Abstract
Migration of the methylene imidazole side chain in the first reported selective drug-like AT2 receptor agonist C21/M024 (1) delivered the AT2 receptor antagonist C38/M132 (2). We now report that the AT2 receptor antagonist compound 4, a biphenyl derivative that is structurally related to 2, is transformed to the agonist 6 by migration of the isobutyl group. The importance of the relative position of the methylene imidazole and the isobutyl substituent is highlighted herein.
Keywords: AT2 receptor, nonpeptide ligands, agonist, antagonist, C21/M024, C38/M132
The sartans (angiotensin II receptor blockers, ABRs) have been on the market as antihypertensives for more than two decades and act as selective antagonists at the angiotensin II AT1 receptor. More recently, the angiotensin AT2 receptor has emerged as a new target for drug intervention.1−3 In 2004, the first selective and drug-like AT2 receptor agonist C21/M024 (1) was disclosed,4 and thereafter, a series of structural analogues with agonistic properties have been reported.5,6 The activation of the AT2 receptor is known to render a large number of diverse biological responses.7,8 For example, receptor activation stimulates neurite outgrowth in neuronal cells, which is one of the first steps in neuronal differentiation.9
In healthy adults the AT2 receptor expression is low and mainly found in renal, cardiovascular, and brain tissues.7,8 Notably, during certain pathological conditions, such as myocardial infarction, vascular injury, brain ischemia, renal failure, and cutaneous wounds, the AT2 receptor expression is significantly up-regulated.7,8
Recently, several studies conducted in experimental disease models have revealed that the selective potent AT2 receptor agonist 1 acts as an anti-inflammatory agent and exerts pronounced tissue protective effects in particular in cardiovascular and renal disease. Hence, the compound has had a large impact on the view of the AT2 receptor as a drug target.1,9−14 In addition, the AT2 receptor is a promising drug target for antagonists. Thus, the AT2 receptor antagonist EMA401 has demonstrated convincing efficacy data in patients suffering from neuropathic pain (postherpetic neuralgia) in a phase II study.3
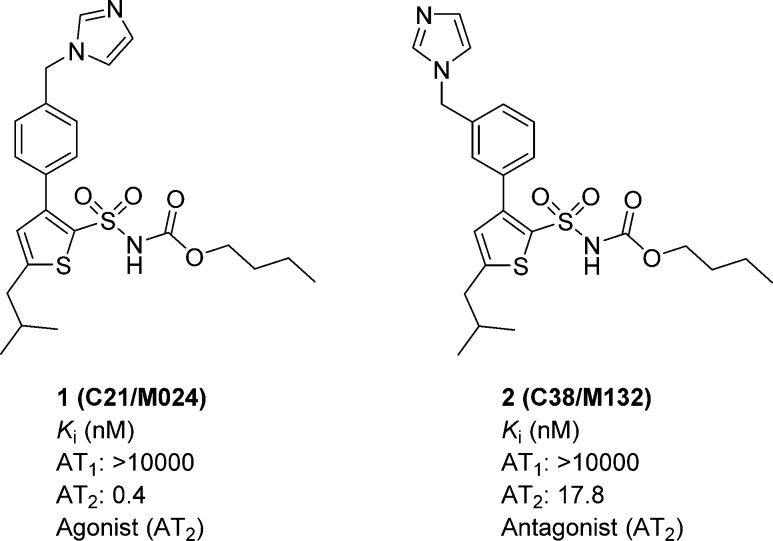
We discovered that migration of the methylene imidazole substituent of 1 from the para to the meta substitution pattern delivered a selective AT2 receptor antagonist, compound C38/M132 (2) (Figure 1).15,16 Thus, a minor structural alteration resulted in the interconversion of a selective AT2 receptor agonist to a selective AT2 receptor antagonist.
Figure 1.
Structures of compounds 1 and 2.
