Abstract
Rodent ovariectomy is an experimental method to eliminate the main source of sexual steroids. This work explored for the first time the ovariectomy temporal changes induced in the hemostatic coagulation markers: prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), and fibrinogen concentration (FIB) along with uterine weight on adult female CD1 mice and Wistar rats. Uterine weight (Uw) was assessed before ovariectomy (control), and 1, 3, 5, 7, 9, 16, and 21 days after surgery. PT, aPTT, TT and FIB were estimated the same days, using reported standard techniques. Ovariectomy decreased Uw, since day 1; and from day 10 to 21 reached the lowest values for both species. After day 1, mice hemostatic parameters changed (PT +10%, P<0.05; aPTT +53%, P<0.05; TT −24%, P<0.05; FIB +67%, P<0.05). Rats showed significant changes only in TT and FIB (TT −13%, P<0.001; FIB +65%, P<0.001). Neither mice PT, aPTT and TT, recovered control values after 21 days. In the rats from day 5 to 16 aPTT diminished (18–23%, P<0.05) recovering to control values on day 21, TT after 9 days and PT on day 16. In both species, FIB returned to its control values after 9 days. Ovariectomy differentially altered the PT hemostatic parameter of mice and rats indicating a non-equivalence among both species behaviour for experimental studies of blood coagulation.
Keywords: blood coagulation, hemostatic parameters, mice, ovariectomy, rat
Introduction
Estrogen therapy is widely used by millions of women worldwide as contraceptives and menopause hormone therapy (MHT) to control menopause symptoms, osteoporosis and dementia in older women [20, 36]. The high contraceptive efficacy and the benefits of MHT are counteracted by the increased risk of cancer [4, 16, 27] and venous thrombosis among users [1].
Estrogen treatment increases coagulation factors, decreases natural anticoagulant activity and also those involved in fibrinolysis [19, 49, 53]. The changes in the plasma coagulation profile produce a prothrombotic shift of the hemostatic balance that may lead to an altered hypercoagulability state that can be favouring thrombogenesis [45]. Recent guidelines recommend that MHT should be prescribed at the lowest effective dose for the shortest possible duration and the regimen regularly reviewed considering the patient’s risk factors [46].
The life expectancy of women has considerably been increased along the last years with longer postmenopausal periods leading the menopause symptoms and complications which consequently reduce the quality of life. These facts reveal the need to search for more specific, efficacious and safer alternative agents with less risk of MHT adverse effects.
Currently, our group has been seeking for new estrogen alternatives safer for MHT users particularly without producing thrombotic risk [28, 36]. We have described the anticoagulant effects produced by 17β-aminoestrogens in mice and rats which contrast with the procoagulant effects exhibited by the natural hormone estradiol (E2) [28,29,30,31].
In healthy young women, the E2 at physiological levels acts as a modulator of transcription factor genes of blood coagulation maintaining the equilibrium between procoagulant and anticoagulant factors preserving the balance of the hemostatic processes [45]. Supra-physiological levels or exogenous pharmacological doses could impair the coagulation system, inducing to an imbalance that could lead to a prothrombotic state predisposing to a thrombosis risk [19, 45, 53].
In vivo, experimental studies with estrogen on the blood coagulation system are a complex issue. In mice and rats, natural and synthetic estrogens produce biphasic effects on blood clotting time. These effects occur depending on the route of administration, the dosage and estrogen used [28]. Additionally, mice and rats also have inter- and intra-species significant differences in some hemostatic parameters [30, 32] that hinder the extrapolation and draw conclusions.
Ovariectomy in mice and rats is an experimental model used in many preclinical studies to remove the primary estrogen source. After ovariectomy, the endogenous ovarian estrogen production ceases, this leads to uterine involution two or three weeks after surgery. The ovariectomized (Ovx) animals are commonly used to test estrogenic activity of natural and synthetic estrogens evaluating their capability to restore uterine weight that is considered a marker for estrogenicity allowing comparing such effects with those of the natural hormone E2. The thrombogenic risk of estrogen derivatives has been associated with the estrogenic content of the formulations [49] however, estrogen effects data in rodents hemostasis is limited [10, 11, 24, 34, 40, 43, 52, 57] and the changes in hemostatic parameters induced after ovariectomy have not been fully explored. In a previous work with mice and rats we found significant differences in the prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT) and fibrinogen concentration (FIB) after 21 days of ovariectomy [31]. These markers are blood clotting parameters commonly used to monitor and detect blood clotting alterations [7, 12, 14, 54]. In the experimental design timing to use the animals after ovariectomy is a critical point to assess the biological response [56], impacting experimental reproducibility. To address these limitations and to characterize E2’s effects and actions on blood clotting, is necessary to know the time course of ovariectomy related to the uterotrophic and blood clotting effects. The data obtained may shed light to determine best usefulness time after ovariectomy, as well as the species to be used. The aim of this study was to explore the effect induced by the ovariectomy on PT, aPTT, TT, and FIB of blood coagulation parameters in adult mice and rats, analyzing the relationship between the time course of the uterine weight marker and the mentioned hemostatic parameters in both species. During this study, we performed ovariectomy to adult female CD1 mice and Wistar rats and evaluated changes in uterine weight and the hemostatic parameters: PT, aPTT, TT, and FIB concentration on days 1, 3, 5, 7, 9, 16 and 21 after ovariectomy. These data will be used as a guide for our pharmacological studies about the effects of estrogen on the blood coagulation basic hemostatic markers.
