Abstract
Background:
The present study was designed to investigate the effect of Saraca indica (SI) flowers extract and different bioactive fraction on in vitro aldose reductase (AR) inhibitory activity, high glucose-induced cataract in goat lens and in vivo streptozotocin (STZ; 45 mg/kg, i.p) induced cataract in rats.
Methods:
Extract of flowers of SI tested for inhibition against rat lens AR. Furthermore, bioactive fraction was investigated against high glucose-induced opacification of the lens in vitro lens culture and STZ induced diabetic cataract in rats. Identification of the bioactive component was attempted through high-performance thin-layer chromatography, high-performance liquid chromatography and liquid chromatography-mass spectrometry analysis.
Results:
Ethyl acetate fraction of S. indica (EASI) produced maximum inhibition that may be due to high phenolic content. Goat lenses in media containing glucose developed a distinctly opaque ring in 72 h and treatment with EASI fraction lowered lens opacity in 72 h. Prolonged treatment with EASI to STZ-induced diabetic rats inhibited the AR activity and delayed cataract progression in a dose dependent manner.
Conclusion:
Ethyl acetate fraction of S. indica fraction has potential to inhibit rat lens AR enzyme and prevent cataractogenesis not only in goat lens model (in vitro), but also in STZ induced diabetic rats (in vivo). This study is suggestive of the anticataract activity of EASI fraction that could be attributed to the phytoconstituents present in the same.
Keywords: Cataractogenesis, Saraca indica, streptozotocin
INTRODUCTION
Cataract, characterized by cloudiness or opacification of the eye lens is the leading cause of blindness in developed and developing countries.[1] Diabetes is one of the prime causes for cataract development. At present, surgery remains to be the sole treatment for removal of cataract.[2] This causes tremendous socioeconomic burden particularly in rural areas where diabetes treatment and cataract surgeries are often neglected. Under normal condition, most of the cellular glucose is metabolized by glycolysis and pentose phosphate pathway. A negligible amount of glucose proceeds through an alternative route of metabolism, that is, polyol pathway.[3] Aldose reductase (AR) is a major and rate limiting enzyme in the polyol pathway, which catalyzes the formation of sorbitol by reducing the aldehyde form of glucose with concomitant conversion of NADPH to NADP+.[4] Depletion of NADPH by activation of the polyol pathway further increases tissue oxidative stress. In addition, some of the sorbitol are oxidized by sorbitol dehydrogenase to fructose, which is substrate for advanced glycation end product. In polyol pathway under hyperglycemic state, >30% of glucose is metabolized in contrast to the normal condition wherein 3% of glucose is subjected to this pathway. This results in the buildup of sorbitol in the tissues further leading to osmosis culminating in oxidative stress related cataract and other diabetic complication.[5] Underlying mechanisms related to glucose toxicity such as oxidative stress, enhanced polyol pathway and processes of nonenzymatic glycation significantly contribute to the development of eye complications in diabetic patients.[6]
Inhibition of AR would serve as a vital target for reducing complications associated with diabetes. There is an uprising search to come up with molecules exhibiting AR inhibitory activity, which are either synthesized or isolated from plant sources. In spite of continuous search, many molecules were unable to reach clinical trials due to their safety and efficacy issues.[7,8] Thus, the development of safe and efficacious inhibitors of AR is the need of the hour.
Saraca indica (SI) is a small evergreen tree native to India, commonly known as Ashoka tree belonging to Caesalpiniaceae family. The flowers are orange-yellow in color. Its various uses to treat various types of ailments have been reported in the traditional system since antiquity. It has widely been used in treating internal piles, diabetes, dyspepsia, indigestion, burning sensation, blood disorders, fractures, tumors, inflammation and in uterine disorders.[9] Apart from its uses in traditional medicine, scientific studies have indicated that it possesses various biological activities such as antioxidant,[10] antibacterial,[11] antiulcer,[12] cardioprotective[13] and hypoglycemic.[14] The flowers of SI have been reported to possess antidiabetic activity. This further instigated us to screen SI flowers and its fraction as a potential AR enzyme inhibitor and preventing cataractogenesis in goat lens model (in vitro), followed by in vivo evaluation of streptozotocin (STZ)-induced cataract formation in diabetic rats. The active fraction was further subjected to various analytical techniques such as high-performance thin-layer chromatography (HPTLC), high performance liquid chromatography (HPLC) and LC-MS in order to identify the compound responsible for the AR inhibitory activity.
Experimental
Chemicals
Streptozotocin was purchased from Sisco research laboratories, Mumbai. The chemical and reagents utilized in this experiment were of analytical grade. Different fractions of SI were dissolved in dimethyl sulfoxide for in vitro studies and suspended in 0.5% sodium carboxymethyl cellulose for in vivo studies.
Animals
Male adult Wistar rats are weighing 180-250 g were used. The experimental protocol was approved by Institutional Animal Ethics Committee (ICT/IAEC/2011/ICT/P56) of Institute of Chemical Technology, Mumbai. The animals were acclimatized for a period of 1 week, ad libitum food and water was provided and 12 h light/dark cycle was maintained.
