Abstract
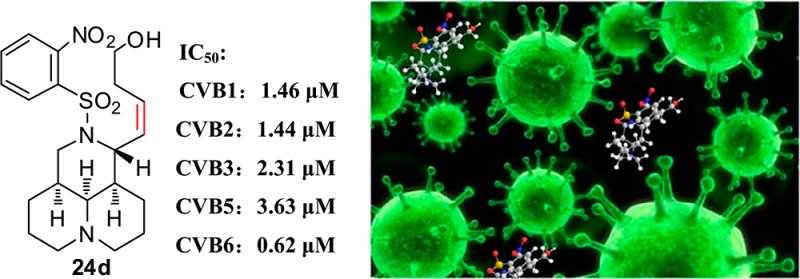
Novel N-benzenesulfonyl sophocarpinic acid/ester and sophocarpinol derivatives were synthesized and evaluated for their antienteroviral activities against coxsackievirus type B3 (CVB3) from sophocarpine (1), a natural medicine isolated from Chinese herb. Structure–activity relationship (SAR) analysis revealed that the double bond and its geometrical configuration and position at the C-11 attachment did not greatly affect the potency. Among these derivatives, sophocarpinol 24d exerted the promising activities against not only CVB3 but also CVB1, CVB2, CVB5, and CVB6 with IC50 ranging from 0.62 to 3.63 μM (SI from 46 to 275), indicating a broad-spectrum antienteroviral characteristic. The SAR results provided the powerful information for further strategic optimization and development of a novel scaffold of broad-spectrum antiviral candidates against enteroviruses.
Keywords: Sophocarpine, sophocarpinol, structure−activity relationship, enteroviruse, coxsakievirus type B3
Enteroviruses are a genus of single-stranded (+) RNA viruses associated with many human diseases.1,2 Among the enteroviruses, coxsackie B viruses (CVB) are important human pathogens causing pleurodynia, myocarditis, hepatitis, and so on.3,4 Coxsackievirus type B3 (CVB3) is an important pathogen that induces acute and chronic viral myocarditis in children and young adults, and eventually leads to cardiomyopathy.5,6 Besides heart infections, CVB3 causes chronic inflammatory diseases of the pancreas and central nervous system as well.7,8 In recent years, the sudden epidemic infections of enteroviruses have caused great concerns of society.9−11 However, there has been no special efficient drug approved for the treatment of the infections caused by CVB3 until now.12 Therefore, there is an urgent need to develop broad-spectrum antiviral candidates against enteroviruses to meet an emergency of sudden infectious diseases.
Sophocarpine (1, Figure 1), a natural medicine extracted from Chinese herb Sophora flavescens, has been used to treat viral myocarditis caused by CVB3 for decades in China with an unknown mechanism of action.13,14 The primary structure–activity relationship (SAR) investigation had been carried out with D-ring opening from compound 1, and SAR results indicated that E-β,γ-sophocarpinic acid (2, Figure 1) with a 3-ring core was more favorable than 1 with a 4-ring scaffold. As described in Figure 1, the representative N-benzenesulfonyl sophocarpinic acids 3 and 4 were identified with higher potency than lead 1,15 and an effort to further improve the potency via modification and optimization was carried out, in order to develop a novel family of antienteroviral candidates against CVB3. In the present study, using 3/4 as the leads, the SAR study was mainly focused on the variations of the carboxyl group and carbon–carbon double bond located at the C-11 attachment, while 12-benzenesulfonyl moiety on the N-12 atom was maintained as a required group for CVB3.15 Herein, we reported the synthesis, SAR analysis, and in vitro anti-CVB3 evaluation of a series of novel N-benzenesulfonyl sophocarpinic acid/ester and sophocarpinol derivatives.
Figure 1.