Material and Methods
The study was performed in the Laboratory of Endocrine Pharmacology of the Department of Pharmacology, at the Faculty of Medicine, UNAM, Mexico D.F. The study was approved by the Local Animal Ethics Committee. All experimental studies were conducted in accordance to the Mexican National Protection Laws of Animal Welfare (NOM-062-Z00-1999).
Animals
Domestic adult female CD1 mice (Mus musculus) seven to eight weeks old, weighing 25–30 g and adult female Wistar rats (Rattus norvegicus) eight weeks old, weighing 200–250 g were bred in our Animal Breeding Division facilities. Starting the experiment, vaginal smears of the animals were obtained and only those showing cornified cells as an indicator of oestrus cycle phase were housed in standard local acrylic cages (mice: 210 × 160 × 130 mm) (rats: 425 × 266 × 150 mm) with autoclaved wood shaving bedding (90–100 g/cage) in groups of four to five. The animals were kept at constant temperature (20–22°C) in a room with 12 h-12 h light-dark, cycle (light: 07:00 to 19:00), and humidity range 50 ± 10%. They were fed with a commercial diet (Nutricubos, Purina) and tap water ad libitum. The mice and rats were free of all viral, bacterial and parasitic pathogens listed according to the Federation of European Laboratory Animal Science Associations recommendations [44].
Ovariectomy
Adult female CD1 mice and Wistar rats were bilateral ovariectomized (Ovx) under chloral hydrate anesthesia (4% solution, 280 mg/kg J. T. Baker, Mexico City, Mexico). Sham animals received anesthesia and an incision on the dorsal back, sectioning the skin and stitching similarly to that of the Ovx animals without extirpation the ovaries. After ovariectomy, the animals were randomly assigned to the different groups. Each experimental group consisted of 10 animals. Uterotrophic activity was evaluated through the uterine wet (Uww) or uterine dry weight (Udw) in Ovx mice and rats. Uterine weights were expressed in milligrams. Sham animals did not show significant differences in the hemostatic parameters with respect to the intact and treated animals on days 0 and day 1.
Experimental design
The different groups of sham and Ovx mice and rats were housed until blood collection, and uterotrophic activity assessment was performed. Animals were caged according to each condition until blood samples were drawn. Data determinations were registered as obtained from day 0 to days 1, 3, 5, 7, 9, 16 up to day 21 post-ovariectomy. On the evaluation day, animals were weighed, the blood samples collected (as described below), their uteri were dissected, blotted, and weighed to obtain the Uww, and then were dried at 70°C for 24 h and weighed again to determine the Udw.
Blood collection
Animals were anaesthetized with intra-peritoneal injection of chloral hydrate anesthesia (4% solution, 280 mg/kg J. T. Baker, Mexico City, Mexico) prior to blood withdrawal. Each animal was placed on its back on a cork surgery table and restrained with string fixed at the corners. Arterial blood was collected by syringe suctioning from the iliac bifurcation, which provided hemolysis-free blood samples from mice (0.5–0.6 ml) or rats (4–7 ml). Blood was immediately put into plastic tubes containing 0.11 M sodium citrate (1:9, v:v). The samples were gently mixed and centrifuged at 2,500 g for 10 min at 4°C. Plasma samples were pipetted, frozen, and stored at −80°C until assayed. On completion of each blood withdrawal allows the euthanization procedure of the animals.
Hemostatic parameters
The coagulation screening tests, PT, aPTT, TT, and FIB, were performed by modifications of the conventional clinical procedures recently reported [17, 29]. For all clotting tests, Dade® Behring reagents were used and assayed according to instructions in the package insert. Reference curves and accuracy controls were set up using the corresponding control plasma of the manufacturer. PT, aPTT, and TT clotting times were recorded using a Behring Fibrintimer BFT II (Dade® Behring). Plasma samples were warmed at 37°C for 60 seconds (s) before adding the indicated reagent. Clot formation was the end point of the reactions, and the results are reported in s. PT determination was performed using rabbit brain thromboplastin C plus (Dade® Behring). Plasma samples (50 µl) were pipetted into cuvettes, and then 100 µl of thromboplastin C was added to activate the reaction. aPTT was assayed using the Actin FS reagent (Dade® Behring) containing purified soy phosphatides. Actin FS (100 µl) was added to plasma samples (50 µl). The mixture was incubated at 37°C for 120 s and 50 µl of 0.02 M CaCl2 (37°C) were added to activate the reaction. TT was determined using bovine thrombin (Dade® Behring) at a concentration of 5 IU/ml. Plasma samples (50 µl) were treated with 50 µl of bovine thrombin to activate the reaction. FIB concentration was evaluated using a mechanical fibrometer (Fibrosystem Becton-Dickinson Mod 5-117V). Bovine thrombin (100 IU/ml; Dade® Behring) was added to plasma samples (50 µl) to induce clot formation. The FIB concentration was obtained from a reference curve calibrated with human plasma fibrinogen and reported in milligrams per deciliter (mg/dl).
