Abstract
Background:
Bupleurum L. (Aspiaceae) species are used as herbal remedy in Chinese traditional medicine.
Objective:
The aim was to investigate the flavonoids in three annual European Bupleurum species, including B. baldense, B. affine and B. flavum, and to test their antioxidant and possible hepatoprotective effects.
Materials and Methods:
Flavonoids from the methanol-aqueous extracts were quantified by solid-phase extraction-high-performance liquid chromatography. Bupleurum extracts (1–220 mg/ml) were tested for their antioxidant activity in DPPH and ABTS assays, as well as on isolated liver rat microsomes. In vitro hepatoprotective activity of B. flavum flavonoid (BFF) mixture and rutin, and narcissin, isolated from the same mixture, were evaluated on carbon tetrachloride (CCl4) and tert-butyl hydroperoxide (t-BuOOH) toxicity models in isolated rat hepatocytes.
Results:
Narcissin was the dominant flavonol glycoside in B. flavum being present at 24.21 ± 0.19 mg/g, whilst the highest content of rutin (28.63 ± 1.57 mg/g) was found in B. baldense. B. flavum possessed the strongest DPPH (IC50 22.12 μg/ml) and ABTS (IC50 118.15 μg/ml) activity. At a concentration 1 mg/ml of BFF (rutin 197.58 mg/g, narcissin 75.74 mg/g), a stronger antioxidant effect in microsomes was evidenced in comparison with silymarin, rutin and narcissin. The hepatoprotective effect of BFF significantly reduced the elevated levels of lactate dehydrogenase and malondialdehyde, and ameliorated glutathione, being most active in t-BuOOH-induced injury model when compared with CCl4 toxicity (P < 0.001).
Conclusion:
In BFF, synergism of rutin and narcissin could be responsible for stronger protection against mitochondrial induced oxidative stress.
Keywords: Antioxidant activity, Bupleurum, flavonoids, hepatocytes, microsomes, solid-phase extraction-high-performance liquid chromatography
INTRODUCTION
Bupleuri radix (roots of Bupleurum L. spp. [Apiaceae]) is one of the most frequently used herbs in Chinese herbal medicine.[1] Ashour and Wink (2011) reviewed the chemistry and pharmacology of the genus Bupleurum.[2] Saponins (the so-called saikosaponins) and polyphenols from Bupleurum spp. are thought to be potential hepatoprotective agents against acute and chronic hepatic injury. For instance, hepatoprotective and antioxidant properties of herbal prescriptions formulated with B. falcatum, B. kaoi, B. scorzonerifolium roots have been shown on carbon tetrachloride- (CCl4), d-galactosamine-, and lipopolysaccharide-induced toxicity in rats.[3,4,5] In addition to the reduction of hepatic enzymes to the normal levels, a significant inhibition of lipid peroxidation in the liver was observed.[6] Other studies have reported scavenging activity of aerial parts from B. kaoi,[7] B. rigidum and B. fruticescens[8] against DPPH, ABTS and superoxide anion radicals.
In the last few years, the studies of in vitro scavenging activity have gained importance because flavonoids are a major class of secondary metabolites present in all species of the genus Bupleurum.[9,10,11] Most flavonoids in the genus are derivatives of the flavonol aglycones kaempferol, isorhamnetin or quercetin.[2] Recently, few quantitative high-performance liquid chromatography-ultraviolet (HPLC-UV) and UPLC-photodiode array methods have been established for simultaneous determination of flavonoids in the aerial parts of several Bupleurum species for quality assessment of the raw materials of traditional Chinese medicines.[10,12,13] However, there are only a few studies with respect to the flavonoid content of annual European Bupleurum species and the antioxidant activity of these taxa.[8,14] A previous study of the aerial parts of B. flavum, a native plant of the Eastern Mediterranean area, led to the isolation of five lupane-type triterpenoids, one lignan, and eight flavonoids.[15]
Based on all these findings, we aimed at investigating the flavonoid variability in three European Bupleurum species, including B. affine Sadler, B. baldense Turra and B. flavum, using a solid-phase extraction-HPLC (SPE-HPLC) method. The radical scavenging activity of hydromethanolic extracts from the assayed species was evaluated by DPPH and ABTS methods.
In the present study, a potential hepatoprotective effect of B. flavum flavonoid (BFF) mixture and the major flavonoids (rutin and narcissin), isolated from the same mixture, on isolated microsomes, CCl4- and tert-butyl hydroperoxide- (t-BuOOH) induced cell toxicity were evaluated.
MATERIALS AND METHODS
Plant material and sample preparation
Aerial parts from B. flavum, B. baldense and B. affine were collected in August 2006, respectively, from the Black Sea (Sv. Vlas region) (42°42’00” N – 27°48’00” E), Topolovgrad region (42°5’4” N – 26°20’12” E) and Radomir region (42°32’20” N – 22°57’31” E) in Bulgaria. These plants were identified by one of us (R.G.)
Air-dried powdered parts of each plant (0.1 g) were extracted with 5 ml 80% methanol (v/v) (×2) by sonication for 15 min at room temperature. Combined extracts were filtered, and the final volume was made up to 10 ml. SPE was accomplished on VARIAN Vac Elut 10 vacuum manifold using cartridges Bond Elut C18, 500 mg, 3 ml and Bond Elut CN, 500 mg, 3 ml (Varian, CA, USA). After loading samples on previously conditioned cartridges, and washing step with 3 ml phosphate buffer (20 mM KH2PO4, pH 3.2) and 3 ml of methanol-phosphate buffer (20:80, v/v), the flavonoids were eluted from the cartridges with 1.5 ml methanol The final eluates were evaporated under gentle nitrogen stream and the residue was dissolved in 1 ml methanol. Ten microlotre aliquots of samples were injected into the chromatographic system.
