Abstract
Background:
Diabetes mellitus is one of the leading chronic diseases worldwide. In patients with poor glycemic control, high blood glucose level may lead to other life-threatening complications. Pandanus amaryllifolius Roxb. (PA) leaves are used in traditional medicine for the treatment of diabetes.
Objective:
This study evaluated the effect of crude extract from PA leaves on blood glucose level and the hypoglycemic mechanisms.
Materials and Methods:
Thirty healthy volunteers were asked to drink PA tea (test-group) or hot water (control group) 15 min after glucose loading (75 g) in a standard oral glucose tolerance test. To study hypoglycemic mechanisms, PA leaves were extracted using two different methods. Method 1; dried PA leaves were extracted with distilled water at 90°C for 15 min, and method 2; dried PA leaves were extracted with 95% ethanol. Both PA extracts were tested for α-glucosidase enzyme inhibition, insulin stimulation, and glucose uptake stimulation.
Results:
The average of blood glucose level in the control group was 5.55 ± 0.98 mmol/l, while in PA treated group was 6.16 ± 0.79 mmol/l which were statistically different (P < 0.001). The results of antihyperglycemic mechanism showed that PA extracts, prepared both methods, could inhibit α-glucosidase enzyme and induce insulin production in rat pancreatic cell (RINm5F) in dose-dependent manner (P < 0.05).
Conclusions:
The knowledge gained from this research can be used as a basis for a new drug discovery for the treatment of diabetes.
Keywords: α-glucosidase inhibitor, antihyperglycemic mechanism, insulin stimulation, Pandanus amaryllifolius Roxb
INTRODUCTION
Diabetes mellitus (DM) is a chronic disease, which rapidly increases due to lifestyle changes and some environmental factors. World Health Organization projected that diabetes will be the 7th leading cause of death in 2030.[1] Diabetes can be found in newborns and the incidence is higher in females than males.[1,2,3] Hyperglycemia, or high blood sugar, is a common effect of uncontrolled diabetes and over time can lead to serious damage to many systems, especially nerves and blood vessels, in organs such as eyes, kidneys, heart, and other organs.[4] There is no cure for diabetes. Treatment for diabetes focuses on glycemic control with proper diet and exercise. There are several groups of oral hypoglycemic drugs for patients with diabetes like alpha-glucosidase inhibitors which stimulate in and may have ulin secretion or increase cellular glucose uptake. However, these drugs are expensive unwanted side effects such as headache, diarrhea, or itchy skin.[5]
In recent years, interests in examining the effects of hypoglycemic herbs, which used in traditional Asian medicine for treatment of diabetes, is on the rise[6] since these herbs are cheaper and have fewer side effects as compared to synthetic drugs. The aromatic pandan (Pandanus amaryllifolius Roxb (PA); Thai name: Toei-hom) is a plant native to Thailand and commonly used in cooking. Peungvicha et al. found that the extracts from roots of the fragrant pandanus could effectively reduce blood glucose levels in streptozotocin-diabetic rats.[7,8] Peungvicha also found that the extract form PA roots might have some toxicity because it raised rat liver enzymes and caused congestion in the lungs, liver, and kidney.[9] In general, we do not consume roots of the aromatic pandanus. On the other hand, pandan leaves are used in daily life in pandan leaf tea, or pandan flavors in food or snacks using pandan leave extract. In our pilot study, we found that an extract of pandan leaves can significantly reduce postpandrial blood sugar in healthy participants (n = 10) using a standard oral glucose tolerance test (OGTT) (P ≤ 0.008, data not shown). However, little is known about antihyperglycemic mechanism of PA leaf extract. Therefore, in this study, we aimed to examine the hypoglycemic mechanisms of crude pandan leaf extract by evaluating inhibition of alpha-glucosidase enzyme, and stimulation of insulin production in RIN-m5F (rat insulinoma cell line). Furthermore, we investigated its postprandial antihyperglycemic effect in healthy volunteer. If the aromatic pandan leaf extract could help lower blood sugar, it is possible that we may use it as an alternative medicine or supplement for the treatment of diabetes.
