Abstract
The melanocortin system regulates many important functions in the body. There are five melanocortin G protein-coupled receptor subtypes known to date. Herein, we report a structure–activity relationship (SAR) study of a tetrapeptide lead discovered through a double substitution strategy at the melanocortin core His-Phe-Arg-Trp sequence. Several compounds were identified with micromolar agonist activity at the mouse melanocortin-1 (mMC1R) and mouse melanocortin-5 receptor (mMC5R) subtypes, weak antagonist activity at the mouse melanocortin-3 receptor (mMC3R), and potent antagonist activity at the mouse melanocortin-4 receptor (mMC4R). Two compounds (2 and 3) were nanomolar mMC4R antagonists with no mMC3R antagonist activity observed. Additionally, we identified three tetrapeptide MC3R antagonists (1, 6, and 7) that possess minimal mMC3R agonist activity only at 100 μM, not commonly observed for mMC3R/mMC4R antagonists. These novel molecular templates have the potential as molecular probes to better differentiate the roles of the centrally expressed MC3 and MC4 receptors.
Keywords: Melanotropin, obesity, feeding behavior, solid phase synthesis, probe
The melanocortin receptors are seven transmembrane spanning α-helical G-protein coupled receptors (GPCRs) that signal through Gs to increase the amount of intracellular cAMP.1−7 Five melanocortin receptors have been cloned to date. The melanocortin-1 receptor (MC1R) is found primarily in the skin and regulates skin pigmentation. The melanocortin-2 receptor (MC2R) is a key component in steroidogenesis and only responds to ACTH. Both the melanocortin-3 (MC3R) and melanocortin-4 (MC4R) are expressed within the central nervous system and involved in energy and weight homeostasis.3,6,7 The MC4R has been more extensively studied than the MC3R in the field of obesity, and mutations of the hMC4R account for one of the largest monogenic determinants of obesity.8 It has been demonstrated that mMC3R and mMC4R agonist ligands reduce food intake, while antagonist ligands at these receptors result in increased food intake upon central administration.9 The use of selective MC4R agonists induce hypertension through a MC4R mediated process, which is currently not completely understood.10 Therefore, targeting the MC3R selectively may be a more suitable target for energy homeostasis therapies, yet very few selective and “clean” MC3R ligands exist in the field, which can be used to fully elucidate the receptor function. The melanocortin-5 receptor (MC5R) is implicated in exocrine gland function in mice with other physiological functions unknown.11
All of the melanocortin receptor subtypes are stimulated by a series of endogenous peptide agonists that are derived from the proopiomelanocortin (POMC) protein.12 The POMC derived α-, β-, and γ-melanocyte stimulating hormones (MSH) bind to and only stimulate the MC1R, MC3R, MC4R, and MC5R subtypes, while adrenocorticotropic hormone (ACTH) has activity at all five of the melanocortin receptor subtypes. Since the MC2R is only stimulated by the ACTH peptide and not any melanocortin-based tetrapeptides, it has been excluded from this study. The MSH hormones contain a highly conserved His-Phe-Arg-Trp motif. Extensive truncation and structure–activity relationship (SAR) studies have identified this as the core melanocortin messaging sequence.13 Multiple studies on the SAR of the tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 using single substitution strategies have been reported by our group.14−21 Similar work has been reported by others studying a linear pentapeptide template for receptor selectivity and potency.22−24 There have been numerous additional studies with cyclic templates targeting the melanocortin receptors. On the basis of these previous studies, a simultaneous double substitution study was conducted around this tetrapeptide scaffold by Todorovic et al.25 The substituted residues had been previously identified as important in dramatically altering potency and the mMC3R and mMC4R selectivity profiles.14,15 Some of these substitutions, such as a DPhe to (pI)DPhe, switched the pharmacology of the ligands from agonist to antagonist at the mMC3R.15,21
