Abstract
In previous studies, we have shown that phosphonium salt diphenyl derivatives are attractive antitrypanosomal hit compounds with EC50 values against Trypanosoma brucei in the nanomolar range. To evaluate the role of the cationic center on the trypanocidal activity and extend the structure–activity relationship (SAR) of this series, trialkylammonium, pyridinium, and quinolinium salt analogues were synthesized and evaluated in vitro against T. b. brucei. Similar SARs were observed with ammonium and phosphonium salts showing that charge dispersion and lipophilic groups around the cationic center are crucial to obtain submicromolar activities. The new compounds were equally effective against wild type (T. b. brucei s427) and resistant strains (TbAT1-KO and TbB48) of trypanosomes indicating that the P2 and high affinity pentamidine transporters (HAPT) are not essential to their trypanocidal action. Similarly to phosphonium salt derivatives, diffusion seems to be the main route of entry into trypanosomes.
Keywords: Trypanosoma brucei, ammonium salt, quinolinium salt, pyridinium salt, P2-transporter, high affinity pentamidine transporter (HAPT)
Parasitic protozoa of the genus Trypanosoma cause human and animal trypanosomiases in Africa. Two subspecies of T. brucei (T. b. gambiense and T. b. rhodesiense) are pathogenic to man and cause human African trypanosomiasis (HAT) in sub-Saharan Africa. In contrast, T. b. brucei, T. congolense, and T. vivax cause veterinary diseases in domestic and wild animals.1,2 African trypanosomiasis is one of the most neglected tropical diseases as shown by the lack of safe drugs to treat both (early and late) stages of the illness. These drugs, which have been used for decades, are subject to treatment failure (drug resistance) and have severe drawbacks such as unacceptable toxicity and parenteral administration.3
Cationic drugs are an important class of trypanocides used for the treatment of human and veterinary trypanosomiasis (e.g., pentamidine, diminazene, homidium, and isomethamidium).4,5 In previous studies we have shown that a new class of cationic compounds, mono- and bisphosphonium salt derivatives with a diphenyl scaffold, have submicromolar activities against T. brucei and Leishmania parasites.6,7 These compounds display a good selectivity index relative to human cell lines, are not cross-resistant with existing trypanocides, and require only a short exposure time for trypanocidal activity.6 Like other dicationic trypanocides,8,9 the bisphosphonium salts are expected to accumulate in the mitochondria. Indeed, a mitochondrial target for these compounds was demonstrated in Leishmania.7
Structure–activity relationship (SAR) studies revealed that bulky substituents on the phosphorus atoms are required to achieve submicromolar EC50 values within this series. In the present work, we wanted to learn whether the nature of the cationic center was important for the observed trypanocidal activity of these hit compounds. Thus, new cationic analogues of the hits with a nitrogen atom as cationic center (i.e., trialkylammonium, pyridinium, and quinolinium salts) were synthesized and tested in vitro against wild type and resistant lines of T. brucei.
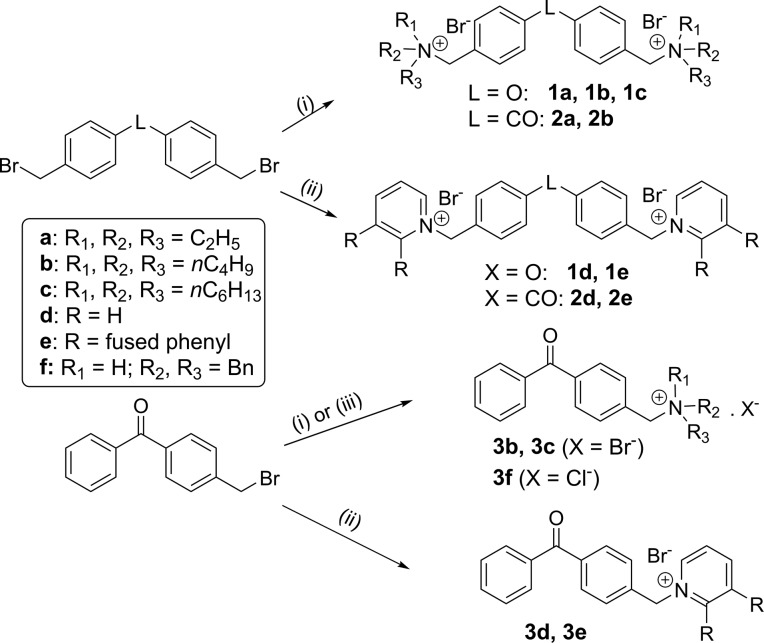
The ammonium (1a–c, 2a–b, 3b–c, and 3f), pyridinium (1d–3d), and quinolinium (1e–3e) derivatives were synthesized by nucleophilic substitution of the (bis)brominated precursors 1–36 with tertiary amines in refluxing acetonitrile (Scheme 1). The compounds were isolated and purified by crystallization.