Collection and authentication of Saraca indica flower
Flowers of SI were collected in the month of April 2011 from ICT campus, Mumbai. The flowers were authenticated by the Dr. Ganesh Iyer, Botany Department, Ruia College, Mumbai and deposited in the herbarium of ICT department as a voucher specimen number ICT 201111.
Extraction and fractionation of Saraca indica flower
Fresh flowers of SI were collected and dried in the oven at 50°C until it attained constant weight and pulverized to a coarse powder. The powdered plant material (100 g) was defatted by using petroleum ether and then subjected to methanolic extraction by soxhlet apparatus, which was filtered and concentrated under reduced pressure using a rotary evaporator till constant weight. Methanolic extract was further successively fractionated with an equal volume of ethyl acetate and water (1:1) to obtain Ethyl acetate fraction of S. indica (EASI) and water fraction separately. Water fraction was refractionated by n-butanol to get n-butanol and water fraction. Each fraction was concentrated by rotary evaporator at 40°C. Fractionation and extraction of SI is shown in Figure 1.
Figure 1.
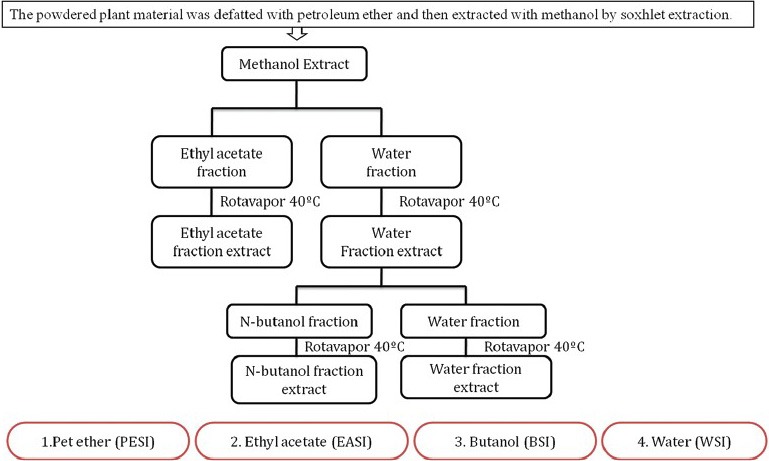
Fractionation scheme for Saraca indica flower
Assay for aldose reductase inhibitory activity
Preparation of crude rat lens aldose reductase enzyme
Eyes of normal Wistar rats were removed immediately after they were sacrificed. The lenses were removed from the eyes, washed with saline and weights of lens were recorded. The lenses were homogenized in sodium phosphate buffer (pH 6.2) containing 10 mM β-mercaptoethanol. The homogenate was then centrifuged at 10,000 rpm at 4°C for 20 min to obtain a clear supernatant, which was frozen until further estimation. Protein content of the lens homogenate was determined by Lowry et al.[15]
Aldose reductase inhibitory activity using xylose as substrate
The AR inhibition studies of methanolic extract of SI and its isolated fractions were carried out. The freshly prepared enzyme, varying the concentration of isolated fractions, 0.067 M buffer (pH 6.2), 0.125 mM NADPH, 400 mM lithium sulfate and 40 mM xylose were taken in a cuvette with total volume of 2 ml. The change in the absorbance over a period of 3 min was measured at 340 nm. Quercetin was used as a standard under similar conditions.[7,16]
%Inhibitory activity = (optical density [OD] control − OD sample)/OD control × 100
The fraction showing maximum activity was subjected to further studies.
In vitro anti-cataract activity of bioactive fraction
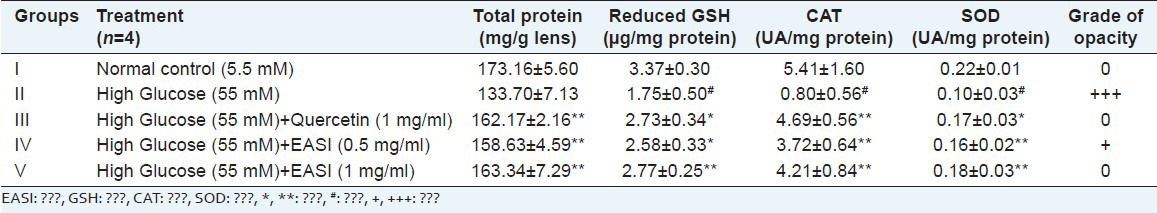
Fresh goat eyeballs were obtained from local slaughter house (Matunga, Mumbai) immediately after sacrifice and were transported to the laboratory under cold conditions (0-4°C). The lenses were removed by extracapsular extraction and incubated in artificial aqueous humor media. Cataract was induced in the goat lens using 55 mM of glucose solution. A total of 20 lenses were divided into the five groups (n = 4) as follows: Group I: Glucose 5.5 mM (normal control), Group II: Glucose 55 mM (cataract control), Group III: Glucose 55 mM + 1 mg/ml Quercetin (Positive control) Group IV: Glucose 55 mM + 0.5 mg/ml EASI (Test Group 1) and Group V: Glucose 55 mM + 1 mg/ml EASI (Test Group 2). After 3 days of incubation period, 10% w/v homogenate of the lenses were prepared using 0.2 M Tris (pH 7.8) containing 0.25 × 10−3 M ethylenediaminetetraacetic acid. The supernatant was obtained after centrifugation of the above homogenate at 10,000 g at 4°C, which was further used in the determination of total lens protein, reduced glutathione,[17] superoxide dismutase (SOD)[18] and catalase activity.[19] The degree of opacity was graded as 0: Absence (Clear lens), +: Slight degree, ++: Diffuse opacity, +++: Extensive thick opacity.[20]
In vivo cataractogenesis in streptozotocin induced diabetes rat Induction of diabetes
Diabetes mellitus was induced by single intraperitoneal injection of freshly prepared STZ (45 mg/kg) in 0.1 M citrate buffer (pH 4.5). After 7 days, the blood was collected from rat tail and the glucose level of each rat was determined. Rats with a fasting blood glucose range of 250–350 mg/dl were considered diabetic and included in the study. Blood glucose level was measured using glucometer (Accu-Check® sensor comfort purchased from Roche Diagnostics, India Pvt. Ltd.).[21]