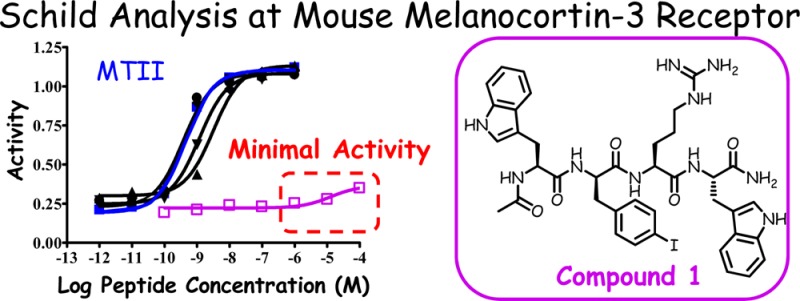
Results from single position scanning studies can be used in double substitution strategies to discover potent, selective, and pharmacologically unique ligands. The concept for this strategy is based upon the hypothesis that additive combinations of substitutions identified in single position scanning studies may produce ligands resulting in distinct pharmacological profiles. Conversely, the possibility of distinct pharmacological profiles may result and be different than simply the additive effect of the single substitutions. The double substitution strategy herein, of the tetrapeptide template, led to the identification of lead compound 1, Ac-Trp-(pI)DPhe-Arg-Trp-NH2.25 This compound had micromolar full agonist activity at the mMC1R and mMC5R with competitive antagonist activity at both the mMC3R and mMC4R (pA2 = 5.4 and 7.8, respectively). What is unique about this compound, however, is that it possessed only minimal agonist activity at 100 μM concentrations at the mMC3R (Figure 1). A survey of human and mouse MC3R and MC4R antagonists, particularly the SHU9119 ligand26 used extensively for in vivo physiological studies, reveals the in vitro pharmacological profile of an MC4R competitive antagonist with no partial agonist activity; however, at the MC3R, partial agonist activity is observed in addition to the competitive antagonism. Figure 1 illustrates the antagonist/partial agonist pharmacological profile of SHU9119 at the mMC3R and mMC4R. The SHU9119 ligand displays competitive antagonist activity at both receptor subtypes, in addition to partial agonist activity, approximately 50% of maximum response, at the mMC3R subtype. The SHU9119 ligand has also been used for in vivo feeding studies and demonstrated an ability to significantly induce feeding in mice.9 Because of the mixed partial agonist/antagonist MC3R pharmacology of ligands like SHU9119, unless melanocortin receptor knockout mice are used in conjunction with such mixed antagonist ligands, deconvolution of the physiological behavior becomes confounding. Thus, the discovery of molecular probes that are “clean” mMC3R and mMC4R antagonists, lacking any partial agonist activities, are still needed in the field. On the basis of this unmet need and the discovery of this novel tetrapeptide template possessing these mMC3R and mMC4R antagonistic pharmacological profiles, additional SAR studies of this Ac-Trp-(pI)DPhe-Arg-Trp-NH2 template are described herein. Using this template, the Trp1 position is held constant, and the Trp4 position is substituted with the following six tryptophan side chain modifications: Phe, β-(3-benzothienyl)-alanine (3Bal), tetrahydroisoquinoline (Tic), (2-naphthyl)-alanine [Nal(2′)], DNal(2′), and 4-4′-biphenylalanine (Bip) (Figure 2). It was hypothesized that, by performing this SAR, potency and selectivity profiles at the mMC3R and mMC4R could be explored with the goal of retaining the unique antagonist pharmacology at the mMC3R observed for the compound Ac-Trp-(pI)DPhe-Arg-Trp-NH2.
Figure 1.
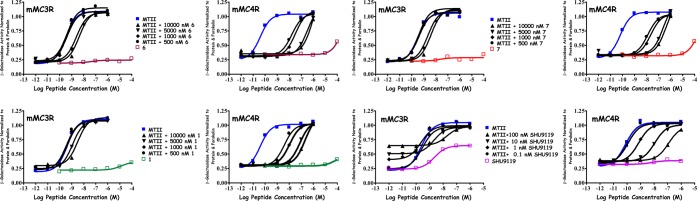
Illustration of the in vitro receptor pharmacology at the mMC3R and mMC4R for tetrapeptides 1 (Trp), 6 [Nal(2′)], and 7 [DNal(2′)], Ac-Xaa-(pI)DPhe-Arg-Trp-NH2 and SHU9119 Ac-Nle-c[Asp-His-DNal(2′)-Arg-Trp-Lys]-NH2. The SHU9119 is a representative mMC3R/mMC4R antagonist that possesses mMC3R partial agonist activity, whereas 1, 6, and 7 possess minimal agonist activity at concentrations of 100 μM.