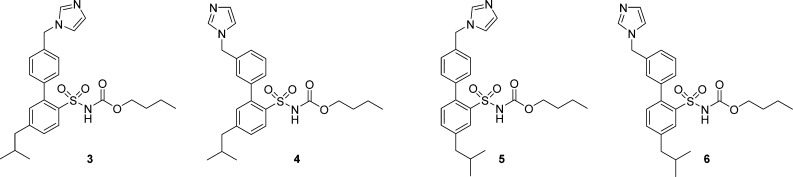
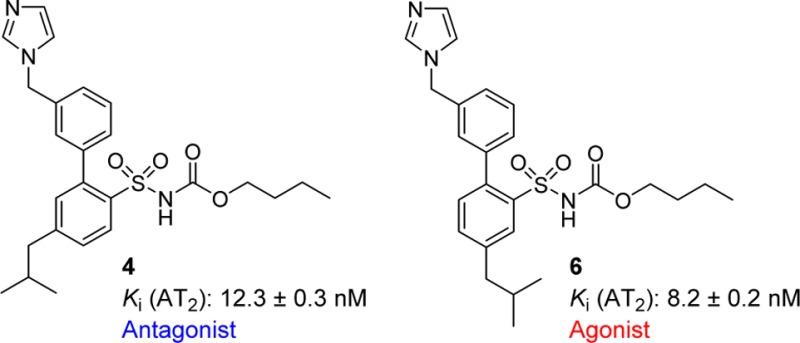
Herein, we report the synthesis and biological evaluation of four AT2 receptor ligands, the biphenyl derivatives, and regioisomers, 3, 4, 5, and 6 (Figure 2), and that the relative position of the imidazole and isobutyl substituent determines the functional outcome.
Figure 2.
Structures of the regioisomers 3, 4, 5, and 6.
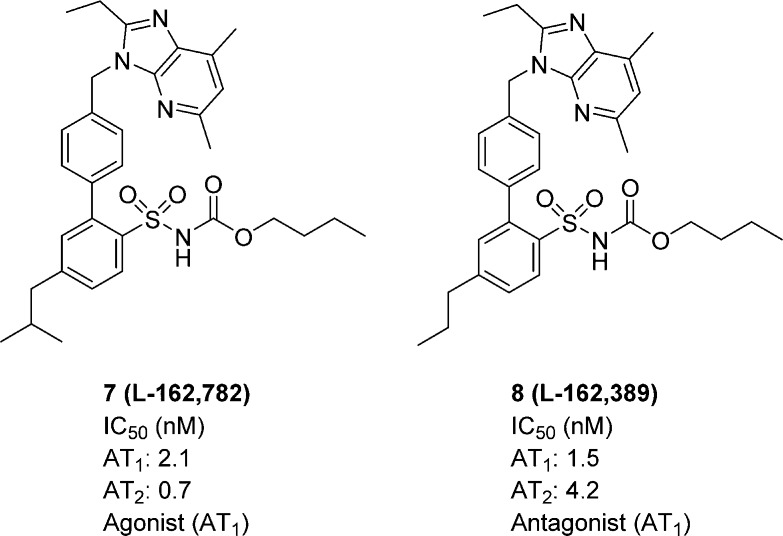
The nonselective Ang II receptor ligand 7 (L-162,782) acts as an AT1 receptor agonist and 8 (L-162,389) acts as an AT1 receptor antagonist (Figure 3).17 Notably, these two structures differ only by a methyl group; the isobutyl group gives rise to agonism, while the n-propyl group renders antagonism.17
Figure 3.
Structures of compounds 7 and 8.
Since the only structural difference between the agonist 1 and the antagonist 2 can be found in the position of the methylene imidazole substituent, it can be hypothesized that the change in functionality could be attributed to a reduced ability to stabilize the activated receptor conformation due to a differently positioned imidazole group in 2. However, as an alternative hypothesis the imidazole rings could have the same receptor interaction point, while the isobutyl groups are forced to be positioned differently and cause the difference in functionality (Figure 4A). If so, the topography and location of the lipophilic side chain of ligands binding to the AT2 receptor is the primary determinant of functionality as was the case at the AT1 receptor (cf. 7 and 8).
Figure 4.