Data analysis
Statistical significance among groups was assessed by one way variance analysis (ANOVA). The significance of the differences between control and Ovx groups was estimated by the pairwise multiple comparison procedures Dunn’s and Holm-Sidak methods as indicated [58]. Results were expressed in means ± standard error (s.e.). Pearson correlation analysis was performed on the hemostatic parameters of CD1 mice or Wistar rats. In order to determine differences between temporal course of hemostatic parameters between mice and rats after ovariectomy, we used multiple linear regression models. The independent variables of the models were: animal species (mice/rats), linear and quadratic effect of time, ovariectomy (yes/no), and the interaction between the linear effect of time and animal species. Values of P<0.05 were considered statistically significant. All the statistical analysis was performed using the Sigma Plot program version
Results
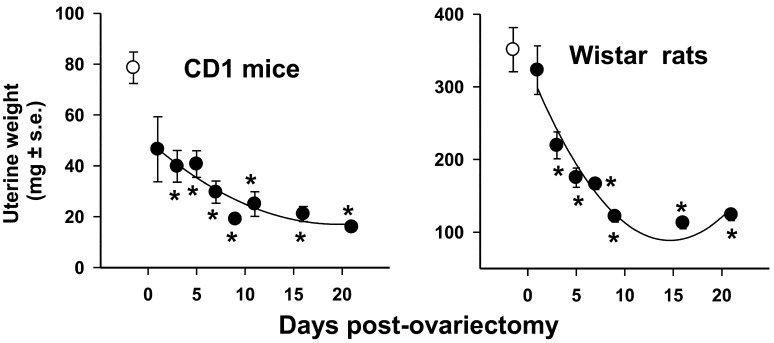
The uterine involution induced by ovariectomy in mice and rats from day 1 (24 h post-ovariectomy) to days 3, 5, 7, 9, 16 until 21 is shown in Fig. 1. Uterine weight was measured in these same days setting Uww and Udw as biological markers for estrogenicity; since the temporal course data from Uww and Udw displayed similar patterns, only the Uww’s were shown (Fig. 1). The variation of blood coagulation parameters PT, aPTT, TT, and FIB concentrations in adult female CD1 mice and Wistar rats after ovariectomy are shown in Figs. 2 and 3.
Fig. 1.
Temporal course of the involution of uterine weight in CD1 mice and Wistar rats. The
results correspond to the Uww.  =control;
=control;  =ovariectomized (Ovx) animals.
Data expressed in mean ± SEM. N=10. The significance of the differences between
control and Ovx groups were obtained by Dunn’s method.
*P<0.05.
=ovariectomized (Ovx) animals.
Data expressed in mean ± SEM. N=10. The significance of the differences between
control and Ovx groups were obtained by Dunn’s method.
*P<0.05.
Fig. 2.
Temporal course of the screening tests, of PT, aPTT, TT, and FIB, in CD1 mice.
 =control;
=control;  =ovariectomized (Ovx) animals
=ovariectomized (Ovx) animals  =sham animals. Data
expressed in mean ± SEM. N=10. *P<0.05. The significance of the
differences between control and Ovx groups were estimated by the pairwise multiple
comparison procedures Dunn’s method.
=sham animals. Data
expressed in mean ± SEM. N=10. *P<0.05. The significance of the
differences between control and Ovx groups were estimated by the pairwise multiple
comparison procedures Dunn’s method.
PT: prothrombin time, PTT: partial thromboplastin time, TT: thrombin time, FIB: fibrinogen concentration.
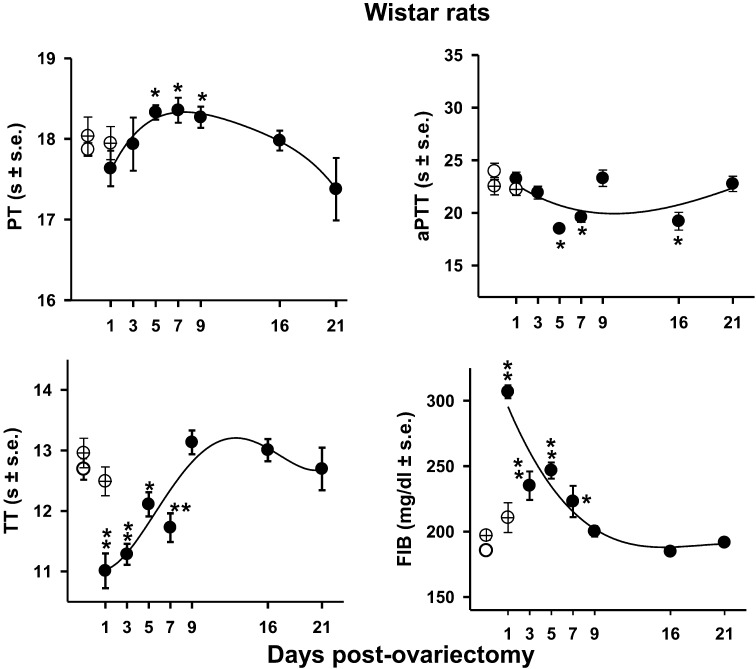
Fig. 3.
Temporal course of the screening tests, of PT, aPTT, TT, and FIB, in Wistar rats.
 =control;
=control;  =ovariectomized (Ovx) animals;
=ovariectomized (Ovx) animals;  =sham animals. Data
expressed in mean ± SEM. N=10. *P<0.05
**P<0.001. The significance of the differences between control
and Ovx groups were estimated by the pairwise multiple comparison procedures Dunn’s
(PT, and aPTT) and Holm-Sidak (TT and FIB) methods.
=sham animals. Data
expressed in mean ± SEM. N=10. *P<0.05
**P<0.001. The significance of the differences between control
and Ovx groups were estimated by the pairwise multiple comparison procedures Dunn’s
(PT, and aPTT) and Holm-Sidak (TT and FIB) methods.
PT: prothrombin time, PTT: partial thromboplastin time, TT: thrombin time, FIB: fibrinogen concentration.
Temporal course of the uterine weight in mice and rats induced by ovariectomy
Ovariectomy to both caused a marked decrease of uterine weight since day 1 to day 9. Their uterine involution from day 9 up to 21 without significant changes, reaching the Uww lowest values for mice and rats (Fig. 1).