Figure 2.
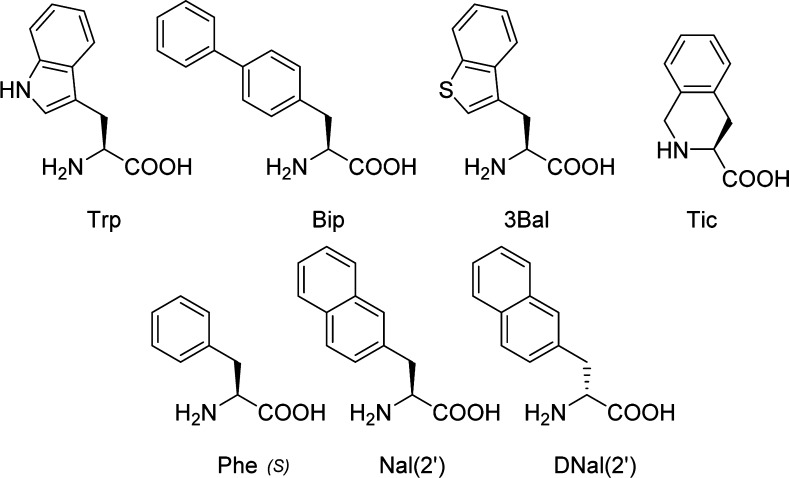
Illustration of the amino acid building blocks incorporated at the Trp4 position in the tetrapeptide template Ac-Trp-(pI)DPhe-Arg-Trp4-NH2. Abbreviations for the nonstandard 20 amino acids are (3Bal) β-(3-benzothienyl)-alanine, (Tic) tetrahydroisoquinoline, [L/D-Nal(2′)], (2-naphthyl)-alanine, and (Bip) 4-4′-biphenylalanine.
Table 1 summarizes the agonist receptor pharmacology at the mMC1R and mMC5R subtypes, and Table 2 summarizes the agonist and antagonist pharmacology at the mMC3R and mMC4R subtypes. Compound 1 possessed micromolar full agonist potency at the mMC1R and mMC5R. At the mMC3R, 1 is a micromolar antagonist with minimal agonist activity at 100 μM concentrations and a nanomolar mMC4R antagonist (Figure 1). This feature is unique to the field since mMC3R mixed partial agonist/antagonist pharmacology is commonly observed for peptides that function as dual mMC3R/mMC4R antagonists. A representative example of this type of mixed pharmacology can be seen with the tetrapeptide Ac-His-(pI)DPhe-Arg-Trp-NH214,21 and illustrated with the SHU911926 mMC3R/mMC4R antagonist in Figure 1. Previous SAR studies of the tetrapeptide template substituted at the Trp4 position resulted in the identification of amino acids at this position that modified MC3R potencies and receptor selectivity profiles; thus, these amino acid modifications (Figure 2) were incorporated into the lead template herein in attempts to increase receptor potency and selectivity while probing the Trp4 side chain moiety. Substitution at the Trp4 position on the tetrapeptide template 1 herein yielded flat SAR at the mMC1R and mMC5R subtypes with full agonist ligand potencies in the micromolar range. At the mMC3R and mMC4R subtypes, more varied SAR resulted. Tetrapeptide 2, containing the Bip4 (4-4′-biphenylalanine) residue, resulted in 10-fold decreased agonist potency at the mMC1R while maintaining equipotent agonist activity at mMC5R, as compared to 1 and within the 3-fold inherent experimental error associated with this assay in our hands. Compound 2 did not possess agonist or antagonist activity at the mMC3R at up to 100 μM concentrations but was a 480 nM mMC4R antagonist, albeit 30-fold less potent than 1. Peptide 3, containing the 3Bal [β-(3-benzothienyl)-alanine] sulfur analogue of Trp, resulted in 4-fold to 8-fold decreased agonist potency at the mMC1R and mMC5R respectively, compared to 1. Compound 3 did not stimulate or antagonize the mMC3R at up to 100 μM concentrations but possessed nanomolar antagonist potency at the mMC4R that was 6-fold less potent than 1. Tetrapeptide 4, containing the