Scheme 1. Synthesis of Ammonium, Pyridinium, and Quinolinium Salts.
Reagents and conditions: (i) R3N (2.2 equiv for 1 and 2; 1.1 equiv for 3), CH3CN, 80 °C; (ii) pyridine or quinoline (2.2 equiv for 1 and 2; 1.1 equiv for 3), CH3CN, 80 °C; (iii) Bn2NH (1.1 equiv), K2CO3 (1.2 equiv), 60 °C then HClg/dioxane.
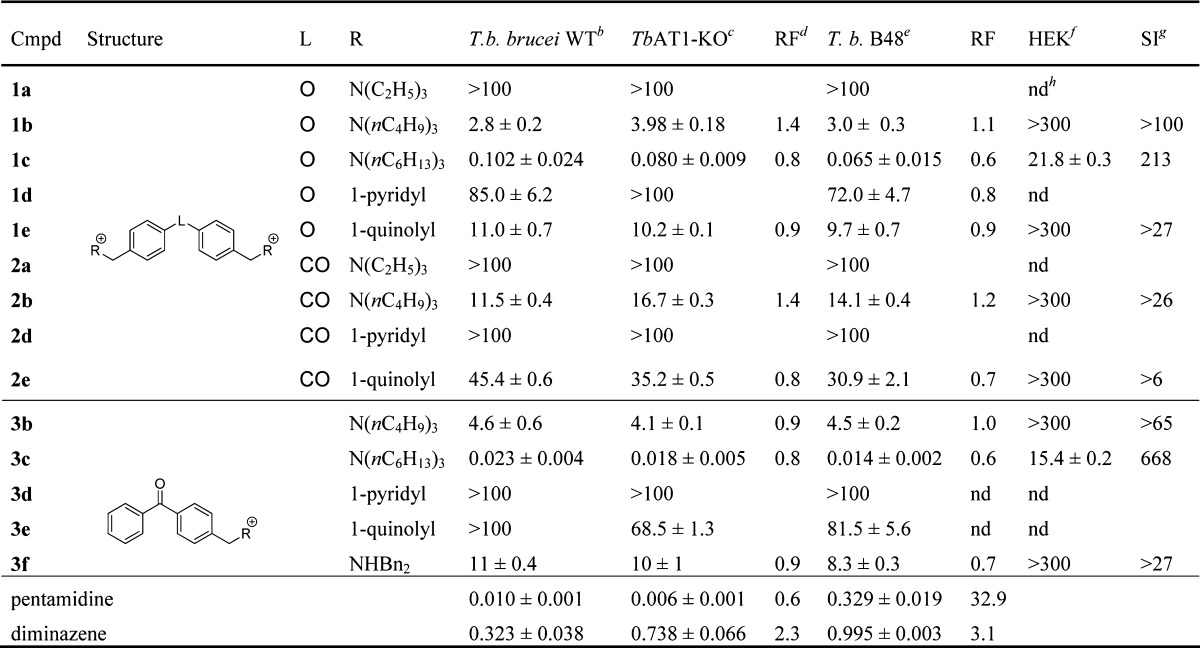
The compounds were evaluated against the T. b. brucei s427 wild type strain and two drug resistant strains, TbAT1-KO and B48, using an alamarBlue-based assay.12,13TbAT1-KO is a trypanosome strain lacking a functional P2-transporter and is resistant to diminazene.10 The B48 strain is a mutant derived from the TbAT1-KO strain11 with a nonfunctional high affinity pentamidine transporter (HAPT).14 This strain is resistant to diminazene, pentamidine, and melaminophenyl arsenicals.11 The resistance factor (RF) with respect to wild type trypanosomes was calculated for both resistant strains. Finally, the cytotoxicity against human endothelial kidney (HEK) cells was also determined,15 and the selectivity indices were calculated (Table 1).
