment for one week, supplied with water, and fed pelleted feed free from any anticoccidial and antibiotic drugs ad libitum before experimentation. Blank, drug-free plasma samples were collected with sodium heparin anticoagulant from adult healthy rabbits and stored at −80°C. All procedures involving animals were in accordance with all regulations of the local ethical committee for research and animal experiments.
Experimental design
The rabbits were divided into four groups (n=8, each group). The rabbits in the control group were fed anticoccidial-free rations throughout the study. According to previous reports concerning the clinically relevant dose of coccidiostats, the rabbits in the other groups were fed rations containing SUL (250 ppm) [12], ROB (66 ppm) [24], or TOL (2 ppm) [21] for 20 consecutive days, respectively. At the end of the 20th day of feeding, a single dose of FFC was injected intravenously at 25 mg/kg body weight (b.w.) into the left auricular vein of each rabbit in all groups as described in a previous study [18]. Blood (approximately 1 ml) samples were collected into heparin-coated tubes from the right auricular vein of each rabbit at 5, 10, 15, 30, and 45 min and 1, 1.5, 2, 4, 6, 8, and 12 h after administration of FFC. The plasma was harvested after centrifugation at 3,000 g for 10 min and stored at −20°C until analysis.
Analytical method
Plasma sample preparation was performed as described in a previous study [19] with a slight modification. Briefly, a 200 µl aliquot of thawed plasma in a 1.5 ml centrifuge tube was spiked with 5 µg of chloramphenicol (internal standard) in 5 µl methanol and added to 800 µl of ethyl acetate, and it was then mixed vigorously for 5 min on a vortex mixer, followed by centrifugation at 3,000 g for 10 min at room temperature. The supernatant was transferred to a new tube, and the subnatant was re-extracted with 800 µl ethyl acetate solution to collect the extract again. The pooled supernatant was evaporated to dryness under a flow of nitrogen at 40°C. The residue was dissolved in 400 µl mobile phase, and centrifuged at 11,200 g for 10 min at 4°C. Finally, a volume of 50 µl of the supernatant was injected into the HPLC system. Chromatography separations were performed with an Agilent 1100 series HPLC system (Agilent Technologies, Palo Alto, CA, USA) on a reverse phase Agilent Poroshell 120 EC-C18 column (4.6 × 50 mm, 2.7 µm particle size) and run with isocratic elution with a mobile phase composed of acetonitrile and water (25:75, v/v) at the flow rate of 1 ml/min. The detection wavelength was set at 224 nm, and the column temperature was 25°C.
A stock solution of FFC (1 mg/ml) was prepared accurately in acetonitrile and stored at −80°C. The calibration samples were determined with seven different concentrations (0.1, 0.5, 1, 2.5, 5, 10, and 25 µg/ml) prepared by spiking 20 µl of the appropriate FFC working solution, the dilution of stock solution with mobile phase, into 180 µl of blank rabbit plasma. Quality control (QC) samples were prepared in the same manner at concentrations of 1, 5, and 10 µg/ml. The pretreatments and analyses performed for the calibration samples or QC samples were the same as those for the unknown plasma samples. The calibration curve for FFC was constructed by plotting the ratio of the peak area (FFC to chloramphenicol) against the nominal concentration of the calibration standards.
Method validation
The specificity of the method was tested by comparing chromatograms of blank plasma samples collected from six different rabbits with the QC samples and unknown plasma samples after administration of FFC to note the absence of interference in the elution position of FFC. Linearity was assessed at the analyte concentration ranging from 0.1 to 25 µg/ml. The correlation coefficient (r2) of the calibration curve was calculated using linear regression analysis that required a correlation coefficient of not less than 0.99. The limit of quantitation (LOQ) was defined as the lowest concentration of the calibration curve and was calculated based on a signal-to-noise ratio (S/N) of 10. The intra- and inter-day assay precision and accuracy were estimated by analyzing six replicates of three QC samples (1, 5, and 10 µg/ml) in three different batches. The assay precisions were expressed as the relative standard deviation (RSD) calculated as follows: RSD (%)=[standard deviation/mean]×100. The accuracy was calculated as the percent recovery of the measured concentration relative to the nominal concentration. The criteria for acceptability of the data included accuracy of 85–115% and precision lower than 15%. The extraction recovery of analytes was expressed as the ratio of the mean peak area of three QC plasma samples to that of the analytes from neat standard samples at equivalent concentrations.