sterically constrained Tic (tetrahydroisoquinoline) substitution (Figure 2), resulted in agonist activity equipotent at the mMC1R and the mMC5R as compared to 1. At up to 10 μM, 4 did not possess antagonist activity at the mMC3R although it possessed some agonist activity (48% of the maximal MTII-induced response) at 100 μM. At the mMC4R, 4 resulted in a 1 μM antagonist that possessed some stimulatory activity (44% of the maximal MTII-induced response at 100 μM) and was a 76-fold less potent antagonist compared to 1. The Phe4 containing substitution (5) resulted in an equipotent agonist, compared to 1, at both the mMC1R and the mMC5R when taking into account the inherent experimental error. Compound 5 lacked any antagonist activity at the mMC3R and possessed nanomolar MC4R antagonist potency, and partial receptor activation was observed at both the mMC3R and the mMC4R at 100 μM concentrations (66% and 44% activation, respectively). Tetrapeptide 6, containing the Nal(2′)4 [(2-naphthyl)-l-alanine] substitution, resulted in an equipotent agonist at the mMC1R and possessed a modest 7-fold decreased agonist activity at mMC5R, as compared to 1. The tetrapeptide did not possess any agonist activity at mMC3R and mMC4R, and similar to the lead compound 1, it did possess equipotent antagonist activity at the mMC3R and mMC4R. The DNal(2′) [(2-naphthyl)-d-alanine] containing tetrapeptide 7 resulted in similar pharmacology compared to its diastereomer compound 6. Tetrapeptide 7 was equipotent for agonist activity at the mMC1R, compared to 1 and 6, but resulted in 7-fold decreased agonist potency at mMC5R as compared to 1 but equipotent with 6. At concentrations up to 100 μM, 7 was unable to stimulate the mMC3R or mMC4R subtypes. However, 7 resulted in an equipotent mMC3R and mMC4R antagonist as compared to 1.
Table 1. Summary of the Tetrapeptide Agonist (EC50) Receptor Pharmacologically at the mMC1R and mMC5R (Mean ± SEM)a.
| analogue | mMC1R agonist EC50 (nM) | mMC5R agonist EC50 (nM) |
|---|---|---|
| MTII | 0.22 ± 0.14 | 0.57 ± 0.07 |
| SHU9119 | 0.67 ± 0.11 | 2.4 ± 0.4 |
| NDP-MSH (6–9) | 71 ± 20 | 4.6 ± 1.2 |
| 1 | 2000 ± 600 | 2800 ± 1100 |
| 2 | 20700 ± 7400 | 7800 ± 2600 |
| 3 | 8300 ± 1900 | 23700 ± 4300 |
| 4 | 2700 ± 600 | 5300 ± 1500 |
| 5 | 6500 ± 1200 | 1800 ± 600 |
| 6 | 5500 ± 300 | 20300 ± 3200 |
| 7 | 5800 ± 1700 | 19700 ± 4400 |
The reported errors are the standard error of the mean of at least three independent experiments. MTII, SHU9119, and NDP-MSH (6–9) are included as control compounds.
Table 2. Tetrapeptide Agonist (EC50) and Antagonist (pA2) Receptor Pharmacologically at the mMC3R and mMC4R (Mean ± SEM)a.
