Abstract
The present study aimed at characterizing the oil extracted from Bertholletia excelsa H.B.K. almond, a native species from the Amazon region. Analytical methods used for oils and fats were employed through pharmacopoeia assays, AOCS (American Oil Chemists Society) standard methods as well as those recommended by ANVISA (National Health Surveillance Agency) such as acidity, peroxide value, saponification index, iodine value and refractive index, pH and relative density, and also thermoanalytical analyses (thermogravimetry, differential thermogravimetry and differential thermal analysis) as well as chromatographic analysis (gas chromatography). The characterization assessments of B. excelsa oil showed results indicating that the oil contains polyunsaturated fatty acids in large proportion. The termoanalytical tests indicated that B.excelsa oil showed thermal stability up to 220 °C, These results showed that the oil extracted from B. excelsa has acceptable characteristics and is of good quality.
Keywords: Brazil nut oil, quality control, oxidative stability
INTRODUCTION
In recent years, there has been a growing trend of using raw materials of natural origin in pharmaceuticals and cosmetics. This has caused the race to oils extracted from Amazon native plants, causing a rapid and significant expansion of the domestic and international market of these products. The oil industrialization is one of the most important activities of Brazilian agribusiness as its products are used in the food, cosmetics, and pharmaceuticals industries.[1]
The Bertholletia excelsa one of the riches of the Amazon forest is an important exporting component in the region. Its exploitation plays a key role in the socioeconomic organization of large areas of the Amazon forest extraction. Food greatly appreciated for its taste, the oil of B. excelsa also has nutritional qualities. The fruit of the chestnut tree, commonly called hedgehog, has a woody and quite hard shell. It can contain 15-24 seeds, whose size varies between 4 cm and 7 cm length. These seeds also have a very hard and rough peel and much appreciated almond.[2] The almond consists of 60-70% fat, 15-20% protein of good biologic quality, liposoluble Vitamins (A, E), and minerals (Ca, Fe, Zn, Na, K and Se).[3] The almond nut contains 13.8% palmitic acid, 8.7% stearic acid, 31.4% linoleic acid, and 45.2% oleic acid,[4] and it also contains a small quantity of palmitoleic and myristic acids.[5]
The oil extraction takes place on an industrial scale by hot or cold pressing. In lab scale, the solid-liquid extraction is commonly employed, with the aid of solvents.[6] The crude oil at room temperature is fluid with light yellow color, with a characteristic pleasant aroma and flavor.[7] The ways in which the oil extraction is processed can influence its characteristics. Factors such as exposure of the almonds to air, light, heat, exposure of the material to reagents, temperature variations, contribute to decrease the quality of the final product.
The stability of the oil may be altered by these factors which contribute to make it less stable to atmosphere changes and temperature rises.[8,9] The thermal stability of vegetable oils is a determining factor in their quality. Thermogravimetry (TG), derivative thermogravimetric curve (DTG), and differential thermal analysis (DTA) are used to evaluate the decomposition profile and the thermal stability of the oil as well as to register enthalpy variations.[10,11] These methods are widely used in quality control of vegetable oils because they quickly provide, data on the stability of the oil, regarding their thermal behavior.[12,13] These techniques have attracted great interest of researchers because of their use for the characterization of drugs both from natural and synthetic source, as well as in food products, polymers, cosmetics, and pharmaceuticals.[14,15,16]
Standard methods for the analysis of oils and fats American Oil Chemists Society (AOCS)[17] and pharmacopoeia assays,[18] such as indexes of acid, saponification, iodine, peroxides, determination of potential of hydrogen (pH), and density are important since they indicate the quality and authenticity of the oil.
The purpose of this study was to characterize the oil extracted from kernels of B. excelsa (Brazil-nut) by standard methods of analysis, chromatographic techniques and thermoanalytical analysis, to assess the quality of the oil to be used as raw material in the technology of products in pharmaceutical and cosmetic segments.