Air-dried powdered parts of B. flavum (25 g) were extracted with aqueous methanol (250 ml, 80%, v/v) by ultrasound assisted extraction (2 × 15 min each time) to yield the crude extract. The crude extract was fractionated by low pressure liquid chromatography over a RP C18 column (310 × 25 mm, 40–63 μm, Merck, Germany) using initial passage of water (100 ml) and then a step-gradient elution to give 8 flavonoid fractions. The binary solvent system consisted of acidified water (0.1% ortho-phosphoric acid) (solvent A [sA]) and methanol (solvent B [sB]), and separation was achieved using the following step-gradient: 20% sB (100 ml), 70% (600 ml) and 100% sB (100 ml). Fractions were concentrated in vacuo at 40°C to give rutin (fraction 4, 20 mg, 93% purity), narcissin (fraction 6, 10 mg, 95% purity) and BFF mixture (fraction 5, 25 mg).
Chemicals and reagents
The standards of flavonoids were purchased from Extrasynthese (Genay, France) except for kaempferid (Fluka, Buchs, Germany). HPLC-grade solvents and analytical-grade chemicals NaCl, KCl, NaHCO3, CaCl2.2H2O, FeSO4, D-glucose, 2,2’-dinitro-5,5’-dithiodibenzoic acid (DTNB) and CCl4 were provided by Merck (Darmstadt, Germany). HEPES, EGTA, albumin, bovine serum fraction V, minimum 98%, 2-thiobarbituric acid (4,6-dihydroxypyrimidine-2-thiol; TBA), and t-BuOOH were provided by Sigma-Aldrich (Germany). In our experiments, pentobarbital sodium (Sanofi, France), KH2PO4 (Scharlau Chemie SA, Spain), MgSO4.7H2O (Fluka AG, Germany), trichloroacetic acid (TCA), ascorbinic acid and glycerol (Valerus, Bulgaria), and lactate dehydrogenase (LDH) kit (Randox, UK) were used.
Chromatographic equipment and conditions
Chromatographic analyses were performed on a Varian (Walnut Creek, CA, USA) apparatus equipped with a tertiary pump model 9012, a UV-VIS detector model 9050, and a Rheodyne injector with a 10 μl sample loop. The chromatograms were recorded at 360 nm. All data were acquired and processed with Varian Star Chromatography software (version 4.5) (Varian, CA, USA). The separation was carried out with a Luna C18 column (150 × 4.6 mm i.d.; 5 μm) (Phenomenex, USA), fitted with a precolumn (30 × 4.6 mm i.d.) dry packed with Perisorb RP-18 (30–40 μm) (Merck, Germany) and periodically changed.
The binary solvent system consisted of sA: (aqueous phosphoric acid, 0.1%) and sB: (acetonitrile with 0.1% ortho-phosphoric acid). Gradient program was performed as follows: 0 min-10% sB; 10 min-20% sB; 25 min-30% sB; 40 min-40% sB; 45 min-40% sB; 60 min-60% sB and then return to the initial conditions in 5 min. The solvents were filtered through Millipore (Watford, Ireland) 0.45 μm filters and degassed in an ultrasonic bath prior to use. The flow rate was 1 ml/min. The oven temperature was set at 35°C.
Quantitative high-performance liquid chromatography analysis and analytical performance
The quantification of flavonoids was carried out using an external standard method. Because of the similar molecular structure, the responses of narcissin were related to rutin, assuming the responses at 360 nm to be equal. The concentrations of astragalin and isorhamnetin 3-glucoside were estimated using the isoquercitrin calibration curve; concentrations of luteolin, kaempferol and isorhamnetin were estimated using the quercetin calibration curve. The amounts of astragalin, isorhamnetin 3-glucoside, luteolin, kaempferol and isorhamnetin are thus relative and not absolute. External standard calibrations were established on seven data points covering the concentration range 0.001–1 mg/ml for rutin, 0.004–0.4 mg/ml for isoquercitrin, and 0.005–0.5 mg/ml for quercetin. The stock standard solutions of appropriate concentration were prepared in methanol and were stored at 4°C in the dark. The working standard solutions of appropriate concentration were prepared by diluting the stock standard solutions with methanol.
High-performance liquid chromatography analyses were performed in triplicate for each concentration and the peak area was detected at 360 nm according to the UV absorption maxima of the compounds. Calibration curve was constructed from peak areas versus analyte concentrations. Slope, intercept, and other statistics of calibration lines were calculated with linear regression program using the Analytik-Software STL statistics program (Leer, Germany). The regression equations were: y = 1.34.107x + 8604, r2 = 0.9999 (rutin); y = 1.57.107x + 44071, r2 = 0.9993 (isoquercitrin), and y = 2.27.107x + 48900, r2 = 0.9995 (quercetin).
Peaks of the studied flavonoids were assigned in the HPLC chromatograms by comparison of individual peak retention times with those of authentic reference standards, as well as by co-chromatography spiking the samples with reference compounds. For each sample, the complete assay procedure was carried out in triplicate, and standard deviation was calculated.
The repeatability was established by injecting the standard solution of rutin, isoquercitrin and quercetin (0.1 mg/ml) 6 times. The reproducibility was estimated over 10 days by three injections per day of the same solutions. The limits of detection (LOD) and limits of quantification (LOQ) were calculated according to ICH recommendation.[16] They were based on a standard deviation of the regression line of specific calibration curve and its slope, using analyte concentrations in the range of LOD and LOQ solutions.