MATERIALS AND METHODS
Reagents and chemicals
The insulin-secreting beta-cell line, RINm5F, was obtained from American Type Culture Collection, Manassas, VA; ATCC numbers: CRL-11169. Ethanol, dimethtyl sulfoxide (DMSO), α-glucosidase from rat intestinal powder, sodium phosphate monobasic, sodium phosphate diabasic, maltose, sucrose, PGO enzyme, o-dianisidine dihydrochloride, acarbose, fetal bovine serum (FBS), krebs-ringer bicarbonate buffer (KRB), sodium bicarbonate, β-nicotinamide adenine dinucleotide phosphate sodium salt, diaphorase from Clostridium kluyveri, resazurin sodium salt, 2-deoxy-d glucose, sodium hydroxide, C, N-diphenyl-N’-4,5-dimethyl thiazoly 2 tetrazolium bromide (MTT) formazan powder, hydrochloric acid, and house serum were obtained from Sigma-Aldrich Co., USA. Dulbecco's Modified Eagle Medium/high glucose medium, RPMI 1640 medium, penicillin-streptomycin solution, and phosphate buffered saline were obtained from thermo scientific hyclone. sodium potassium tartate, HEPES free acid, triethanolamine (TEA) hydrochloride, potassium chloride, calcium chloride, magnesium sulfate, metformin were purchased from Merck Co., Germany. 2-deoxy-glucose-6-phosphate sodium salt was purchased from Santa Cruz biotechnology Inc., USA. High range rat insulin ELISA kit was purchased from Mercodia, Sweden. All other reagents used were of the ACS grade available.
Participants
A total of 30 healthy participants (15 males, 15 females) aged 18-25 years old without a history of diabetes or other diseases was recruited from the staff and students of Faculty of Allied Health Sciences, Chulalongkorn University, Thailand. All participants were screened for diabetes using fasting plasma glucose concentration of 3.89-5.55 mmol/l as criteria to recruit healthy volunteers into the study. This study was approved by the Ethics Review Committee for Research Involving Human Research Subjects, Health Science Group, Chulalongkorn University and written informed consents were obtained from all participants.
Plant material
Aromatic pandan leaves used in this research have been harvested from Siri Ruckhachati Nature Park Nakhon Pathom Province. The scientific name is PA Roxb. in family Pandanaceae. The voucher specimen is BCU 013409 and was approved by Professor Dr Thaweesak Bunkoed from the Department of Botany, Faculty of Sciences, Chulalongkorn University. The collection record was kept at the herbarium of the Botany Department. Fresh aromatic pandan leaves were used for extraction in this study.
Preparation of plant extract
Pandan leaves were cut into small pieces (1.0-1.5 cm), washed with distilled water, and air – dried for 5 days. The extracts were prepared by soaking 30 g of dried PA leaf powder in 300 ml, 90°C water for 15 min (PA tea). Air-dried powdered leaves were also extracted with 100% ethanol (1 g: 10 ml) at 37°C by maceration method for 48 h. The ethanolic extracts were filtered with Whatman filter paper no. 1. The process was repeated for 3 times, and the solvent was removed by evaporation. Stock solution (100 mg/ml) of the extract was prepared in DMSO, and stored at −20°C until use.
Postprandial antihyperglycemic effect of Pandanus amaryllifolius tea in human
The study was designed as a before/after experiment using a standard OGTT to help evaluate antihyperglycemic activity. The “before” or control group was a group of 30 healthy volunteers (15 female, 15 male) who were subjected to OGTT without PA tea consumption. The “after” or treated group was the same group of subjects who were subjected to OGTT in combination with PA tea consumption, PA tea was prepared by mixing 30 g of PA powder in 0.3 l of 90°C distilled water for 15 min. The participants were asked to drink PA tea (treated group) or hot water (control group) 15 min after glucose loading by drinking glucose solution (75 g glucose in 0.3 l water). Plasma glucose level was measured at 0, 30, 60, and 120 min after the oral glucose loading. Plasma glucose concentration was determined by glucose oxidase method.[10] Before and after test were conducted on a separate day with at least one week interval.