MATERIALS AND METHODS
Raw material
The B. excelsa oil was purchased from Amazon Oil Industry (Ananindeua, Pará, Brazil). The crude oil sample without the addition of preservatives, batch number OCA 001/10 whose extraction occurred on 10/01/2010 was performed by cold pressing (press expeller coupled with a kettle, 500 kg/h) from the milled almond nut. The analytical tests for the characterization of the oil were performed at the Laboratory for Research and Analysis of fuels, Chemistry Department and Drugs Quality Control Laboratory, Faculty of Pharmacy, Federal University of Pará, UFPA, from March to December 2010.
Determination of physico-chemical characteristics
Determination of relative density
Analyses were carried out by direct reading in glass hydrometer (pycnometry) at 25°C. The method used was recommended by the Brazilian Pharmacopoeia V edition.[18]
Refractive index-performed by a 2WAJ Abbe model refractometer. Method recommended by the Brazilian Pharmacopoeia.[17] It is specific for each oil, within certain limits. It is related to the degree of binding saturation, but it is affected by other factors such as free fatty acids content, oxidation, and thermal treatment.
Peroxide values-expressed in milliequivalents of active oxygen/kg of oil, calculated from the iodine released from potassium iodide, operating under the conditions indicated in the method proposed by AOCS Cd 8–53.[17] The value found is an evaluation criterion for incipient rancidity (pre-rancidity, characterized by the formation of unstable peroxides) and indicates the conservation state of fatty matter.
Iodine index
It is the measure of oils and fats unsaturation and is defined as the amount of iodine in grams calculated as the iodine absorbed by 100 g of the sample. The method AOCS Cd 1-25 was used for this determination.[17]
Saponification value
It is defined by the amount in milligrams of potassium hydroxide required to saponify 1g of oil or fat. The method recommended by the AOCS Cd 3c-91 was used.[17]
Acid value
This index is expressed as the number of milligrams of potassium hydroxide required to neutralize the free acids of 1 g of sample. Free fatty acids are determined in oil in methanol solution, by titration of sodium hydroxide solution using phenolphthalein as indicator. The content of free fatty acid was calculated based on the molecular weight of the predominant acid. The method recommended by AOCS Cd 3d-63 was used.[17]
Determination of pH
The determination of the pH was performed without previous dilution of the oil and performed by inserting the electrode directly into the samples at a temperature of 25°C ± 2°C. The potentiometer model pH 21/mv m (Hanna) was used. Pharmacopeial method recommended by the National Agency for Sanitary Surveillance.[19]
Fatty acid composition of the oil
Esterification was performed by the method Khan and Scheinmann, followed by the determination through gas chromatography, AOAC method Ce 1-62.[17] A SHIMADZU CG 14A gas chromatograph equipped with a flame ionization detector was used. A capillary column CP WA × 52 CB (25 m × 0.25 mm); DF 1 μm was used. Carrier gas flow (He) 1.0 mL/min. Injector temperature 200°C, detector temperature 250°C and the column temperature 190°C for 60 s, with increasing ratio of 2°C/min until reaching the maximum temperature of 250°C, standing there for 35 min.[20]
Thermoanalytical assays
The curves were obtained from approximately 4 mg sample using an alumina crucible and subjecting them to a temperature range between 25°C and 600°C under dynamic atmosphere (flow of 50 ml/min), heating rate 10°C/min.[21] A thermal analyzer Shimadzu TGA-50 was used.
RESULTS AND DISCUSSION
Physicochemical characteristics
Table 1 shows the results of the physico-chemical analysis of B. excelsa oil. According to the results found in this study, we observed that the refractive index and density at 25°C for B. excelsa oil were 1.466 and 0.911 g/ml, respectively, which are in accordance with the values found by Ferreira et al.[22] As these indexes vary inversely with the temperature, these results can be considered satisfactory. The iodine value was 95.0, allowing the analysis of the degree of the unsaturation of the oil and providing the amount of iodine absorbed per 100 g of the sample within the expected range. The pH value found was 3.80 which characterized as acid. The saponification number was 178.5 indicating that the oil contains polyunsaturated fatty acids in a large proportion. The value obtained in this study was lower than the one found by Ferreira et al.,[22] whose value was 198.5 mgKOH/g.