The recovery of the analyte was evaluated by applying the entire SPE procedure to a control plant matrix (B. flavum) that had been spiked with a standard solution of rutin, isoquercitrin and quercetin, and measured in triplicate. The percentage recovery was determined as described by ICH recommendation.[16]
The accuracy of the overall method was assessed analyzing analytes (rutin, isoquercitrin, and quercetin) added to the control plant matrix and tested in triplicate. The obtained peak areas were corrected using the values recorded for the control matrix, and the amounts of standards were determined from the corresponding calibration curves. The accuracy of the method (expressed as a percentage) was calculated by dividing the deviation of the mean concentrations found from the nominal value by the nominal value of the analyte.[17]
Antioxidant assays
Free radicals scavenging activities were measured by using DPPH and ABTS methods with slight modifications.[18] The inhibitory effect of different concentrations of extracts (10–220 μg/ml) on DPPH and ABTS was measured by recording the absorbance of the reaction mixture at 517 and 734 nm, respectively. IC50 values of samples were determined.[19]
Protective effects of flavonoids on toxicity models in vitro
Animals
Animals were purchased from the National Breeding Center, Sofia, Bulgaria. At least 7 days of acclimatization were allowed before the commencement of the study. The health was regularly monitored by a veterinary physician. The vivarium (certificate of registration of farm No. 0072/01.08.2007) was inspected by the Bulgarian Drug Agency in order to check the husbandry conditions (No. A-11-1081/03.11.2011). All performed procedures were approved by the Institutional Animal Care Committee and made according Ordinance No. 15/2006 for humaneness behavior to experimental animals.
Isolation of liver microsomes
Liver is perfused with 1.15% KCl and homogenized with four volumes of ice-cold 0.1 M potassium phosphate buffer, pH = 7,4. The liver homogenate was centrifuged at 9 000 × g for 30 min at 4°C and the resulting postmitochondrial fraction (S9) was centrifuged again at 105 000 × g for 60 min at 4°C. The microsomal pellets were re-suspended in 0.1 M potassium phosphate buffer, pH = 7.4, containing 20% glycerol. Aliquots of liver microsomes were stored at − 70°C until use.[20] The content of microsomal protein was determined according to the method of Lowry using bovine serum albumin as a standard.[21]
FeSO4/ascorbinic acid-induced lipid peroxidation in vitro
As a system, in which metabolic activation may not be required in the production of lipid peroxide, 20 μM FeSO4 and 500 μM ascorbinic acid were added directly into rat liver microsomes and incubated for 20 min at 37°C.[18]
Lipid peroxidation in microsomes
After incubation of microsomes (1 mg/ml) with the compounds, we added to the microsomes 1 ml 25% (w/v) TCA and 1 ml 0.67% 2-TBA. The mixture is heated at 100°C for 20 min. The absorbance was measured at 535 nm, and the amount of malondialdehyde (MDA) was calculated using a molar extinction coefficient of 1.56 × 105/M/ cm. The calculations were made by using the formula – MDA nmol/mg protein = E × 12.8.[22]
Isolation and incubation of hepatocytes
An optimized in situ liver perfusion using less reagents, and shorter time of cell isolation was performed.[23] The method resulted in the higher amount of live and metabolically active hepatocytes.
The cell viability was measured by using trypan blue (0.05%) as a reagent.[24] Initial viability averaged 89–90%.
Lactate dehydrogenase release
Lactate dehydrogenase release in isolated rat hepatocytes was measured by using LDH kit. The calculations were made by using the formula – LDH μmol/min/mill cells = E × 1.34.[25]
Glutathione depletion
The content of reduced glutathione (GSH) was measured spectrophotometrically at 412 nm by using DTNB. The calculations were made by using the formula– GSH nmol/mill cells = E × 187.21.[24]
Malondialdehyde assay
The production of MDA was measured by method of Fau et al. by using 2-TBA. The calculations were made by using the formula – MDA nmol/mill cells = E × 1.4.[24]
Statistical analysis
Statistical analysis was performed using statistical program “MEDCALC.” Results are expressed as mean ± SEM for 6 experiments. The significance of the data was assessed using the nonparametric Mann–Whitney test. Values of P ≤ 0.05; P ≤ 0.01 and P ≤ 0.001 were considered statistically significant. Three parallel samples were used.
RESULTS
Quantitative determination of flavonoids by solid-phase extraction-high-performance liquid chromatography
The extraction efficiency was tested with solvents containing different percentages of methanol in water (50 and 80%, v/v), 80% methanol in aqueous phosphoric acid (0.1%) and pure methanol. It was found that 80% methanol in water was the most efficient for B. flavum extract and was, therefore, the extraction solvent used in this study.
A method for purification and isolation of flavonoids from aerial parts of the studied Bupleurum species was developed by SPE. We examined the effect of three eluents, methanol, 75% methanol or tetrahydrofuran, on analytical recovery. The optimum elution of the flavonol glycosides was achieved with pure methanol, and it was used in all of the following experiments.
The quantification of the main compounds in the flavonoid mixture isolated by SPE was performed using an improved HPLC-UV method. Based on the common approach, HPLC analysis of flavonoids from three Bupleurum species were performed with mobile phase composed of acetonitrile and water (0.1% phosphoric acid), and with UV detection at 360 nm.[26]
For triplicate analysis of both standards and plant samples, RSDs of the retention times were ≤0.34% for the determined compounds (n = 6). The proposed method was found to be linear in the studied concentration ranges of rutin, isoquercitrin, quercetin. The RSDs of the repeatability and the reproducibility were estimated to be ≤2.47% and ≤2.87%, respectively. The LODs and LOQs were 1.2 μg/ml and 3.5 μg/ml (rutin), 0.7 μg/ml and 2.2 μg/ml (isoquercitrin), 0.5 μg/ml and 1.5 μg/ml (quercetin), respectively. Based on the above results, the accuracy and recovery of the overall method were assessed employing the selected SPE conditions. The accuracy values varied between ± 1.93% (rutin) to ± 6.75 (isoquercitrin) indicating good agreement between spiked and determined concentrations. Mean recoveries of the studied flavonoids in the spiked control samples were 98.88 ± 2.07% (rutin), 104.22 ± 1.45% (isoquercitrin) and 104.02 ± 1.54% (quercetin) (RSD <2.09%).
It should be noted that the results of the overall method are acceptable.[27]
Typical HPLC profiles of Bupleurum species are presented in Figure 1. The content of flavonoids in the assayed samples is shown in Table 1.
Figure 1.