Cell culture
The RINm5F, rat pancreatic beta cell line (ATCC), was cultured under 5% CO2/95% air at 37°C in the RPMI-1640 medium (10% v/v) containing 11.2 mM glucose and 2 mmol/l L-glutamine supplemented with 10% FBS, 100 U/ml penicillin. All experiments were performed using RINm5F cells between the 30th and 40th passages. Cells were plated 3 days before each experiment and in a 6-multiwell plate at a density of 5 × 105 cells per well in the presence of glucose (15 mM).
Cell viability test using C, N-diphenyl-N’-4,5-dimethyl thiazoly 2 tetrazolium bromide assay
RINm5F cells (1 × 104 cells/well) were cultured in a 96-well microplate in RPMI-1640 medium containing 10% (v/v) heat-inactivated FBS supplemented with 100 U/ml penicillin at 37°C in 5% CO2. Cells were washed twice with phosphate buffer saline and cultured in medium containing various concentrations of PA extract (various concentration in DMSO) for another 24 h. MTT assay was performed and modified as described by Mosmann, 1983.[11] After 24 h, cells were incubated with MTT (Sigma, St Louis, MO) for 4 h at 37°C under 5% CO2. Plates were the centrifuge at 2000 rpm for 20 min. All the supernatants were removed. The formazan crystals in each well were dissolved in DMSO. The amount of purple formazan was measured in a spectrophotometer at 540 nm. Cell viability was expressed as a percentage of cell viability using the following formula.
% Cell viability = 100 × [(Atc − Ab)/(Auc − Ab)]
Where Atc is absorbance of treated cell, Ab is absorbance of blank and Auc is absorbance of untreated cell. Serum-free medium was used as a control. Cells treated with 0.01, 0.1% DMSO were used as solvent controls. Wells with no cells were used as blanks. Assays were repeated in three times. Data were expressed as mean ± standard error of the mean (SEM).
Determination of insulin secretion
One day before an insulin release experiment, the glucose concentration in the medium was reduced to 5 mM. Cultured medium was replaced using the medium with KRB buffer, pH 7.4, containing 3 mM glucose. After washing cells twice with KRB containing 0.1% bovine serum albumin, insulin secretion assay was performed in 2 ml of the same buffer solution in the presence or absence of PA extract (1 mg in 1 ml of 100% DMSO) and incubated with RINm5F cells for 3 h at 37°C. Insulin secretion was measured, using cells treated with 0.01% DMSO alone as negative controls. After 3 h of incubation at 37°C, the solution was collected and centrifuged at 25°C, 200 g for 20 min. The supernatant was collected for insulin measurement. Insulin concentrations were measured by high range rat insulin ELISA kit. Data were expressed as the fold increase of two independent experiments.
Determination of alpha-glucosidase inhibitory activity
Sucrose was dissolved 0.05 ml in 120 mM in 0.1M potassium phosphate buffer (pH 7, 0.2 ml) and was mixed with 0.01 ml of PA extracts (1-5 mg/ml) in DMSO. Maltose was 0.07 ml of 37 mM in 0.1M potassium phosphate buffer (pH 7, 0.2 ml) incubated at 37°C for 5 min with rat intestinal powder (Sigma, St Louis, MO) that contained alpha-glucosidase enzyme. Acarbose and DMSO were used as positive and negative control, respectively. After incubated at 37°C for 15 min, the reaction was stopped by addition of boiling water followed by 10 min incubation. Alpha-glucosidase activity was measured at 450 nm, and the inhibitory activity was calculated by the following formula: Enzyme inhibition (%) =100 × (1-[absorbance of the treatment group/absorbance of control]).
Statistical analysis
Data were expressed as mean ± standard deviation (SEM) from three independent experiments. Data analysis was performed using Student's t-test or pair t-test. A P ≤ 0.05 was considered as statistically significance.