Table 1.
Physic-chemical characteristic of B. excelsa oil
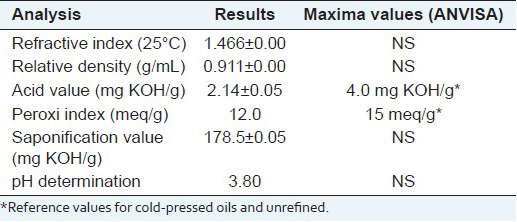
The acid and peroxide indexes are parameters that demonstrate the quality of the oil. The acid value obtained was 2.14 mgKOH/g, which is within the range of 4.0 mgKOH established by the National Agency of Sanitary Surveillance (ANVISA).[23] The acidity is due to the hydrolytic rancidity process during the storage of the oil of B. excelsa almond. The peroxide value was 12.0 mEq/kg, a value close to 15.0 meq/kg established by the ANVISA[23] and the above the limit of 10.0 mEq/kg established by the Codex Alimentarius.[24]
Peroxide value showed a slightly high value, possibly due to variations in the conditions of the oil conservation, such as changes in storage time, time for performing the assessments, as well as particularities during the extraction process, which resulted in an increased oxidation of the oil.[6]
According to Table 2, 11 different fatty acids were identified, and the predominant was the unsaturated ones such as oleic acid 38.5% (C18:1) and linoleic acid 31.26% (C18:2). Saturated fatty acids represented 25.55% of total fatty acids, mainly C16: 0-14.28%, C18: 0-10.61%; other saturated fatty acids such as C12:0 and C20:0 were also identified in trace amounts. Unsaturated fatty acids represented 70.19% of total fatty acids. C18:1 (oleic acid) - 38.50%; C18:2 (linoleic acid) - 31.26% were about 69.76% of total unsaturated fatty acids. Chromatogram of the B. excelsa oil is shown in Figure 1.
Table 2.
Fatty acids profile of B. excelsa oil
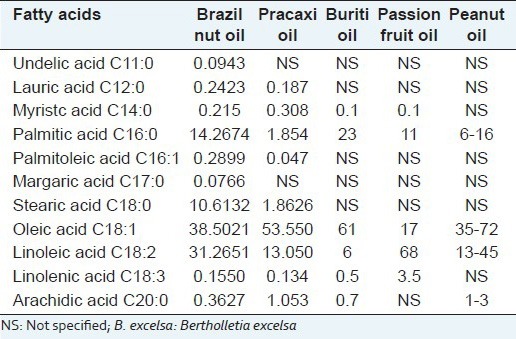
Figure 1.
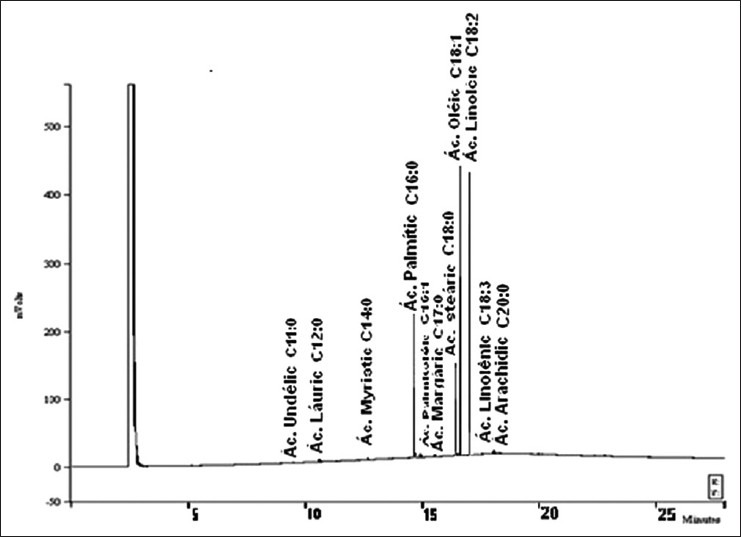
Bertholletia excelsa oil by gas chromatography.