Temporal course of hemostatic parameters in mice
Mice ovariectomy results are shown in Fig. 2. The control (C) and sham (S) on day 0 and one day after ovariectomy did not show significant differences [PT: day 0: C=11.95 ± 0.29 S=11.25 ± 0.58 day 1: S=11.38 ± 0.56); aPPT: day 0: C=39.18 ± 1.61 S=35.79 ± 1.49 day 1: S=42.63 ± 2.33; TT: day 0: C=12.79 ± 0.37 S=12.12 ± 0.40 day 1: S=11.95 ± 0.49; FIB: day 0: C=188.32 ± 6.39 S=172.50 ± 9.96 day 1: S=180.88 ± 13.52]. A biphasic response was displayed in PT and aPTT: On day 1 post-ovariectomy, PT increased 10% (P<0.05) followed by significant (P<0.05) decreases through days: 5, 7, 9, 16, and 21 from 9% to 11%. With the same pattern, aPTT at day 1 post-ovariectomy depicted an important increase of 53% (P<0.05); decreasing afterwards to 14% (day 7; P<0.05), 15% (day 9; P<0.05), and 24% (day 21; P<0.05). TT values decreased 24%, 15%, 20%, 16% and 14% (P<0.05) on days 1, 3, 5, 7, and 21, respectively. FIB concentration increased significantly on day 1, 67%, (P<0.05) then declined 46, 49, and 28% (P<0.05) on days 3, 5, 7 post-ovariectomy respectively. After day 9, no significant differences were found with respect to the control group.
Temporal course of hemostatic parameters in rats
The effect of ovariectomy on PT aPTT, TT, and FIB concentrations in rats are shown in Fig. 3. The control (C) and sham (S) on day 0 and one day after ovariectomy did not show significant differences [PT: day 0: C=17.87 ± 0.08 S=18.04 ± 0.24 day 1: S=17.95 ± 0.21; aPPT: day 0: C=23.94 ± 0.77 S=22.53 ± 0.82 day 1: S=22.22 ± 0.57; TT: day 0: C=12.69 ± 0.18 S=12.96 ± 0.24 day 1: S=12.49 ± 0.54; FIB: day 0: C=185.41 ± 3.47 S=197 ± 4.46 day 1: S=211 ± 11.32]. On days 5, 7, and 9 after surgery, PT increased slightly, around 3% (P<0.05). aPTT decreased 23%, 18%, and 20% (P<0.05) on days 5, 7, and 16, respectively. The rat’s one way analysis of variance of TT and FIB values passed normality and equal variance tests, because of that the Holm-Sidak method was applied. TT also decreased 13% (P<0.001), 11% (P<0.001), 5% (P<0.05), and 8% (P<0.001) on days 1, 3, 5, and 7, respectively. As observed in mice, FIB concentration was the most affected parameter by the surgery procedure increasing 65% (P<0.001) on day 1, at day 7 the increase was maintained in 20% (P<0.005) and returned to control values on day 9. In all cases after day 21 from ovariectomy, the hemostatic parameters differences between the ovariectomized the control and sham animals were not statistically significant (Fig. 3).
The PT temporal course values after ovariectomy of mice and rats were analyzed by a linear regression model with the explanatory variables: animal species, ovariectomy, the linear and quadratic effect of time. The interaction between the linear effect of time and the animal species explained 95% of the total variance observed in PT. All the explanatory variables were significantly associated with PT (P ≤ 0.002). The interaction indicates that the effect of time on PT depends on the animal species and evidenced in Figs. 2 and 3.
Discussion
Ovaries are the main source of E2 production into the body and to assess E2’s mechanisms in experimental studies; ovariectomy in rats and mice is the typical approach to address them. In this study, the ovariectomy of mice and rats produce a gradual decrease in uterine weight until the ninth day, with no further significant changes. Uterine weight loss is associated with the diestrus stage which is accompanied by increases in LH levels and reductions in plasma circulating levels of E2 [3, 12, 33]. The uterotrophic effects of E2 are primarily mediated by the binding and the subsequent activation of its specific nuclear receptors ERα and ERß that act as transcription factors modulating the gene expression, promoting protein synthesis and factors that ensure the reproductive functionality [8, 35].
Hormone replacement with E2 in the Ovx mice and rats models produced dose-dependent uterotrophic effects which in the mice occurred at lower concentrations than in the rats [30]. Such difference among these two species may be divergent expression pattern profiles of genes regulated by estrogens in uterus [25].
Moreover, the influence of ovariectomy alters the hemostatic parameters here studied in both, mice and rats. The surgical procedure per se (anesthesia, skin and muscle cutting and stitching) would be expected to affect similarly in the two species the explored hemostatic markers. Since the blood parameters of the sham group evaluated paralleled on day 0 and one day after surgery, although they showed greater data variability, their values showed no significant differences compared with the intact rats control group.
Mice, however, showed significant changes in all the evaluated hemostatic parameters one day after the ovariectomy, while significant changes were detected in rats only in TT and FIB, indicating sensitivity differences in their hemostatic variables between the two species.
After the surgery, inflammation processes influence the blood clotting course. These complex interactions have been reviewed and are a crosstalk between the coagulation cascade, the complement system, the fibrinolysis and the immune system which communicate through many direct and bidirectional interactions [9].
The ovarian hormones are considered global regulators of immune system function and surgical ovarian hormone removal impacts it delaying atrophied myofibre mass recovery. E2 is involved in the initial blood interaction with the vascular wall lesion conducting to the alteration of platelet activation and aggregation to generate the clot [21], as well as in the repair processes, wound healing of damaged tissue and restoring blood supply to ischemic tissues [37]. Ovariectomy induced time-related changes in some haemostatic parameters of the Ovx animals. The more significant changes in time courses after surgery between the two species were mainly in PT and aPTT.