Experimental design
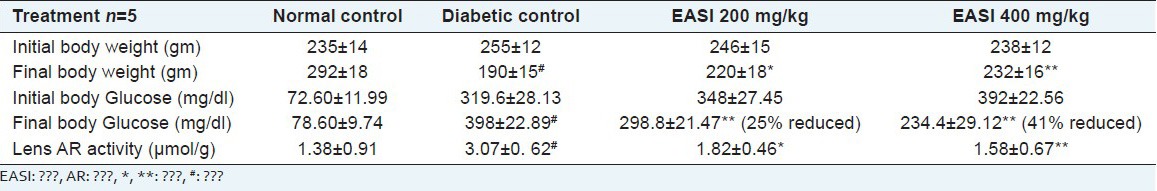
The animals were divided into four groups (n = 5) viz. normal control, diabetic control, diabetic animals treated with 200 mg/kg body weight of EASI, and diabetic animals treated with 400 mg/kg body weight of EASI. The treatment continued for 8 weeks. The body weight and blood glucose levels were monitored, and cataract formation in each animal was observed.[22] At the end of the experiment eyes of rats were enucleated, incision was made anteriorly to the ora serrata across the circumference of the eye in order to obtain the cornea and iris. Application of gentle pressure to the sclera resulted in the removal of the lens from the rat eye ball, which was further deprived of vitreous tissue. The lenses were weighed and homogenized with of 50 mM phosphate buffer (pH 7.6). The AR activity in the lens was determined in terms of the decrease in NADPH absorbance using a ultraviolet-visible (UV-VIS) spectrophotometer at 340 nm.[23] AR activity is expressed as μ moles NADPH oxidized/g protein.
Eye examination and cataract grading
Eyes were examined every week on dilated pupils. Initiation and development of goat lens opacity was evaluated according to the grading scale provided by Suryanarayana et al. briefly, the scoring pattern used is as follows:
Stage 0: Clear lenses with no vacuoles;
Stage 1: Vacuoles cover approximately one-half of the surface of the anterior pole;
Stage 2: Disappearance of few vacuoles and cortex haziness;
Stage 3: A hazy cortex and dense nuclear opacity;
Stage 4: A mature cataract.[24]
Acute oral toxicity study
Acute oral toxicity study was performed according to OECD 425 guideline.[25] Female Wistar were administered with a single dose (2000 mg/kg; p.o.) of most active fraction of SI and observed for mortality during 14 day's study period. The LD50 of the active fraction was calculated.
Phytochemical analysis and identification of phytoconstituents in ethyl acetate fraction of Saraca indica fraction
The preliminary phytochemical screening of the active fraction of EASI was carried out for the detection of secondary metabolites such as phenols, flavonoids, glycosides, alkaloids, steroids, triterpenoids and saponins using standard chemical tests as elaborated by Khandelwal.[26]
Determination of total phenolic content
Total phenolic content (TPC) of the active fraction of SI was determined by using folin-ciocalteau reagent.[27] EASI and standard gallic acid (GA) were dissolved in methanol. Briefly, 100 μL of EASI (1 mg/mL) was added to a test tube containing 50 μL phenol reagent (1M). The volume was adjusted with 1.85 mL distilled deionized water and vortexed. After 8 min, 300 μL Na2CO3 (20% in water, v/v) was added. The solution was vortexed, and the final volume (4 mL) was adjusted by adding 1.7 mL distilled deionized water. A reagent blank was prepared using distilled deionized water instead of test sample. The final mixture was vortexed and then incubated for 1 h in the dark at room temperature. The absorbance was measured at 725 nm using a Shimadzu UV-VIS spectrophotometer. A standard curve was prepared using various concentrations of GA against the absorbance. The results were expressed as μg GA equivalents/mg of dry extract.
High-performance thin-layer chromatography analysis of ethyl acetate fraction of Saraca indica fraction
High-performance thin-layer chromatography (CAMAG make Germany) was performed on aluminum backed plates coated with silica gel 60F254 (Merck, Mumbai, India). The camag glass twin-trough chamber was saturated using a mobile phase comprising of toluene: Ethyl acetate: Formic acid, 5:4:1 (v/v/v), wherein the development was done in the ascending front at room temperature. After development, thin-layer chromatography plate was sprayed with ferric chloride reagent.[26]
High performance liquid chromatography analysis of ethyl acetate fraction of Saraca indica fraction
Agilent-1200 series HPLC (Agilent Technologies, Wald-bronn, Germany) equipped with UV detector (at 254 nm) was used for analysis. Method was developed using Agilent Zorbax Eclipse C18 column (4.6 × 250 mm) with mobile phase composition acetonitrile: 1% formic acid (20:80), operated at 1 ml/min flow rate. The column was maintained at ambient temperature (20°C). Each sample was run for 10 min. Methanol was used for the preparation of sample solution.