| mMC3R |
mMC4R |
||||
|---|---|---|---|---|---|
| analogue | structure | EC50 (nM) | pA2 | EC50 (nM) | pA2 |
| MTII | Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 | 0.35 ± 0.02 | none | 0.05 ± 0.01 | none |
| SHU9119 | Ac-Nle-c[Asp-His-DNal(2′)-Arg-Trp-Lys]-NH2 | 54% at 1 μM | 8.7 ± 0.3 | >100,000 | 9.7 ± 0.2 |
| 3.0 ± 0.7 | |||||
| NDP-MSH (6–9) | Ac-His-DPhe-Arg-Trp-NH2 | 64 ± 19 | none | 5.4 ± 2.0 | none |
| 1 | Ac-Trp-(pI)DPhe-Arg-Trp-NH2 | >100,000 | 5.4 ± 0.2 | >100,000 | 7.8 ± 0.1 |
| 2 | Ac-Trp-(pI)DPhe-Arg-Bip-NH2 | >100,000 | none | >100,000 | 6.3 ± 0.1 |
| 3 | Ac-Trp-(pI)DPhe-Arg-3Bal-NH2 | >100,000 | none | >100,000 | 7.1 ± 0.1 |
| 4 | Ac-Trp-(pI)DPhe-Arg-Tic-NH2 | 48% at 100 μM | none | 44% at 100 μM | 6.0 ± 0.2 |
| 5 | Ac-Trp-(pI)DPhe-Arg-Phe-NH2 | 66% at 100 μM | >100,000 | 44% at 100 μM | 6.8 ± 0.2 |
| 6 | Ac-Trp-(pI)DPhe-Arg-Nal(2′)-NH2 | >100,000 | 5.9 ± 0.2 | >100,000 | 8.3 ± 0.1 |
| 7 | Ac-Trp-(pI)DPhe-Arg-DNal(2′)-NH2 | >100,000 | 5.7 ± 0.1 | >100,000 | 8.3 ± 0.1 |
The reported errors are the standard error of the mean of at least three independent experiments. The pA2 values were calculated by a Schild analysis and used MTII as the agonist compound. A value of >100,000 nM indicates that no agonist activity was observed at the concentrations tested. A % at 100 μM indicates some agonist activity was observed with the corresponding percentage indicating the amount of activity relative to the MTII-induced maximal response.
In conclusion, a focused seven-membered library of tetrapeptides was designed and synthesized based on the scaffold Ac-Trp-(pI)DPhe-Arg-Xaa-NH2. Three of the tetrapeptides, 1, 6, and 7 possessed distinct antagonist pharmacology at the mMC3R. These peptides possessed antagonist activity with minimal agonist activity at 100 μM concentrations and, to the best of our knowledge, are the first low molecular weight (MW < 900) antagonist mMC3R ligands that display this in vitro pharmacological profile. The tetrapeptides possessed potent antagonist activity at the mMC4R, and two of them, compounds 6 and 7, had pA2 values greater than 8.3 (Ki < 6 nM). Tetrapeptides 2 and 3 are selective antagonists for the mMC4R versus the mMC3R. The distinct pharmacology ascertained from this small library may aid in the future development of more potent and selective antagonists for the melanocortin receptors to more clearly identify in vivo roles of the various receptors.
Acknowledgments
We thank Anamika Singh, Srinivasa Tala, Katie Freeman, Mark Ericson, and Cody Lensing for the critical reading and revision of our manuscript.
Glossary
Abbreviations
- cAMP
cyclic 5′-adenosine monophos-phate
- MSH
melanocyte stimulating hormone
- POMC
proopiomelanocortin
Supporting Information Available
Details about the peptide synthetic procedures, >95% purity analytical characterization, experimental biological assays, and a speculative discussion about the mouse versus human MC4R SAR similarities. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of the first (S.R.D.) and senior (C.H.L.) authors. Compounds were designed and synthesized by all the contributing authors.