PT temporal course patterns of mice showed to be opposite to those of rats. The curves analysis of PT values by a multiple linear regression model showed significant differences (P ≤ 0.002 see results) due to the species. So it could be thought that mice and rats might differ in the expression and activation of one or more of factors II, V, VII, and X, and prothrombin, since PT is clinically used to detect their deficiencies [13, 15].
The behavioral pattern of aPTT after surgery was similar between both species, though being more pronounced differences in mice. It is important to consider that during the surgical procedure; injured tissues interact with negatively charged surfaces of the surgical instruments and activate the FXII (Hageman factor). aPTT can detect changes in this factor and also in others like the V, VIII, IX, X, XI, those of contact prekallikrein and high molecular weight kininogen (HMWK) [15].
The administration of E2 in Ovx rats increases FXII stimulating the transcription of genes involved [18]. This factor plays a key role in the cascade proteolytic pathways, coagulation activation, fibrinolysis, and the production of kininogen during inflammation processes and is involved in maintaining the hemostatic balance [6]. The temporal curves of aPTT of mice and rats did not show significant statistical differences. Even when the surgery affected aPTT in both models, rats showed minor changes related to the controls, reflecting less sensitivity.
The present data extend and confirm some previous observations about inter- and intra-species and also the data of 21 days ovariectomized animals, which also showed significant differences in the PT and aPTT hemostatic variables [32].
In general the mice hemostatic parameters were of greater magnitude and their baseline recovery values relative to the controls did not occur except the FIB’s, contrasting with the rat’s profile that did recover their baseline values. These facts may be due to the inherent differences of each species reflected in the hemostatic parameters studied. Substantial differences in protein coagulation reactivity and the fibrinolytic system among different species, including humans have been reported [23].
Unfortunately, it has not been established a differential genetic profile related to the hemostasis in these two species allowing comparing discrepancies between them. Information about the natural estrogen E2 effect on the hemostatic system in rodents is limited, in part due to its rapid hepatic metabolism. Instead, is commonly preferred the evaluation of synthetic estrogens such as the 17α-ethinyl estradiol (EE) which undergoes less biotransformation and possesses high estrogenicity. Oral administration of EE to Ovx mice alters their plasma and hepatic coagulation profile, affecting the transcription of many genes involved in blood clotting. All these effects have been mainly assigned to the ERα estrogen receptor mediation [6, 40]. In Ovx rats EE oral treatment reduced the plasma essential anticoagulant tissue factor pathway inhibitor (TFPI) such effect was blocked by co-administration of the estrogen receptor antagonist ICI 182,780, indicating that these responses are also mediated by estrogen receptors [47].
Furthermore, the E2 sources in the body, in addition to the ovaries are the adrenal glands and adipose tissue. After menopause, these estrogen sources are important [2, 26]. Probably after the rodents ovariectomy these alternative estrogen sources can balance some of the studied parameters. In the rats, which showed to be less sensitive to ovariectomy than mice, it was possible to detect the recovery of all the hematological parameters baselines; while mice being more sensitive, recovered only their FIB baseline values.
Until now, there is no reference about the optimal time to use ovariectomized mice or rats. In different experimental reports Ovx animals are used immediately, few days, or 1 to 4 weeks after ovariectomy [22, 51]. The current report indicates that there is a critical surgery recovery period to be considered for the experimentation with Ovx animals to minimize hematological changes due to the surgery that could impact experimental results [48]. Ovariectomy in rodents has proven to be a useful model for menopause, however, the time of ovariectomy and timing of hormone replacement influence importantly the evaluation of the E2 target effects. Walf and Frey [55] have reported differences in measurements of anxiety in rats, depending on how long ago they were ovariectomized with or without E2 replacement [14, 56]. If this time is very short, the animals will not have adequate recovery. On the other hand, prolonged deficiency of sex hormones could produce deficiency in the receptors synthesis which can induce desensitization in target tissues [38, 39].
Our results showed that the most important changes in blood clotting occurred during the nine days after the animals’ surgery. These findings are online with those recently reported by Toth et al. [50] who found early 3–7 days post-surgery changes in hematological parameters of implanted dogs. The critical importance of this first week was associated with both, inflammatory and acute phase reactions associated with increased FIB and progressive increase in platelet counts where the possibility of thrombotic events increases [41, 50].
Finally, it should be noted that the measurement of the hemostatic markers here evaluated are a guide to detect abnormal clotting of the blood in these two rodent species. These parameters provide indirect information about the route and the factors affected by ovariectomy. PT measurements give information about the initial thrombin formation necessary for the formation of plasmatic fibrin and do not reflect the coagulation activity as a whole [42]. Further work in these two rodent species will be necessary to establish the genetic hemostatic profiles to validate the equivalence and non-equivalence of both models that allow more assertive experimental studies related to the influence of sex hormones on the coagulation of blood.