RESULTS
Postprandial antihyperglycemic effect of Pandanus amaryllifolius tea in human
The antihyperglycemic effect of water extracts of PA Roxb. (PA tea) in the human were performed in 30 healthy participants, using a standard OGTT test. The participants consumed PA tea (0.1 g/ml), 15 min after glucose loading. The plasma glucose was determined by glucose-oxidase method. As shown in Figure 1, results showed that the average postprandial plasma glucose concentration peaks in treated (“after”) group (6.16 ± 0.79 mmol/l) was significantly lower than that in control (“before”) group (6.94 ± 0.98 mmol/l: P <0.001) suggesting that PA tea can effectively decreased postprandial blood sugar.
Figure 1.
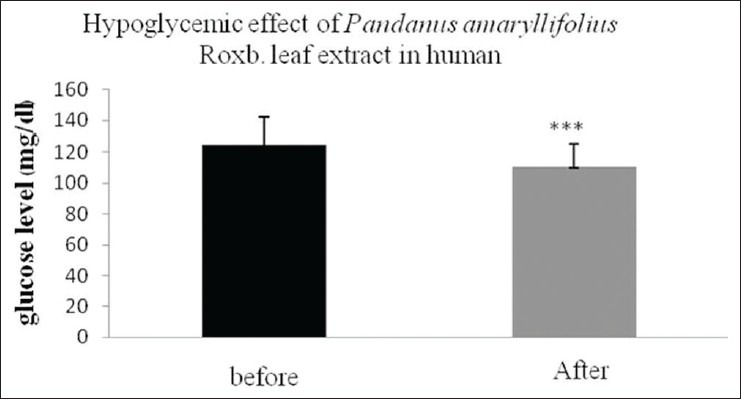
The effect of Pandanus amaryllifolius Roxb. tea in human. Thirty participants were treated with PA tea (0.1 g/ml), 15 min after glucose loaded. Data were shown as means ± standard error of the mean of triple determinations. (***P < 0.001)
Cell viability test by C, N-diphenyl-N’-4,5-dimethyl thiazoly 2 tetrazolium bromide assay
To determine cytotoxicity of PA crude extracts, RINm5F rat insulinoma cells were incubated with different concentrations of distilled water and ethanolic PA extracts (from 0 to 100 μg/ml) for 24 h. MTT assay was performed to test for cell viability. As shown in Figure 2, the data indicated that aqueous extract of PA at all concentrations from 0 to 100 μg/ml, had little to effect on cell viability with more than 80% of viable cells as compared to that of control (DMSO). Ethanolic PA extract treatment at the concentrations of 0 to 25 μg/ml showed more than 80% cell viability [Figure 3]. Thus, PA ethanolic extract at concentrations of 0 to 25 μg/ml of were selected for insulin secretion stimulation assays.
Figure 2.
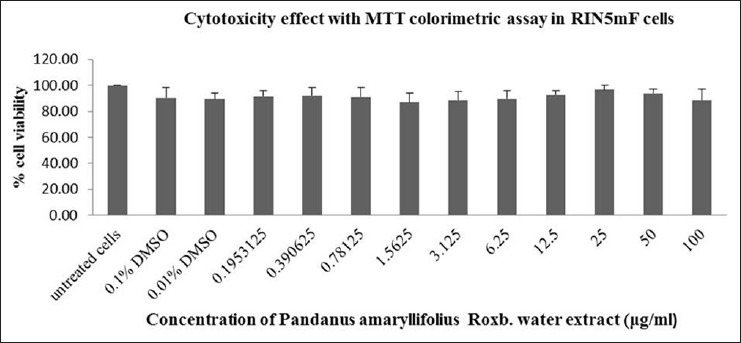
Cytotoxicity test of Pandanus amaryllifolius aqueous extract on RINm5F cells. The PA aqueous at all concentrations from 0 to 100 μg/ml, showed little to no effect on cell viability up to 80% of viable cells after treatment as compared to that of control (dimethtyl sulfoxide)
Figure 3.