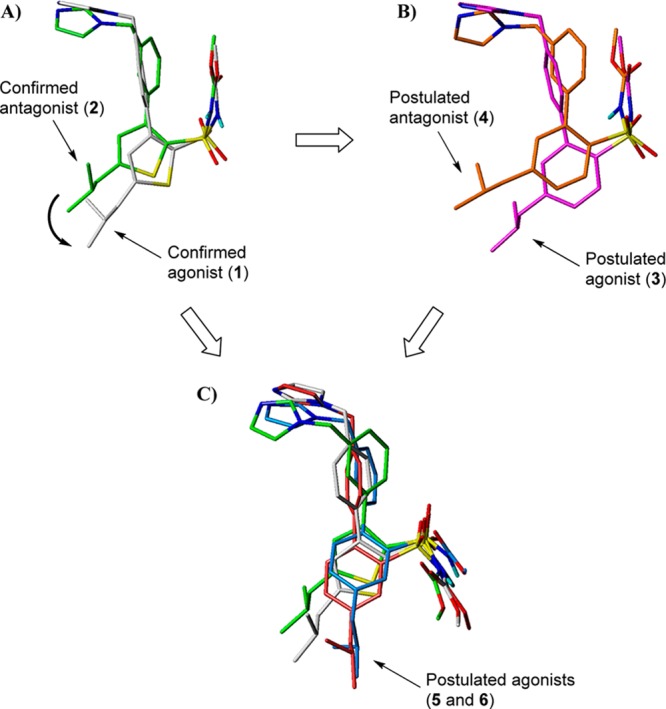
(A) Possible binding mode of two model compounds corresponding to structures 1 (white) and 2 (green). (B) Corresponding hypothesized binding mode for the two model compounds corresponding to the biphenyl analogues, 3 (purple) and 4 (orange), and postulated functions at the AT2 receptor. (C) Comparison of the suggested binding modes of 1 and 2 with the analogues of 3 and 4, where the isobutyl have been moved to the meta-position, the two model compounds corresponding to compounds 5 (red) and 6 (blue), respectively.
In order to explore this alternative hypothesis and to identify a potential binding mode, a computational analysis was performed. Potential pharmacophore groups, including the imidazole rings, of 1 and 2 were superimposed using the pharmacophore modeling program DISCOtech18 in Sybyl19 (see Supporting Information for details). In order to reduce the number of conformations, model structures with methyl instead of butyl substituents on the sulfonylcarbamate group were used in the modeling. From the suggested binding mode model identifide, shown in Figure 4A, the isobutyl groups are oriented differently in the two thiophene compounds, which could be of importance for their ability to induce either the agonistic or antagonistic response. On the basis of this model, we hypothesized that it should be possible to convert the meta-substituted antagonist 2 to an agonist if the isobutyl chain could adopt a similar orientation as in the para-substituted agonist 1, illustrated in Figure 4A. To investigate this hypothesis we performed further modeling where we replaced the thiophene ring with a phenyl group to obtain biphenyl analogues allowing better assessment of the impact of relative positions of the isobutyl side chain (i.e., 3 and 4, see Figure 2). Accordingly, superimposition of 3 and 4 produced a model, depicted in Figure 4B, which was very similar to that shown in Figure 4A. The corresponding analogues with an isobutyl group meta to the sulfonylcarbamate, compounds 5 and 6 (Figure 2), were also modeled and compared with the suggested binding modes of 1 and 2 as seen in Figure 4C. Although the position of the isobutyl group in 6 did not superimpose optimally to the corresponding group in 1, it was clearly closer to this binding mode than that of 2, where the imidazole and the sulfonylcarbamate also could reach the same interactions (Figure 4C). According to the model in Figure 4C, compound 5, although having a meta-methylene imidazole substitution, could adopt a conformation that allows a similar binding mode as 6 and was therefore also hypothesized to act as an agonist. In order to explore this binding mode hypothesis we set out to synthesize regioisomers 3–6 and determine their AT2 receptor binding affinity and, in particular, their functional activity at the AT2 receptor.