The percentage of oleic acid found in Brazil-nut oil is two times higher than that found in passion fruit oil, however its value is lower when compared with Buriti and Pracaxi oils.[25] Oleic acid is a long chain fatty acid, consisting 18 carbon atoms containing a double bond between carbons and it is considered an essential fatty acid (Omega 9) which is involved in human metabolism and the synthesis of hormones. Other acids present in Brazil-nut oil, such as linoleic acid (Omega 6) and linolenic acid (Omega 3) are also required in the human diet since they have beneficial effects in the prevention of cardiovascular diseases (arrhythmia and blood clotting) and hyperinsulinemia.[26]
The high content of unsaturated fatty acids present in the oil, mainly oleic, and linoleic acid favor the occurrence of oxidative degradation reactions. The results are similar to the results obtained by Gonçalves et al.[27] for polyunsaturated fatty acids, such as oleic acid in the proportion of 37.42%, as well as 24.83% of saturated acids such as palmitic and stearic, with 13.15% and 10.36%, respectively. The proportion of polyunsaturated linoleic acid found in the assessment resembles the results found by Tateo[4] which was 31.4%.
According to the fatty acid composition, the sample is within the limits established for vegetable oils by the RDC 270[22] and Codex Alimentarius.[24]
Thermal analysis
Figure 2 demonstrates the TG profile of B. excelsa oil showing its thermal behavior under dynamic conditions. The TG curve shows the thermal stability of the oil to a temperature of 220°C, similar to other experiments.[6,28] The decomposition and carbonization processes occurred in three phases of the curve ending at a temperature of 580°C. At this temperature, the mass loss reached 97%. In DTG curve [Figure 2], it can be seen more clearly that the thermal decomposition of the oil occurred in three steps, with loss of initial mass at the range of approximately 220°C and then a greater loss of mass in the range of 270–580°C. The values of mass loss are shown in Table 3.
Figure 2.
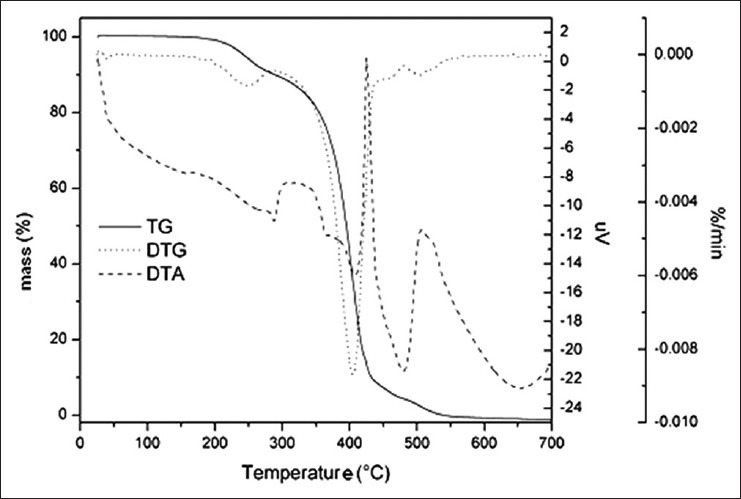
TG/DTG and DTA curves of the thermal behavior of Bertholletia excelsa oil
Table 3.
Mass loss of B. excelsa oil by thermogravimetric analysis

The first step is considered as the most important one and represents an early stage of the degradation of triglycerides, mainly composed of polyunsaturated fatty acids. At this stage, the oxidation of polyunsaturated fatty acids occurs. The loss of mass is complete at 580°C, leaving a residue of 3%, corresponding to the content of inorganic material or mineral salts. The mass loss at this stage corresponded to 97% of the original mass. High temperatures catalyze hydrolysis and oxidation reactions in oils.[29]
The graph corresponding to the DTA curve [Figure 2] shows one exothermic peak at a temperature range 400-450°C. At oxygen flow, the curves keep the standard of mass loss of <1% at the temperature range 200-220°C; in the second stage the temperature range stood at around 270-580°C with mass loss of 97% [Table 3].