Pharmacokinetic and statistical analysis
The pharmacokinetic software DAS 2.0 (issued by the State Food and Drug Administration of China) was applied to calculate the pharmacokinetic parameters for FFC based on the statistical moment method, a non-compartmental method of analysis. Briefly, the elimination rate constant (λz) was estimated by linear regression of the terminal data points, and the terminal elimination half-life (t1/2z) was calculated with the equation t1/2z=0.693/λz. The area under the concentration–time curve (AUC) was calculated using the trapezoidal rule method. The total body clearance (CLz) was calculated from CLz=Dose/AUC. The area under the first moment curve (AUMC) was defined as the area under the product of the time and drug concentration–time curve and also calculated using the trapezoidal rule method. The mean residence time (MRT) was calculated with the equation MRT=AUMC/AUC, and the apparent steady-state volume of distribution (Vss) was calculated with the equation Vss=CLz·MRT.
Statistical analysis was performed with the SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The differences in main pharmacokinetic parameters between FFC alone and co-administration of FFC with SUL, ROB, or TOL were statistically analyzed by using the Student’s t-test [3, 10]. Statistical significance was assigned at P<0.05.
Results
In blank plasma, no interference was observed during the retention time of FFC and the internal standard. This indicated that the method was specific and selective. The working calibration curves showed good linearity over the concentration range of 0.1–25 µg/ml. A typical regression equation was y=8.653 x −0.946 with a correlation coefficient (r2) of 0.999. A reproducible linear relationship between concentration and response with a correlation coefficient (r2) of ≥0.995 was obtained in all analytical runs. LOQ was validated at 0.1 µg/ml (S/N=10), which is sensitive enough to investigate the pharmacokinetic profile of FFC in rabbits. The results for assay precision and accuracy are summarized in Table 1. The intra- and inter- day assay accuracy values at three QC levels ranged from 97.06 to 101.64%. All observed data for the intra- and inter-day assay precisions were below 6%. The average extraction recoveries of FFC at three QC levels were 75.4–78.2% with the precision below 10%, which indicates that the extraction efficiency for FFC was satisfactory, consistent, and concentration independent. So, this method was successfully established for a pharmacokinetic study of FFC.
Table 1. The precision and accuracy for FFC in rabbit plasma.
| Nominal concentration (μg/ml) | Intra-day (n=6) | Inter-day (n=18, 3 days) | |||||
|---|---|---|---|---|---|---|---|
| Determined concentration (μg/ml) |
Accuracy (%) | Precision(%) | Determined concentration (μg/ml) |
Accuracy (%) | Precision(%) | ||
| 1 | 1.01 ± 0.01 | 101.23 | 1.19 | 0.97 ± 0.05 | 97.3 | 5.04 | |
| 5 | 5.08 ± 0.04 | 101.64 | 0.83 | 5.04 ± 0.11 | 100.76 | 2.24 | |
| 10 | 9.72 ± 0.07 | 97.2 | 0.76 | 9.71 ± 0.02 | 97.06 | 0.18 | |
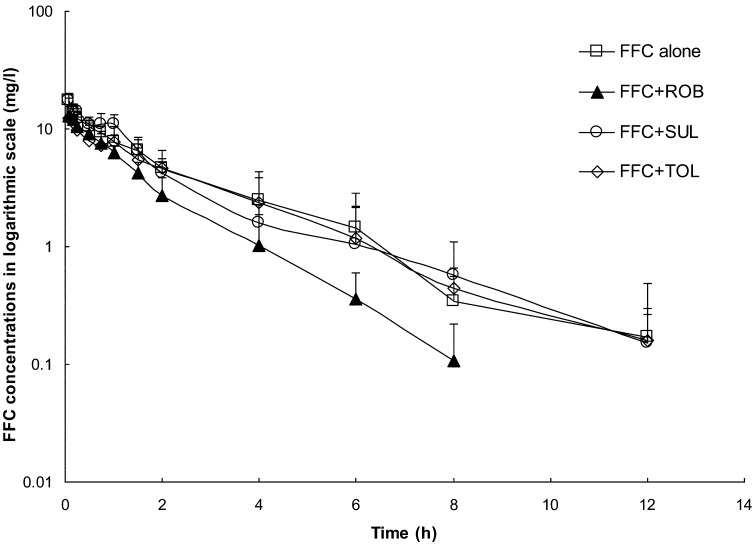