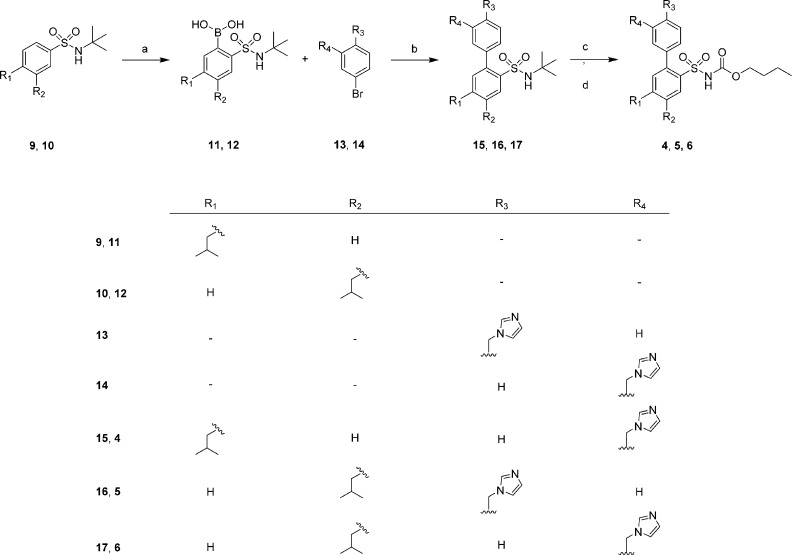
The synthesis of 3 has been previously described.5 The synthesis of compounds 4–6 are outlined in Scheme 1 and started with the benzenesulfonamides 9 and 10, prepared as previously described by Wallinder et al.20 Compound 13 and 14 were prepared by N-alkylation of imidazole by 3- and 4-bromobenzyl bromide in DMF. The crude boronic acids (11 and 12) were coupled with compounds 13 and 14 under Suzuki coupling conditions with Pd(PPh3)4 as catalyst and Na2CO3 as base using microwave irradiation (150 °C, 5 min) to form compounds 15–17 in good yields (75–87%). Compounds 4–6 were afforded after deprotection of the sulfonamide with BCl3 and subsequent acylation with n-butyl chloroformate in DCM, water, and Na2CO3 to yield the final products in 27–51% yield, after purification by preparative HPLC.
Scheme 1.
Compounds 3–6 were evaluated for affinity in a radioligand-binding assay by displacement of [125I]Ang II from AT2 receptors in pig uterus membranes as described previously by Nielsen et al.21 The natural substrate Ang II and the selective AT1 receptor antagonist losartan were used as reference substances.22 The results of the binding study are presented in Table 1. Only AT2 receptor affinity was evaluated since no AT1 receptor binding have been seen for these methylene imidazoles even upon modifications of the lower part of the structures. This is also verified by compound 3, which previously has been tested for AT1 receptor affinity (Ki > 10 000 nM).5
Table 1. Biological Activities at the AT2 Receptor.
| compd | Ki ± SD (nM) | function (10 nM) |
|---|---|---|
| 3 | 0.7 ± 0.1 | agoinst |
| 4 | 12.3 ± 0.4 | antagonist |
| 5 | 10.1 ± 0.4 | agonist |
| 6 | 8.2 ± 0.2 | agonist |
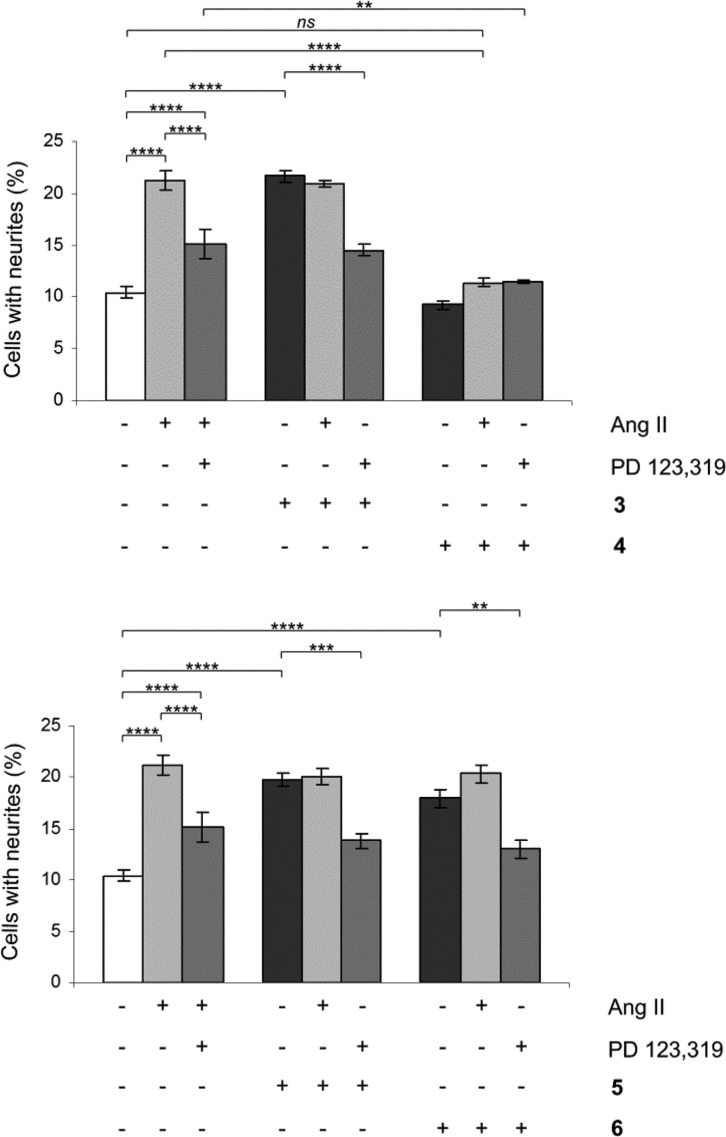