Liquid chromatography-mass spectrometry analysis of ethyl acetate fraction of Saraca indica fraction
Liquid chromatography-mass spectrometry (LC-MS) system (Model triple Quadrupole LC-MS Agilent 6410/Agilent 1200) was used to identify the structure of the phytoconstituent in EASI. The electrospray ionization mass spectrum in the negative ion mode was acquired using a capillary voltage of − 4.5 kV, fragmentation voltage of 135 V and desolvation gas (nitrogen) was heated at 325°C.
Electrospray ionization tandem mass spectra was obtained by mass-selecting the target ion by the use of quadrupole mass analyzer with subsequent use of 25 eV collision induced dissociation using nitrogen in a collision cell. Active fraction of SI (EASI) was dissolved in methanol, filtered (0.22 mm), and 10 μL of the solution was used for the LC-MS analysis. The gas nebulizer pressure was 45 psi. The fragmentation information from mass spectra was used to assess the molecular structure of the active component.
Statistical analysis
Statistical evaluation of the data was performed using t-test or one-way analysis of variance followed by Dunnett's multiple comparison tests using GraphPad Prism software version 5. The minimum level of significance considered was P < 0.05.
RESULTS
Aldose reductase inhibition activity using xylose as substrate
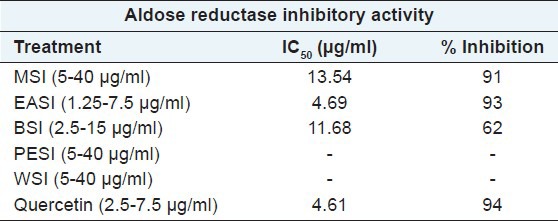
The methanolic extract of SI and its various fractions were analyzed for their inhibitory activity on crude AR enzyme by using xylose as the substrate [Table 1]. Amongst all the fractions investigated, EASI fraction (inhibitory concentration [IC50] =4.69 μg/ml) showed higher percentage of inhibition at less concentration, followed by methanolic extract (IC50 = 13.54 μg/ml). Butanol fraction was found to have the least inhibition potential against AR enzyme. However, petroleum ether and water fraction were devoid of AR inhibitory activity. Quercetin was used as a positive control under similar assay conditions.
Table 1.
Inhibition on crude aldose reductase inhibitory activity by MSI and its different fractions
Anti-cataract activity of ethyl acetate fraction of Saraca indica in sugar induced cataract
Following the positive in vitro inhibitory results of the EASI fraction, we decided to evaluate its effect on hyperglycemia induced cataract in goat lens model. Representative photos of goat eyes incubated with glucose are shown in Figure 2. Incubation of goat lenses with 55 mM glucose caused cataract progression within 24 h. All lenses in normal control group remained transparent whilst all lenses in high glucose group became cloudy and lost transparency. The opacity gradually increased towards the center with full opacification by 72 h. EASI at 0.5 mg/ml and 1 mg/ml retarded the development of opacity when compared with cataract control. The grade of opacity is shown in Table 2. Incubation of lens with 55 mM of glucose (Group II) showed significantly (P < 0.05) decreased glutathione, SOD and catalase levels when compared to the normal control group [Table 2]. EASI at the concentrations of 0.5 mg/ml and 1 mg/ml showed significant restoration of activities of lens reduced glutathione, SOD and catalase when compared to the cataract group. EASI (1 mg/ml) group showed comparable activity to 1 mg/ml of quercetin group.
Figure 2.
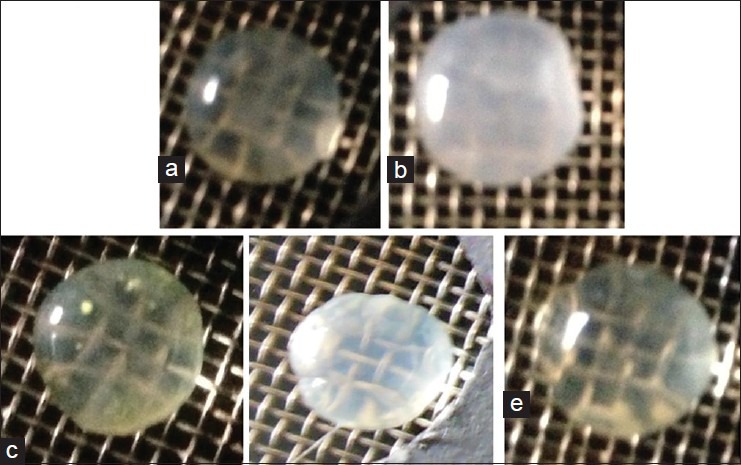
Representative digitized illustrations of the anticataract activity of ethyl acetate fraction of Saraca indica (EASI) lenses for incubated (a) with 5.5 mM glucose (Normal control) (b) high glucose (c) high glucose + Quercetin (1 mg/ml) (d) high glucose + EASI (0.5 mg/ml) (e) high glucose + EASI (1 mg/ml)
Table 2.