Acknowledgments
The authors are grateful to Dr. Rebeca Aguirre from our Department for her statistical analysis advice and comments. This study was supported by a grant PAPIIT IN218813-2. UNAM
References
- 1.Archer D.F., Oger E.2012. Estrogen and progestogen effect on venous thromboembolism in menopausal women. Climacteric 15: 235–240. doi: 10.3109/13697137.2012.664401 [DOI] [PubMed] [Google Scholar]
- 2.Bagger Y.Z., Tankó L.B., Alexandersen P., Qin G., Christiansen C.2004. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obes. Res. 12: 1519–1526. doi: 10.1038/oby.2004.189 [DOI] [PubMed] [Google Scholar]
- 3.Cavalcanti-de-Albuquerque J.P., Salvador I.C., Martins E.L., Jardim-Messeder D., Werneck-de-Castro J.P., Galina A., Carvalho D.P.2014. Role of estrogen on skeletal muscle mitochondrial function in ovariectomized rats: a time course study in different fiber types. J. Appl. Physiol. 116: 779–789. doi: 10.1152/japplphysiol.00121.2013 [DOI] [PubMed] [Google Scholar]
- 4.Chen W.Y., Colditz G.A., Rosner B., Hankinson S.E., Hunter D.J., Manson J.E., Stampfer M.J., Willett W.C., Speizer F.E.2002. Use of postmenopausal hormones, alcohol, and risk for invasive breast cancer. Ann. Intern. Med. 137: 798–804. doi: 10.7326/0003-4819-137-10-200211190-00008 [DOI] [PubMed] [Google Scholar]
- 5.Citarella F., Misiti S., Felici A., Farsetti A., Pontecorvi A., Fantoni A.1996. Estrogen induction and contact phase activation of human factor XII. Steroids 61: 270–276. doi: 10.1016/0039-128X(96)00037-2 [DOI] [PubMed] [Google Scholar]
- 6.Cleuren A.C.A., Van der Linden I.K., De Visser Y.P., Wagenaar G.T.M., Reitsma P.H., Van Vlijmen B.J.M.2010. 17α-Ethinylestradiol rapidly alters transcript levels of murine coagulation genes via estrogen receptor α. J. Thromb. Haemost. 8: 1838–1846. doi: 10.1111/j.1538-7836.2010.03930.x [DOI] [PubMed] [Google Scholar]
- 7.Cushman M., Folsom A.R., Wang L., Aleksic N., Rosamond W.D., Tracy R.P., Heckbert S.R.2003. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood 101: 1243–1248. doi: 10.1182/blood-2002-05-1416 [DOI] [PubMed] [Google Scholar]
- 8.Dahlman-Wright K., Cavailles V., Fuqua S.A., Jordan V.C., Katzenellenbogen J.A., Korach K.S., Maggi A., Muramatsu M., Parker M.G., Gustafsson J.A.2006. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol. Rev. 58: 773–781. doi: 10.1124/pr.58.4.8 [DOI] [PubMed] [Google Scholar]
- 9.Danckwardt S., Hentze M.W., Kulozik A.E.2013. Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond. J. Mol. Med. 91: 1257–1271. doi: 10.1007/s00109-013-1074-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emms H., Lewis G.P.1985. Sex and hormonal influences on platelet sensitivity and coagulation in the rat. Br. J. Pharmacol. 86: 557–563. doi: 10.1111/j.1476-5381.1985.tb08931.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farsetti A., Misiti S., Citarella F., Felici A., Andreoli M., Fantoni A., Sacchi A., Pontecorvi A.1995. Molecular basis of estrogen regulation of Hageman factor XII gene expression. Endocrinology 136: 5076–5083. [DOI] [PubMed] [Google Scholar]
- 12.Finkelman R.D., Bell N.H., Strongt D.D., Demerstt L.M., Baylinkti D.J.1992. Ovariectomy selectively reduces the concentration of transforming growth factor p8 in rat bone: Implications for estrogen deficiency-associated bone loss. Proc. Natl. Acad. Sci. USA 89: 12190–12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom A.R.1995. Epidemiology of fibrinogen. Eur. Heart J. 16:(Suppl A): 21–23, discussion 23–24. doi: 10.1093/eurheartj/16.suppl_A.21 [DOI] [PubMed] [Google Scholar]
- 14.Frye C.A., Walf A.A., Paris J.J.2010. Conjugated equine estrogen, with medroxyprogesterone acetate, enhances formation of 5α-reduced progestogens and reduces anxiety-like behavior of middle-aged rats. Behav. Pharmacol. 21: 530–539. doi: 10.1097/FBP.0b013e32833e0a23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furie B., Furie B.C.1988. The molecular basis of blood coagulation. Cell 53: 505–518. doi: 10.1016/0092-8674(88)90567-3 [DOI] [PubMed] [Google Scholar]
- 16.Furness S., Roberts H., Marjoribanks J., Lethaby A., Hickey M., Farquhar C.2009. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst. Rev. 15: CD000402 doi: 10.1002/14651858.CD000402.pub3 [DOI] [PubMed] [Google Scholar]
- 17.García-Manzano A., González-Llaven J., Jaimez R., Franco Y., Estela Avila M., Rubio-Póo C., Lemini C.2002. Changes on hemostatic parameters induced by 17β-estradiol, ethinylestradiol, and the 17β-aminoestrogen pentolame in the male Wistar rat. Steroids 67: 1129–1135. doi: 10.1016/S0039-128X(02)00067-3 [DOI] [PubMed] [Google Scholar]
- 18.Gordon E.M., Douglas J.G., Ratnoff O.D., Arafah B.M.1985. The influence of estrogen and prolactin on Hageman factor (factor XII) titer in ovariectomized and hypophysectomized rats. Blood 66: 602–605. [PubMed] [Google Scholar]