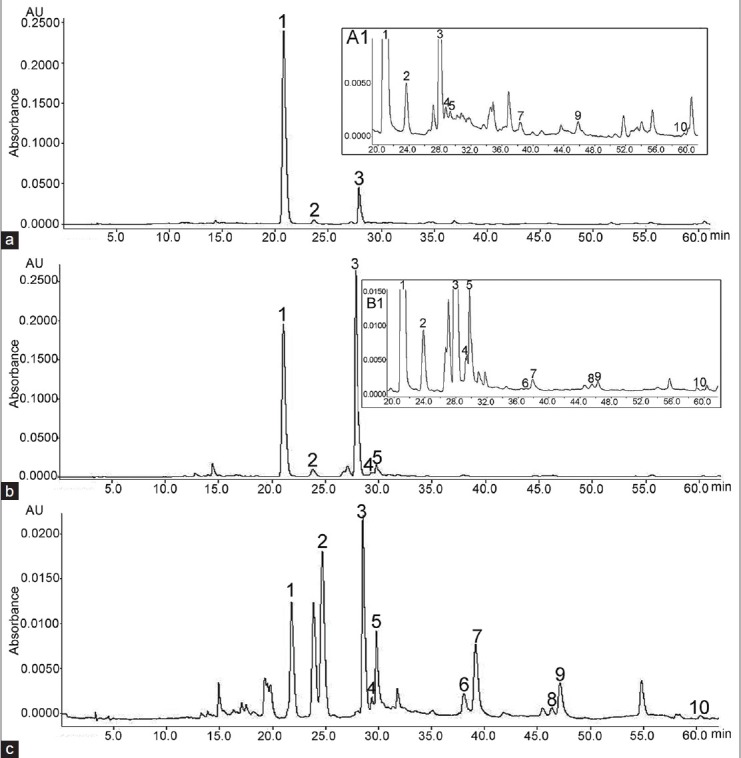
High-performance liquid chromatography chromatograms of the assayed Bupleurum species after solid-phase extraction: (a) B. baldense (a1 – the same chromatogram from 20 to 60 min); (b) B. flavum (b1 – the same chromatogram from 20 to 60 min) and (c) B. affine. Key to peaks identities: 1 – Rutin; 2 – Isoquercitrin; 3 – Narcissin; 4 – Astragalin; 5 – Isorhamnetin 3-glucoside; 6 – Luteolin; 7 – Quercetin; 8 – Kaempferol; 9 – Isorhamnetin; 10 – Kaempferid (for chromatographic protocol see Chromatographic equipment and conditions)
Table 1.
Content of flavonoids in aerial parts of assayed Bupleurum species (mg/g dry weight) (n=3)
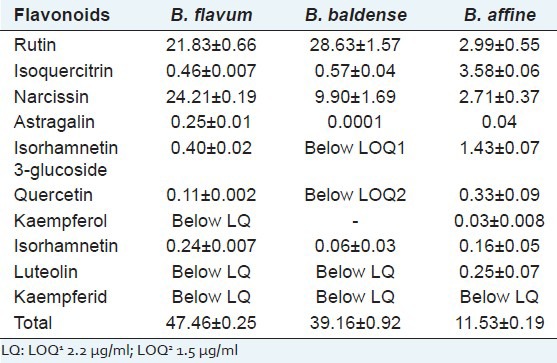
With respect to the flavonol glycosides, rutin was present in the highest amount in B. baldense, followed by B. flavum, while its content was considerably lower in B. affine [Table 1]. Narcissin was the dominant flavonol glycoside in B. flavum being present at 24.21 ± 0.19 mg/g [Figure 1b]. The content of the isoquercitrin was substantially lower; astragalin and isorhamnetin 3-glucoside (as isoquercitrin equivalents) were presented in small concentrations or below the LOQ in both B. flavum and B. baldense. However, in B. affine isorhamnetin 3-glucoside occurred in relatively higher concentration [Table 1 and Figure 1c]. In particular, the highest content of aglycones (as quercetin equivalents) was found in B. affine. B. flavum aerial parts contained an appreciable total amount of the studied flavonoids 47.46 ± 0.25 mg/g. Chromatographic purification of B. flavum crude extract resulted in the isolation of two major flavonoids. The identity of isolated rutin and narcissin was confirmed by HPLC and the purity was assessed to be 93% (rutin) and 95% (narcissin). In addition, flavonoid mixture, consisted of rutin 197.58 mg/g and narcissin 75.74 mg/g (as rutin equivalents) was isolated from B. flavum crude extract.
Antioxydant activity of crude Bupleurum extracts
DPPH and ABTS activities of the studied Bupleurum extracts were investigated, and the IC50 values (expressed as μg/ml) are presented in Table 2.
Table 2.
Antioxidant activity of assayed Bupleurum species
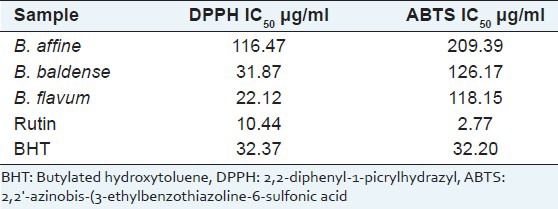
B. flavum demonstrated stronger DPPH and ABTS radical scavenging activity compared to the other Bupleurum species with IC50 values of 22.12 μg/ml and 118.15 μg/ml, respectively. However, the antioxidant activity of all tested species was less effective than pure rutin. Correlation coefficients (r2) between the total flavonoid content of Bupleurum extracts and DPPH and ABTS radical scavenging activities were 0.9918 and 0.9898, respectively.