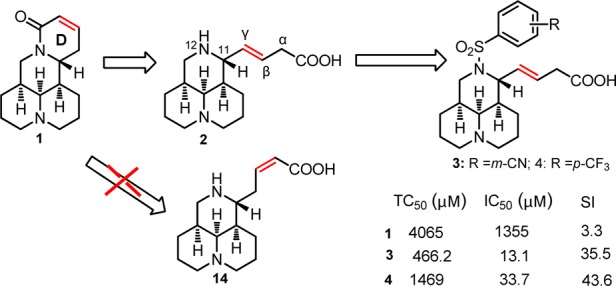
Chemical structures of sophocarpine (1); E-β,γ-sophocarpinic acid (2); E-β,γ-12-N-m-cyanobenzene-sulfonyl sophocarpinic acid (3); E-β,γ-12-N-p-trifluoromethy-benzenesulfonyl sophocarpinic acid (4); and Z-α,β-sophocarpinic acid (14).
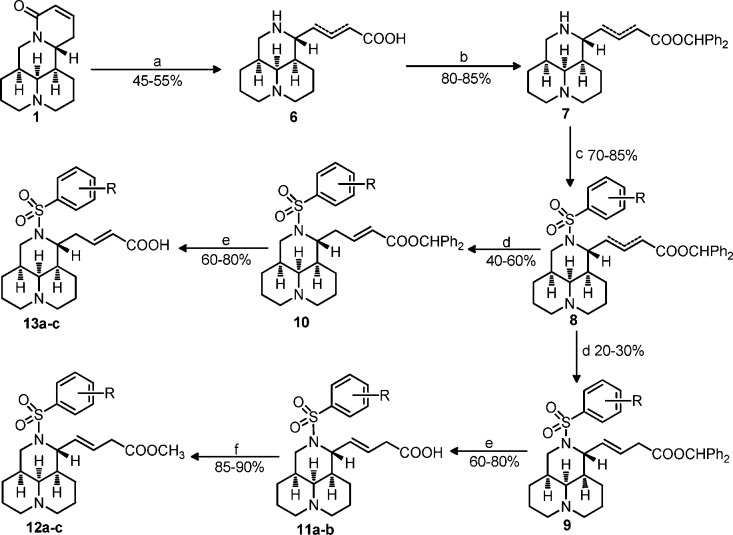
Forty-one target compounds were prepared with commercially available 1, lehmannine (5), or matrine (25) as the starting material as described in Schemes 1–3, respectively. The D-ring in compound 1 was opened in alkaline conditions to produce an isomer mixture of E-sophocarpinic acid (6), and diphenyldiazomethane was then chosen as the protective agent to facilitate the separation of mixture 8 into its two isomers 9 and 10 with good yields. The desired acid products in 11 and 13 series were acquired by deprotection of 9 or 10 in m-cresol with overall yields of 5–12% as reported previously.15,16 The sophocarpinic esters 12a–c were obtained via methyl esterification of 11 at refluxing temperature in 2 N HCl/CH3OH in 85–90% yields.
Scheme 1.
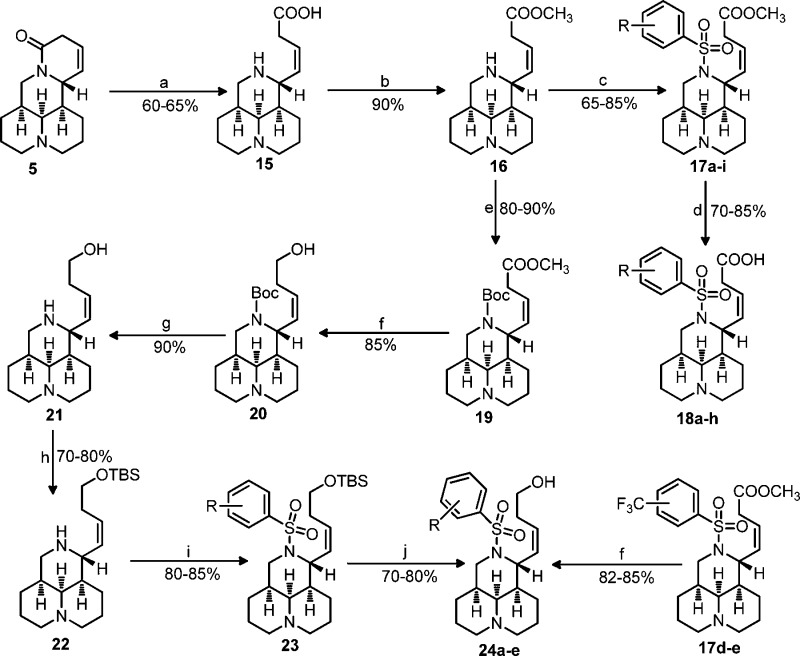
Scheme 3.