The semilogarithmic plot of the mean plasma concentration-time curves of FFC following a single intravenous injection (25 mg/kg) into rabbits after anticoccidial-free (FFC alone group), SUL (FFC+SUL group), ROB (FFC+ROB group), or TOL (FFC+TOL group) feeding for 20 days is presented in Fig. 1. Following intravenous injection of FFC in rabbits at a dose of 25 mg/kg, the drug was detected at the LOQ level (0.1 µg/ml) at 12 h postinjection in the FFC alone, FFC+SUL, and FFC+TOL group, whereas it was detected at 8h postinjection in the FFC+ROB group. No FFC was detected thereafter. Rabbits in the FFC+ROB group showed an obvious decrease in plasma FFC concentration level as compared with the FFC alone group, but no significant change was observed in the FFC+SUL or FFC+TOL group. The pharmacokinetic parameters for FFC in all groups are shown in Table 2. In the FFC+ROB group, the t1/2z, AUC, AUMC, and MRT of FFC were lower, whereas the λz and CLz of FFC were higher than that in the FFC alone group. There was no significant change in the FFC pharmacokinetic profile in the FFC+SUL or FFC+TOL group as compared with the FFC alone group. This suggested that the DDIs of the pharmacokinetic profile occurred in the process of FFC+ROB treatment, but not in the FFC+ SUL or FFC+ TOL group.
Fig. 1.
The semilogarithmic plot of the mean plasma concentration-time curves of florfenicol (FFC) following a single intravenous injection (25 mg/kg) into rabbits after anticoccidial-free (FFC alone group), SUL (FFC+SUL group), ROB (FFC+ROB group), and TOL (FFC+TOL group) feeding for 20 days. Results are presented as the mean ± SD (n=8).
Table 2. Effects of three coccidiostats on the pharmacokinetics of florfenicol.
| Parameter | Unit | FFC Alone | FFC + SUL | FFC + ROB | FFC + TOL |
|---|---|---|---|---|---|
| λz | 1/h | 0.46 ± 0.18 | 0.38 ± 0.14 | 0.61 ± 0.13* | 0.36 ± 0.13 |
| t1/2z | h | 1.72 ± 0.67 | 2.09 ± 0.88 | 1.19 ± 0.29* | 2.20 ± 0.88 |
| AUC(0-t) | μg•h /ml | 31.49 ± 6.40 | 27.72 ± 8.26 | 19.51 ± 4.30** | 28.25 ± 6.47 |
| AUC(0-∞) | μg•h /ml | 32.34 ± 6.64 | 28.69 ± 8.00 | 19.85 ± 4.30** | 28.89 ± 6.06 |
| AUMC(0-∞) | μg•h2/ml | 83.55 ± 43.51 | 79.44 ± 33.68 | 31.89 ± 14.01* | 79.46 ± 22.87 |
| MRT(0-∞) | h | 2.49 ± 1.02 | 2.91 ± 1.48 | 1.57 ± 0.49* | 2.88 ± 1.24 |
| CLz | L/kg/h | 0.81 ± 0.19 | 0.94 ± 0.28 | 1.31 ± 0.25** | 0.91 ± 0.22 |
| Vss | L/kg | 1.92 ± 0.62 | 2.91 ± 2.25 | 2.00 ± 0.61 | 2.79 ± 2.08 |
Pharmacokinetics parameters of florfenicol (FFC) after a single intravenous injection (25 mg/kg) into rabbits which fed normal feed (anticoccidial-free) and the feed containing sulfaquinoxaline (SUL, 250 ppm), robenidine (ROB, 66 ppm), or toltrazuril (TOL, 2 ppm) for 20 days. Values are presented as the mean ± SD (n=8). λz, elimination rate constant; t1/2z, terminal elimination half-life; AUC, area under the plasma concentration–time curve; AUMC, area under the first moment curve; MRT, mean residence time; CLz, total body clearance; Vss, apparent steady-state volume of distribution; *P<0.05 and **P<0.01; significant difference from the FFC alone group.