All compounds were evaluated for functionality in a neurite outgrowth assay with NG108-15 cells. The NG108-15 cells express mainly the AT2 receptor in their undifferentiated state, and a three-day treatment with Ang II or the selective peptidic AT2 receptor agonist CGP-42112 (Nα-nicotinoyl-Tyr-(Nα-Cbz-Arg)-Lys-His-Pro-Ile)23,24 induce neurite outgrowth.16,25 The signaling pathways involve a sustained increase in Rap1/B-Raf/p42/p44mapk activity and activation of the nitric oxide/guanylyl cyclase/cGMP pathway.26,27 Cells were treated in the absence or presence of Ang II, 3, 4, 5, and 6. After 3 days of treatment, cells were examined under a phase-contrast microscope and micrographs were taken. To establish an adequate test concentration, the compounds were tested at various concentrations ranging from 0.1 to 100 nM. No evidence of cell death was observed. All compounds were tested at a concentration of 10 nM, compound 4 was also tested at 100 nM to verify that the lack of neurite outgrowth was not due to too low concentration (data not shown). Coincubation with the selective AT2 receptor antagonist PD 123,319 reduced neurite outgrowth, verifying that the effect was mediated through the AT2 receptor. Treatment with PD 123,319 alone did not alter the morphology compared to untreated cells (data not shown), as we have shown previously.16 The antagonistic effect was verified through coincubation with Ang II resulting in reduced neurite outgrowth. The functionalities are summarized in Table 1 and the neurite outgrowth results are shown in Figure 5.
Figure 5.
Effect of compounds 3–6 on neurite outgrowth in NG108-15 cells. The cells were plated at a cell density of 3.6 × 104 cells/Petri dish (35 mm) and were cultured for 3 days in the absence or presence of 100 nM Ang II, 10 nM 3, 10 nM 4, 10 nM 5, or 10 nM 6 alone or in combination with 10 μM PD 123,319 or 100 nM Ang II. Cells with at least one neurite longer than a cell body were counted as positive for neurite outgrowth. The number of cells with neurites was expressed as the percentage of the total number in the micrographs (at least 400 cells according to the experiment). The results are significant according to two-way ANOVA: ****, p < 0.0001; ***, p < 0.001; **, p < 0.01; ns = not significant.