The results obtained by TG/DTG and DTA in a dynamic atmosphere clearly showed the thermal behavior of the analyzed oil. By using these methods, it was possible to determine the thermal stability of this material, which is the determining factor in quality control of oils and fats during processing, storage, and industrial use.[28]
CONCLUSION
The results obtained by the physicochemical analyses carried out in B. excelsa oil are within the parameters established by the Brazilian legislation; the acidic characteristics, the high degree of unsaturation, indicated that the oil contains polyunsaturated fatty acids to a large extent. The techniques, by which assays were performed, are useful for quality control of the oil in the study and allowed obtaining satisfactory results which ratified its use as raw material for the pharmaceutical and cosmetic industries.
ACKNOWLEDGMENTS
The authors thank the Research Support Foundation of the State of Pará, the Research and Development Foundation and PROPESP/UFPA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Zague V, Polacow ML, Pires-de-Campos MS, Ribeiro MC, Leonardi GR. Evaluation of jojoba oil effect in the cutaneous regeneration. Acta Farm Bonaer. 2005;24:85–8. [Google Scholar]
- 2.Vilhena MR. 711c. São Paulo, Brazil: College Food Engineering, State University of Campinas; 2004. Science, technology and development of the Brazil-nuts economy: Industrial transformation of Brazil nut in COMARU - South of the Amapá; Region; p. 120. [Google Scholar]
- 3.Cardarelli HR, Oliveira AJ. Conservation milk Brazil nuts. Sci Agric. 2000;57:617–22. [Google Scholar]
- 4.Tateo F. La composizione acidica della matera guesta estratte daí semi di Bertholletia excelsa. Ind Alimentari Pinerolo. 1971;10:68–70. [Google Scholar]
- 5.Gutierrez EM, Regitano D’arce MA, Rauen-Miguel AM. Oxidative stability of crude oil from Brazil nuts. Ciênc Tecnol Aliment. 1997;17:22–7. [Google Scholar]
- 6.Santos OV, Corrêa NC, Soares FA, Gioielli LA, Costa CE, Lannes SC. Chemical evaluation and thermal behavior of Brazil nut oil obtained by different extraction processes. Food Res Int. 2012;47:253–8. [Google Scholar]
- 7.Pechnik E, Borges P, Siqueira R. Study on Para-nut. Arq Bras Nutr. 1950;7:7–41. [Google Scholar]
- 8.Mothé CG, Azevedo AD. São Paulo: School of Chemistry – UFRJ; 2002. Análise térmica de materiais. [Google Scholar]
- 9.Neto SH, Matos JR. Thermal analysis and comportability studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;7:367–74. [Google Scholar]
- 10.Wendlandt WW. 3rd ed. Vol. 19. New York: John Wiley and Sons; 1986. Thermal Analysis. Chemical Analysis; p. 1. [Google Scholar]
- 11.Machado LD, Matos JR. Differential thermal analysis and differential scanning calorimetry. In: Canevarolo SV Jr, editor. Techniques for characterizing polymers. São Paulo: Artliber; 2004. pp. 229–60. [Google Scholar]
- 12.Ochocka RJ, Wesolowski M, Lamparczyk H. Thermal analysis supported by principal component analysis of essential oil samples. Termochim Acta. 1990;173:199–210. [Google Scholar]
- 13.Wesolowski M, Erecinska J. Thermal analysis in quality assessment of rapeseed oils. Thermochim Acta. 1998;323:137–43. [Google Scholar]
- 14.Alves TV, Tavares EJ, Aquada FA, Negrão CA, Oliveira ME, Duarte JA, et al. Thermal analysis characterization of PAAm-co MC hydrogels. J Therm Anal Calorim. 2011;106:717–24. [Google Scholar]
- 15.Santos AL, Chierice GO, Alexander KS, Riga A. Characterization of the row essential oil eugenol extracted from Syzygium aromaticum L. J Therm Anal Calorim. 2009;5:96–821. [Google Scholar]
- 16.Rodrigues FH, Costa AM, Souza JR, Ricardo NM, Feitosa JP. PE, Brazil: Porto de Galinas; 2008. [Last accessed on 2013 Mar 14]. Physicochemical properties of the liquid from cashew nut (icc) technical and natural. 18th Brazilian Congress of Engineering and Materials Science. Available from: http//www.cbecimat.com.br/cbecimat/resumospolimericos.asp . [Google Scholar]
- 17.Oficial methods and recommended practices of the American oil Chemists Society. 27th ed. Arlington: A.O.C.S. Official Method Ce; 1993. American Oil Chemists Society; pp. 1–62. [Google Scholar]
- 18.Brazilian Pharmacopoeia. 5th ed [Published in 2010].