Discussion
In some documents about pharmacokinetic studies of FFC, the disposition of FFC was analyzed by the one-compartment open model [18, 20], two-compartment open model [3, 32], and non-compartmental analysis [1, 23]. In this study, we found that due to the presence of individual differences in the plasma concentration-time data, some data were fit for the one-compartment model, but others appear to be fit for the multi-compartment model. To overcome this problem, we used a non-compartmental analysis based on the statistical moment theory. After intravenous injection of FFC in the FFC alone group, the t1/2z, MRT, CLz, and Vss of FFC were 1.72 ± 0.67 h, 2.49 ± 1.02 h, 0.81 ± 0.19 l/kg/h, and 1.92 ± 0.62 l/kg, respectively, which were similar to those in previous reports [1, 23].
DDIs in pharmacokinetics may be unfavourable for the prevention and cure of disease, which resulted in an increase or decrease in the plasma drug concentration in the body as a consequence of co-administration with another drug (s) [5]. Recent studies showed that co-administration of some anthelmintics (ivermectin, albendazole, and rafoxanide) with FFC in goats [3] and supplementation of some polyether ionophore anticoccidial drugs (salinomycin, monensin, and maduramycin) as feed additives in broiler chickens [32] can affect the disposition kinetics of FFC.
Sulfaquinoxaline (SUL), a sulfa antimicrobial agent, is a traditional antibiotic drug widely used to control coccidiosis in poultry and rabbits. SUL has high activity against Eimeria maxima, Eimeria brunetti, and Eimeria acervulina but a narrow therapeutic index [13]. Robenidine (ROB) is used widely to prevent or cure poultry and rabbit coccidiosis and showed high therapeutical effects against most types of Eimeria infection in rabbits [24]. Toltrazuril (TOL), a triazinone antimicrobial agent, has broad-spectrum anticoccidial activity, high efficacy, and low toxicity, and is often used in poultry, rabbits, goats, and pigs [21]. So far, little is known about whether use of anticoccidial drugs as feed additives influences the pharmacokinetic profile of FFC in rabbits. In the present study, we observed that the t1/2z, AUC, AUMC, and MRT of FFC were reduced, whereas λz and CLz of FFC were increased in the process of co-administration of ROB with FFC in rabbits. However, SUL and TOL have no effect on elimination of FFC in rabbits. Our results suggested that chronic treatment with ROB can accelerate the elimination of FFC and reduce the exposure of FFC in rabbits.
Variation in the pharmacokinetic parameters of FFC may result from many reasons in the process of co-administration of ROB with FFC. A previous study reported that inhibition of cytochrome P450 3A4 (CYP3A4) by ketoconazole could increase the AUC and decrease the elimination of FFC significantly, which suggests that CYP3A4 is critical in the metabolism of FFC in rabbits [20]. Therefore, we conjectured that ROB may be a CYP3A4 enzyme inducer resulting in much higher metabolism of FFC by CYP3A4 in the liver, which could explain why ROB accelerates the elimination of FFC and further decreases the parent drug concentration in vivo. It was previously reported that in veal calves, renal excretion is the main route of elimination of FFC as the parent form (64%) and that some FFC was excreted as urinary metabolites including florfenicol amine, florfenicol alcohol, florfenicol oxamic acids, and monochloroflorfenicol [31]. So, if renal excretion is also the main route of excretion in rabbits, another reason for the variation in the pharmacokinetic parameters of FFC may be that chronic treatment with ROB may enhance the excretion of FFC in urine through some mechanism such as adjustment of the pH of urine or promotion of the excretion of FFC in kidney tubules. P-gp is a transporter present in many tissues and is also critical in the disposition of FFC in rabbits [20]. For example, P-gp may be related to fecal excretion of the substrates in intestinal tissues, bile secretion of the substrates in the liver, and renal excretion of the substrates in the kidney. So, if FFC is a P-gp substrate, ROB might enhance the renal excretion of FFC by increasing the expression of P-gp in the kidney. Which mechanism is actually involved needs to be further studied.