Protective effects of flavonoids from Bupleurum flavum on toxicity models in vitro
Effect of narcissin, rutin and Bupleurum flavum flavonoid on malondialdehyde activity in isolated microsomes
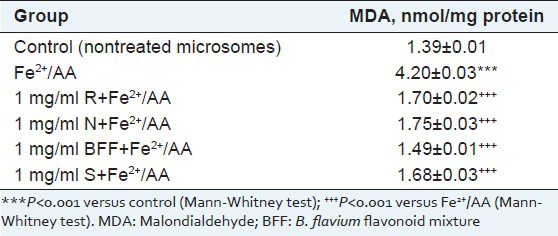
Microsomes incubation with Fe2+/AA, resulted in statistically significant increase of the amount of MDA with 202% vs. control. In nonenzyme-induced lipid peroxidation model, pretreatment with BFF, rutin and narcissin at concentration 1 mg/ml significantly reduced lipid damage by 65, 60 and 58%, respectively, as compared to the toxic agent (Fe2+/AA) [Table 3]. At the same concentration, silymarin, used as a control, lowered MDA formation by 60%.
Table 3.
Effect of rutin (1 mg/ml) (R), narcissin (1 mg/ml) (N), B. flavum flavonoid mixture (1 mg/ml) (BFF) and silymarin (1 mg/ml) (S) in nonenzyme-induced (Fe2+/AA) lipid peroxidation in isolated rat liver microsomes
Effect of narcissin, rutin and Bupleurum flavum flavonoid on markers of carbon tetrachloride-induced hepatic injury
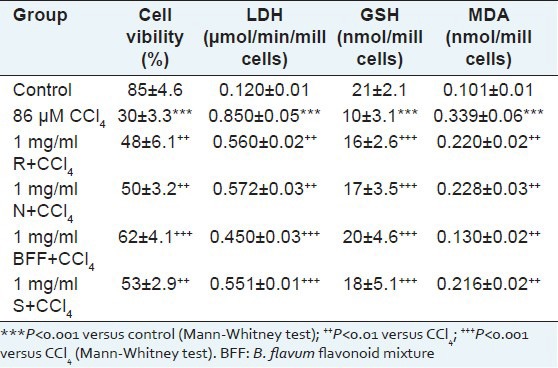
Hepatocytes incubation with CCl4 resulted in statistically significant reduction of cell viability by 65%, increased LDH activity by 608%, depletion of cell GSH by 52% and increased lipid peroxidation by 236%, compared with the control.
After preincubation of hepatocytes with 1 mg/ml of individual flavonoids, the Trypan blue exclusion test showed a cell viability preservation by 60 and 67% when rutin and narcissin were used, respectively [Table 4]. BFF seems to be the most active (P < 0.001) with 107% viability preservation while silymarin exhibited activity comparable with that of pure flavonoids (77%). Rutin, narcissin and silymarin significantly lowered LDH and MDA enzymes activities when compared to the toxicant (P < 0.01). BFF showed the highest activity: LDH and MDA were decreased by 47 and 62% (P < 0.001 and P < 0.01), respectively. Compensative increase of GSH was observed after preincubation with CCl4. BFF exerted a higher protective effect (depletion is preserved by 100%) when compared with silymarin (80%).
Table 4.
Effect of rutin (1 mg/ml) (R), narcissin (1 mg/ml) (N), B. flavum flavonoid mixture (1 mg/ml) (BFF) and silymarin (1 mg/ml) (S) in CCl4-induced hepatotoxicity on cell viability, LDH activity, GSH depletion and MDA level in isolated rat hepatocytes
Effect of narcissin, rutin and Bupleurum flavum flavonoid on markers of tert-butyl hydroperoxide-induced oxidative stress
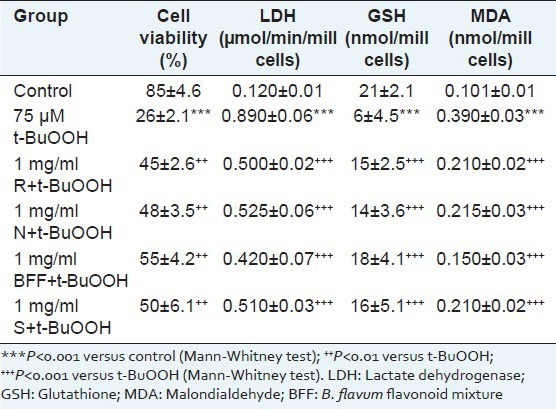
Similar experiments were conducted to test (individually) the activities of BFF and studied compounds against t-BuOOH-induced oxidative stress in isolated rat hepatocyteses. The toxic effects of t-BuOOH on hepatocytes are stronger than the toxic effects of CCl4: significant reduction of cell viability by 69%, increased LDH activity by 642%, depletion of cell GSH by 71% and increased lipid peroxidation by 286%, compared to the control. There was a dramatic increase in the enzyme level of LDH and MDA in t-BuOOH treated cells. Cell viability preservation with BFF was found to be 112% while narcissin and rutin had similar effect (P < 0.01) [Table 5]. The levels of LDH, GSH and MDA in cells subjected to the different treatment are shown in Table 4. Pretreatment with narcissin and rutin attenuated the increase in LDH and MDA activities, but results were comparable to that of silymarin. After incubation of cells with BFF, GSH was dramatically increased by 200% when compared to t-BuOOH treated cells (P < 0.001). Narcissin and rutin exerted hepatoprotective effect as evidenced by enhance of GSH enzyme activity (by 133 and 150%) being less potent than silymarin (167%).
Table 5.
Effect of rutin (1 mg/ml) (R), narcissin (1 mg/ml) (N), B. flavum flavonoid mixture (1 mg/ml) (BFF) and silymarin (1 mg/ml) (S) in t-BuOOH-induced oxidative stress on cell viability, LDH activity, GSH depletion and MDA level in isolated rat hepatocytes
DISCUSSION
In the present study, we show for the first time that extracts from B. baldense and B. affine possess antioxidant activity and BFF mixture, narcissin, and rutin, isolated from the same mixture, are potent hepatoprotective agents in sub-cellular and cellular in vitro systems.
Bupleurum extracts are characterized by means of their high-performance liquid chromatography flavonoid profiles
In this study, the content of flavonoids in three annual European Bupleurum species, including B. flavum, B. baldense and B. affine, is reported. HPLC flavonoid profiles of B. baldense and B. affine were characterized for the first time. In comparison with HPLC methods for the analysis of flavonol derivatives in Bupleurum species as reported above, the SPE-HPLC method optimized in this study allowed a good recovery of the compounds of interest.