Table 1. In Vitro Antitrypanosomal Activity (EC50, μM)a against Wild Type and Resistant Strains of T. b. brucei and Cytotoxicity against HEK Cells (CC50, μM).
Mean of three experiments ± SEM.
T. b. brucei s427 trypomastigotes.
T. b. brucei knockout strain10 lacking a functional P2-transporter and resistant to diminazene aceturate.
Resistance factor compared to WT.
The B48 strain11 is a mutant derived from the TbAT1-KO strain with a nonfunctional high affinity pentamidine transporter (HAPT). This strain is resistant to diminazene, pentamidine, and melaminophenyl arsenicals.
Human endothelial kidney cells.
Selectivity index = [CC50/EC50 (T.b.b.WT)].
Not determined.
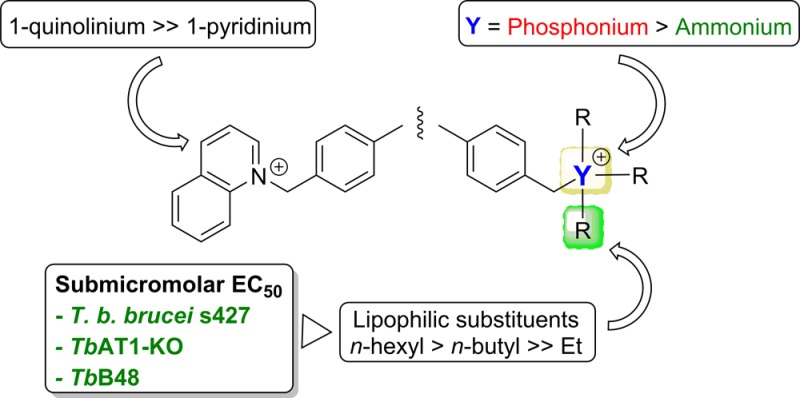
The trialkylammonium salts with long alkane chains (n-C6H13 > n-C4H9 ≫ n-C2H5) and the dibenzyl derivative 3f were the most potent of the new series with EC50 values against T. brucei in the low micromolar (1b, 2b, and 3b) to nanomolar range (1c and 3c). The monocationic trihexylammonium salt 3c (EC50 (s427) = 23 nM) was two times less active than pentamidine (EC50 (s427) = 10 nM) but 14 times more active than diminazene in this assay. More importantly, this compound was equally active against the TbAT1-KO and B48 resistant strains (RF = 0.8 and 0.6, respectively) contrary to pentamidine (RF = 0.6 and 32.9) and diminazene (RF = 2.3 and 3.1).
In contrast, the quinolinium salts (1e and 2e) displayed micromolar range activities (>10 μM), whereas the pyridinium salts (1d–3d) were mostly inactive (EC50 > 50 μM). Importantly, all the compounds had similar activities against wild type and resistant lines of T. brucei (i.e., RF ≈ 1), which indicates that the P2 and HAPT transporters are not essential for the uptake of these compounds by the parasite. Hence, cross-resistance with existing trypanocides is not likely to appear with these compounds. The compounds showed low cytotoxicity against HEK cells, which turned into selectivity indices >25 for 1e, 2b, 3b, and 3f and >100 for 1b, 1c, and 3c.
The comparison of these data with previous results obtained with phosphonium salt analogues6 informed on the influence of P ↔ N replacement on the antitrypanosomal activity of these series (Table 2). In most cases, phosphonium salts have EC50 against T. brucei from 2- to 24-times lower than the corresponding ammonium salts, but the cytotoxicity of both series against HEK cells is similar, which means that phosphonium salts generally have higher therapeutic indices than ammonium and quinolinium salts. In both series, more lipophilic alkane substituents (i.e., alkyl chains with >4 methylene units) were favored and produced compounds with submicromolar EC50 values.