Until now, the minimum inhibitory concentrations (MICs) of FFC for bacteria isolated from rabbits and the pharmacokinetic/pharmacodynamic (PK/PD) properties of FFC in rabbits have not been reported. However, the MICs of FFC for many bacteria pathogens in other animals ranged from 0.4 to 8 µg/ml in various studies [11, 26, 28,29,30]. On the other hand, it has been generally considered that the bacterium may be sensitive to an antibiotic when the average concentration in blood is 2–4 times more than its MIC. So, we conjectured that 1–16 µg/ml may be the effective drug plasma concentration for treatment of various bacteria pathogens in vivo. Recent studies showed that the antibacterial action of FFC has the characteristics of concentration dependency against M. haemolytica and codependency (on time and concentration) against P. multocida in calves. The main assessment parameter for efficacy of FFC may be Cmax/MIC, AUC/MIC, or the time above the MIC (T>MIC) [15, 29]. In this study, we found the time that the mean plasma concentration exceeded the concentration of 1 µg/ml was approximately 6 h in the FFC alone group; however, it was lowered to 4 h by ROB pretreatment (Fig. 1), which resulted in the insufficient maintenance time for the effective FFC concentration in vivo, and this may lower the therapeutic efficiency of this drug. Due to the fact that maximum plasma concentration (initial plasma concentration) of FFC was not changed by ROB pretreatment, we suggest that when FFC is co-administrated with ROB in clinical use, more frequent dosing of FFC is needed to maintain therapeutic efficiency.
In summary, the elimination of FFC is accelerated by co-administration with ROB in rabbits, which suggests that attention needs to be paid to the DDIs when ROB is co-administrated with FFC.
Conflicts of Interest
All authors declare that they have no conflicts of interest regarding this article.
Acknowledgments
This study was financially supported by the Priority Academic Program for Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1.Abd El-Aty A.M., Goudah A., Abo El-Sooud K., El-Zorba H.Y., Shimoda M., Zhou H.H.2004. Pharmacokinetics and bioavailability of florfenicol following intravenous, intramuscular and oral administrations in rabbits. Vet. Res. Commun. 28: 515–524. doi: 10.1023/B:VERC.0000040241.06642.49 [DOI] [PubMed] [Google Scholar]
- 2.Angelos J.A., Dueger E.L., George L.W., Carrier T.K., Mihalyi J.E., Cosgrove S.B., Johnson J.C.2000. Efficacy of florfenicol for treatment of naturally occurring infectious bovine keratoconjunctivitis. J. Am. Vet. Med. Assoc. 216: 62–64. doi: 10.2460/javma.2000.216.62 [DOI] [PubMed] [Google Scholar]
- 3.Atef M., El-Gendi A.Y., Amer A.M., Abd El-Aty A.M.2010. Effect of three anthelmentics on disposition kinetics of florfenicol in goats. Food Chem. Toxicol. 48: 3340–3344. doi: 10.1016/j.fct.2010.08.039 [DOI] [PubMed] [Google Scholar]
- 4.Ayling R.D., Baker S.E., Peek M.L., Simon A.J., Nicholas R.A.2000. Comparison of in vitro activity of danofloxacin, florfenicol, oxytetracycline, spectinomycin and tilmicosin against recent field isolates of Mycoplasma bovis. Vet. Rec. 146: 745–747. doi: 10.1136/vr.146.26.745 [DOI] [PubMed] [Google Scholar]