This work was supported by NIH R01 grant R01DK091906 (to C.H.L.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Chhajlani V.; Wikberg J. E. S. Molecular Cloning and Expression of the Human Melanocyte Stimulating Hormone Receptor cDNA. FEBS Lett. 1992, 309, 417–420. [DOI] [PubMed] [Google Scholar]
- Gantz I.; Konda Y.; Tashiro T.; Shimoto Y.; Miwa H.; Munzert G.; Watson S. J.; DelValle J.; Yamada T. Molecular Cloning of a Novel Melanocortin Receptor. J. Biol. Chem. 1993, 268, 8246–8250. [PubMed] [Google Scholar]
- Gantz I.; Miwa H.; Konda Y.; Shimoto Y.; Tashiro T.; Watson S. J.; DelValle J.; Yamada T. Molecular Cloning, Expression, and Gene Localization of a Fourth Melanocortin Receptor. J. Biol. Chem. 1993, 268, 15174–15179. [PubMed] [Google Scholar]
- Gantz I.; Tashiro T.; Barcroft C.; Konda Y.; Shimoto Y.; Miwa H.; Glover T.; Munzert G.; Yamada T. Localization of the Genes Encoding the Melanocortin-2 (Adrenocorticotropic Hormone) and Melanocortin-3 Receptors to Chromosomes 18p11.2 and 20q13.2-q13.3 by Fluorescence In Situ Hybridization. Genomics 1993, 18, 166–167. [DOI] [PubMed] [Google Scholar]
- Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Cone R. D. The Cloning of a Family of Genes that Encode the Melanocortin Receptors. Science 1992, 257, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Mountjoy K. G.; Mortrud M. T.; Low M. J.; Simerly R. B.; Cone R. D. Localization of the Melanocortin-4 Receptor (MC4-R) in Neuroendocrine and Autonomic Control Circuits in the Brain. Mol. Endocrinol. 1994, 8, 1298–1308. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L.; Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Low M. J.; Tatro J. B.; Entwistle M. L.; Simerly R. B.; Cone R. D. Identification of a Receptor for g Melanotropin and Other Proopiomelanocortin Peptides in the Hypothalamus and Limbic System. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi I. S.; Keogh J. M.; Yeo G. S.; Lank E. J.; Cheetham T.; O’Rahilly S. Clinical Spectrum of Obesity and Mutations in the Melanocortin 4 Receptor Gene. N. Engl. J. Med. 2003, 348, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Irani B. G.; Xiang Z.; Yarandi H. N.; Holder J. R.; Moore M. C.; Bauzo R. M.; Proneth B.; Shaw A. M.; Millard W. J.; Chambers J. B.; Benoit S. C.; Clegg D. J.; Haskell-Luevano C. Implication of the Melanocortin-3 Receptor in the Regulation of Food Intake. Eur. J. Pharmacol. 2011, 660, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield J. R.; Miller J. W.; Keogh J. M.; Henning E.; Satterwhite J. H.; Cameron G. S.; Astruc B.; Mayer J. P.; Brage S.; See T. C.; Lomas D. J.; O’Rahilly S.; Farooqi I. S. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N. Engl. J. Med. 2009, 360, 44–52. [DOI] [PubMed] [Google Scholar]
- Chen W.; Kelly M. A.; Opitz-Araya X.; Thomas R. E.; Low M. J.; Cone R. D. Exocrine Gland Dysfunction In MC5-R Deficient Mice: Evidence For Coordinated Regulation Of Exocrine Gland Functions By Melanocortin Peptides. Cell 1997, 91, 789–798. [DOI] [PubMed] [Google Scholar]
- Eipper B. A.; Mains R. E. Structure and Biosynthesis of Pro-ACTH/Endorphin and Related Peptides. Endocrin. Rev. 1980, 1, 1–26. [DOI] [PubMed] [Google Scholar]
- Hruby V. J.; Wilkes B. C.; Hadley M. E.; Al-Obeidi F.; Sawyer T. K.; Staples D. J.; DeVaux A.; Dym O.; Castrucci A. M.; Hintz M. F.; Riehm J. P.; Rao K. R. a-Melanotropin: The Minimal Active Sequence in the Frog Skin Bioassay. J. Med. Chem. 1987, 30, 2126–2130. [DOI] [PubMed] [Google Scholar]
- Holder J. R.; Bauzo R. M.; Xiang Z.; Haskell-Luevano C. Structure-Activity Relationships of the Melanocortin Tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the Mouse Melanocortin Receptors: I Modifications at the His Position. J. Med. Chem. 2002, 45, 2801–2810. [DOI] [PubMed] [Google Scholar]