Table 2. SAR Studies: Effect of P ↔ N Replacement on Antitrypanosomal Activity and Cytotoxicity.
|
T.
b. brucei WT EC50 (μM) |
HEK
cells CC50 (μM) |
||||
|---|---|---|---|---|---|
| cmpd | R | NR3 | PR3a | NR3 | PR3a |
| 1a | C2H5 | >100 | 36.5 ± 3.5 | nd | >300 |
| 1b | n–C4H9 | 2.8 ± 0.2 | 0.59 ± 0.06b | >300 | >300b |
| 1c | n-C6H13 | 0.102 ± 0.024 | 0.037 ± 0.004 | 21.8 ± 0.3 | 22.1 ± 1.1 |
| 2a | C2H5 | >100 | >100 | nd | >300 |
| 2b | n-C4H9 | 11.5 ± 0.4 | 0.48 ± 0.11 | >300 | >300 |
| 3b | n-C4H9 | 4.6 ± 0.6 | 5.9 ± 0.7b | >300 | >300b |
| 3c | n-C6H13 | 0.023 ± 0.004 | 0.011 ± 0.004 | 15.4 ± 0.2 | 13.4 ± 0.3 |
Data taken from ref (6).
EC50 value for R = i-C4H9 (isobutyl).
Phosphonium salt derivatives based on the diphenyl scaffolds 1–3 are promising compounds active in vitro against wild type and resistant lines of T. brucei. They also display a good selectivity index versus human cell lines.6 In the present study, we synthesized new ammonium, pyridinium, and quinolinium salt derivatives using the same diphenyl scaffolds to check the importance of the phosphorus atom in these series. Replacement of the cationic center from phosphorus to biososteric nitrogen atom led to compounds with disparate activities. The trialkylammonium salt derivatives displayed antiparasitic activities with the same order of magnitude as the trialkylphosphonium salts, showing no improvement in selectivity. We have shown earlier that in this series increased activity is obtained with positive charge dispersion over a large surface.6 Hence, the 2- to 5-fold higher activity of the phosphonium salt derivatives relative to the ammonium salts may possibly be related to the lower charge density of the phosphorus atom that arises from a larger atomic radius.16 The 2- to 7-fold higher activities of the quinolinium salts (i.e., charge delocalized over two aromatic rings) compared with the pyridinium salts reported here also support this view.
As for phosphonium analogues6 the anti-T. brucei activity of the trialkylammonium compounds requires a substantial level of lipophilicity around the cationic N atom. It is also not dependent on entry through the P2 and HAPT transporters. These results, similar to a series of dicationic choline-derived compounds,9 indicate that diffusion is the most probable mechanism of uptake into trypanosomes, although the presence of an unknown transporter for lipophilic cations cannot be excluded. However, it is likely that the lipophilic ammonium and phosphonium compounds would have to pass both the plasma membrane and the mitochondrial membrane, as such compounds are generally believed to accumulate, and act, in the mitochondria (De Koning, manuscript in preparation).7
In summary, modulation of the cationic center (from phosphorus to nitrogen) of the diphenylether and benzophenone class of trypanocides resulted in 2- to 5-times lower activities against wild type and resistant T. b. brucei lines. Nevertheless, two new potent and selective trypanocidal compounds were obtained, 1c and 3c, which displayed nanomolar EC50 and SI > 100. Similar SARs were observed with ammonium and phosphonium salts showing that charge dispersion and lipophilic groups around the cationic center are crucial to obtain submicromolar activities. Nitrogen and phosphorus cationic compounds are taken up into trypanosomes independently from the known T. b. brucei drug transporters. This indicates that the dicationic compounds reported here should not be prone to cross-resistance with clinically used trypanocidal drugs such as melarsoprol, diminazene, and pentamidine, as extensive evidence from laboratory models and clinical studies link resistance to these drugs to the P2 and HAPT1 transporters10,11,14,17 absent from the test strains utilized here.