Effect of EASI on antioxidant enzymes and grade of opacity on isolated goat lens model
In vivo cataractogenesis in streptozotocin induced diabetes rat
To affirm the protective effect of EASI in the hyperglycemia mediated goat lens opacification in vitro organ culture, we investigated whether in vivo administration of EASI can prevent or delay STZ induced diabetic cataract in rats. Representative photos of eye taken from the experimental animals are shown in Figure 3. Also, at the end of 8 weeks of EASI treatment a dose dependent decrease in AR activity in the diabetic rats was observed [Table 3].
Figure 3.
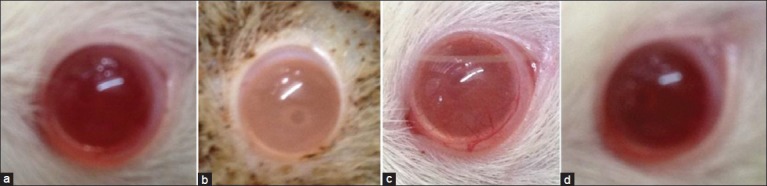
Representative photograph lens of (a) normal control (b) diabetic control (c) ethyl acetate fraction of Saraca indica (EASI) 200 mg/kg treatment (d) EASI 400 mg/kg treatment
Table 3.
Effect of the EASI fraction on body weight, blood glucose and aldose reductase in streptozotocin-induced diabetic rats
Cataract study
Eye examination showed that normal control rat lenses were in stage 0 throughout the duration of the experimental period. The onset of cataract due to hyperglycemia was observed in diabetic animals between 4 and 5 weeks of STZ injection and progressed to mature cataract by 8 weeks in untreated diabetic rats [Figure 4]. The values of the average stage of the cataract score at the end of 8 weeks were lower in the EASI treatment than in the diabetic control group, indicating that the EASI delayed the maturation of diabetic cataract.
Figure 4.
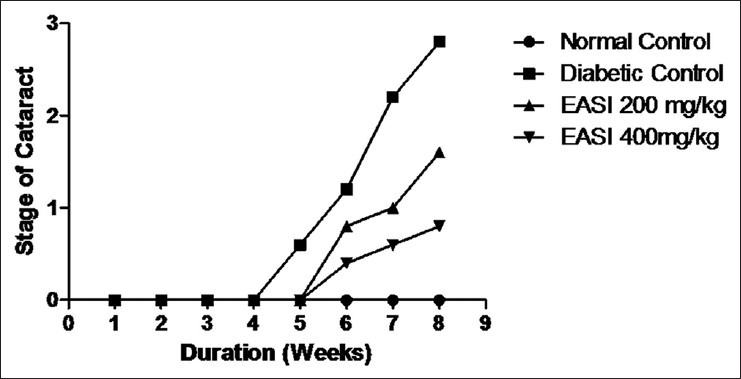
The effect of ethyl acetate fraction of Saraca indica fraction on streptozotocin-induced diabetic cataract. Representation of cataract progression in each group with the duration of diabetes in weeks. Stage of cataract in each group was averaged at a given time and the average stage of cataract was plotted as a function of time
Effect of ethyl acetate fraction of Saraca indica on blood glucose levels in diabetic rat
In this study, the effect of EASI fraction on glucose levels in STZ induced diabetic rats was monitored to understand whether EASI ameliorated cataract onset and its progression by reducing STZ induced hyperglycemia. The blood glucose levels were found to be elevated in the STZ induced diabetic rats to 398, whereas in the case of normal control animals it was found to be 78. However, the EASI treatment for 8 weeks at the dose of 200 and 400 mg/kg exhibited 25% and 41% reduced in the blood glucose level respectively [Table 3]. Treatment with EASI caused a marginal (200 mg/kg) to moderate (400 mg/kg) decrease in the levels of glucose in diabetic rats.
Phytochemical analysis and identification of phytoconstituents from ethyl acetate fraction of Saraca indica fraction
The TPC was 118.06 ± 0.16 μg GA equivalent/mg dry extract. Thus, EASI was found to contain high TPC. Phytochemical investigations confirmed the presence of phenol, flavonoid, glycosides triterpenoid and sterols in the EASI fraction. Further, the fraction was subjected to various analytical techniques such as HPTLC, HPLC and LC-MS to identify the compounds responsible for their AR inhibitory activity. HPTLC-analysis of EASI revealed three component spots with the retention factor (Rf) values ranging from 0.24 to 0.65 [Figure 5]. The HPLC chromatograms of the mixture of polyphenolic compounds (GA and ellagic acid) and EASI are shown in [Figure 6a and b] respectively. HPLC analysis exhibited presence of GA (Rt 1.9) by comparing with the retention time of the reference compound. To characterize the bioactive components responsible for inhibition of AR LC-MS profile of EASI was undertaken. Anthocyanidin pigments; pelargonidin-3, 5-diglucoside and cyanidin-3,5-diglucoside and β-sitosterol in EASI were identified and separated from its respective mixture by LC-MS and the analysis of the fragmentation pattern allowed us to corroborate the structure [Table 4 and Figure 7].