- 19.Gottsäter A., Rendell M., Hulthén U.L., Berntorp E., Mattiasson I.2001. Hormone replacement therapy in healthy postmenopausal women: a randomized, placebo-controlled study of effects on coagulation and fibrinolytic factors. J. Intern. Med. 249: 237–246. doi: 10.1046/j.1365-2796.2001.00797.x [DOI] [PubMed] [Google Scholar]
- 20.Hogervorst E., Bandelow S.2010. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas 66: 56–71. doi: 10.1016/j.maturitas.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Jayachandran M., Mukherjee R., Steinkamp T., LaBreche P., Bracamonte M.P., Okano H., Owen W.G., Miller V.M.2005. Differential effects of 17β-estradiol, conjugated equine estrogen, and raloxifene on mRNA expression, aggregation, and secretion in platelets. Am. J. Physiol. Heart Circ. Physiol. 288: H2355–H2362. doi: 10.1152/ajpheart.01108.2004 [DOI] [PubMed] [Google Scholar]
- 22.Kararigas G., Nguyen B.T., Jarry H.2014. Estrogen modulates cardiac growth through an estrogen receptor α-dependent mechanism in healthy ovariectomized mice. Mol. Cell. Endocrinol. 382: 909–914. doi: 10.1016/j.mce.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Karges H.E., Funk K.A., Ronneberger H.1994. Activity of coagulation and fibrinolysis parameters in animals. Arzeim-Forsch. Drug Res. 44: 793–797. [PubMed] [Google Scholar]
- 24.Kita E., Lamartiniere C.A.1981. Pituitary control of blood coagulation in the rat. J. Endocrinol. 91: 367–373. doi: 10.1677/joe.0.0910367 [DOI] [PubMed] [Google Scholar]
- 25.Kwekel J.C., Burgoon L.D., Burt J.W., Harkema J.R., Zacharewski T.R.2005. A cross-species analysis of the rodent uterotrophic program: elucidation of conserved responses and targets of estrogen signaling. Physiol. Genomics 23: 327–342. doi: 10.1152/physiolgenomics.00175.2005 [DOI] [PubMed] [Google Scholar]
- 26.Labrie F.2010. DHEA, important source of sex steroids in men and even more in women. Progress in Brain Research 182: 97–147. ISSN: 0079–6123 Copyright Elsevier B.V. L. Martini (Eds.) doi: 10.1016/S0079-6123(10)82004-7 [DOI] [PubMed]
- 27.Lacey J.V., Jr, Mink P.J., Lubin J.H., Sherman M.E., Troisi R., Hartge P., Schatzkin A., Schairer C.2002. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 288: 334–341. doi: 10.1001/jama.288.3.334 [DOI] [PubMed] [Google Scholar]
- 28.Lemini C., Rubio-Póo C., Silva G., García-Mondragon J., Zavala E., Mendoza-Patiño N., Castro D., Cruz-Almanza R., Mandoki J.J.1993. Anticogulant and estrogenic effects of two new 17β-aminoestrogens butolame [17β-(4-hydroxy-1-butylamino)-1,3,5(10)-estratrien-3-ol] and pentolame [17β-(5-hydroxy-1-pentylamino)-1,3,5(10)-estratrien-3-ol]. Steroids 58: 457–461. doi: 10.1016/0039-128X(93)90002-5 [DOI] [PubMed] [Google Scholar]
- 29.Lemini C., Franco Y., Avila M.E., Jaimez R.2005a. Contrasting effects of estradiol and 17 β-aminoestrogens on blood clotting time in rats and mice. Eur. J. Pharmacol. 510: 229–233. doi: 10.1016/j.ejphar.2005.01.027 [DOI] [PubMed] [Google Scholar]
- 30.Lemini C., Franco Y., Avila M.E., Jaimez R.2005b. Estrogenic effects of 17 β-aminoestrogens assessed in uteri of rats and mice. Eur. J. Pharmacol. 510: 235–239. doi: 10.1016/j.ejphar.2005.01.028 [DOI] [PubMed] [Google Scholar]
- 31.Lemini C., Jaimez R., Medina-Jiménez M., Ávila M.E.2012. Gender differences in response to chronic treatment with 17β-oestradiol and 17β-aminoestrogen pentolame on hemostasis in rats. Indian J. Pharmacol. 44: 749–753. doi: 10.4103/0253-7613.103287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemini C., Jaimez R., Franco Y.2007. Gender and inter-species influence on coagulation tests of rats and mice. Thromb. Res. 120: 415–419. doi: 10.1016/j.thromres.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 33.Lemus A.E., Jaimez R., Lemini C., Menjivar M., Silva G., Rubio-Poo C., Valenzuela F., Larrea F.1998. Estrogenic effects of the synthetic aminoestrogen 17 β-(5-hydroxy-1-pentylamino)-1,3,5(10)-estratrien-3-ol (pentolame). Steroids 63: 433–438. doi: 10.1016/S0039-128X(98)00046-4 [DOI] [PubMed] [Google Scholar]
- 34.Mandoki J.J., Zavala E., Silva G., Mendoza-Patiño N., Rubio-Póo C., Medina-Martínez S., Domínguez-Escoto P.1983. The dual effects of estrogens on blood clotting time. Proc. West. Pharmacol. Soc. 26: 45–48. [PubMed] [Google Scholar]
- 35.Marino M., Acconcia F., Ascenzi P.2005. Estrogen receptor signalling: bases for drug actions. Curr. Drug Targets Immune Endocr. Metabol. Disord. 5: 305–314. doi: 10.2174/1568008054863763 [DOI] [PubMed] [Google Scholar]
- 36.Marjoribanks J., Farquhar C., Roberts H., Lethaby A.2012. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 11;7:CD004143. doi: 10.1002/14651858.CD004143.pub4) Clin. Hemorheol. Microcirc. 50: 197–211. doi: [DOI] [PubMed]
- 37.McClung J.M., Davis J.M., Carson J.A.2007. Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp. Physiol. 92: 219–232. doi: 10.1113/expphysiol.2006.035071 [DOI] [PubMed] [Google Scholar]
- 38.Mendelsohn M.E., Karas R.H. HRT and the young at heart. 2007. N. Engl. J. Med. 356: 2639–2641. [DOI] [PubMed] [Google Scholar]