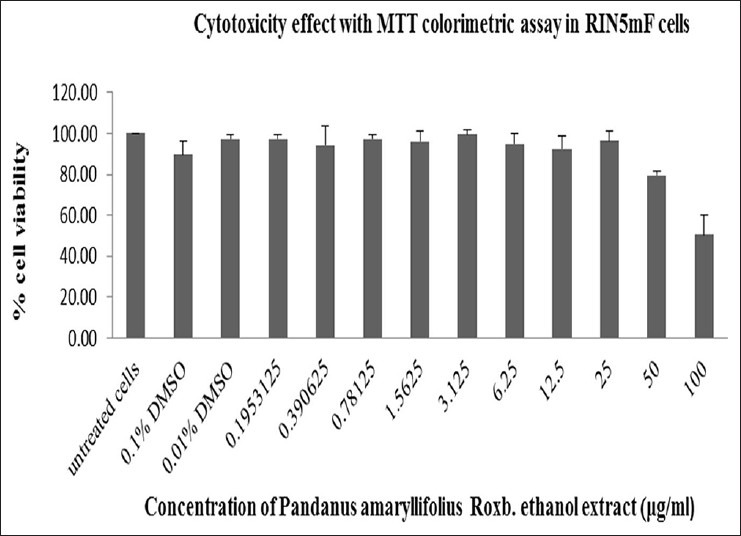
Cytotoxicity test of Pandanus amaryllifolius (PA) ethanolic extract on RINm5F cells. PA ethanolic extract at all concentrations from 0 to 25 μg/ml, showed little to no effect on cell viability up to 80% of viable cells after treatment as compared to that of control dimethtyl sulfoxide
Effects of Pandanus amaryllifolius aqueous and ethanolic extracts on insulin secretion
To demonstrate the effect of PA extracts on insulin secretion, RINm5F, rat insulinoma cells were incubated with PA extracts for 4 h. Insulin concentrations in the supernatants were quantified by ELISA test kit as described in Materials and Methods. Data showed that both aqueous and ethanolic extracts of PA leaf can dose-dependently stimulate insulin secretion in RINm5F cells [Figure 4].
Figure 4.
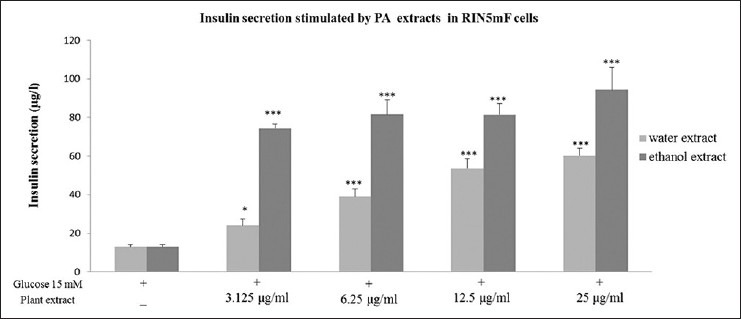
The effect of Pandanus amaryllifolius aqueous and ethanolic extracts on stimulation of insulin secretion in RINm5F cells. Cells were treated with various concentrations of PA extracts and insulin concentrations in culture medium were quantified by ELISA. Data were expressed as means ± standard error of the mean from triplicate wells. (*P < 0.05, ***P < 0.001)
Effects of Pandanus amaryllifolius aqueous and ethanolic extracts on inhibition of alpha-glucosidase activity
To study the effect of PA extract on alpha-glucosidase enzyme activity, PA extract was added into alpha-glucosidase activity assay as described in Materials and Methods. Sucrase and maltase were used as examples of alpha-glucosidase enzymes in these studies [Figures 5 and 6]. Acrabose and DMSO were used as positive and negative control, respectively. We found that both PA aqueous and ethanolic extracts could dose-dependently inhibit alpha-glucosidase enzyme activity.
Figure 5.