In keeping with the reports of Zhang et al., 2010, our results indicated that B. baldense and B. flavum aerial parts contained significant quantities of rutin.[10] Several differences were observed in the qualitative and quantitative pattern of flavonoids in Chinese and European Bupleurum species. The contents of individual flavonoids reported here were generally higher than those reported by Zhang et al., 2010.[10] The main finding of this study is that the Bulgarian B. flavum has a higher total content of assayed compounds and higher combined levels of rutin and narcissin compared to the other species.
Our SPE-HPLC analysis revealed the presence of higher amounts of rutin [Table 3] than those reported for Chinese species (up to 0.40 mg/g for B. chinense and B. longicaule).[10] The contents of isorhamnetin and quercetin were in the same order of magnitude as previously reported (up to 0.70 and 0.35 mg/g, respectively in B. yunnanense).[10] In the present study, narcissin, calculated as rutin equivalents, was the dominant flavonoid in the B. flavum extract.
Recently, acacetin 7-rutinoside (linarin) and apigenin 6, 8-di-C-β-D-glucopyranoside (vicenin-2), both flavon glycosides, have proved to be useful chemotaxonomic markers for B. chinense.[9] In contrast, our HPLC analysis revealed that B. flavum is different with a high yield of flavonol glycoside narcissin. B. affine was noticeably separated by the highest content of isoquercitrin. At present, there are no data on the quantitative determination of narcissin in Bupleurum species.
Antioxidant activity of Bupleurum species and in vitro hepatoprotective activity of Bupleurum flavum
To test the biological effects of Bupleurum extracts, we used DPPH and ABTS assays as well as isolated microsomes and hepatocytes. In vitro systems play an important role for the investigation of xenobiotic biotransformation and reveal the possible mechanisms of toxic stress and its prevention.
In the present study, the evaluation of Bupleurum extracts for antioxidant activity was carried out. Previous investigations revealed DPPH radical scavenging activity of hexane extracts from B. lancifolium with IC50 435 mg/ml (leaves) and 135 mg/ml (seeds), in comparison with ascorbic acid (IC50 27 mg/ml).[28] Results for DPPH assays of ethanol root extracts from endemic Bupleurum species (B. sulphureum, B. lycaonicum, B. turcicum, B. heldreichii, B. pauciradiatum) ranged from 57.37 ± 2.67 μg/ml to 443 ± 2.76 μg ml.[29] According to the results presented here, B. flavum extract demonstrated stronger antioxidant activity than previously studied Bupleurum species probably due to the differences in the methods used for extraction and the high levels of flavonoids. However, the highest DPPH and ABTS activity was presented by rutin.
One of the most suitable sub-cellular in vitro systems for investigation of drug metabolism is isolated microsomes.
The microsomal fraction, which is prepared by differential centrifugation, contents fragments from the endoplasmic reticulum and preserve the enzyme activity, mostly cytochrome P450 enzymes. Microsomes are used as a model of lipid membrane in experiments, related to the process of lipid peroxidation.[30] Here, we show that narcissin and rutin revealed statistically significant antioxidant effect, similar to that of the classical hepatoprotector silymarin, in non-enzyme-induced lipid peroxidation in isolated microsomes. Our results demonstrated that MDA level in the samples treated with BFF, was markedly decreased, which was consistent with the observed high effect of this flavonoid mixture on the following in vitro toxicity models.
It's known that CCl4 is bioactivated by CYP2E1, as well as CYP2B1 and possibly CYP3A, to form the trichlormethyl radical (●CCl3), which initiates the chain reaction of lipid peroxidation.[31] Preincubation of the hepatocytes with the studied compounds resulted in protection against CCl4 toxicity. There are data that in human liver microsomes, some flavonoids exert inhibitory effects on CYP3A,[32] CYP1A2, CYP3A4 and CYP2E1 activity.[33]
Another model in isolated rat hepatocytes, used for oxidative stress, is t-BuOOH. The metabolism of t-BuOOH to free radicals undergoes through several steps, which lead to the production of different radicals. All these radicals caused lipid peroxidation process and decreased the level of reduced GSH – a known cell protector.[34]
Narcissin and to a lesser extent rutin, increased cell viability in both CCl4 and t-BuOOH-induced injury models [Tables 3 and 4]. Pretreatment with narcissin or rutin significantly decreased LDH and MDA. It has been reported that rutin has free radical scavenging property and inhibits the lipid peroxidation.[33] As our study showed that both narcissin and BFF lowered MDA formation as shown in Tables 4 and 5, it could be conclude that lipid peroxidation induced by free radicals was reduced. Since GSH activity did not decrease after pretreatment with BFF and individual flavonoids, it is assumed that the hepatoprotective effect of BFF or narcissin (rutin) might not decrease the activity of endogenous antioxidant enzymes. This clearly shows that both narcissin and rutin might have a direct scavenging activity or protective effect on the antioxidant enzymes. Here, we show for the first time that the narcissin revealed statistically significant antioxidant and hepatoprotective effect, similar to that of silymarin and rutin. In addition, narcissin in BFF was found to produce a synergistic effect with rutin in the protection of hepatic cells against oxidative damage. It was reported previously that rutin exerted a similar effect in CCl4 and paracetamol treated rats.[33,35] Narcissin and rutin also have direct antioxidant effects as they belong to flavonoids that contain one or more phenolic hydroxyl groups in their moiety responsible for their antioxidant activity.[36] Here, we showed for the first time the hepatoprotective effect of rutin and narcissin in t-BuOOH-induced oxidative stress in isolated rat hepatocytes. The main finding of this study is that BFF was more potent cytoprotective and antioxidant agent as compared with the control drug silymarin at an equal concentration.