The biphenyl analogues of 1 and 2, compounds 3 and 4, respectively, exhibited a similar biological response both in affinity to and functional activity at the AT2 receptor, indicating that the biphenyl scaffold is a bioisoster to the thienylphenyl scaffold in this series of compounds. It is worth noting that the antagonist 4 exhibited a better inhibition of Ang II-induced neurite outgrowth compared to PD 123,319, even thought the concentration of 4 was a 1000-fold lower compared to PD 123,319 (10 nM vs 10 μM), as also seen with 2.15,16 Ang II stimulation after pretreatment with 4 resulted in neurite outgrowth no longer significantly different compared to untreated cells, while Ang II stimulation after pretreatment with PD 123,319 showed a neurite outgrowth significantly (****, p < 0.0001) higher compared to untreated cells. This indicates that compound 4 is a more efficient AT2 receptor antagonist than PD 123,319 in the cell assay.
Compound 3 that has been previously synthesized and assessed as an AT2 receptor ligand5 acts as an agonist (Table 1 and Figure 5). When the substitution pattern for the isobutyl chain of 3 was changed to give 5, the affinity dropped approximately 10 times, but the agonistic property was retained (Table 1 and Figure 5). When the same structural modification was performed with 4 to deliver compound 6 the affinity was slightly increased, but more interestingly, the antagonistic property of 4 was interconverted to agonism (Table 1 and Figure 5). Thus, independent of the methylene imidazole position, both an agonist and an antagonist could be obtained by moving the isobutyl side chain. This suggests that the structural feature responsible for functionality is the spatial relationship between the imidazole ring and the isobutyl side chain.
When looking at the peptide-activated GPCRs there seems to be a trend that minor structural alterations in small nonpeptidic ligands can interconvert the functional activity, cf. 7 and 8.17 Small changes in the effector domain of peptides can frequently also have large impact on functionality, as exemplified by [Ile8]AngII, which acts as an antagonist at the AT1 receptor (or partial agonist), while the native [Phe8]AngII acts as an agonist.28 Likely it is the same effects on the equilibrium between the active and inactive receptor conformation that we see from these small nonpeptidic ligands.
The presented investigation highlights the importance of the relative position of the methylene imidazole and the isobutyl substituent for functional activity in this class of small molecule AT2 receptor ligands. Thus, regardless of the position of the methylene imidazole group that previously was proposed to serve as an important determinant for agonism, both agonists and antagonists can be obtained by a short distance migration of the isobutyl group attached to the biphenyl scaffold. This suggests that the structural feature responsible for functionality is the spatial relationship between the imidazole ring and the isobutyl side chain. The data gained from molecular modeling provides a good foundation in the future design of selective AT2 receptor agonists and antagonists.
Acknowledgments
We thank Dr. Luke R. Odell for linguistic advice.
Supporting Information Available
Synthetic procedure and analytical data of the synthesized compounds, biological evaluation methods, and computational methods. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors.
We gratefully acknowledge support from the Swedish Research Council (to V.R.), the Swedish Foundation for Strategic Research to (S.S.F.), Kjell and Märta Beijer Foundation, Knut and Alice Wallenberg Foundation, the Canadian Institutes of Health Research to N.G.P., M.D. Payet (MOP 27912), and Vicore Pharma AB. N.G.P. is a holder of a Canadian Research Chair in Endocrinology of the Adrenal Gland.
The authors declare no competing financial interest.