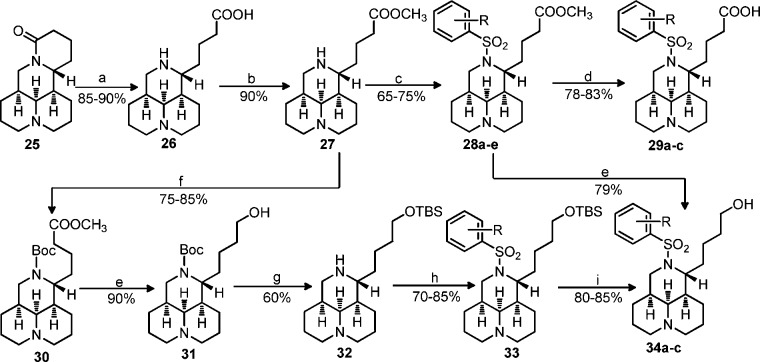
As shown in Scheme 2, methanol was used as the protective agent in compound 16 so as to conveniently obtain the target products 17 with high yields as previously reported.17 Sophocarpinols 24b–c bearing a CF3 substitution were directly gained through selective reduction of 17d–e using LiAlH4 as the reducing agent in THF in 82–85% yields.17 In another synthetic route, the key intermediate 21 was acquired via N-tert-butoxycarbonyl (Boc) protection, ester reduction with LiAlH4, and Boc removal in 2 N HCl from 16 with good yields. The sophocarpinols 24a and 24d–e possessing CN or NO2 substitution were successfully achieved through hydroxyl protection of 21 with tert-butyldimethylsilyl (TBS), 12-N-benzenesulfonyl substitution, and deprotection of 23 in 2 N HCl with overall yields of 26–32%. Similarly, as depicted in Scheme 3, matrinic acids/esters (28a–e and 29a–c) were semisynthesized from matrine with good yields as reported previously.16,17 As mentioned above, matrinol 34b possessing CF3 substitution was directly acquired from 28b; while compounds 34a and 34c with CN or NO2 were obtained via a six-step sequence from compound 27 in high yields.
Scheme 2.
All the new target compounds were measured for their in vitro anti-CVB3 activities in African green monkey kidney (Vero) cells using viral cytopathogenic effect (CPE) assay with ribavirin (RBV) as the positive control.18 The potency against CVB3 of each tested compound was evaluated by the combination of its IC50 and selectivity index (SI) value as the important therapeutic indication.
The influence of the carboxyl group at the C-11 attachment was first explored. As shown in Table 1, E-β,γ-sophocarpinic esters (12a–c) exerted potent anti-CVB3 activities with IC50 between 7.3 and 11.9 μM and high cytotoxic activities (TC50 = 38–80 μM) to give SI values 3.2–9.9. Their corresponding sophocarpinic acid (3 and 11a–b) with weaker activities and toxicities gave SI values ranging from 15.6 to 35.5. Then, E-α,β-sophocarpinic acids (13a–c) were generated to investigate whether the position of double bond would affect the activity. Compounds 13b–c afforded activities comparable to their geometric isomers; while compound 13a lost its activity, suggesting the double bond located in β,γ-position is a little more beneficial than that in α,β-position.
Table 1. Structure–Activity Relationship of Newly Synthesized Compounds against CVB3.