- 39.Miller V.M., Duckles S.P.2008. Vascular actions of estrogens: functional implications. Pharmacol. Rev. 60: 210–241. doi: 10.1124/pr.107.08002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Movérare S., Skrtic S., Lindberg M.K., Dahlman-Wright K., Ohlsson C.2004. Estrogen increases coagulation factor V mRNA levels via both estrogen receptor-alpha and -beta in murine bone marrow/bone. Eur. J. Endocrinol. 151: 259–263. doi: 10.1530/eje.0.1510259 [DOI] [PubMed] [Google Scholar]
- 41.Nemeth N., Kiss F., Hever T., Brath E., Sajtos E., Furka I., Miko I.2012. Hemorheological consequences of hind limb ischemia-reperfusion differs in normal and gonadectomized male and female rats. Clin. Hemorheol. Microcirc. 50: 197–211 doi: 10.3233/CH-2011-1427. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi R., Sugimura M., Kanayama N.2003. Estrogen administration enhances thrombin generation in rats. Thromb. Res. 112: 325–328. doi: 10.1016/j.thromres.2003.11.014 [DOI] [PubMed] [Google Scholar]
- 43.Owens M.R., Cimino C.D., Donnelly J.1986. Measurements of rat plasma coagulation proteins during prolonged exposure to diethylstilbesterol. Thromb. Res. 42: 343–354. doi: 10.1016/0049-3848(86)90263-X [DOI] [PubMed] [Google Scholar]
- 44.Rehbinder C., Baneux P., Forbes D., van Herck H., Nicklas W., Rugaya Z., Winkler G.1996. FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health accepted by the FELASA Board of Management, November 1995. Lab. Anim. 30: 193–208. doi: 10.1258/002367796780684881 [DOI] [PubMed] [Google Scholar]
- 45.Rosendaal F.R., Helmerhorst F.M., Vandenbroucke J.P.2001. Oral contraceptives, hormone replacement therapy and thrombosis. Thromb. Haemost. 86: 112–123. [PubMed] [Google Scholar]
- 46.Santen R.J., Allred D.C., Ardoin S.P., Archer D.F., Boyd N., Braunstein G.D., Burger H.G., Colditz G.A., Davis S.R., Gambacciani M., Gower B.A., Henderson V.W., Jarjour W.N., Karas R.H., Kleerekoper M., Lobo R.A., Manson J.E., Marsden J., Martin K.A., Martin L., Pinkerton J.V., Rubinow D.R., Teede H., Thiboutot D.M., Utian W.H.Endocrine Society2010. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 95:(Suppl 1): s1–s66. doi: 10.1210/jc.2009-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirk R.A., Zhang Z., Winneker R.C.2005. Differential effects of estrogens and progestins on the anticoagulant tissue factor pathway inhibitor in the rat. J. Steroid Biochem. Mol. Biol. 94: 361–368. doi: 10.1016/j.jsbmb.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 48.Szokoly M., Nemeth N., Furka I., Miko I.2009. Hematological and hemostaseological alterations after warm and cold limb ischemia-reperfusion in a canine model. Acta Cir. Bras. 24: 338–346. doi: 10.1590/S0102-86502009000500002 [DOI] [PubMed] [Google Scholar]
- 49.Tchaikovski S.N., Rosing J.2010. Mechanisms of estrogen-induced venous thromboembolism. Thromb. Res. 126: 5–11. doi: 10.1016/j.thromres.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 50.Toth C., Klarik Z., Kiss F., Toth E., Hargitai Z., Nemeth N.2014. Early postoperative changes in hematological, erythrocyte aggregation and blood coagulation parameters after unilateral implantation of polytetrafluoroethylene vascular graft in the femoral artery of beagle dogs. Acta Cir. Bras. 29: 320–327. doi: 10.1590/S0102-86502014000500006 [DOI] [PubMed] [Google Scholar]
- 51.Torres M., Palomer X., Montserrat J.M., Vázquez-Carrera M., Farré R.2014. Effect of ovariectomy on inflammation induced by intermittent hypoxia in a mouse model of sleep apnea. Respir. Physiol. Neurobiol. 202: 71–74. [DOI] [PubMed] [Google Scholar]
- 52.Unruh M., Grunow A., Gottstein C.2005. Systemic coagulation parameters in mice after treatment with vascular targeting agents. Thromb. J. 3: 21–32. doi: 10.1186/1477-9560-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Hylckama Vlieg A., Rosendaal F.R.2003. Interaction between oral contraceptive use and coagulation factor levels in deep venous thrombosis. J. Thromb. Haemost. 1: 2186–2190. doi: 10.1046/j.1538-7836.2003.00406.x [DOI] [PubMed] [Google Scholar]
- 54.Verhovsek M., Moffat K.A., Hayward C.P.M.2008. Laboratory testing for fibrinogen abnormalities. Am. J. Hematol. 83: 928–931. doi: 10.1002/ajh.21293 [DOI] [PubMed] [Google Scholar]
- 55.Walf A.A., Frye C.A.A.2006. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology 31: 1097–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walf A.A., Frye C.A.2010. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol. Behav. 99: 169–174. doi: 10.1016/j.physbeh.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winn M.J, Park B.K.1987. The effects of 17 β-oestradiol or testosterone on the response to S-warfarin in castrated male rats. J. Pharm. Pharmacol. 39: 958–960. doi: 10.1111/j.2042-7158.1987.tb03140.x [DOI] [PubMed] [Google Scholar]
- 58.Zar J.H.1984. Bio-statistical Analysis. New Jersey: Prentice-Hall Inc Englewood Cliffs. [Google Scholar]