- Holder J. R.; Bauzo R. M.; Xiang Z.; Haskell-Luevano C. Structure-Activity Relationships of the Melanocortin Tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the Mouse Melanocortin Receptors: Part 2 Modifications at the Phe Position. J. Med. Chem. 2002, 45, 3073–3081. [DOI] [PubMed] [Google Scholar]
- Holder J. R.; Xiang Z.; Bauzo R. M.; Haskell-Luevano C. Structure-Activity Relationships of the Melanocortin Tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the Mouse Melanocortin Receptors: Part 4 Modifications at the Trp Position. J. Med. Chem. 2002, 45, 5736–5744. [DOI] [PubMed] [Google Scholar]
- Holder J. R.; Xiang Z.; Bauzo R. M.; Haskell-Luevano C. Structure-Activity Relationships of the Melanocortin Tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the Mouse Melanocortin Receptors: Part 3 Modifications at the Arg Position. Peptides 2003, 24, 73–82. [DOI] [PubMed] [Google Scholar]
- Holder J. R.; Marques F. F.; Xiang Z.; Bauzo R. M.; Haskell-Luevano C. Characterization of Aliphatic, Cyclic, and Aromatic N-Terminally “Capped” His-DPhe-Arg-Trp-NH2Melanocortin Tetrapeptides at the Melanocortin Receptors. Eur. J. Pharmacol. 2003, 462, 41–52. [DOI] [PubMed] [Google Scholar]
- Todorovic A.; Holder J. R.; Scott J. W.; Haskell-Luevano C. Synthesis and Activity of the Melanocortin Xaa-DPhe-Arg-Trp-NH2 Tetrapeptides with Amide Bond Modifications. J. Pept. Res. 2004, 63, 270–278. [DOI] [PubMed] [Google Scholar]
- Todorovic A.; Holder J. R.; Bauzo R. M.; Scott J. W.; Kavanagh R.; Abdel-Malek Z.; Haskell-Luevano C. N-Terminal Fatty Acylated His-DPhe-Arg-Trp-NH2 Tetrapeptides: Influence of Fatty Acid Chain Length on Potency and Selectivity at the Mouse Melanocortin Receptors and Human Melanocytes. J. Med. Chem. 2005, 48, 3328–3336. [DOI] [PubMed] [Google Scholar]
- Proneth B.; Pogozheva I. D.; Portillo F. P.; Mosberg H. I.; Haskell-Luevano C. Melanocortin Tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 Modified at the Para Position of the Benzyl Side Chain (DPhe): Importance for Mouse Melanocortin-3 Receptor Agonist Versus Antagonist Activity. J. Med. Chem. 2008, 51, 5585–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.; Danho W.; Swistok J.; Qi L.; Kurylko G.; Franco L.; Yagaloff K.; Chen L. Structure-Activity Relationship of Linear Peptide Bu-His-DPhe-Arg-Trp-Gly-NH(2) at the Human Melanocortin-1 and −4 Receptors: Arginine Substitution. Bioorg. Med. Chem. Lett. 2002, 12, 2407–2410. [DOI] [PubMed] [Google Scholar]
- Cheung A. W.; Danho W.; Swistok J.; Qi L.; Kurylko G.; Rowan K.; Yeon M.; Franco L.; Chu X. J.; Chen L.; Yagaloff K. Structure-Activity Relationship of Linear Peptide Bu-His-DPhe-Arg-Trp-Gly-NH(2) at the Human Melanocortin-1 and −4 Receptors: Histidine Substitution. Bioorg. Med. Chem. Lett. 2003, 13, 133–137. [DOI] [PubMed] [Google Scholar]
- Danho W.; Swistok J.; Wai-Hing Cheung A.; Kurylko G.; Franco L.; Chu X. J.; Chen L.; Yagaloff K. Structure-Activity Relationship of Linear Peptide Bu-His(6)-DPhe(7)-Arg(8)-Trp(9)-Gly(10)-NH(2) at the Human Melanocortin-1 and −4 Receptors: DPhe(7) and Trp(9) Substitution. Bioorg. Med. Chem. Lett. 2003, 13, 649–52. [DOI] [PubMed] [Google Scholar]
- Todorovic A. Peptide, Peptidomimetic, and Small Molecule Based Ligands Targeting Melanocortin Receptor System. Ph.D. Thesis, University of Florida, 2006. [Google Scholar]
- Hruby V. J.; Lu D.; Sharma S. D.; Castrucci A. M. L.; Kesterson R. A.; Al-Obeidi F. A.; Hadley M. E.; Cone R. D. Cyclic Lactam a-Melanotropin Analogues of Ac-Nle4-c[Asp5, DPhe7, Lys10]-a-MSH(4–10)-NH2 With Bulky Aromatic Amino Acids at Position 7 Show High Antagonist Potency and Selectivity at Specific Melanocortin Receptors. J. Med. Chem. 1995, 38, 3454–3461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.