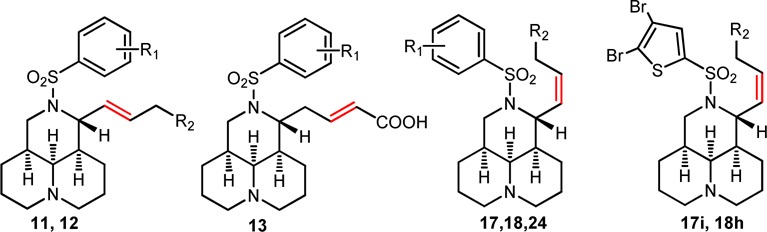
| compd | R1 | R2 | TC50 (μM)a | IC50 (μM)b | SIc |
|---|---|---|---|---|---|
| 3 | m-CN | COOH | 466.2 | 13.1 | 35.5 |
| 4 | p-CF3 | COOH | 1469 | 33.7 | 43.6 |
| 11a | o-CN | COOH | 466.2 | 22.7 | 20.5 |
| 11b | o-CF3 | COOH | 393.3 | 11.1 | 15.6 |
| 12a | m-CN | CO2CH3 | 72.4 | 7.30 | 9.9 |
| 12b | o-CN | CO2CH3 | 80.3 | 8.92 | 9.0 |
| 12c | o-CF3 | CO2CH3 | 38.1 | 11.9 | 3.2 |
| 13a | o-CN | COOH | 466.2 | >155.4 | |
| 13b | p-CN | COOH | >466.2 | 29.9 | >15.7 |
| 13c | o-CF3 | COOH | 423.7 | 27.2 | 35.5 |
| 17a | o-CN | CO2CH3 | 289.2 | 4.21 | 68.7 |
| 17b | m-CN | CO2CH3 | 542.6 | 3.07 | 176.7 |
| 17c | p-CN | CO2CH3 | 292.0 | 8.04 | 36.3 |
| 17d | m-CF3 | CO2CH3 | 198.0 | 2.24 | 88.3 |
| 17e | p-CF3 | CO2CH3 | 54.7 | 8.17 | 6.7 |
| 17f | o-NO2 | CO2CH3 | 481.2 | 5.23 | 92.1 |
| 17g | m-NO2 | CO2CH3 | 111.8 | 9.24 | 12.1 |
| 17h | p-NO2 | CO2CH3 | 185.5 | 7.02 | 26.4 |
| 17i | CO2CH3 | 21.1 | 1.19 | 17.7 | |
| 18a | o-CN | COOH | 429.6 | 27. 6 | 15.6 |
| 18b | m-CN | COOH | 744.8 | 17.4 | 42.7 |
| 18c | p-CN | COOH | 744.8 | 52.4 | 14.2 |
| 18d | p-CF3 | COOH | 272.4 | 18.6 | 14.7 |
| 18e | o-NO2 | COOH | 594.6 | 16.2 | 36.8 |
| 18f | m-NO2 | COOH | 445.4 | 63.6 | 7.0 |
| 18g | p-NO2 | COOH | 308.8 | 49.8 | 6.2 |
| 18h | COOH | 191.2 | 53.1 | 3.6 | |
| 24a | m-CN | CH2OH | 147.7 | 5.47 | 27.0 |
| 24b | m-CF3 | CH2OH | 93.5 | 15.0 | 6.2 |
| 24c | p-CF3 | CH2OH | 93.5 | 4.17 | 22.4 |
| 24d | o-NO2 | CH2OH | 353.5 | 2.31 | 152.9 |
| 24e | p-NO2 | CH2OH | 265.4 | 17.03 | 15.6 |
| RBV | 8197 | 694.6 | 11.8 |
Cytotoxic concentration required to inhibit Vero cell growth by 50%.
Concentration required to inhibit CVB3 growth by 50%.
Selectivity index: TC50/IC50.