Experimental Methods
Chemistry
All dry solvents were purchased from Aldrich or Fluka in Sure/Seal bottles. All reactions requiring anhydrous conditions were performed under argon atmosphere. The reactions were performed in screw-capped Kimax tubes and monitored by HPLC–MS. Analytical HPLC–MS was run with an Xbridge C18–3.5 μm (2.1 × 100 mm) column on a Waters 2695 separation module coupled with a Waters Micromass ZQ spectrometer using electrospray ionization (ESI+). All compounds are >95% pure by HPLC otherwise noted. 1H and 13C NMR spectra were recorded on a Bruker Advance 300 spectrometer. 1H NMR chemical shifts were internally referenced to the residual proton resonance of the deuterated solvent for CD3OD (δH = 3.49 ppm) or TMS (δH = 0 ppm) for CDCl3. Peak multiplicity is given as singlet (s), doublet (d), triplet (t), quadruplet (q), m (multiplet), and br (broad peak). J values are given in Hz. 13C NMR spectra were referenced to the chemical shift of the deuterated solvent: CD3OD (δC = 49.0 ppm) and CDCl3 (δC = 77.16 ppm). Peak assignments are noted as follows: aromatic H (ArH), pyridine H (PyrH), and quinoline H (QuinH). Melting points (uncorrected values) were determined in open capillary tubes with a Mettler Toledo MP70 melting point system or with a Reichert Jung Thermovar apparatus.
General Procedure for the Synthesis of Ammonium, Pyridinium, and Quinolinium Salts
The reactions were carried out under argon in Kimax tubes stopped with a screwcap. To a solution of linker 1–3 (1 equiv) in anhydrous CH3CN (0.8 mL) was added 2.2 equiv (1.1 equiv for linker 3) of the corresponding tertiary amine. The tube was stopped up and heated at 80 °C for 75 min. The product was purified by crystallization as specified in each case. Representative examples (1c, 1d, and 1e) are given below. The synthesis and characterization of 1a, 1b, 2a, 2b, 2d, 2e, and 3b–3f will be reported elsewhere.
N,N′-((Oxybis(4,1-phenylene))bis(methylene))bis(N,N-dihexylhexan-1-aminium) Bromide (1c)
The reaction was carried out by following the general procedure starting from 4,4′-oxybis((bromomethyl)benzene) (54.6 mg, 0.153 mmol) and trihexylamine (114 μL, 0.337 mmol). Then, diethyl ether (4 mL) was added to the cold reaction mixture, and the tube was allowed to stand at 4 °C overnight. The supernatant was removed and the sticky precipitate was covered with Et2O and triturated with a spatula until formation of a white solid. The product was recrystallized from CH2Cl2/Et2O, rinsed with Et2O, and dried under vacuum. Off-white amorphous solid (29 mg, 21%); HPLC > 90% pure; 1H NMR (300 MHz, CDCl3) δ 7.58 (d, J = 8.15, 4H, ArH), 6.89 (d, J = 8.15, 4H, ArH), 4.98 (s, 4H, PhCH2), 3.27 (brm, 12H, NCH2), 1.75 (brm, 12H, NCH2CH2), 1.27 (brs, 36H, (CH2)3CH3), 0.82 (t, J = 6.6, 18H, CH3). 13C NMR (75 MHz, CDCl3) δ 157.87, 134.83, 123.10, 119.34, 59.04, 31.31, 26.27, 22.89, 22.53, 13.97. LRMS (ES+ mode) m/z 367.5 [(M2+), 100%].