Figure 5.
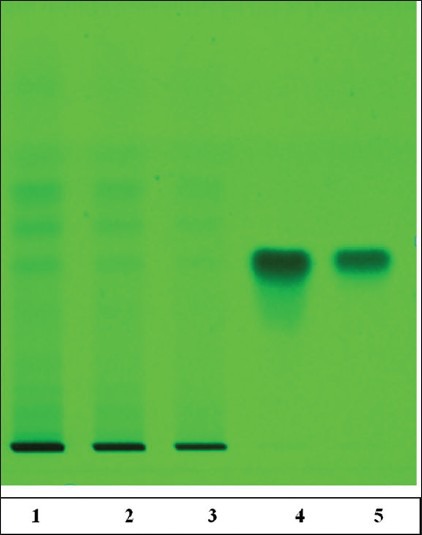
High-performance thin-layer chromatography (HPTLC) fingerprint of ethyl acetate fraction of Saraca indica (EASI) fraction (image at 580 nm, after derivatization). HPTLC finger print of EASI showed presence of gallic acid (GA) at Rf = 4.1, EASI of the individual samples (2 mg/ml) was spotted onto the activated thin-layer chromatography plate. Toluene: Ethyl acetate: Formic acid 5:4:1 (v/v/v) as solvent system. Ferric chloride was used as derivatizing agent. (The track number 1-EASI 5 μl, 2-EASI 10 μl, 3-EASI 20 μl, 4-GA 5 μl, 5-GA 10 μl)
Figure 6.
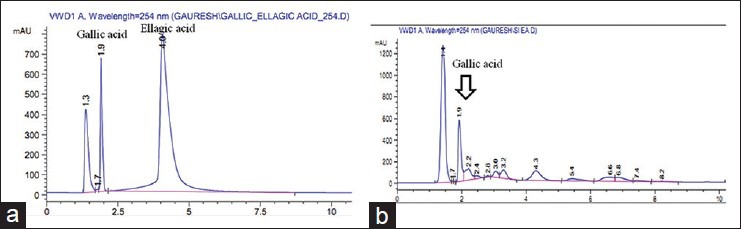
High performance liquid chromatography chromatogram for reference standard gallic acid (GA) and ellagic acid (a) and ethyl acetate fraction of Saraca indica (EASI) fraction (b). Both peaks have similar retention time of about 1.9 min indicating that EASI fraction sample contains GA as the active phytoconstituent
Table 4.
Retention time and the mass spectral data of the different phytoconstituents present in the EASI
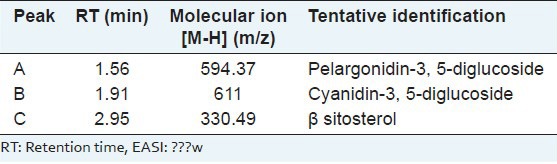
Figure 7.
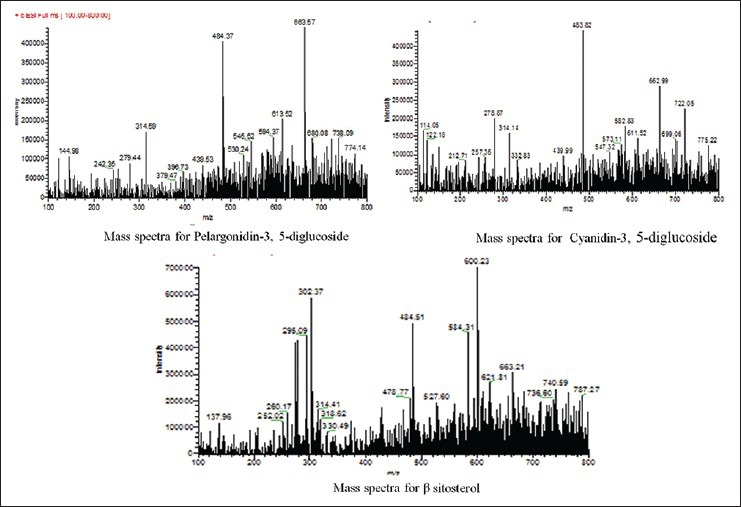
Mass spectral data of the different phytoconstituents present in the ethyl acetate fraction of Saraca indica (Tentative identification)
DISCUSSION
Cataract formation is a multifunctional process, and the majority of studies have indicated that hyperglycemia and duration of diabetes pose major risk factors for the development of the same.[28] Alteration of polyol pathway, nonenzymatic glycation and oxidative stress pathways are implicated in the pathogenesis of development of cataract.[29] Hyperglycemia increases the production of reactive oxygen species leading to severe oxidative stress. Alterations in the levels of antioxidant enzymes are associated with an increase in the reactive radicals resulting into detrimental effects like loss of cell membrane integrity and its function.[5]
The crude methanolic extract of SI and its different fractions were employed in the current study for AR inhibitory activity. Among them, EASI exhibited significant inhibition of AR enzyme, followed by methanol extract. The butanol fraction was found to possess the least inhibitory potential. It was further observed that petroleum ether and water fraction were devoid of any inhibitory activity towards AR. EASI inhibited AR enzyme using xylose as a substrate with IC50 = 4.69 μg/ml which was comparable to standard drug quercetin (IC50 = 4.61 μg/ml). This fraction was further subjected to in vitro and vivo screening for hyperglycemia induced cataract.