Next, SAR was moved on the impact of Z-configuration of double bond on the anti-CVB3 effect. Among Z-sophacarpinic ester analogues (17a–i), all of them displayed excellent activities with IC50 between 1.19 and 9.24 μM, much better than that of their corresponding sophacarpinic acids 18a–h. Especially, compound 17b had a promising potency with an IC50 of 3.07 μM and SI value of 176. Sophocarpinols 24a–e showed good potency with IC50 below 17.0 μM, particularly, compound 24d exhibited an excellent potency with an IC50 of 2.31 (SI = 153), suggesting that the o-NO2 substituent might be helpful for the improved activity. The SAR results indicated that E- or Z-configuration of the double-bond was not an important factor for potency. Then, the double-bond was removed, and a couple of matrinic acids/esters (28a–e and 29a–c) and matrinols (34a–c) were constructed. As shown in Table 2, all of them displayed a favorable activity against CVB3 with SI ranging from 5.2 to 92, indicating that the double bond might not play the key role for keeping good potency.
Table 2. Structure–Activity Relationship for CVB3 of Some Aimed Compounds.
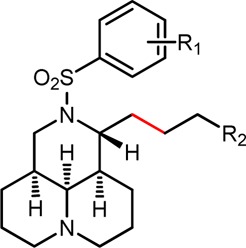
| compd | R1 | R2 | TC50 (μM)a | IC50 (μM)b | SIc |
|---|---|---|---|---|---|
| 28a | m-CN | CO2CH3 | 288.0 | 11.7 | 24.6 |
| 28b | p-CF3 | CO2CH3 | 73.4 | 2.54 | 28.9 |
| 28c | o-NO2 | CO2CH3 | 398.8 | 23.9 | 16.7 |
| 28d | m-NO2 | CO2CH3 | 68.9 | 3.51 | 19.7 |
| 28e | m-CF3 | CO2CH3 | 193.3 | 1.98 | 92.4 |
| 29a | m-CN | COOH | 427.8 | 82.3 | 5.2 |
| 29b | p-CF3 | COOH | 391.7 | 75.4 | 5.2 |
| 29c | o-NO2 | COOH | 410.3 | 78.9 | 5.2 |
| 34a | m-CN | CH2OH | 147.0 | 11.3 | 13.0 |
| 34b | p-CF3 | CH2OH | 232.6 | 6.55 | 35.5 |
| 34c | o-NO2 | CH2OH | 243.9 | 35.7 | 6.8 |
| RBV | 8197 | 694.6 | 11.8 |
Cytotoxic concentration required to inhibit Vero cell growth by 50%.
Concentration required to inhibit CVB3 growth by 50%.
Selectivity index: TC50/IC50.
Out of the 41 new derivatives, compounds 17b, 24d, and 28e demonstrated the promising anti-CVB3 effects with SI over 92.4, and then all of them were chosen for next investigation. Their antienteroviral activities against another four coxsackievirus B subtypes, including CVB1, CVB2, CVB5, and CVB6, were carried out using RBV as the positive control. As described in Table 3, compounds 17b and 24d afforded potencies against the four tested CVBs with IC50 ranging from 0.62 to 12.6 μM (SI from 39 and 275), indicating broad-spectrum antienteroviral activities against different types of CVB. It was noteworthy that compound 24d exhibited a promising broad-spectrum antienteroviral effect against five coxsakievirus B subtypes with average IC50 of 1.4 μM (average SI = 142), much better than that of RBV with average SI of 6.7, and was thus selected for further investigation.