1,1′-((Oxybis(4,1-phenylene))bis(methylene))bis(pyridin-1-ium) Bromide (1d)
The reaction was carried out by following the general procedure starting from 4,4′-oxybis((bromomethyl)benzene) (25 mg, 0.07 mmol) and pyridine (12.5 μL, 0.15 mmol). Then, the white precipitate was collected by filtration, rinsed with acetonitrile and ether, and dried under vacuum to yield a white solid (12 mg). The filtrate and washings were mixed and evaporated under vacuum. The resulting residue dissolved in a little methanol was treated with diethyl ether, and the flask was allowed to stand at 4 °C overnight. The resulting white solid was collected and rinsed with ether to yield another 15.6 mg of the product. White amorphous solid (27.6 mg, 76%); HPLC > 95% pure. 1H NMR (300 MHz, CD3OD) δ 9.09 (d, J = 6.6, 4H, PyrH-2, H-6), 8.65 (m, 2H, PyrH-4), 8.14 (m, 4H, PyrH-3 and H-5), 7.57 (d, J = 8.7, 4H, ArH), 7.11 (d, J = 8.7, 4H, ArH), 5.86 (s, 4H, CH2). 13C NMR (75 MHz, CD3OD) δ 159.36, 147.30, 145.90, 132.43, 129.87, 129.75, 120.91, 65.07. LRMS (ES+) m/z 117.4 [(M2+), 100%].
1,1′-((Oxybis(4,1-phenylene))bis(methylene))bis(quinolin-1-ium) Bromide (1e)
The reaction was carried out by following the general procedure starting from 4,4′-oxybis((bromomethyl)benzene) (27 mg, 0.076 mmol) and quinoline (20 μL, 0.167 mmol). The yellow precipitate was collected and rinsed with CH3CN and diethyl ether, successively, to yield a yellow solid (24.6 mg). The filtrate was evaporated under vacuum, and the residue dissolved in MeOH was treated with diethyl ether. The flask was allowed to stand at 4 °C overnight. The precipitate was collected and rinsed with diethyl ether yielding a yellowish solid (21 mg). Both solids were mixed and recrystallized in boiling dichloromethane. The pure product was dried under high-vacuum at 70 °C. Yellowish solid (30 mg, 64%); mp 228.4 °C; HPLC = 95% pure; 1H NMR (300 MHz, CD3OD) δ 9.58 (d, J = 6.9, 2H, QuinH-4), 9.30 (d, J = 8.4, 2H, QuinH-2), 8.58 (d, J = 9.0, 2H, QuinH-8), 8.46 (d, J = 8.6, 2H, QuinH-5), 8.20 (m, 4H, QuinH-3 and H-7), 8.03 (t, J = 7.7, 2H, QuinH-6), 7.45 (d, J = 8.5, 4H, ArH), 7.03 (d, J = 8.6, 4H, ArH), 6.35 (s, 4H, CH2). 13C NMR (75 MHz, CD3OD) δ 158.88, 150.77, 149.68, 139.62, 137.33, 132.19, 131.90, 131.45, 130.94, 129.54, 123.28, 120.77, 120.26, 61.48. LRMS (ES+ mode) m/z 227.4 [(M2+), 100%].
In Vitro Activity against T. b. brucei
The in vitro trypanocidal and cytotoxic activities were determined using an alamarBlue-based assay13,18 exactly as described.9,12,15
Glossary
Abbreviations
- BBB
blood–brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- HAPT
high affinity pentamidine transporter
- HAT
human African trypanosomiasis
- HEK cells
human endothelial kidney cells
- LAPT
low affinity pentamidine transporter
- RF
resistance factor
- WT
wild type
Author Contributions
The manuscript was written through contributions of all authors.
Support of the Government of Saudi Arabia (Studenship to A.A.M.A., Aljouf University, Saudi Arabia) is acknowledged. This work was supported by grants from the Spanish Ministerio de Ciencia e Innovación (Grant SAF2009–10399) to C.D.
The authors declare no competing financial interest.