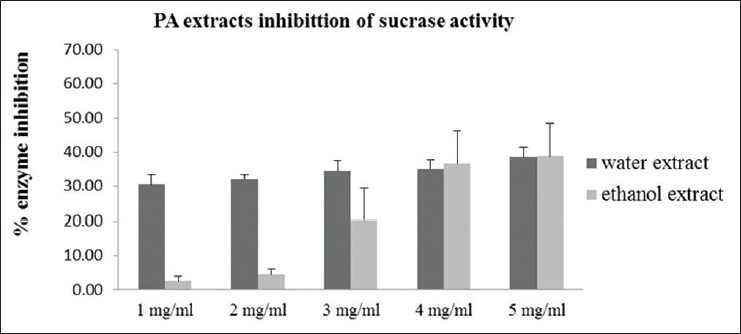
Effect of Pandanus amaryllifolius aqueous and ethanolic extracts at 1-5 mg/ml on sucrase activity. Enzyme inhibition (%) = 100 × (1-[absorbance of treatment group/absorbance of control])
Figure 6.
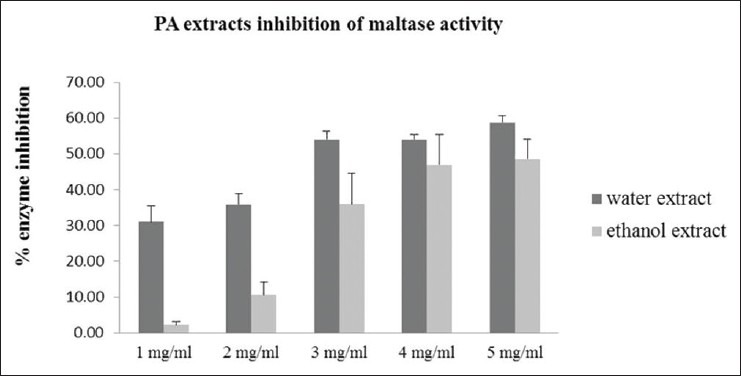
Effect of Pandanus amaryllifolius aqueous and ethanolic extracts at 1-5 mg/ml on maltase activity. Enzyme inhibition (%) = 100 × (1-[absorbance of treatment group/absorbance of control])
DISCUSSION
The potential uses of PA Roxb. leaf extract as a natural antihyperglycemic agent was investigated in this study. Our data demonstrated that PA tea (30 g in 300 ml boiling water) could significantly reduce postprandial blood glucose levels in healthy participants (P < 0.001) by average of 16.7%. Sasidharan and co-workers also found that combination of ethanolic extract of Carica papaya and Pandanus amaryfollius leaf could reduce blood sugar levels in straptozotocin-induced diabetic mice.[12] Similar results were obtained in a study conducted by Peungvicha et al. using only Pandanus odorus Ridl. root aqueous extract on normal rats, however, the antihyperglycemic mechanism remained unclear.[7,8] Two possible antihyperglycemic mechanisms were investigated in this study. The α-glucosidase enzyme (EC 3.2.1.20) is an enzyme which digests starch and disaccharides to glucose. We found that the α-glucosidase enzyme, such as maltase and sucrase, activity was effectively inhibited by PA extract suggesting that PA extract could prevent the hydrolysis of carbohydrates into monosaccharide and absorbed through the intestinal. These data indicated that PA extracts might suppress an increase in postpandrial blood sugar through inhibition of alpha-glucosidase activity. The stimulation of insulin secretion from insulinotropic rat cells (RINm5F) by PA extract was also observed in this study. PA aqueous and ethanolic extracts could significantly stimulate insulin secretion of rat beta cells in a dose-dependent manner. Our results were in agreement with results obtained in Peungvicha's study using 4-hydroxibenzoic acid derived from P. odorus root extract to stimulate insulin secretion in diabetic rats.
Pandanus amaryllifolius Roxb. left extract contained several compounds that act as a natural antioxidant and antidiabetic agents, such as essential oils, tocopherols, tocotrienols, alkaloid fatty acids, esters non-specific lipid transfer protein, and carotinoids and flavonoids.[13,14] PA leaf extract demonstrated higher antioxidant activity than that of root extract. The major flavonoid in plant is qurcetin, which belongs to a flavonol class. Tadera et al. found that quercetin could inhibit α-glucosidase enzyme by acting as a noncompetitive inhibitor.[15] Youl et al. demonstrated that quercetin could stimulate insulin secretion from the pancreas by activation of ERK ½ pathway resulting in Ca2+ influx into cells, or inhibition of sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) pump resulting in Ca2+ release from cells.[16] Strobel et al. and Bazuine et al. found that six flavonoid compounds; apigenin, luteolin, kaempferol, quercetin, genistein, and fisetin could inhibit glucose uptake in mouse fat cells.[17,18] In a future study, pure flavonol compounds in PA leaf extracts should be investigated to further identify key ingredients that possess antihyperglycemic activity.