Table 3. Antienteroviral Activities against Four CVB Subtypes of Representative Compounds.
|
17b |
24d |
28e |
RBV |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compd | TC50 (μM)a | IC50 (μM)b | SIc | TC50 (μM)a | IC50 (μM)b | SIc | TC50 (μM)a | IC50 (μM)b | SIc | TC50 (μM)a | IC50 (μM)b | SIc |
| CVB1 | 489.8 | 2.60 | 188.4 | 169.8 | 1.46 | 116.0 | 183.3 | 1.28 | 143.5 | 8196 | 1951 | 4.2 |
| CVB2 | 489.8 | 12.6 | 38.9 | 169.8 | 1.44 | 117.2 | 127.1 | 7.61 | 16.7 | 8196 | 2101 | 3.9 |
| CVB5 | 489.8 | 10.5 | 46.7 | 169.8 | 3.63 | 46.8 | 73.4 | 8196 | 910.8 | 9.0 | ||
| CVB6 | 489.8 | 2.81 | 174.3 | 169.8 | 0.62 | 275.5 | 61.1 | 0.44 | 139.4 | 8196 | 1708 | 4.8 |
Cytotoxic concentration required to inhibit Vero cell growth by 50%.
Concentration required to inhibit CVB growth by 50%.
Selectivity index: TC50/IC50.
Taken together, 41 new N-benzenesulfonyl sophocarpinic acid/ester and sophocarpinol derivatives were designed, synthesized, and evaluated for their antienterovirus activities against CVB3. SAR analysis revealed that (i) the double-bond and its configuration and position at the 11-attachment could not greatly affect the potency; (ii) the replacement of carboxyl with ester or alcohol might significantly improve the activity against CVB3. Among them, sophocarpinol 24d exhibited the highest potency against CVB3 as well as CVB1, CVB2, CVB5, and CVB6, indicating a broad-spectrum antienteroviral feature. The SAR results provided the powerful information on further strategic modification and optimization. Overall, N-benzenesulfonyl matrinic acid derivatives, as a new series of compounds, offer an attractive and promising starting point for further optimization and development of a novel scaffold of broad-spectrum antiviral agents against enteroviruses.
Glossary
Abbreviations
- CVB
coxsackievirus B
- SAR
structure–activity relationship
- THF
tetrahydrofuran
- TLC
thin layer chromatography
- TBS
tert-butyldimethylsilyl
- Boc2O
di-tert-butyl pyrocarbonate
- TEA
triethylamine
- RBV
ribavirin
- SI
selective index
- Vero cells
African green monkey kidney cells
- CPE
viral cytopathogenic effect
Supporting Information Available
Synthetic procedure, analytical data, antiviral assays, and cytotoxicity assay. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
§ S.T. and L.K contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (81321004 and 81402799), the Beijing Natural Science Foundation (7142107), and 863 Youth Project (SS2015AA020910).
The authors declare no competing financial interest.
Supplementary Material
References
- Derbyshire J. B.; Jessett D. M. Receptor activity for porcine enteroviruses in pig tissues. J. Med. Microbiol. 1969, 2, 489–493. [DOI] [PubMed] [Google Scholar]
- Grist N. R.; Bell E. J.; Assaad F. Enteroviruses in human disease. Prog. Med. Virol. 1978, 24, 114–157. [PubMed] [Google Scholar]
- Chawareewong S.; Kiangsiri S.; Lokaphadhana K.; Wasi C.; Pacharee P.; Chavanich L.; Thongcharoen P. Neonatal herpangina caused by Coxsackie A-5 virus. J. Pediatr. 1978, 93, 492–494. [DOI] [PubMed] [Google Scholar]
- Downing C.; Ramirez-Fort M. K.; Doan H. Q.; Benoist F.; Oberste M. S.; Khan F.; Tyring S. K. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J. Clin. Virol. 2014, 60, 381–386. [DOI] [PubMed] [Google Scholar]