- 5.Bibi Z.2008. Role of cytochrome P450 in drug interactions. Nutr. Metab. (Lond) 5: 27. doi: 10.1186/1743-7075-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Booker C.W., Jim G.K., Guichon P.T., Schunicht O.C., Thorlakson B.E., Lockwood P.W.1997. Evaluation of florfenicol for the treatment of undifferentiated fever in feedlot calves in western Canada. Can. Vet. J. 38: 555–560. [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon M., Harford S., Davies J.1990. A comparative study on the inhibitory actions of chloramphenicol, thiamphenicol and some fluorinated derivatives. J. Antimicrob. Chemother. 26: 307–317. doi: 10.1093/jac/26.3.307 [DOI] [PubMed] [Google Scholar]
- 8.Deeb B.J., DiGiacomo R.F.2000. Respiratory diseases of rabbits. Vet. Clin. North Am. Exot. Anim. Pract. 3: 465–480, vi–vii. [DOI] [PubMed] [Google Scholar]
- 9.Dorne J.L., Fernández-Cruz M.L., Bertelsen U., Renshaw D.W., Peltonen K., Anadon A., Feil A., Sanders P., Wester P., Fink-Gremmels J.2013. Risk assessment of coccidostatics during feed cross-contamination: animal and human health aspects. Toxicol. Appl. Pharmacol. 270: 196–208. doi: 10.1016/j.taap.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 10.El-Gendi A.Y., Atef M., Amer A.M., Kamel G.M.2010. Pharmacokinetic and tissue distribution of doxycycline in broiler chickens pretreated with either: diclazuril or halofuginone. Food Chem. Toxicol. 48: 3209–3214. doi: 10.1016/j.fct.2010.08.024 [DOI] [PubMed] [Google Scholar]
- 11.Gharaibeh S., Al Rifai R., Al-Majali A.2010. Molecular typing and antimicrobial susceptibility of Clostridium perfringens from broiler chickens. Anaerobe 16: 586–589. doi: 10.1016/j.anaerobe.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Hagen K.W., Jr1958. The effects of continuous sulfaquinoxaline feeding on rabbit mortality. Am. J. Vet. Res. 19: 494–496. [PubMed] [Google Scholar]
- 13.Hagen K.W., Jr1961. Hepatic coccidiosis in domestic rabbits treated with 2 nitrofuran compounds and sulfaquinoxaline. J. Am. Vet. Med. Assoc. 138: 99–100. [PubMed] [Google Scholar]
- 14.Ho S.P., Hsu T.Y., Che M.H., Wang W.S.2000. Antibacterial effect of chloramphenicol, thiamphenicol and florfenicol against aquatic animal bacteria. J. Vet. Med. Sci. 62: 479–485. doi: 10.1292/jvms.62.479 [DOI] [PubMed] [Google Scholar]
- 15.Illambas J., Potter T., Sidhu P., Rycroft A.N., Cheng Z., Lees P.2013. Pharmacodynamics of florfenicol for calf pneumonia pathogens. Vet. Rec. 172: 340. doi: 10.1136/vr.101155 [DOI] [PubMed] [Google Scholar]
- 16.Jim G.K., Booker C.W., Guichon P.T., Schunicht O.C., Wildman B.K., Johnson J.C., Lockwood P.W.1999. A comparison of florfenicol and tilmicosin for the treatment of undifferentiated fever in feedlot calves in western Canada. Can. Vet. J. 40: 179–184. [PMC free article] [PubMed] [Google Scholar]
- 17.Jones F.T., Ricke S.C.2003. Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult. Sci. 82: 613–617. doi: 10.1093/ps/82.4.613 [DOI] [PubMed] [Google Scholar]
- 18.Koc F., Ozturk M., Kadioglu Y., Dogan E., Yanmaz L.E., Okumus Z.2009. Pharmacokinetics of florfenicol after intravenous and intramuscular administration in New Zealand White rabbits. Res. Vet. Sci. 87: 102–105. doi: 10.1016/j.rvsc.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 19.Kowalski P., Konieczna L., Chmielewska A., Oledzka I., Plenis A., Bieniecki M., Lamparczyk H.2005. Comparative evaluation between capillary electrophoresis and high-performance liquid chromatography for the analysis of florfenicol in plasma. J. Pharm. Biomed. Anal. 39: 983–989. doi: 10.1016/j.jpba.2005.05.032 [DOI] [PubMed] [Google Scholar]