CONCLUSION
We demonstrated that PA Roxb. extracts could reduce postprandial blood glucose, stimulate insulin secretion from rat pancreatic beta cell line and inhibit alpha glycosidase enzyme activity. Our study showed that PA Roxb. leaf has a potential to serve as a natural source of antihyperglycemic agents.
ACKNOWLEDGMENT
The 90th anniversary of Chulalongkorn university fund (Ratchadaphiseksomphot Endowment Fund).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Honeycutt AA, Boyle JP, Broglio KR, Thompson TJ, Hoerger TJ, Geiss LS, et al. A dynamic Markov model for forecasting diabetes prevalence in the United States through 2050. Health Care Manag Sci. 2003;6:155–64. doi: 10.1023/a:1024467522972. [DOI] [PubMed] [Google Scholar]
- 3.Rathmann W, Giani G. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:2568–9. doi: 10.2337/diacare.27.10.2568. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Program: Baseline characteristics of the randomized cohort. The Diabetes Prevention Program Research Group. Diabetes Care. 2000;23:1619–29. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheen AJ. Antidiabetic agents in subjects with mild dysglycaemia: Prevention or early treatment of type 2 diabetes? Diabetes Metab. 2007;33:3–12. doi: 10.1016/j.diabet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–94. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 7.Peungvicha P, Thirawarapan SS, Watanabe H. Possible mechanism of hypoglycemic effect of 4-hydroxybenzoic acid, a constituent of Pandanus odorus root. Jpn J Pharmacol. 1998;78:395–8. doi: 10.1254/jjp.78.395. [DOI] [PubMed] [Google Scholar]
- 8.Peungvicha P, Temsiririrkkul R, Prasain JK, Tezuka Y, Kadota S, Thirawarapan SS, et al. 4-Hydroxybenzoic acid: A hypoglycemic constituent of aqueous extract of Pandanus odorus root. J Ethnopharmacol. 1998;62:79–84. doi: 10.1016/s0378-8741(98)00061-0. [DOI] [PubMed] [Google Scholar]
- 9.Peungvicha P. Toxicity and Phytochemical Screening of Water extract of Pandanus Odorus Ridl. The Japanese Society for Promotion of Science (JSPS) National Research Council of Thailand (NRCT) 1992 [Google Scholar]
- 10.Raba J, Mottola HA. Glucose oxidase as an analytical reagent. Crit Rev Anal Chem. 1995;25:1–42. [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Sasidharan S, Sumathi V, Jegathambigai NR, Latha LY. Antihyperglycaemic effects of ethanol extracts of Carica papaya and Pandanus amaryfollius leaf in streptozotocin-induced diabetic mice. Nat Prod Res. 2011;25:1982–7. doi: 10.1080/14786419.2010.523703. [DOI] [PubMed] [Google Scholar]
- 13.Van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–63. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 14.Nor FM, Mohamed S, Idris NA, Ismail R. Antioxidative properties of Pandanus amaryllifolius leaf extracts in accelerated oxidation and deep frying studies. Food Chem. 2008;110:319–27. doi: 10.1016/j.foodchem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol (Tokyo) 2006;52:149–53. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- 16.Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic ß-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol. 2010;161:799–814. doi: 10.1111/j.1476-5381.2010.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strobel P, Allard C, Perez-Acle T, Calderon R, Aldunate R, Leighton F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem J. 2005;386:471–8. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazuine M, van den Broek PJ, Maassen JA. Genistein directly inhibits GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;326:511–4. doi: 10.1016/j.bbrc.2004.11.055. [DOI] [PubMed] [Google Scholar]