- Yang L.; He D.; Tang M.; Li Z.; Liu C.; Xu L.; Chen Y.; Du H.; Zhao Q.; Zhang J.; Cheng T.; Xia N. Development of an enzyme-linked immunosorbent spot assay to measure serum-neutralizing antibodies against coxsackievirus B3. Clin. Vaccine Immunol. 2014, 21, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zhu H.; Ye G.; Huang C.; Yang Y.; Chen R.; Yu Y.; Cui X. Antiviral effects of sophoridine against coxsackievirus B3 and its pharmacokinetics in rats. Life Sci. 2006, 78, 1998–2005. [DOI] [PubMed] [Google Scholar]
- Huber S.; Ramsingh A. I. Coxsackievirus-induced pancreatitis. Viral Immunol. 2004, 17, 358–369. [DOI] [PubMed] [Google Scholar]
- Feuer R.; Ruller C. M.; An N.; Tabor-Godwin J. M.; Rhoades R. E.; Maciejewski S.; Pagarigan R. R.; Cornell C. T.; Crocker S. J.; Kiosses W. B.; Pham-Mitchell N.; Campbell I. L.; Whitton J. L. Viral persistence and chronic immunopathology in the adult central nervous system following Coxsackievirus infection during the neonatal period. J. Virol. 2009, 83, 9356–9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C. M.; Jackson M. A.; Selvarangan R.; Turabelidze G.; Obringer E.; Johnson D.; Giles B. L.; Patel A.; Echols F.; Oberste M. S.; Nix W. A.; Watson J. T.; Gerber S. I. Severe respiratory illness associated with enterovirus d68-missouri and illinois, 2014. Morb. Mortal. Wkly. Rep. 2014, 63, 798–799. [PMC free article] [PubMed] [Google Scholar]
- Biswas T. Enterovirus 71 causes hand, foot and mouth disease outbreak in Cambodia. Natl. Med. J. India 2012, 25, 316. [PubMed] [Google Scholar]
- Osterback R.; Vuorinen T.; Linna M.; Susi P.; Hyypia T.; Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg. Infect. Dis. 2009, 15, 1485–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge M.; Wang H. Q.; Zhang G. J.; Yu S. S.; Li Y. H. The antiviral effec to fjiadifenoic acids C against coxsackievirusB3. Acta Pharm. Sin. B 2014, 4, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Chen M.; Qian F.; Xie L. A clinical research of sophocarpine in treatment of viral myocarditis. J. Clin. Cardiol. 2005, 21, 608–610. [Google Scholar]
- Guo B.; Li C.; Deng Z.; Chen S.; Ji Z.; Zhang J.; Chen M.; Xu F. A new method for measurement of (−)-sophocarpine, a candidate therapeutic for viral myocarditis, in plasma: application to a toxicokinetic study in beagle dogs. Rapid Commun. Mass. Spectrom. 2005, 19, 2840–2848. [DOI] [PubMed] [Google Scholar]
- Gao L. M.; Tang S.; Wang Y. X.; Gao R. M.; Zhang X.; Peng Z. G.; Li J. R.; Jiang J. D.; Li Y. H.; Song D. Q. Synthesis and biological evaluation of N-substituted sophocarpinic acid derivatives as coxsackievirus B3 inhibitors. ChemMedChem 2013, 8, 1545–1553. [DOI] [PubMed] [Google Scholar]
- Du N. N.; Li X.; Wang Y. P.; Liu F.; Liu Y. X.; Li C. X.; Peng Z. G.; Gao L. M.; Jiang J. D.; Song D. Q. Synthesis, structure-activity relationship and biological evaluation of novel N-substituted matrinic acid derivatives as host heat-stress cognate 70 (Hsc70) down-regulators. Bioorg. Med. Chem. Lett. 2011, 21, 4732–4735. [DOI] [PubMed] [Google Scholar]
- Du N. N.; Peng Z. G.; Bi C. W.; Tang S.; Li Y. H.; Li J. R.; Zhu Y. P.; Zhang J. P.; Wang Y. X.; Jiang J. D.; Song D. Q. N-substituted benzyl matrinic acid derivatives inhibit hepatitis C virus (HCV) replication through down-regulating host heat-stress cognate 70 (Hsc70) expression. PLoS One 2013, 8, e58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotarelo M.; Catalan P.; Sanchez-Carrillo C.; Menasalvas A.; Cercenado E.; Tenorio A.; Bouza E. Cytopathic effect inhibition assay for determining the in-vitro susceptibility of herpes simplex virus to antiviral agents. J. Antimicrob. Chemother. 1999, 44, 705–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.