- 20.Liu N., Guo M., Mo F., Sun Y.H., Yuan Z., Cao L.H., Jiang S.X.2012. Involvement of P-glycoprotein and cytochrome P450 3A in the metabolism of florfenicol of rabbits. J. Vet. Pharmacol. Ther. 35: 202–205. doi: 10.1111/j.1365-2885.2011.01310.x [DOI] [PubMed] [Google Scholar]
- 21.Mathis G.F., Froyman R., Irion T., Kennedy T.2003. Coccidiosis control with toltrazuril in conjunction with anticoccidial medicated or nonmedicated feed. Avian Dis. 47: 463–469. doi: 10.1637/0005-2086(2003)047[0463:CCWTIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22.Paape M.J., Miller R.H., Ziv G.1990. Effects of florfenicol, chloramphenicol, and thiamphenicol on phagocytosis, chemiluminescence, and morphology of bovine polymorphonuclear neutrophil leukocytes. J. Dairy Sci. 73: 1734–1744. doi: 10.3168/jds.S0022-0302(90)78850-9 [DOI] [PubMed] [Google Scholar]
- 23.Park B.K., Lim J.H., Kim M.S., Hwang Y.H., Yun H.I.2007. Pharmacokinetics of florfenicol and its major metabolite, florfenicol amine, in rabbits. J. Vet. Pharmacol. Ther. 30: 32–36. doi: 10.1111/j.1365-2885.2007.00809.x [DOI] [PubMed] [Google Scholar]
- 24.Peeters J.E., Halen P.1980. Robenidine treatment of rabbits naturally infected with coccidia. Lab. Anim. 14: 53–54. doi: 10.1258/002367780780943024 [DOI] [PubMed] [Google Scholar]
- 25.Pentecost R.L., Niehaus A.J., Werle N.A., Lakritz J.2013. Pharmacokinetics of florfenicol after intravenous and intramuscular dosing in llamas. Res. Vet. Sci. 95: 594–599. doi: 10.1016/j.rvsc.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 26.Priebe S., Schwarz S.2003. In vitro activities of florfenicol against bovine and porcine respiratory tract pathogens. Antimicrob. Agents Chemother. 47: 2703–2705. doi: 10.1128/AAC.47.8.2703-2705.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren S.Y., Geng Y., Wang K.Y., Zhou Z.Y., Liu X.X., He M., Peng X., Wu C.Y., Lai W.M.2014. Streptococcus agalactiae Infection in Domestic Rabbits, Oryctolagus cuniculus. Transbound. Emerg. Dis. 61: e92–e95. doi: 10.1111/tbed.12073 [DOI] [PubMed] [Google Scholar]
- 28.Salmon S.A., Watts J.L.2000. Minimum inhibitory concentration determinations for various antimicrobial agents against 1570 bacterial isolates from turkey poults. Avian Dis. 44: 85–98. doi: 10.2307/1592511 [DOI] [PubMed] [Google Scholar]
- 29.Sidhu P., Rassouli A., Illambas J., Potter T., Pelligand L., Rycroft A., Lees P.2014. Pharmacokinetic-pharmacodynamic integration and modelling of florfenicol in calves. J. Vet. Pharmacol. Ther. 37: 231–242. doi: 10.1111/jvp.12093 [DOI] [PubMed] [Google Scholar]
- 30.Ueda Y., Suenaga I.1995. In vitro antibacterial activity of florfenicol against Actinobacillus pleuropneumoniae. J. Vet. Med. Sci. 57: 363–364. doi: 10.1292/jvms.57.363 [DOI] [PubMed] [Google Scholar]
- 31.Varma K.J., Adams P.E., Powers T.E., Powers J.D., Lamendola J.F.1986. Pharmacokinetics of florfenicol in veal calves. J. Vet. Pharmacol. Ther. 9: 412–425. doi: 10.1111/j.1365-2885.1986.tb00062.x [DOI] [PubMed] [Google Scholar]
- 32.Wang G.Y., Tu P., Chen X., Guo Y.G., Jiang S.X.2013. Effect of three polyether ionophores on pharmacokinetics of florfenicol in male broilers. J. Vet. Pharmacol. Ther. 36: 494–501. doi: 10.1111/jvp.12020 [DOI] [PubMed] [Google Scholar]