In vitro lens organ culture is well-known model for hyperglycemia induced cataract.[30] The bioactive fraction was further subjected to in vitro cataract model wherein goat lenses were incubated with 55 mM glucose, which resulted in the loss of transparency (opacification) after 72 h. In this study, the levels of SOD, catalase and reduced glutathione were found to be reduced in the lens of high glucose group as compared to the lens of normal control. The lenses treated with EASI (1 mg/ml) were able to effectively reduce the protein aggregation and further exhibited an increase in the SOD, catalase and reduced glutathione levels after 72 h of treatment. GSH is a vital antioxidant in the goat lens which maintains the protein content in reduced form.
The oxidation of sulfhydryl component is one of the deleterious effects, which results in the formation of disulfide cross linkage and protein precipitation, followed by lens opacification.[7] It was also observed visually that while the Group II lenses showed opacification in 72 h, no such effect was seen in the EASI treated lenses, demonstrating its capacity to prevent cataract formation.
To confirm the activity of EASI against cataractogenesis it was subjected to STZ induced diabetic cataract model. The onset of cataract in STZ induced diabetic animals were observed between 4 and 5 weeks and the development of the mature cataract by 8 weeks in diabetic control group [Figure 4]. At the end of 8 weeks, treatment with EASI delayed the progression of cataract formation in diabetic rats in a dose-dependent manner [Figure 3]. A significant increase in the AR activity in the eye lens was observed in diabetic rats while treatment with EASI showed significant dose-dependent decrease of AR activity. Over the course of the experimental period, the eyes of animals appeared to be normal and free of opacities in both the EASI fraction treated group. Blood glucose levels were monitored to understand whether EASI ameliorated cataract progression by reducing STZ induced hyperglycemia. Although, blood glucose levels were decreased to a marginal level after EASI treatment, delay of cataract progression in diabetic rats were not due to glucose lowering capacity of EASI, but could be attributed to its potential to modulate other pathways associated with the development of diabetic cataract. Therefore, extensive studies are required to identify and evaluate EASI for its therapeutic value to prevent the onset and/or delay progression of diabetic cataract.
An acute toxicity study of EASI was carried out and it was observed that the animals did not exhibit any signs of adverse effect up to the dose level of 2000 mg/kg body weight. There were no signs of tremors, salivation, diarrhea, sleep, coma, death or any unusual behaviors. The LD50 of EASI fraction was found to be >2000 mg/kg body weight and hence EASI fraction was found to be safe for administration.
In phytochemical investigation, the presence of phenol, flavonoid, glycoside, triterpenoid and sterols were confirmed in EASI fraction. It has been reported that plants that are rich in polyphenols have ability to inhibit AR activity.[31] EASI was subjected to quantitative phytochemical analysis for TPC. The study revealed that the EASI fraction was rich in phenolic content. Thus, AR inhibitory activity of EASI would be due to the presence of phenols.[32] Further, the fraction was subjected to various analytical techniques such as HPTLC, HPLC and LC-MS to identify the compound responsible for the activity. The HPTLC chromatogram indicated the presence of at least three compounds in EASI. HPLC analysis exhibited the presence of GA as the major phenolic component. LC-MS study, revealed the presence of anthocyanidin; pelargonidin-3, 5-diglucoside and cyanidin-3, 5-diglucoside and β-sitosterol (tentative identification).
In our study, the EASI fraction containing a mixture of phenolic compounds viz., GA; β-sitosterol and anthocyanidin; pelargonidin-3, 5-diglucoside and cyanidin-3, 5-diglucoside showed significant inhibition of in vitro AR, lens opacification in goat lens and also delayed progression of cataract in diabetic rats.[33] The activity could be attributed to the presence of above mentioned individual compounds or due to their synergistic activity in the extract.