References
- Brun R.; Blum J.; Chappuis F.; Burri C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [DOI] [PubMed] [Google Scholar]
- Barrett M. P.; Burchmore R. J.; Stich A.; Lazzari J. O.; Frasch A. C.; Cazzulo J. J.; Krishna S. The trypanosomiases. Lancet 2003, 362, 1469–80. [DOI] [PubMed] [Google Scholar]
- WHO/TDR. Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. Technical Report no. 975; World Health Organization: Geneva, Switzerland, 2012. [PubMed] [Google Scholar]
- Holmes H. P.; Eisler M. C.; Geerts S.. Current Chemotherapy of Animal Trypanosomiasis. In The Trypanosomiases; Maudlin I.; Holmes H. P.; Miles M. A., Eds.; CABI Publishing: Wallingford, U.K., 2004; pp 431–444. [Google Scholar]
- Burri C.; Stich G.; Brun R.. Current Chemotherapy of Human African Trypanosomiasis. In The Trypanosomiasis; Maudlin I.; Holmes P.; Miles M., Eds.; CABI Publishing: Wallingford, U.K., 2004. [Google Scholar]
- Taladriz A.; Healy A.; Flores Pérez E. J.; Herrero García V.; Ríos Martínez C.; Alkhaldi A. A. M.; Eze A. A.; Kaiser M.; De Koning H. P.; Chana A.; Dardonville C. Synthesis and structure-activity analysis of new phosphonium salts with potent activity against African trypanosomes. J. Med. Chem. 2012, 55, 2606–2622. [DOI] [PubMed] [Google Scholar]
- Luque-Ortega J. R.; Reuther P.; Rivas L.; Dardonville C. New benzophenone-derived bisphosphonium salts as leishmanicidal leads targeting mitochondria through inhibition of respiratory complex II. J. Med. Chem. 2010, 53, 1788–1798. [DOI] [PubMed] [Google Scholar]
- Lanteri C. A.; Tidwell R. R.; Meshnick S. R. The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob. Agents Chemother. 2008, 52, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim H. M. S.; Al-Salabi M. I.; Sabbagh N. E.; Quashie N. B.; Alkhaldi A. A. M.; Escale R.; Smith T. K.; Vial H. J.; De Koning H. P. Symmetrical choline-derived dications display strong anti-kinetoplastid activity. J. Antimicrob. Chemother. 2011, 66, 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenko B.; Van Der Burg A. M.; Wanner M. J.; Kaiser M.; Brun R.; Gould M.; De Koning H. P.; Koomen G. J. 2,N6-disubstituted adenosine analogs with antitrypanosomal and antimalarial activities. Antimicrob. Agents Chemother. 2007, 51, 3796–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räz B.; Iten M.; Grether-Bühler Y.; Kaminsky R.; Brun R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [DOI] [PubMed] [Google Scholar]
- Matovu E.; Stewart M. L.; Geiser F.; Brun R.; Mäser P.; Wallace L. J. M.; Burchmore R. J.; Enyaru J. C. K.; Barrett M. P.; Kaminsky R.; Seebeck T.; De Koning H. P. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryotic Cell 2003, 2, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D. J.; Gould M. K.; Nerima B.; Mäser P.; Burchmore R. J. S.; De Koning H. P. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol. Pharmacol. 2007, 71, 1098–1108. [DOI] [PubMed] [Google Scholar]
- Munday J. C.; Eze A. A.; Baker N.; Glover L.; Clucas C.; Aguinaga Andrés D.; Natto M. J.; Teka I. A.; McDonald J.; Lee R. S.; Graf F. E.; Ludin P.; Burchmore R. J. S.; Turner C. M. R.; Tait A.; MacLeod A.; Mäser P.; Barrett M. P.; Horn D.; De Koning H. P. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changtam C.; De Koning H. P.; Ibrahim H.; Sajid M. S.; Gould M. K.; Suksamrarn A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur. J. Med. Chem. 2010, 45, 941–956. [DOI] [PubMed] [Google Scholar]
- Blundell R. K.; Licence P. Quaternary ammonium and phosphonium based ionic liquids: a comparison of common anions. Phys. Chem. Chem. Phys. 2014, 16, 15278–15288. [DOI] [PubMed] [Google Scholar]
- Graf F. E.; Ludin P.; Wenzler T.; Kaiser M.; Brun R.; Pyana P. P.; Buscher P.; De Koning H. P.; Horn D.; Mäser P. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl. Trop. Dis. 2013, 7, e2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J.; Wilson I.; Orton T.; Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [DOI] [PubMed] [Google Scholar]