Supplementary Material
References
- Tamargo J.; López-Sendón J. Novel therapeutic targets for the treatment of heart failure. Nat. Rev. Drug Discovery 2011, 10, 536–555. [DOI] [PubMed] [Google Scholar]
- Foulquier S.; Steckelings U. M.; Unger T. Perspective: A tale of two receptors. Nature 2013, 493, S9. [DOI] [PubMed] [Google Scholar]
- Rice A. S. C.; Dworkin R. H.; McCarthy T. D.; Anand P.; Bountra C.; McCloud P. I.; Hill J.; Cutter G.; Kitson G.; Desem N.; Raff M. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2014, 383, 1637–1647. [DOI] [PubMed] [Google Scholar]
- Wan Y.; Wallinder C.; Plouffe B.; Beaudry H.; Mahalingam A. K.; Wu X.; Johansson B.; Holm M.; Botros M.; Karlen A.; Pettersson A.; Nyberg F.; Faendriks L.; Gallo-Payet N.; Hallberg A.; Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 2004, 47, 5995–6008. [DOI] [PubMed] [Google Scholar]
- Wu X.; Wan Y.; Mahalingam A. K.; Murugaiah A. M. S.; Plouffe B.; Botros M.; Karlén A.; Hallberg M.; Gallo-Payet N.; Alterman M. Selective angiotensin II AT2 receptor agonists: Arylbenzylimidazole structure-activity relationships. J. Med. Chem. 2006, 49, 7160–7168. [DOI] [PubMed] [Google Scholar]
- Murugaiah A. M. S.; Wallinder C.; Mahalingam A. K.; Wu X.; Wan Y.; Plouffe B.; Botros M.; Karlén A.; Hallberg M.; Gallo-Payet N.; Alterman M. Selective angiotensin II AT2 receptor agonists devoid of the imidazole ring system. Bioorg. Med. Chem. 2007, 15, 7166–7183. [DOI] [PubMed] [Google Scholar]
- Steckelings U. M.; Kaschina E.; Unger T. The AT2 receptor-a matter of love and hate. Peptides 2005, 26, 1401–9. [DOI] [PubMed] [Google Scholar]
- Padia S.; Carey R. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch. Eur. J. Physiol. 2013, 465, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme L.; Gasparo M.; Gallo J. M.; Payet M. D.; Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108–15 cells. Effect counteracted by the AT1 receptors. J. Biol. Chem. 1996, 271, 22729–22735. [DOI] [PubMed] [Google Scholar]
- Kaschina E.; Grzesiak A.; Li J.; Foryst-Ludwig A.; Timm M.; Rompe F.; Sommerfeld M.; Kemnitz U. R.; Curato C.; Namsolleck P.; Tschope C.; Hallberg A.; Alterman M.; Hucko T.; Paetsch I.; Dietrich T.; Schnackenburg B.; Graf K.; Dahlof B.; Kintscher U.; Unger T.; Steckelings U. M. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction?. Circulation 2008, 118, 2523–2532. [DOI] [PubMed] [Google Scholar]
- McCarthy C.; Widdop R.; Denton K.; Jones E. Update on the angiotensin AT2 receptor. Curr. Hypertens. Rep. 2013, 15, 25–30. [DOI] [PubMed] [Google Scholar]
- Lauer D.; Slavic S.; Sommerfeld M.; Thöne-Reineke C.; Sharkovska Y.; Hallberg A.; Dahlöf B.; Kintscher U.; Unger T.; Steckelings U. M.; Kaschina E. Angiotensin type 2 receptor stimulation ameliorates left ventricular fibrosis and dysfunction via regulation of tissue inhibitor of matrix metalloproteinase 1/matrix metalloproteinase 9 axis and transforming growth factor β1 in the rat heart. Hypertension 2014, 63, e60–e67. [DOI] [PubMed] [Google Scholar]
- Gelosa P.; Pignieri A.; Fandriks L.; de Gasparo M.; Hallberg A.; Banfi C.; Castiglioni L.; Turolo L.; Guerrini U.; Tremoli E.; Sironi L. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J. Hypertens. 2009, 27, 2444–2451. [DOI] [PubMed] [Google Scholar]
- Guimond M.-O.; Battista M.-C.; Nikjouitavabi F.; Carmel M.; Barres V.; Doueik A. A.; Fazli L.; Gleave M.; Sabbagh R.; Gallo-Payet N. Expression and role of the angiotensin II AT2 receptor in human prostate tissue: In search of a new therapeutic option for prostate cancer. Prostate 2013, 73, 1057–1068. [DOI] [PubMed] [Google Scholar]