Indeed, saikosaponins and polysaccharides from Bupleurum roots have been reported to display hepatoprotective activity.[2] It has also been shown that the extracts of B. kaoi roots and Chinese herbal medicines with Bupleurum roots protect the liver against CCl4 or D-galactosamine-induced hepatotoxicity.[3,4,6] Furthermore, in vitro hepatoprotective activity of B. kaoi leaf infusion was demonstrated in CCl4- and paracetamol-induced toxicity in rat liver cells.[7] However, saikosaponins are a mixture of structurally related forms with similar polarities, their separation is time consuming process and remains a challenge.[5]
Recently, a number of flavonoids have been shown to be promising agents in the protection of hepatic cells against oxidative damage.[33,35,37] Evidence came from the dramatic increase of endogenous liver antioxidant enzymes and protection effect on serum enzyme levels by rutin,[33] quercetin[37] or by synergic action of rutin and naringin.[38] In accordance with these findings it may be hypothesized that protective activity of the assayed flavonoids could be due to the influence of the metabolism of t-BuOOH on a microsomal and mitochondrial level.
CONCLUSION
A rapid validated SPE-HPLC method with excellent recovery was developed for quantitative determination of flavonoids in Bupleurum species. Here, flavonoid profiles of B. baldense and B. affine were characterized for the first time and antioxidant activity was tested. From the pharmaceutical point of view, B. flavum might be of interest, considering the high amount of flavonoids determined in its aerial parts. In addition, we showed for the first time synergistic effect of narcissin in the hepatoprotective activity of rutin from B. flavum. Our results clearly demonstrated that both narcissin and rutin could suppress hepatic injury from CCl4 and t-BuOOH application. The hepatoprotective effect of BFF and assayed pure flavonoids against CCl4-induced toxicity correlated with that against t-BuOOH-induced injury, with a stronger activity in t-BuOOH toxicity model. It is apparent that BFF, narcissin and rutin not only scavenge the free radicals but also inhibit generation of free radicals. Therefore, BFF is worth investigating for the possible development of hepatoprotective agent.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bauer R, Franz G. Modern European monographs for quality control of Chinese herbs. Planta Med. 2010;76:2004–11. doi: 10.1055/s-0030-1250532. [DOI] [PubMed] [Google Scholar]
- 2.Ashour ML, Wink M. Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol. 2011;63:305–21. doi: 10.1111/j.2042-7158.2010.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshikawa M, Murakami T, Hirano K, Inadzuki M, Ninomiya K, Matsuda H. Scorzonerosides A, B, and C, novel triterpene ologoglycosides with hepatoprotective effect from Chinese Bupleuri Radix, the roots of Bupleurum scorzonerifolium Willd. Tetrahedron Lett. 1997;38:7395–8. [Google Scholar]
- 4.Wu CY, Weng YM, Liu CT, Chuang PT, Liu SY, Tseng CY. Hepatoprotective and antioxidative properties of Chinese herbal medicine xiao-chai-hu-tang formulated with Bupleurum kaoi Liu on carbon tetrachloride–induced acute hepatotoxicity in rats. J Food Drug Anal. 2010;18:425–33. [Google Scholar]
- 5.Nakahara Y, Okawa M, Kinjo J, Nohara T. Oleanene glycosides of the aerial parts and seeds of Bupleurum falcatum and the aerial parts of Bupleurum rotundifolium, and their evaluation as anti-hepatitis agents. Chem Pharm Bull (Tokyo) 2011;59:1329–39. doi: 10.1248/cpb.59.1329. [DOI] [PubMed] [Google Scholar]
- 6.Wu CY, Liu CT, Weng YM, Tseng CY. Antioxidant activities and bioactive compounds of Xiao-Chai-Hu-Tang prepared with Bupleurum kaoi. Taiwan J Agric Chem Food Sci. 2005;43:217–23. [Google Scholar]
- 7.Liu CT, Chuang PT, Wu CY, Weng YM, Chen W, Tseng CY. Antioxidative and in vitro hepatoprotective activity of Bupleurum kaoi leaf infusion. Phytother Res. 2006;20:1003–8. doi: 10.1002/ptr.1946. [DOI] [PubMed] [Google Scholar]
- 8.Prieto JM, Ogunsina MO, Novak A, Joshi A, Kokai J, Rocha Ida C, et al. Comparative study of the in vitro bioactivities of Bupleurum rigidum and B. fruticescens. Nat Prod Commun. 2012;7:757–60. [PubMed] [Google Scholar]
- 9.Zhang TT, Zhou J, Wang Q. Flavonoids from aerial part of Bupleurum chinense DC. Biochem Syst Ecol. 2007;35:801–4. [Google Scholar]
- 10.Zhang TT, Zhou JS, Wang Q. HPLC analysis of flavonoids from the aerial parts of Bupleurum species. Chin J Nat Med. 2010;8:107–13. [Google Scholar]
- 11.Bencheraiet R, Kabouche A, Kabouche Z, Touzani R, Jay M. Flavonol 3-O-glycosides from three Algerian Bupleurum species. Rec Nat Prod. 2012;6:171–4. [Google Scholar]
- 12.Tang F, Cai G, Yuan B, Zhang Z. Determination of flavones in different origin and parts of Bupleurum smithii var. parvifoliaum by UPLC-PDA. Zhongguo Zhong Yao Za Zhi. 2010;35:2874–6. [PubMed] [Google Scholar]
- 13.Mei Z, Yang J, Fan HJ, Yang HJ, Lin F, Wang Q. Determination of flavonoids from the aerial parts of 4 species of Bupleurum plants. Chin J New Drugs. 2011;20:932–5. [Google Scholar]
- 14.Gevrenova R, Zheleva-Dimitrova D. Evaluation of flavonoid content and biological activity of Bupleurum flavum Forsk. C R Acad Bulg Sci. 2013;66:1481–6. [Google Scholar]