- 19.1st ed. Brasilia: 2004. Brazil Ministry of Health. Sanitary National Agency. Guide to Stability of Cosmetic Products; p. 52. [Google Scholar]
- 20.Atnan U, İpek S, Sinem A, İlkay EO, Murat K, Nazim S, et al. Variations in fatty acid compositions of the seed oil of Eruca sativa Mill. caused by different sowing periods and nitrogen forms. Pharmacogn Mag. 2010;6:305–8. doi: 10.4103/0973-1296.71801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barberán FA. Capillary electrophoresis: A New Technique in the analysis of plant secondary metabolites. Phytochem Anal. 1995;6:131–77. [Google Scholar]
- 22.Ferreira ES, Silveira CS, Lucien VG, Amaral AS. Characterization Physicist-Chemistry almond, residue and composition fatty acid majoritary of the oil brute of Brazil nut (Bertholletia excels) Aliment. Nutr Araraquara. 2006;17:203–8. [Google Scholar]
- 23.Section 1. Brasília, DF: Official Daily of the Federative Republic of Brazil; 2005. Sep 23, September 22, 2005. Technical Regulation for vegetable oils, fats vegetables and vegetable cream. Brazil. RDC/ANVISA/MS Nº 270. [Google Scholar]
- 24.pAI-47 Food and Agriculture Organization of the United Nations. 2nd ed. Vol. 8. Rome: World Health Organization; 1993. Codex Alimentarius-Codex Stan 33-1981-Fats, oils and related products, Joint FAO/WHO Food Standards Programme – Codexalimentarius Comission. [Google Scholar]
- 25.Costa MN, Muniz MA, Brito-Negrão CA, Costa CE, Lamarão ML, Luiz M, et al. Characterizacion of Pentaclethra macroloba oil: Thermal Stability, gas chromatography and Rancimat. J Therm Anal Calorim. 2014;115:2269–75. [Google Scholar]
- 26.Novello D, Franceschini P, Quintiliano AD. The ω-3 and ω-6 fattyacids importance for disease prevention and in the human health. Rev Salus- Guarapuava PR. 2008;2:45–54. [Google Scholar]
- 27.Gonçalves JF, Fernandes AV, Oliveira AF, Rodrigues LF, Marenco R. Primary from Brasilian Amazon tree species. Braz J. 2002;139:142. [Google Scholar]
- 28.Lima CR, Almeida MM, Zanolini C, Botelho TS, Quenca-Guillen JS. São Paulo, Brazil: 7 th Brazilian Congress on Thermal Analysis and Calorimetry; 2010. Thermal behavior study of the Brazil nut oil in cosmetic formulations using thermal analysis. [Google Scholar]
- 29.Ferrari RA, Oliveira VS, Scabio A. Biodiesel from soybean: characterization and consumption in an energy generator. Quim Nova. 2005;28:19–23. [Google Scholar]