- Murugaiah A. M. S.; Wu X.; Wallinder C.; Mahalingam A. K.; Wan Y.; Sköld C.; Botros M.; Guimond M.-O.; Joshi A.; Nyberg F.; Gallo-Payet N.; Hallberg A.; Alterman M. From the first selective non-peptide AT2 receptor agonist to structurally related antagonists. J. Med. Chem. 2012, 55, 2265–2278. [DOI] [PubMed] [Google Scholar]
- Guimond M.-O.; Wallinder C.; Alterman M.; Hallberg A.; Gallo-Payet N. Comparative functional properties of two structurally similar selective nonpeptide drug-like ligands for the angiotensin II type-2 (AT2) receptor. Effects on neurite outgrowth in NG108–15 cells. Eur. J. Pharmacol. 2013, 699, 160–171. [DOI] [PubMed] [Google Scholar]
- Perlman S.; Costa-Neto C. M.; Miyakawa A. A.; Schambye H. T.; Hjort S. A.; Paiva A. C. M.; Rivero R. A.; Greenlee W. J.; Schwartz T. W. Dual agonistic and antagonistic property of nonpeptide angiotensin AT1 ligands: susceptibility to receptor mutations. Mol. Pharmacol. 1997, 51, 301–311. [DOI] [PubMed] [Google Scholar]
- Martin Y. C.; Bures M. G.; Danaher E. A.; DeLazzer J.; Lico I.; Pavlik P. A. A fast new approach to pharmacophore mapping and its application to dopaminergic and benzodiazepine agonists. J. Comput.-Aided Mol. Des. 1993, 7, 83–102. [DOI] [PubMed] [Google Scholar]
- SYBYL 7.3; Tripos International: St. Louis, MO. [Google Scholar]
- Wallinder C.; Botros M.; Rosenström U.; Guimond M.-O.; Beaudry H.; Nyberg F.; Gallo-Payet N.; Hallberg A.; Alterman M. Selective angiotensin II AT2 receptor agonists: Benzamide structure-activity relationships. Bioorg. Med. Chem. 2008, 16, 6841–6849. [DOI] [PubMed] [Google Scholar]
- Nielsen A. H.; Schauser K.; Winther H.; Dantzer V.; Poulsen K. Angiotensin II receptors and renin in the porcine uterus: myometrial AT2 and endometrial AT1 receptors are down-regulated during gestation. Clin. Exp. Pharmacol. Physiol. 1997, 24, 309–14. [DOI] [PubMed] [Google Scholar]
- Carini D. J.; Duncia J. V.; Aldrich P. E.; Chiu A. T.; Johnson A. L.; Pierce M. E.; Price W. A.; Santella J. B. I.; Wells G. J.; Wexler R. R.; Wong P. C.; Yoo S.-E.; Timmermans P. B. M. W. M. Nonpeptide angiotensin II receptor antagonists: the discovery of a series of N-(biphenylylmethyl)imidazoles as potent, orally active antihypertensives. J. Med. Chem. 1991, 34, 2525–2547. [DOI] [PubMed] [Google Scholar]
- Buisson B.; Bottari S. P.; de Gasparo M.; Gallo-Payet N.; Payet M. D. The angiotensin AT2 receptor modulates T-type calcium current in non-differentiated NG108-15 cells. FEBS Lett. 1992, 309, 161–4. [DOI] [PubMed] [Google Scholar]
- Brechler V.; Jones P. W.; Levens N. R.; de Gasparo M.; Bottari S. P. Agonistic and antagonistic properties of angiotensin analogs at the AT2 receptor in PC12W cells. Regul. Pept. 1993, 44, 207–213. [DOI] [PubMed] [Google Scholar]
- Laflamme L.; de Gasparo M.; Gallo J. M.; Payet M. D.; Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 cells. Effect counteracted by the AT1 receptors. J. Biol. Chem. 1996, 271, 22729–35. [DOI] [PubMed] [Google Scholar]
- Gendron L.; Laflamme L.; Rivard N.; Asselin C.; Payet M. D.; Gallo-Payet N. Signals from the AT2 (angiotensin type 2) receptor of angiotensin II Inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol. Endocrinol. 1999, 13, 1615–26. [DOI] [PubMed] [Google Scholar]
- Gendron L.; Coté F.; Payet M. D.; Gallo-Payet N. Nitric oxide and cyclic GMP are involved in angiotensin II AT(2) receptor effects on neurite outgrowth in NG108-15 cells. Neuroendocrinology 2002, 75, 70–81. [DOI] [PubMed] [Google Scholar]
- Khosla M. C.; Leese R. A.; Maloy W. L.; Ferreira A. T.; Smeby R. R.; Bumpus F. M. Synthesis of some analogs of angiotensin II as specific antagonists of the parent hormone. J. Med. Chem. 1972, 15, 792–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.