- 15.Pistelli L, Noccioli C, Giachi I, Dimitrova B, Gevrenova R, Morelli I, et al. Lupane-triterpenes from Bupleurum flavum. Nat Prod Res. 2005;19:783–8. doi: 10.1080/14786410500045119. [DOI] [PubMed] [Google Scholar]
- 16.International Conference on Harmonisation. Draft Guideline on Validation of Analytical Procedures: Definitions and Terminology. Fed Regist. 1995;60:11260. [Google Scholar]
- 17.Causon R. Validation of chromatographic methods in biomedical analysis. Viewpoint and discussion. J Chromatogr B Biomed Sci Appl. 1997;689:175–80. doi: 10.1016/s0378-4347(96)00297-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Chun YJ, Park JD, Kim SI, Roh JK, Jeong TC. Protection of rat liver microsomes against carbon tetrachloride-induced lipid peroxidation by red ginseng saponin through cytochrome P450 inhibition. Planta Med. 1997;63:415–8. doi: 10.1055/s-2006-957724. [DOI] [PubMed] [Google Scholar]
- 19.Zheleva-Dimitrova D, Balabanova V. Antioxidant and acetylcholinesterase inhibitory potential of Arnica montana cultivated in Bulgaria. Turk J Biol. 2012;36:732–7. [Google Scholar]
- 20.Simeonova RL, Vitcheva VB, Kondeva-Burdina MS, Krasteva IN, Nikolov SD, Mitcheva MK. Effect of purified saponin mixture from Astragalus corniculatus on enzyme- and non-enzyme-induced lipid peroxidation in liver microsomes from spontaneously hypertensive rats and normotensive rats. Phytomedicine. 2010;17:346–9. doi: 10.1016/j.phymed.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 22.Deby C, Goutier R. New perspectives on the biochemistry of superoxide anion and the efficiency of superoxide dismutases. Biochem Pharmacol. 1990;39:399–405. doi: 10.1016/0006-2952(90)90043-k. [DOI] [PubMed] [Google Scholar]
- 23.Mitcheva M, Kondeva M, Vitcheva V, Nedialkov P, Kitanov G. Effect of benzophenones from Hypericum annulatum on carbon tetrachloride-induced toxicity in freshly isolated rat hepatocytes. Redox Rep. 2006;11:3–8. doi: 10.1179/135100006X100968. [DOI] [PubMed] [Google Scholar]
- 24.Fau D, Berson A, Eugene D, Fromenty B, Fisch C, Pessayre D. Mechanism for the hepatotoxicity of the antiandrogen, nilutamide. Evidence suggesting that redox cycling of this nitroaromatic drug leads to oxidative stress in isolated hepatocytes. J Pharmacol Exp Ther. 1992;263:69–77. [PubMed] [Google Scholar]
- 25.Bergmeyer HU, Gawehn K, Grassl M. In: Methods of Enzymatic Analysis. Bergmeyer HU, editor. Vol. 1. Wienheim: Verlag Chemie; 1974. pp. 481–2. [Google Scholar]
- 26.Wang SP, Huang KJ. Determination of flavonoids by high-performance liquid chromatography and capillary electrophoresis. J Chromatogr A. 2004;1032:273–9. doi: 10.1016/j.chroma.2003.11.099. [DOI] [PubMed] [Google Scholar]
- 27.Huber L. Validation of analytical methods: Review and strategy. LC GC Mag. 1997;1:1–18. [Google Scholar]
- 28.Shafaghat A. Antioxidant, antimicrobial activities and fatty acid components of leaf and seed of Bupleurum lancifolium Hornem. J Med Plants Res. 2011;5:3758–62. [Google Scholar]
- 29.Kars G, Kars DM, Akin M, Saracoglu TH, Gunduz U. Determination of saikosaponin phenolic and podophyllotoxin contents of five endemic Bupleurum root extracts and their effects on MCF-7 cells. J Med Plant Res. 2012;6:825–32. [Google Scholar]
- 30.Zaidi SI, Agarwal R, Eichler G, Rihter BD, Kenney ME, Mukhtar H. Photodynamic effects of new silicon phthalocyanines: in vitro studies utilizing rat hepatic microsomes and human erythrocyte ghosts as model membrane sources. Photochem Photobiol. 1993;58:204–10. doi: 10.1111/j.1751-1097.1993.tb09550.x. [DOI] [PubMed] [Google Scholar]
- 31.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–36. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 32.Tsujimoto M, Horie M, Honda H, Takara K, Nishiguchi K. The structure-activity correlation on the inhibitory effects of flavonoids on cytochrome P450 3A activity. Biol Pharm Bull. 2009;32:671–6. doi: 10.1248/bpb.32.671. [DOI] [PubMed] [Google Scholar]
- 33.Khan RA, Khan MR, Sahreen S. CCl4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement Altern Med. 2012;12:178. doi: 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ollinger K, Brunk UT. Cellular injury induced by oxidative stress is mediated through lysosomal damage. Free Radic Biol Med. 1995;19:565–74. doi: 10.1016/0891-5849(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 35.Ziaee A, Zamansoltani F, Nassiri-Asl M, Hadigol T, Ghasemi M. Study of hepatoprotective effects of rutin on acetaminophen and carbon tetrachloride-induced liver injury in rats. Pharm Sci. 2011;7:35–42. [Google Scholar]
- 36.Symonowicz M, Kolanek M. Flavonoids and their properties to form chelate complexes. Biotechnol Food Sci. 2012;76:35–41. [Google Scholar]
- 37.Domitrovic R, Jakovac H, Vasiljev Marchesi V, Vladimir-Kneževic S, Cvijanovic O, Tadic Z, et al. Differential hepatoprotective mechanisms of rutin and quercetin in CCl (4)-intoxicated BALB/cN mice. Acta Pharmacol Sin. 2012;33:1260–70. doi: 10.1038/aps.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmoud M. Naringin and rutin prevent d-galactosamine-induced hepatic injury in rats via attenuation of the inflammatory cascade and oxidative stress. Eur Sci J. 2013;9:141–55. [Google Scholar]