CONCLUSION
Ethyl acetate fraction of S. indica has the ability to inhibit AR in vitro and in vivo and help in delaying the progression of diabetic cataract. Further mechanistic studies of EASI are required to establish it as a prospective pharmacological candidate against diabetic complications especially cataract.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–60. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 2.Pollreisz A, Schmidt-Erfurth U. Diabetic cataract: Pathogenesis, epidemiology and treatment. J Ophthalmol 2010. 2010:1–8. doi: 10.1155/2010/608751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinoshita JH. A thirty year journey in the polyol pathway. Exp Eye Res. 1990;50:567–73. doi: 10.1016/0014-4835(90)90096-d. [DOI] [PubMed] [Google Scholar]
- 4.Yabe-Nishimura C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21–33. [PubMed] [Google Scholar]
- 5.Chatzopoulou M, Alexiou P, Kotsampasakou E, Demopoulos VJ. Novel aldose reductase inhibitors: A patent survey (2006 – present) Expert Opin Ther Pat. 2012;22:1303–23. doi: 10.1517/13543776.2012.726615. [DOI] [PubMed] [Google Scholar]
- 6.Kyselova Z, Stefek M, Bauer V. Pharmacological prevention of diabetic cataract. J Diabetes Complications. 2004;18:129–40. doi: 10.1016/S1056-8727(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 7.Dongare V, Kulkarni C, Kondawar M, Magdum C, Haldavnekar V, Arvindekar A. Inhibition of aldose reductase and anti-cataract action of trans-anethole isolated from Foeniculum vulgare Mill. Fruits. Food Chem. 2012;132:385–90. doi: 10.1016/j.foodchem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Kador PF. Overview of the current attempts toward the medical treatment of cataract. Ophthalmology. 1983;90:352–64. doi: 10.1016/s0161-6420(83)34562-0. [DOI] [PubMed] [Google Scholar]
- 9.Khare CP. New York, USA: Springer Verlag; 2007. Indian Medicinal Plants: An Illustrated Dictionary; pp. 583–4. [Google Scholar]
- 10.Sen S, Chakraborty R, Verma A, De B, Devanna N, Dey BK. Evaluation of antioxidant activity of Saraca indica leaves extracts using in vitro and ex vivo models. J Sci Ind Res. 2014;73:157–62. [Google Scholar]
- 11.Nayak S, Sahoo AM, Chakraborti CK, Haque MN. Antibacterial activity study of Saraca indica leaves extract. Int J Pharm Res Dev. 2011;3:16. [Google Scholar]
- 12.Maruthappan V, Sakthi SK. Antiulcer activity of aqueous suspension of Saraca indica flower against gastric ulcers in albino rats. J Pharm Res. 2010;3:17–20. [Google Scholar]
- 13.Viswanatha Swamy AH, Patel UM, Koti BC, Gadad PC, Patel NL, Thippeswamy AH. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol. 2013;45:44–8. doi: 10.4103/0253-7613.106434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preethi F, Fernandes J, Pricilla K. Hypoglycemic activity of Saraca indica Linn barks. J Pharmacol Res. 2010;3:491. [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 16.Chethan S, Dharmesh SM, Malleshi NG. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorg Med Chem. 2008;16:10085–90. doi: 10.1016/j.bmc.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 19.Aebi H. New York: Academic Press; 1974. Catalase. Methods of Enzymatic Analysis; pp. 673–67. [Google Scholar]
- 20.Nithya S, Sumadhuri S, Anbu J, Sumithra M. in vitro prevention of cataract by Ginseng on isolated goat eye Lens. Pharmacogn Commun. 2012;2:40–3. [Google Scholar]
- 21.Orhan N, Aslan M, Hosbas S, Deliorman O. Antidiabetic effect and antioxidant potential of Rosa canina fruits. Pharmacogn Mag. 2009;5:309–15. [Google Scholar]
- 22.Farswan M, Mazumder M, Parcha V, Upaganlawar A. Modulatory effect of Syzygium cumini seeds and its isolated compound on biochemical parameters in diabetic rats. Pharmacogn Mag. 2009;5:127–33. [Google Scholar]
- 23.Akileshwari C, Raghu G, Muthenna P, Mueller NH, Suryanaryana P, Reddy GB, et al. Bioflavonoid ellagic acid inhibits aldose reductase: Implications for prevention of diabetic complications. J Funct Foods. 2013;6:374–83. [Google Scholar]
- 24.Suryanarayana P, Kumar PA, Saraswat M, Petrash JM, Reddy GB. Inhibition of aldose reductase by tannoid principles of Emblica officinalis: Implications for the prevention of sugar cataract. Mol Vis. 2004;10:148–54. [PubMed] [Google Scholar]
- 25.Acute Oral Toxicity Test. Paris: Organization of Economic Co-operation Development; 2001. OECD. The OECD Guidelines for Testing of Chemicals, 425. [Google Scholar]
- 26.Khandelwal K. 9th ed. Pune: Nirali Prakashan; 2002. Practical Pharmacognosy; pp. 149–56. [Google Scholar]
- 27.Somani G, Chaudhari R, Sancheti J, Sathaye S. Inhibition of carbohydrate hydrolysing enzymes by methanolic extract of Couroupita guianensis leaves. Int J Pharm Bio Sci. 2012;3:511–20. [Google Scholar]
- 28.Crabbe MJ. Aldose reductase inhibitors and cataract. Int Ophthalmol. 1991;15:25–36. doi: 10.1007/BF00150976. [DOI] [PubMed] [Google Scholar]
- 29.Obrosova IG, Chung SS, Kador PF. Diabetic cataracts: Mechanisms and management. Diabetes Metab Res Rev. 2010;26:172–80. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 30.Chandorkar AG, Albal MV, Bulakh PM, Muley MP. Lens organ culture. Methodology and preliminary observations on viability and maintenance of transparency. Indian J Ophthalmol. 1981;29:151–2. [PubMed] [Google Scholar]
- 31.Gacche RN, Dhole NA. Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: An attempt to standardize the botanicals for amelioration of diabetes complications. Food Chem Toxicol. 2011;49:1806–13. doi: 10.1016/j.fct.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 32.Stefek M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscip Toxicol. 2011;4:69–77. doi: 10.2478/v10102-011-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaiswal V, DerMarderosian A, Porter R. Anthocyanins and polyphenol oxidase from dried arils of pomegranate Punica granatum L. Food Chem. 2010;118:11–6. [Google Scholar]


